Abstract
Background and Purpose:
In this era of endovascular therapy (EVT) with early, complete recanalization and reperfusion, we have observed an even more rapid ADC normalization within the acute ischemic lesion compared to the natural history or IV-tPA-treated patient. In this study, we aimed to evaluate the effect of revascularization on ADC evolution within the core lesion in the first 24 hours in acute ischemic stroke patients.
Methods:
This retrospective study included anterior circulation acute ischemic stroke patients treated with EVT with or without intravenous tPA (IVT) from 2015–2017 compared to a consecutive cohort of IVT-only patients treated before 2015. DWI and ADC maps were used to quantify baseline core lesions. Median ADC value change and core reversal were determined at 24-hours. DWI lesion growth was measured at 24-hours and 5-days. Good clinical outcome was defined as modified Rankin Scale 0–2 at 90-days.
Results:
Twenty-five patients (50%) received IVT while the other 25 patients received EVT (50%) with or without IVT. Between these patient groups, there were no differences in age, sex, baseline NIHSS, inter-hospital transfer, or IVT rates. Thirty-two patients (64%) revascularized with 69% receiving EVT. There was a significant increase in median ADC value of the core lesion at 24-hours in patients who revascularized compared to further ADC reduction in non-revascularization patients. Revascularization patients had a significantly higher rate of good clinical outcome at 90-days, 63% versus 9% (p=0.003). Core reversal at 24-hours was significantly higher in revascularization patients, 69% versus 22% (p=0.002).
Conclusions:
ADC evolution in acute ischemic stroke patients with early, complete revascularization, now more commonly seen with EVT, is strikingly different from our historical understanding. The early ADC normalization we have observed in this setting may include a component of secondary injury and serve as a potential imaging biomarker for the development of future adjunctive therapies.
Indexing Terms: Ischemic stroke, endovascular therapy, MRI, revascularization, reperfusion, core lesion reversal
Search Terms: Ischemic stroke, endovascular therapy, MRI, revascularization, reperfusion, core lesion reversal
Introduction
Two MRI contrast mechanisms contribute to the increase in signal intensity seen on diffusion weighted imaging (DWI) of the brain following acute stroke; i) a decrease in the apparent diffusion coefficient (ADC), presumably due to cytotoxic edema1 and ii) an increase the transverse relaxation (T2) time-constant, presumably due to vasogenic edema.2 The physics of these two mechanisms are largely independent, however the biology of cerebral ischemia and the temporal progression couple the two.3, 4
At the earliest times from stroke onset, the ADC decreases with little change in T2, suggesting cytotoxic edema dominates the first minutes to hours. A substantial decrease in the ADC is believed to represent the most severely ischemic tissue, i.e. the area at greatest risk of cell death, and is the premise for delineating the “ischemic core”1, 5, 6 currently used in clinical decision making.7–10 Similarly, the lack of a significant change in T2 suggests early time from stroke onset, which is the premise for thrombolytic therapy in patients with unknown onset time.11, 12
The natural evolution of the ischemic core has been characterized as low ADC for the first 7 days with a nadir at ~3–4 days, followed by “normalization” at 7–10 days, followed by increase in ADC above that of healthy tissue.13–20 Each time-epoch suggests a different phase in the progression from acute ischemia to infarction. As time from onset increases, the probability of a change in T2 increases, presumably as water moves from the vasculature into the brain tissue and may be indicative of a compromised blood-brain barrier. During the first 24 hours, T2 begins to become elevated and contributes to increased conspicuity of the lesion on T2-weighted imaging, on T2-FLAIR imaging, and on DWI (historically referred to as “T2-shine through”). In a patient imaged 24 hours after stroke onset, it is not uncommon for the ischemic core to be conspicuously dark on ADC, and bright on DWI and T2-FLAIR.
In patients treated with IV tPA and undergoing associated accelerated reperfusion of ischemic tissue, this time course of ADC evolution has been demonstrated to occur somewhat faster with earlier normalization of ADC at 5 days (+/−2 d) instead of 7 days (+/− 6 d)..21–25 In this new era of endovascular therapy with early rapid and complete recanalization and reperfusion, we have observed an even more rapid normalization of the ADC with earlier increase in intensity compared to the natural history or IV-tPA-treated acute ischemic stroke. While the ADC of the core appears to normalize, a lesion remains conspicuous on DWI as a result of the change in T2. It remains unclear if earlier renormalization of the ADC has prognostic value. Regardless, following early recanalization, the ischemic core may instead appear normal on ADC, and bright on DWI and T2-FLAIR.
