Abstract
Antibody mediated transplant rejection (AMR) is a major cause of long-term allograft failure, and currently available treatments are of limited efficacy for treating the disease. AMR is caused by donor specific antibodies (DSA) that bind to antigens within the transplanted organ. DSA usually activate the classical pathway of complement within the allograft, and complement activation is believed to be an important cause of tissue injury in AMR. Several new clinical assays may improve our ability to identify patients at risk of AMR. Complement inhibitory drugs have also been tested in selected patients and in small series. Better understanding of the role of complement activation in the pathogenesis of AMR will likely improve our ability to diagnose the disease and to develop novel treatments.
Keywords: Antibody mediated rejection, transplant, complement, immunoglobulin
1. Introduction
Solid organ transplantation is used to treat irreversible failure of the kidneys, heart, liver, and lungs. A primary obstacle to organ transplantation is immunologic rejection of the allograft – i.e. destruction of the organ by the recipient’s immune system. Transplant rejection can be considered to be either acute or chronic, and it is also frequently defined as T cell-mediated rejection (TCMR) or AMR. Specific criteria for diagnosing and distinguishing these various types of rejection have been developed, although there is some overlap in their mechanisms and histologic features. For example, DSA reactive against the transplanted organ are a hallmark of AMR, but are also sometimes present in patients with TCMR (Randhawa, 2015). Tissue infiltrating T cells, on the other hand, are a principal finding in TCMR, but they can also be detected within some organs with AMR. Nevertheless, TCMR and AMR are distinct entities, as evidenced by their different prognoses and responses to treatment.
AMR is caused by donor DSA reactive against polymorphic proteins that are antigenically different between the donor and recipient. DSA are usually reactive against type 1 or type 2 human leukocyte antigens (HLA) and ABO blood group antigens, but other target antigens have been identified, including major-histocompatibility-complex (MHC) class I-related chain A (MICA), angiotensin II type 1 receptor (AT1R), vimentin, and perlecan (Zhang and Reed, 2016; Zou et al., 2007). Once bound to target antigens in the allograft, DSA cause organ damage through several mechanisms, including complement activation, Fc receptor ligation, NK cell activation, and antigen cross-linking (Hidalgo et al., 2012). Complement activation by DSA bound to endothelial cell antigens in the allograft are associated with fixation of C4 to the tissue. Tissue-bound C4d provides an important biomarker of AMR, and transplant biopsies are now routinely stained for C4d.
AMR remains a significant cause of allograft failure. It accounts for up to 50% of acute rejection and more than 50% of chronic rejection episodes (Baldwin et al., 2015; Lefaucheur et al., 2013). As many as 30% of transplant patients develop AMR at some point (Chehade and Pascual, 2016). Furthermore, even though short-term transplant outcomes have improved, allograft loss after the first year has remained largely unchanged over the past 25 years (Lamb et al., 2011), and it is believed that DSA causes much of the chronic injury. The immunosuppressive drugs that are routinely used to prevent transplant rejection include corticosteroids, mycophenolate mofetil, and calcineurin inhibitors. These drugs have a strong effect on T cell function, but they are less effective at blocking humoral immunity. Once AMR is diagnosed, therefore, additional treatments are usually employed with the goal of directly removing pathogenic antibodies. This typically involves plasma exchange and IVIg. Drugs that deplete B cells (rituximab) and plasma cells (bortezomib) have also been tested, although these have not shown a clear-cut benefit in patients with acute AMR.
Given the important role that AMR likely plays in long-term allograft failure, new strategies are needed for preventing humoral immunity against the transplant, reducing the production of DSA, or directly blocking the pathogenic effects of DSA. Complement inhibitors can block some of the inflammatory effects of DSA within the allograft. Complement inhibition may also indirectly affect humoral immunity. Complement activation within the allograft increases HLA expression, for example, and deposited C3 fragments can lower the threshold for B cell signaling. Inhibition of this process, therefore, may suppress the inflammatory effects of existing DSA and also potentially reduce stimulation of B cells and plasma cells to produce additional DSA. There are published case reports and small case series in which therapeutic complement inhibitors were used for treatment AMR. Nevertheless, the role of the role for this class of drugs in the treatment of AMR remains uncertain, and several studies are ongoing to test whether this approach is effective.
2. Antibody-mediated complement activation
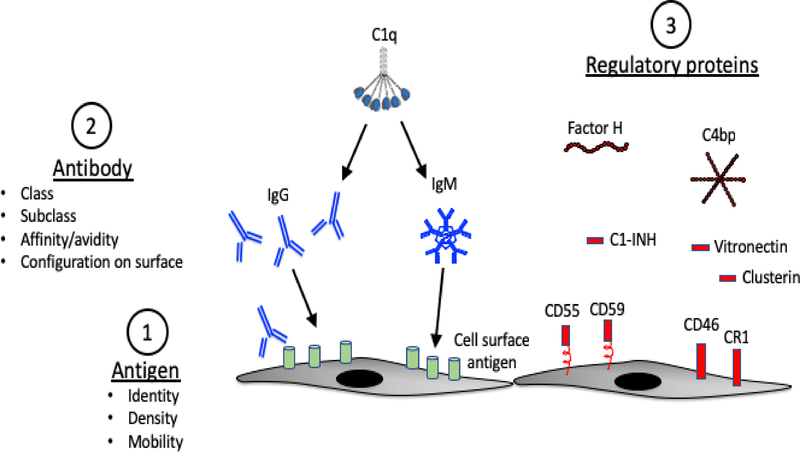
The complement cascade mediates many of the downstream effects of antibodies. Multiple different variables affect classical pathway activation, including antigen density and configuration, antibody abundance, antibody titer and isotype, and complement regulation by the target tissue. Classical pathway activation is initiated when IgG or IgM immunoglobulin binds to its cognate ligand and exposes the C1q binding site(s) within the Fc domain. C1q is a circulating protein that has six globular heads, each of which can bind to specific sites on immunoglobulin heavy chains – the Cγ2 region of IgG or the Cμ3 region of IgM (Kojouharova et al., 2010). The avidity of C1q is greatest when all six of its globular heads are engaged with immunoglobulin. Thus, C1q binds well to pentameric or hexameric IgM molecules that are complexed with target antigen, or with six IgG molecules that have bound to antigen and clustered on a target surface such that all of the Fc regions can be engaged by a single C1q (Diebolder et al., 2014). Consequently, the density of the antibody and the target antigens affects the affinity with which C1q binds to immunoglobulin and the efficiency with which the classical pathway is activated (Figure 1). Once C1q binds to IgG or IgM containing immune-complexes, the C1r and C1s serine proteases are transactivated, acquiring the ability to cleave C4 into C4a and C4b fragments. C4b becomes covalently bound to target surfaces and is subsequently cleaved to form C4d. Tissue-bound C4d is a durable marker of classical pathway activation at that location.
Figure 1. Antibody mediated complement activation.
Multiple different elements influence complement activation on a target surface. 1) Antigen density will affect the clustering of bound IgG and IgM. Mobility of the antigen on the target surface may also affect clustering of the bound immunoglobulin. 2) IgG and IgM can activate the classical pathway. Some subclasses of IgG are better activators than others. In general IgG3 > IgG1 > IgG2 >> IgG4. C1q binds efficiently to six clustered IgG molecules or to a single IgM that is complexed to antigen. 3) Complement regulatory proteins on the target surface and in plasma control complement pathway activation by the immune complexes. Alternative pathway regulators, such as factor H, decrease amplification through the alternative pathway.
IgM is the best activator of the classical pathway due to its ability to engage all of the binding sites on a C1q molecule. The subclass of IgG is also important for classical pathway activation. Among the IgG subclasses, IgG3 is the strongest activator, followed by IgG1 and IgG2. IgG4, on the other hand, does not activate the classical complement pathway (Koneczny, 2018). In transplant patients the total level of DSA in serum is often measured using clinical assays (discussed below). The mean fluorescence intensity (MFI) of the antibodies detected by DSA assays reflect antibody titer, and a higher MFI is associated with increased risk of AMR (Eskandary et al., 2017). This readout does not incorporate information about the isotype or tissue antigen density, however, and it does not always correlate with the presence of AMR. Additional antibody characteristics, such as isotype or complement binding, have been examined in the research setting, although these antibody characteristics are not usually evaluated in the clinical setting.
Another important variable that affects complement activation within tissues is the expression of complement regulatory proteins (Figure 1). Experiments have shown that in some settings antibody can activate the classical pathway and cause C4 deposition on targets, but that activation is controlled so effectively that little C3 is deposited at those sites (Goetz et al., 2018; Riley-Vargas et al., 2005). This indicates that generation of complement activation fragments downstream of the classical pathway is probably dependent upon local complement regulation. Classical pathway activation is limited by CD46 and CD55 expression on target surfaces. C1q esterase inhibitor (C1-INH) and C4 binding proteins (C4bp) are soluble proteins that help control classical pathway activation (Zipfel and Skerka, 2009). Factor H plays an important role in limiting amplification through the alternative pathway in some immune-complex diseases (Alexander et al., 2005), although a specific role for factor H in controlling AMR has not been described.
Studies in xenografts (organs transplanted between different species) have revealed that under some conditions allografts can survive for long periods of time in spite of high DSA levels, a condition referred to as “accommodation” (Dorling, 2012). Accomodation is believed to be the result of resistance of endothelial cells to antibody-mediated injury (in part through upregulation of complement regulatory proteins), altered antigen expression by the allograft, or due to changes in the recipient immune response and antibodies. It is not known how important accommodation is for protecting allografts. It is very likely that generation of DSA is only one of the factors necessary to cause AMR, however, and that other responses within the allograft or other aspects of systemic immunity are important for the development of or resistance to AMR.
3. Risk factors for AMR
The primary risk factors for developing AMR are ABO and HLA incompatibility between donor and recipient. ABO incompatibility is such a strong risk factor for hyper-acute rejection that it was previously regarded as a contraindication to transplantation. However, a growing number of centers now perform transplants across blood groups after “desensitizing” the recipient – i.e. treating the patients to lower DSA levels. The results using this approach are very good with one-year graft survival of 96% reported in a recent meta-analysis of published studies, although ABO incompatible patients did have a higher rate of AMR compared to control patients (de Weerd and Betjes, 2018).
Pre-transplant DSA is also a risk factor for acute AMR, and high DSA levels are associated with worse overall outcomes (Montgomery et al., 2018; Orandi et al., 2015). Patients can have DSA prior to transplantation as a result of prior transplants, blood transfusions, mechanical assist devices, and pregnancies. Patients are tested for DSA prior to transplantation in order to identify recipients who have high levels of antibody against specific donor HLA. Organs are generally not transplanted into patients who have high levels of DSA reactive to the donor HLA type. Desensitization protocols can lower DSA levels, thereby allowing patients to receive mismatched organs. Even with these interventions, however, DSA-positivity is associated with AMR and with shorter allograft survival (Schwaiger et al., 2016). DSA can also develop post-transplantation in patients who previously had absent or only low levels of DSA. The development of de novo DSA after transplantation can be precipitated by TCMR episodes, infections, and medication non-compliance. De novo DSA are also associated with worse transplant outcomes, consistent with a pathogenic role for the antibodies.