This apparent divergent behavior of the ADC, potentially as the result of early recanalization and reperfusion, has multiple ramifications to the clinical interpretation of MRI, the understanding of the pathophysiological mechanisms, and development of future therapeutic strategies. In this study, we aimed to evaluate the effect of revascularization26 on the evolution of ADC within the ischemic core lesion in the first 24 hours in acute ischemic stroke patients.
Materials and Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Patients
This retrospective study included patients with admission diagnosis of imaging-positive anterior circulation acute ischemic stroke who were either treated with endovascular therapy (EVT) with or without intravenous tPA treatment (IVT) from January 2015 to July 2017 or treated with standard IVT only prior to January 2015 at two regional stroke centers, MedStar Washington Hospital Center (Washington, DC) and Suburban Hospital (Bethesda, MD). These admission dates were selected to reflect the periods prior to and after the release of the results from the successful endovascular therapy stroke trials. Two different patient groups were utilized to provide contrast in ADC lesion reversal and reperfusion injury incidences when revascularization occurred, independent of therapy. Patients were also required to have consented to the NIH Natural History of Stroke Study. The appropriate Ethics and Institutional Review Boards (NINDS/NIH IRB for Suburban Hospital, Johns Hopkins Medicine, Bethesda, MD; and MedStar Washington Hospital Center, Washington Hospital Center, Washington, DC IRB) approved the study (). For imaging selection, all patients were required to have a sufficient quality baseline MRI obtained prior to EVT or IVT and a follow-up MRI at 24-hours. Consecutive IVT patients were selected by matching to an EVT patient using equal, or as close to as possible, age, sex, and baseline NIHSS.
Image Acquisition
Clinical 3T scanners were utilized at both hospital sites (Siemens Skyra, Siemens AG, Munich, Germany and Philips Achieva, Philips Healthcare, Best, the Netherlands) using established acute stroke imaging protocols. In brief, diffusion tensor imaging (DTI) using repetition time (TR) 4293–7800 ms, echo time (TE) 63–81 ms, 3.5- slice thickness, 12–16 directions, 40 slices, b=0 and b=1000 s/mm2 was performed to generate an isotropic map (DWI) and corresponding apparent diffusion coefficient (ADC) map. Time-of-flight magnetic resonance angiography (MRA) was performed centered on the Circle of Willis (COW), using TR 18–39 ms, TE 2.1–6.9 ms, 0.75–1.6 mm slice thickness, 86–95 slices. If no contraindication, dynamic susceptibility perfusion-weighted imaging (PWI) was performed using TR 1000–2000 ms, TE 25–45 ms, 7 mm slice thickness, 20 slices, 70–120 dynamic acquisitions. Time-to-peak (TTP) and mean transmit time (MTT) maps were calculated on the scanner. A dosage of 0.1 mmol/kg of gadolinium-based contrast agent (GBCA) was administered using a power injector for the PWI.
Large Vessel Occlusion Analysis
All the EVT patients in this study were brought to the IR suite based on clinical and\or imaging evidence of anterior circulation Large Vessel Occlusion (LVO). For this analysis, documentation of LVO by angiogram in the IR suite prior to mechanical intervention was used but also confirmed by visual evaluation of the baseline MRA COW that was obtained prior to EVT. Likewise, for the IVT patients, visual evaluation of the baseline MRA COW obtained prior to IVT was performed in this study. LVO location was documented for each patient using categories of intracranial ICA, M1, or other including extracranial ICA, M2, and M3. If no LVO was visualized on baseline MRA COW, then this was also noted.
Revascularization Analysis
Complete revascularization was defined as TICI score of 2B or 3 in the IR suite upon completion of the procedure or complete resolution of the perfusion deficit at 24-hours using the scanner-provided, delay-independent, TTP and MTT images, comparing to the baseline perfusion lesion. Tmax maps using > 6 seconds threshold, and associated volumes were generated from the PWI at baseline using Perfusion Mismatch Analyzer (PMA) ASIST-Japan software. The TTP, MTT, and Tmax maps were calculated using selection of the arterial input function and deconvolution was performed. Complete resolution of the perfusion deficit was assessed by two separate imaging readers with a third reader if necessary as tie-breaker. The consensus data was used for all analyses.