DSA are evaluated using several laboratory assays, described below.
3.1. Cross-matching
Cross-matching involves in vitro testing to test whether recipient serum contains antibodies that bind to donor HLA. The original test used donor lymphocytes and recipient serum. Complement dependent cytotoxicity of the lymphocytes identified lysis of target cells by anti-HLA antibodies (Patel and Terasaki, 1969). Flow cytometric cross-matching, a currently used form of this test (Schinstock et al., 2016), detects immunoglobulin bound to HLA 1 and 2 on donor T cells and B cells.
3.2. Single antigen bead assays for DSA measurement
Single antigen bead (SAB) assays utilize microbeads upon which HLA proteins are immobilized. Each bead is coated with a single HLA antigen that can be identified by a distinct combination of two fluorophores (Lachmann et al., 2013). The beads are incubated with patient serum, and immunoglobulin bound to the HLA on each bead is then detected by flow cytometry using a secondary antibody. The beads can be designed to measure reactivity to various panels of HLA class 1 and/or class 2 antigens.
3.4. Assays that measure antibody subclass
The SAB assay described above can also be modified to identify the subclass of antibody bound to the HLA antigen displayed on each bead. DSA of the IgG3 subclass seems to be associated with a greater risk of AMR and worse transplant outcomes, consistent with its ability to potently activate the classical pathway (Everly et al., 2014; Kaneku et al., 2012; Lefaucheur et al., 2016). Although DSA subclass is probably an important determinant of its pathogenicity, the subclass and the specific target antigen of a DSA may change over time. Identification of subclass is not usually tested in the clinical setting.
3.4. Complement-binding DSA assays
The single bead assay described above has been further modified so that it not only identifies DSA against specific HLA types, but also tests whether the reactive antibodies bind to complement. Assays that measure binding of DSA to C1q (A. Loupy et al., 2013) and C3d (Sicard et al., 2015) have been developed. In addition to identifying the HLA antigen specificity of a patient’s DSA, these assays may more accurately predict the antibody’s pathogenicity than the standard SAB assay. DSA that bind to either C1q of C3d are associated with a greater likelihood of developing AMR as well as with worse transplant outcomes than DSA that do not bind to these complement proteins. It is not clear, however, whether complement binding is simply a reflection of antibody subclass and titer (Schaub et al., 2014). Assays to determine whether DSA bind complement are clinically available, but they are not widely performed as part of standard clinical care.
Although all of the above assays provide useful information about circulating DSA and are of prognostic value, these tests do not provide any information about the abundance of antigen in target tissues, complement activation within tissues, or actual tissue injury. Thus, these DSA assays may reflect a patient’s risk of developing AMR, but they do not indicate active AMR in a given patient and the definitive diagnosis of AMR requires histologic examination of a biopsy. A recent study also retrospectively examined whether DSA in the absence of TCMR or AMR predicted allograft failure (Parajuli et al., 2019). In this setting, there was no difference in the incidence of rejection or allograft failure between patients who were DSA+ and DSA−. An accompanying editorial pointed out that many of the index biopsies were performed “for cause”, so that some subjects in the control group may have had undetected pathology in the allografts (Crew and Fajardo, 2019). Nevertheless, these findings underscore some of the limitations DSA as a biomarker of AMR.
4. Mechanisms of complement-mediated allograft injury
DSA-mediated complement activation on the endothelial cells of transplanted organs generates C3a, C3b, C5a, and C5b-9. Each of these molecules may contribute to allograft inflammation, although specific information about the role of each complement fragment in AMR is limited. The roles of the various complement fragments are important to identify insofar as drugs that target specific complement components (such as antibodies to C5 or small molecule inhibitors of C5a) may not prevent all of detrimental effects caused by complement activation within an allograft.
The C3a and C5a receptors are not ordinarily expressed on endothelial cells, although expression may increase in the setting of inflammation (Laumonnier et al., 2017). Furthermore, these molecules may have important effects on myeloid cell chemoattraction and lymphocyte activation, thereby affecting organ rejection (Llaudo et al., 2018). C4a is not typically thought of as an important pro-inflammatory molecule. Interestingly, however, a recent study demonstrated that C4a can activate proteinase-activated receptors (PARs)-1 and 4 on endothelial cells, raising the possibility that it may contribute to vascular activation and injury in AMR (Wang et al., 2017). Sub-lytic C5b-9 formed on the surface of endothelial cells also activates the cells, stimulating NF- κb activation and production of IL-1α and IL-8 (Brunn et al., 2006; Kilgore et al., 1997). IL-1α can then elicits production of other proinflammatory cytokines by endothelial cells in a paracrine fashion (Saadi et al., 2000). The importance of C5b-9 has also been demonstrated in xenograft models. C6 deficiency and inhibitory antibodies to C6 are protective, for example, highlighting a specific role of C5b-9 in AMR (Dorling, 2012; Suhr et al., 2007).
Non-complement-mediated mechanisms of injury are also likely important in AMR. DSA can interact with Fc receptors on myeloid cells. Cross-linking of HLA 1 and 2 by DSA on the endothelial cell surface can stimulate the cells to produce cytokines in a complement-independent fashion (Jane-Wit et al., 2013; Lion et al., 2016). Antibodies to other endothelial receptors can also serve as agonists, triggering an inflammatory response. Antibodies to AT1R, for example, activate signaling pathways in endothelial cells and induce the release of tissue factor (Dragun et al., 2005).
5. Diagnosis of AMR
Currently, the diagnosis of AMR requires a tissue biopsy. In kidney allografts, active AMR is characterized by microvascular inflammation (glomerulitis and/or peritubular capillaritis; Figure 2A) and capillary thrombosis. Inflammation of larger arteries may also be evident (“arteritis”). This is in contrast with TCMR, in which T cells infiltrate the tubulointerstitium resulting in interstitial inflammation and tubulitis. Chronically, AMR in the kidney leads to a glomerular pattern referred to as transplant glomerulopathy (TG). TG is characterized by reduplication of the glomerular basement membrane and development of “double contours.” The peritubular capillaries also show development of multi-layering in the basement membrane in biopsies from patients with chronic AMR.
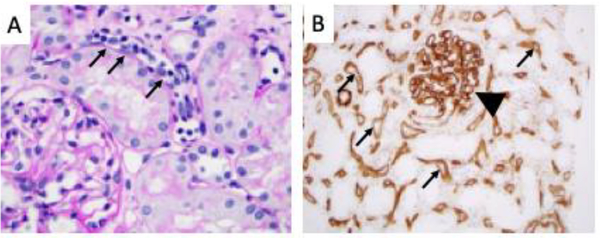
Figure 2. Histologic findings in antibody mediated rejection.
A) By light microscopy the peritubular capillaries are congested with leukocytes (“capillaritis”, small arrows). The adjacent tubules are intact and there is little infiltration of the tubules by leukocytes. B) Immunohistochemistry for C4d. C4d staining (brown) can be seen throughout the peritubular capillaries (small arrows) and within the glomerular capillaries (arrowhead).
The original consensus criteria for the diagnosis of AMR required detection of microvascular inflammation on biopsy, presence of DSA in the serum, and detection of C4d in the peritubular capillaries by immunofluorescence microscopy [Figure 2B, (Racusen et al., 2003)]. More recently the criteria were updated to include C4d-negative AMR in cases with evidence of AMR by histology (microvascular inflammation) and serologically (DSA), but with negative or minimal C4d deposition within peritubular capillaries (Haas et al., 2014; Sapir-Pichhadze et al., 2015). This change was made in response to the observation that C4d is not always detected in patients who have peritubular inflammation (A. Loupy et al., 2011; Sis et al., 2007; Sis and Halloran, 2010; Tible et al., 2013). Patients with C4d-negative AMR still generally have tissue dysfunction (which is generally what prompted the biopsy), as well as decreased allograft survival (A. Loupy et al., 2011).
C4d deposition in an allograft does not always correlate with C3 deposition. Fragments of C3 and C4 should both be fixed to target tissues, so it is not clear why there is sometimes discrepant staining for these different proteins. Possible explanations include control of complement activation at the level of C4, deposition of C3 via the alternative pathway, or technical differences in the staining methods. Immunostaining of biopsies for C3 fragments is not currently included in the diagnosis and evaluation of acute or chronic AMR. A recent study did show, however, that glomerular C3 deposition in patients with TG is associated with worse clinical outcomes (Panzer et al., 2019).
6. Treatment of AMR
Therapies for AMR can target the B cells and plasma cells that generate DSA, remove the DSA, or block complement activation downstream of the DSA. Most treatment protocols for acute AMR include multiple interventions, such as high dose corticosteroids, plasmapheresis, and IVIg. There are currently no treatments of proven benefit in chronic AMR.
6.1. Strategies to prevent production of DSA
Rituximab is a monoclonal antibody to CD20 that depletes mature B cells, although it does not affect plasma cells. Although rituximab is frequently included in treatment protocols to desensitize patients prior to transplantation or to treat acute AMR, studies testing its efficacy have been mixed (Macklin et al., 2014). Rituximab may dampen antibody production in patients who undergo plasmapheresis (Jackson et al., 2015), but it is difficult to see a specific benefit in most studies since multiple treatments are administered (Lefaucheur et al., 2009; Sautenet et al., 2016). A randomized controlled trial of IVIg and rituximab versus placebo in patients with chronic AMR did not show any benefit in either the rate of decline in kidney function or in proteinuria, although the sample size was small (n = 13 in the rituximab group and 12 in the control group (Moreso et al., 2018).
Bortezomib is a proteasome inhibitor that can induce plasma cell apoptosis. Studies have shown that bortezomib may be beneficial when added to other treatments that deplete antibodies in patients with acute AMR (Everly et al., 2008), but it does not seem to be beneficial in patients with chronic AMR (Gupta et al., 2014).
6.2. Strategies to deplete DSA
Plasmapheresis is frequently used to remove pre-existing DSA in patient with AMR, although studies of its efficacy are inconclusive (Roberts et al., 2012). The treatment is typically used in combination with therapies aimed at stopping production of new DSA. Immunoadsorption has also been used to remove the pathologic IgG (Bohmig et al., 2007). A new strategy to deplete DSA was recently tested in patients with AMR. IgG degrading enzyme of Streptococcus pyogenes (IdeS) cleaves IgG, essentially depleting the body of IgG. This agent was tested in 25 highly sensitized recipients prior to transplantation with HLA-incompatible allografts (Jordan et al., 2017). Ten of the treated patients developed AMR in spite of this treatment, although all of them subsequently responded to standard therapy.
6.3. Complement inhibition
Several case reports have suggested that eculizumab may be effective for treating AMR, leading to its evaluation in several clinical trials. In the first reported case, eculizumab was used in conjunction with plasmapheresis, rituximab, and IVIg in a patient with severe AMR (Locke et al., 2009). The treatment led to a reduction in C5b-9 deposition and improvement in renal function. This initial report was followed by several uncontrolled studies of highly sensitized patients. In a recent uncontrolled study, treatment of sensitized patients with eculizumab resulted in better outcomes at 9 weeks compared to historical controls (Glotz et al., 2019).
In another study, sensitized patients were treated with eculizumab for four weeks, and treatment continued in those patients with high DSA titers until the crossmatch became negative (Stegall et al., 2011). The addition of eculizumab was associated with a lower incidence of early AMR compared to historical controls, but it did not reduce chronic AMR or transplant glomerulopathy 1–2 years post transplantation. It is possible that longer treatment - similar what is typically done for atypical hemolytic uremic syndrome - would have produced better outcomes (Cornell et al., 2015). It is possible that DSA measurements did not detect persistent antibody within the tissue, or fluctuating levels of DSA during the follow up period.
Two subsequent phase II randomized trials of eculizumab have shown mixed results. When the drug was added to a desensitization protocol in highly sensitized patients who received kidneys from living donors the treatment did not reduce the incidence of AMR, death, or graft loss at 9 weeks (Marks et al., 2019). In a separate study, however, treatment did reduce the incidence of AMR in recipients of kidneys from living donors at one year. These latter results have been presented in abstract form and in reviews, but are not yet published (Alexandre Loupy and Lefaucheur, 2018; Montgomery et al., 2018). Eculizumab was also tested in combination with splenectomy for patients with active, severe AMR (Orandi et al., 2014). In these patients the combination of eculizumab and splenectomy appeared to be more effective than either treatment alone.
It may be that larger studies are needed to accurately assess whether eculizumab is beneficial in AMR. It is also notable that eculizumab was not effective at preventing or treating in two patients who had C4d-negative acute AMR (Burbach et al., 2014). This raises the possibility that that detection of complement-binding DSA or complement deposition within the biopsy may be useful for identifying patients most likely to benefit from complement inhibition.
C1-INH disrupts the classical pathway C1 complex, preventing classical pathway activation. Purified and recombinant forms of C1-INH have been developed as replacement therapies for patients with hereditary angioedema, and have also been tested for prevention or treatment of AMR in small trials (Vo et al., 2015). One study treated six patients who had AMR non-responsive to conventional therapy with C1-INH and IVIg (Viglietti et al., 2015). The treatment protocol was associated with an improvement in GFR and with reduced C4d deposition within the allografts. An ongoing randomized controlled trial is testing whether the addition of C1-INH to standard of care treatment improves outcomes in patients with acute AMR (ClinicalTrials.gov NCT03221842).
7. Conclusions
Improved immunosuppressive protocols have reduced the incidence of TCMR and have improved short-term outcomes after transplantation of kidneys, hearts, lungs, and livers. Unfortunately, long-term allograft survival has not shown significant improvement in recent years, and AMR is probably the most important determinant of chronic transplant failure. This may be due to fact that the immunomodulatory drugs typically used in transplant patients have limited efficacy for preventing production of DSA by B cells and plasma cells, or for blocking the downstream inflammatory effects of DSA in the transplanted organ. The complement system is probably an important mediator of AMR, providing a rationale for testing whether the addition of complement inhibitory drugs to the standard of care protocols is beneficial in patients with acute and chronic AMR. The available clinical data, however, does not shown a clear-cut benefit in AMR patients who have been treated with the available therapeutic complement inhibitors (eculizumab and C1-INH).
Testing new drugs for preventing or treating AMR is challenging. The effects of new treatments need to be evaluated on the background of multiple other interventions, including maintenance immunosuppression, plasmapheresis, IVIg, and rituximab. Furthermore, there are probably complement-dependent and complement-independent mechanisms of injury in AMR, and the balance of these factors may differ from patient to patient. A feature of the complement cascade that may be advantageous for future trial design is that complement activation generates multiple soluble and tissue-bound biomarkers. Furthermore, patients can be stratified based on additional risk factors, such as high levels of complement-binding DSA. By incorporating new complement analyses into patient evaluation and selection, future clinical trials may be able to more accurately select appropriate patients for treatment with complement inhibitory drugs and detect a response to treatment.
Highlights.
Antibody mediated transplant rejection is an important cause of tissue injury in solid organ transplants.
Antibody mediated transplant rejection is caused by donor antibodies reactive against proteins expressed in the transplant.
Donor antibodies reactive to antigens within the transplant can activate the classical pathway of complement, and C4d can often be detected in the allograft capillaries.
Complement inhibitory drugs have been tested in small cohorts of transplant recipients at risk for antibody mediated rejection, but the efficacy of this approach is unclear.
Acknowledgements
This work was supported by National Institutes of Health Grants R01DK076690 and R01DK113586 (JMT). The authors would like to thank Weixiong Zhong, MD, PhD, for his assistance with renal pathology images.
Abbreviations
- AMR
Antibody mediated transplant rejection
- DSA
donor specific antibodies
- TCMR
T cell-mediated rejection
- HLA
human leukocyte antigens
- MHC
major-histocompatibility-complex
- MICA
MHC class I-related chain A
- AT1R
angiotensin II type 1 receptor
- MFI
mean fluorescence intensity
- SAB
single antigen bead
- C1-INH
C1q esterase inhibitor
- PAR
proteinase-activated receptor
- TG
transplant glomerulopathy
Footnotes
Declaration of interest
JMT receives royalties from Alexion Pharmaceuticals, Inc. JMT is also a consultant for AdMIRx, Inc., a company developing complement inhibitors. He holds stocks and will receive royalty income from AdMIRx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander JJ, Pickering MC, Haas M, Osawe I, Quigg RJ, 2005. Complement factor h limits immune complex deposition and prevents inflammation and scarring in glomeruli of mice with chronic serum sickness. J Am Soc Nephrol 16, 52–57. [DOI] [PubMed] [Google Scholar]
- Baldwin WM 3rd, Valujskikh A, Fairchild RL, 2015. Mechanisms of antibody-mediated acute and chronic rejection of kidney allografts. Current opinion in organ transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmig GA, Wahrmann M, Regele H, Exner M, Robl B, Derfler K, Soliman T, Bauer P, Mullner M, Druml W, 2007. Immunoadsorption in severe C4d-positive acute kidney allograft rejection: a randomized controlled trial. Am J Transplant 7, 117–121. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Saadi S, Platt JL, 2006. Differential regulation of endothelial cell activation by complement and interleukin 1alpha. Circ Res 98, 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach M, Suberbielle C, Brocheriou I, Ridel C, Mesnard L, Dahan K, Rondeau E, Hertig A, 2014. Report of the inefficacy of eculizumab in two cases of severe antibody-mediated rejection of renal grafts. Transplantation 98, 1056–1059. [DOI] [PubMed] [Google Scholar]
- Chehade H, Pascual M, 2016. The Challenge of Acute Antibody-Mediated Rejection in Kidney Transplantation. Transplantation 100, 264–265. [DOI] [PubMed] [Google Scholar]
- Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD, 2015. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant 15, 1293–1302. [DOI] [PubMed] [Google Scholar]
- Crew RJ, Fajardo M, 2019. How do we interpret the presence of donor specific antibodies when there is no rejection? Kidney Int Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerd AE, Betjes MGH, 2018. ABO-Incompatible Kidney Transplant Outcomes: A Meta-Analysis. Clinical journal of the American Society of Nephrology : CJASN 13, 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, van de Winkel JG, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PW, 2014. Complement is activated by IgG hexamers assembled at the cell surface. Science 343, 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling A, 2012. Transplant accommodation--are the lessons learned from xenotransplantation pertinent for clinical allotransplantation? Am J Transplant 12, 545–553. [DOI] [PubMed] [Google Scholar]
- Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G, 2005. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352, 558–569. [DOI] [PubMed] [Google Scholar]
- Eskandary F, Bond G, Kozakowski N, Regele H, Marinova L, Wahrmann M, Kikic Z, Haslacher H, Rasoul-Rockenschaub S, Kaltenecker CC, Konig F, Hidalgo LG, Oberbauer R, Halloran PF, Bohmig GA, 2017. Diagnostic Contribution of Donor-Specific Antibody Characteristics to Uncover Late Silent Antibody-Mediated Rejection-Results of a CrossSectional Screening Study. Transplantation 101, 631–641. [DOI] [PubMed] [Google Scholar]
- Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, Roy-Chaudhury P, Govil A, Mogilishetty G, Rike AH, Cardi M, Wadih G, Tevar A, Woodle ES, 2008. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation 86, 1754–1761. [DOI] [PubMed] [Google Scholar]
- Everly MJ, Rebellato LM, Haisch CE, Briley KP, Bolin P, Kendrick WT, Kendrick SA, Morgan C, Maldonado AQ, Harland RC, Terasaki PI, 2014. Impact of IgM and IgG3 anti-HLA alloantibodies in primary renal allograft recipients. Transplantation 97, 494–501. [DOI] [PubMed] [Google Scholar]
- Glotz D, Russ G, Rostaing L, Legendre C, Tufveson G, Chadban S, Grinyo J, Mamode N, Rigotti P, Couzi L, Buchler M, Sandrini S, Dain B, Garfield M, Ogawa M, Richard T, Marks WH, group C.s., 2019. Safety and efficacy of eculizumab for the prevention of antibody-mediated rejection after deceased-donor kidney transplantation in patients with preformed donor-specific antibodies. Am J Transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz L, Laskowski J, Renner B, Pickering MC, Kulik L, Klawitter J, Stites E, Christians U, van der Vlag J, Ravichandran K, Holers VM, Thurman JM, 2018. Complement factor H protects mice from ischemic acute kidney injury but is not critical for controlling complement activation by glomerular IgM. Eur J Immunol 48, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Abu Jawdeh BG, Racusen LC, Bhasin B, Arend LJ, Trollinger B, Kraus E, Rabb H, Zachary AA, Montgomery RA, Alachkar N, 2014. Late antibody-mediated rejection in renal allografts: outcome after conventional and novel therapies. Transplantation 97, 1240–1246. [DOI] [PubMed] [Google Scholar]
- Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, DavidNeto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing, c., 2014. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14, 272–283. [DOI] [PubMed] [Google Scholar]
- Hidalgo LG, Sellares J, Sis B, Mengel M, Chang J, Halloran PF, 2012. Interpreting NK cell transcripts versus T cell transcripts in renal transplant biopsies. Am J Transplant 12, 1180–1191. [DOI] [PubMed] [Google Scholar]
- Jackson AM, Kraus ES, Orandi BJ, Segev DL, Montgomery RA, Zachary AA, 2015. A closer look at rituximab induction on HLA antibody rebound following HLA-incompatible kidney transplantation. Kidney Int 87, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane-Wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, Abrahimi P, Devalliere J, Moeckel G, Kulkarni S, Tellides G, Pober JS, 2013. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factorkappaB signaling in endothelial cells. Circulation 128, 2504–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan SC, Lorant T, Choi J, Kjellman C, Winstedt L, Bengtsson M, Zhang X, Eich T, Toyoda M, Eriksson BM, Ge S, Peng A, Jarnum S, Wood KJ, Lundgren T, Wennberg L, Backman L, Larsson E, Villicana R, Kahwaji J, Louie S, Kang A, Haas M, Nast C, Vo A, Tufveson G, 2017. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N Engl J Med 377, 442–453. [DOI] [PubMed] [Google Scholar]
- Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB, 2012. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transpl 18, 984–992. [DOI] [PubMed] [Google Scholar]
- Kilgore KS, Schmid E, Shanley TP, Flory CM, Maheswari V, Tramontini NL, Cohen H, Ward PA, Friedl HP, Warren JS, 1997. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am J Pathol 150, 2019–2031. [PMC free article] [PubMed] [Google Scholar]
- Kojouharova M, Reid K, Gadjeva M, 2010. New insights into the molecular mechanisms of classical complement activation. Mol Immunol 47, 2154–2160. [DOI] [PubMed] [Google Scholar]
- Koneczny I, 2018. A New Classification System for IgG4 Autoantibodies. Frontiers in immunology 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann N, Todorova K, Schulze H, Schonemann C, 2013. Luminex((R)) and its applications for solid organ transplantation, hematopoietic stem cell transplantation, and transfusion. Transfus Med Hemother 40, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb KE, Lodhi S, Meier-Kriesche HU, 2011. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant 11, 450–462. [DOI] [PubMed] [Google Scholar]
- Laumonnier Y, Karsten CM, Kohl J, 2017. Novel insights into the expression pattern of anaphylatoxin receptors in mice and men. Mol Immunol 89, 44–58. [DOI] [PubMed] [Google Scholar]
- Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, Verine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahousse M, Empana JP, Hill GS, Glotz D, Legendre C, Jouven X, 2013. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet 381, 313–319. [DOI] [PubMed] [Google Scholar]
- Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C, 2009. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 9, 1099–1107. [DOI] [PubMed] [Google Scholar]
- Lefaucheur C, Viglietti D, Bentlejewski C, Duong van Huyen JP, Vernerey D, Aubert O, Verine J, Jouven X, Legendre C, Glotz D, Loupy A, Zeevi A, 2016. IgG Donor-Specific Anti-Human HLA Antibody Subclasses and Kidney Allograft Antibody-Mediated Injury. J Am Soc Nephrol 27, 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion J, Taflin C, Cross AR, Robledo-Sarmiento M, Mariotto E, Savenay A, Carmagnat M, Suberbielle C, Charron D, Haziot A, Glotz D, Mooney N, 2016. HLA Class II Antibody Activation of Endothelial Cells Promotes Th17 and Disrupts Regulatory T Lymphocyte Expansion. Am J Transplant 16, 1408–1420. [DOI] [PubMed] [Google Scholar]
- Llaudo I, Fribourg M, Edward Medof M, Conde P, Ochando J, Heeger PS, 2018. C5aR1 regulates migration of suppressive myeloid cells required for costimulatory blockade-induced murine allograft survival. Am J Transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, King KE, Kraus E, Lees LM, Melancon JK, Stewart ZA, Warren DS, Zachary AA, Montgomery RA, 2009. The use of antibody to complement protein C5 for salvage treatment of severe antibodymediated rejection. Am J Transplant 9, 231–235. [DOI] [PubMed] [Google Scholar]
- Loupy A, Hill GS, Suberbielle C, Charron D, Anglicheau D, Zuber J, Timsit MO, Duong JP, Bruneval P, Vernerey D, Empana JP, Jouven X, Nochy D, Legendre CH, 2011. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant 11, 56–65. [DOI] [PubMed] [Google Scholar]
- Loupy A, Lefaucheur C, 2018. Antibody-Mediated Rejection of Solid-Organ Allografts. New England Journal of Medicine (NEJM) 379, 1150–1160. [DOI] [PubMed] [Google Scholar]
- Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Fremeaux-Bacchi V, Mejean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X, 2013. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369, 1215–1226. [DOI] [PubMed] [Google Scholar]
- Macklin PS, Morris PJ, Knight SR, 2014. A systematic review of the use of rituximab for desensitization in renal transplantation. Transplantation 98, 794–805. [DOI] [PubMed] [Google Scholar]
- Marks WH, Mamode N, Montgomery R, Stegall MD, Ratner LE, Cornell LD, Rowshani AT, Colvin RB, Dain B, Boice JA, Glotz D, Group CS, 2019. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am J Transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RA, Loupy A, Segev DL, 2018. Antibody-mediated rejection: New approaches in prevention and management. Am J Transplant 18 Suppl 3, 3–17. [DOI] [PubMed] [Google Scholar]
- Moreso F, Crespo M, Ruiz JC, Torres A, Gutierrez-Dalmau A, Osuna A, Perello M, Pascual J, Torres IB, Redondo-Pachon D, Rodrigo E, Lopez-Hoyos M, Seron D, 2018. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double-blind clinical trial. Am J Transplant 18, 927–935. [DOI] [PubMed] [Google Scholar]
- Orandi BJ, Chow EH, Hsu A, Gupta N, Van Arendonk KJ, Garonzik-Wang JM, Montgomery JR, Wickliffe C, Lonze BE, Bagnasco SM, Alachkar N, Kraus ES, Jackson AM, Montgomery RA, Segev DL, 2015. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant 15, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orandi BJ, Zachary AA, Dagher NN, Bagnasco SM, Garonzik-Wang JM, Van Arendonk KJ, Gupta N, Lonze BE, Alachkar N, Kraus ES, Desai NM, Locke JE, Racusen LC, Segev DL, Montgomery RA, 2014. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 98, 857–863. [DOI] [PubMed] [Google Scholar]
- Panzer SE, Joachim E, Parajuli S, Zhong W, Astor BC, Djamali A, 2019. Glomerular C3 Deposition Is an Independent Risk Factor for Allograft Failure in Kidney Transplant Recipients With Transplant Glomerulopathy. Kidney Int Rep 4, 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli S, Joachim E, Alagusundaramoorthy S, Aziz F, Blazel J, Garg N, Muth B, Mohamed M, R.R. R, Mandelbrot DA, Zhong W, Djamali A, 2019. Donor-Specific Antibodies in the Absence of Rejection Are Not a Risk Factor for Allograft Failure. Kidney Int Rep. Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Terasaki PI, 1969. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280, 735–739. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K, 2003. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3, 708–714. [DOI] [PubMed] [Google Scholar]
- Randhawa P, 2015. T-cell-mediated rejection of the kidney in the era of donor-specific antibodies: diagnostic challenges and clinical significance. Current opinion in organ transplantation 20, 325–332. [DOI] [PubMed] [Google Scholar]
- Riley-Vargas RC, Lanzendorf S, Atkinson JP, 2005. Targeted and restricted complement activation on acrosome-reacted spermatozoa. J Clin Invest 115, 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Jiang SH, Chadban SJ, 2012. The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation 94, 775–783. [DOI] [PubMed] [Google Scholar]
- Saadi S, Holzknecht RA, Patte CP, Platt JL, 2000. Endothelial cell activation by pore-forming structures: pivotal role for interleukin-1alpha. Circulation 101, 1867–1873. [DOI] [PubMed] [Google Scholar]
- Sapir-Pichhadze R, Curran SP, John R, Tricco AC, Uleryk E, Laupacis A, Tinckam K, Sis B, Beyene J, Logan AG, Kim SJ, 2015. A systematic review of the role of C4d in the diagnosis of acute antibody-mediated rejection. Kidney Int 87, 182–194. [DOI] [PubMed] [Google Scholar]
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, Ducloux D, Chatelet V, Moulin B, Freguin C, Hazzan M, Lang P, Legendre C, Merville P, Mourad G, Mousson C, Pouteil-Noble C, Purgus R, Rerolle JP, Sayegh J, Westeel PF, Zaoui P, Boivin H, Le Gouge A, Lebranchu Y, 2016. One-year Results of the Effects of Rituximab on Acute Antibody-Mediated Rejection in Renal Transplantation: RITUX ERAH, a Multicenter Double-blind Randomized Placebo-controlled Trial. Transplantation 100, 391–399. [DOI] [PubMed] [Google Scholar]
- Schaub S, Honger G, Koller MT, Liwski R, Amico P, 2014. Determinants of C1q binding in the single antigen bead assay. Transplantation 98, 387–393. [DOI] [PubMed] [Google Scholar]
- Schinstock CA, Gandhi MJ, Stegall MD, 2016. Interpreting Anti-HLA Antibody Testing Data: A Practical Guide for Physicians. Transplantation 100, 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger E, Eskandary F, Kozakowski N, Bond G, Kikic Z, Yoo D, Rasoul-Rockenschaub S, Oberbauer R, Bohmig GA, 2016. Deceased donor kidney transplantation across donor-specific antibody barriers: predictors of antibody-mediated rejection. Nephrol Dial Transplant 31, 1342–1351. [DOI] [PubMed] [Google Scholar]
- Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, Scoazec JY, Bachelet T, Lepreux S, Visentin J, Merville P, Fremeaux-Bacchi V, Morelon E, Taupin JL, Dubois V, Thaunat O, 2015. Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol 26, 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sis B, Campbell PM, Mueller T, Hunter C, Cockfield SM, Cruz J, Meng C, Wishart D, Solez K, Halloran PF, 2007. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant 7, 1743–1752. [DOI] [PubMed] [Google Scholar]
- Sis B, Halloran PF, 2010. Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Current opinion in organ transplantation 15, 42–48. [DOI] [PubMed] [Google Scholar]
- Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM, 2011. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11, 2405–2413. [DOI] [PubMed] [Google Scholar]
- Suhr BD, Black SM, Guzman-Paz M, Matas AJ, Dalmasso AP, 2007. Inhibition of the membrane attack complex of complement for induction of accommodation in the hamsterto-rat heart transplant model. Xenotransplantation 14, 572–579. [DOI] [PubMed] [Google Scholar]
- Tible M, Loupy A, Vernerey D, Suberbielle C, Beuscart T, Cazes A, Guillemain R, Amrein C, Pezzella V, Fabiani JN, Nochy D, Hill G, Empana JP, Jouven X, Charron D, Bruneval P, Duong Van Huyen JP, 2013. Pathologic classification of antibody-mediated rejection correlates with donor-specific antibodies and endothelial cell activation. J Heart Lung Transplant 32, 769–776. [DOI] [PubMed] [Google Scholar]
- Viglietti D, Gosset C, Loupy A, Deville L, Verine J, Zeevi A, Glotz D, Lefaucheur C, 2015. C1-Inhibitor in Acute Antibody-Mediated Rejection Non-Responsive to Conventional Therapy in Kidney Transplant Recipients: A Pilot Study. Am J Transplant 16, 1596–1603. [DOI] [PubMed] [Google Scholar]
- Vo AA, Zeevi A, Choi J, Cisneros K, Toyoda M, Kahwaji J, Peng A, Villicana R, Puliyanda D, Reinsmoen N, Haas M, Jordan SC, 2015. A phase I/II placebo-controlled trial of C1inhibitor for prevention of antibody-mediated rejection in HLA sensitized patients. Transplantation 99, 299–308. [DOI] [PubMed] [Google Scholar]
- Wang H, Ricklin D, Lambris JD, 2017. Complement-activation fragment C4a mediates effector functions by binding as untethered agonist to protease-activated receptors 1 and 4. Proc Natl Acad Sci U S A 114, 10948–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Reed EF, 2016. The importance of non-HLA antibodies in transplantation. Nature reviews. Nephrology 12, 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C, 2009. Complement regulators and inhibitory proteins. Nat Rev Immunol 9, 729–740. [DOI] [PubMed] [Google Scholar]
- Zou Y, Stastny P, Susal C, Dohler B, Opelz G, 2007. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med 357, 1293–1300. [DOI] [PubMed] [Google Scholar]