Core Lesion Analysis
An initial lesion mask was created by outlining all DWI-positive regions consistent with the acute ischemia on the baseline MRI using Mipav™, an image processing package with semi-automated segmentation tools and processes. This lesion mask was then applied to the registered ADC images to automatically threshold just the voxels contained in the DWI mask that were ≤620 μm2/s to define the core lesion.27 The core lesion mask was saved and used to generate the core lesion volume at baseline and the median signal intensity values of the ADC images at baseline and 24-hours. The percent of the baseline core lesion still ≤620 μm2/s at 24-hours was calculated to define significant core reversal. All follow-up DWI images were co-registered to the baseline DWI prior to any measurements using the optimized automatic registration in Mipav™ with options of rigid-6 degrees of freedom and trilinear interpolation. The resultant transformation matrix was then applied to co-register the follow-up ADC images. Significant reversal of the baseline core was achieved if >50% of the baseline core was >620 μm2/s at 24-hours. The volume of the DWI-positive regions at baseline, 24-hours, and 5-days were also measured.
Clinical Outcome Analysis
Early neurologic improvement (ENI) was defined as a ≥8-point decrease or a 0–1 value on the NIHSS at 24-hours. Good clinical outcome was defined as 0–2 on the modified Rankin Score (mRS) at 90-days.
Statistical Analysis
The software SPSS v19.0 was used to perform descriptor statistics for this study. Values are reported as mean (±SD) or median (interquartile range percentiles [25–75]) as noted. Nonparametric tests (Mann Whitney U or Chi-squared) were used to compare the distributions and classifications of variables as appropriate. Adjustment based on baseline median ADC value using the Bonferroni method and complete revascularization or significant ADC reversal status was performed for these comparisons.
Results
Baseline Characteristics
Fifty patients diagnosed with acute ischemic stroke of the anterior circulation were included in this study with median age of 66 years, 68% female, and median baseline NIHSS of 18. Table 1 summarizes the clinical and imaging characteristics of all patients and with patients stratified by whether revascularization was achieved by 24-hours.
Table 1.
Characteristics of all patients versus patients stratified by complete revascularization
| All patients (n=50) | Patients achieving complete revascularization (n=32) | Patients not achieving complete revascularization (n=18) | p-value | |
|---|---|---|---|---|
| Age, median [IQR] | 66 [50–74] | 64 [50–74] | 69 [50–78] | 0.944 |
| Women (%) | 34 (68%) | 22 (69%) | 13 (68%) | 0.942 |
| Transferred from outside hospital | 15 (30%) | 12 (38%) | 3 (16%) | 0.107 |
| Baseline NIHSS, median [IQR] | 18 [11–22] | 18 [7–22] | 18 [13–21] | 0.172 |
| IV tPA administered | 40 (80%) | 24 (75%) | 17 (89%) | 0.239 |
| Time Onset to IV tPA (min), median [IQR] | 148 [103–182] | 132 [83–177] | 165 [132–189] | 0.151 |
| Endovascular therapy administered | 25 (50%) | 22 (69%) | 3 (17%) | <0.001 |
| Large vessel occlusion (LVO) location on cerebral angiogram or COW MRA (%) ^ | ||||
| Intracranial ICA | 5 (10%) | 3 (9%) | 2 (12%) | 0.114 |
| MCA: M1 | 32 (65%) | 24 (75%) | 8 (47%) | |
| MCA: M2 or other | 12 (25%) | 5 (16%) | 7 (41%) | |
| Baseline ADC Core lesion (mL), with ADC ≤620 μm2/sec, median [IQR] | 18 [1–53] | 9 [1–43] | 35 [3–96] | 0.115 |
| Baseline ADC intensity value of Core lesion, median [IQR] | 485 [449–518] | 496 [456–524] | 466 [405–506] | 0.041 |
| Baseline Tmax>6 sec volume (mL), median [IQR] | 178 [110–267] | 212 [120–266] | 128 [73–272] | 0.236 |
| Percent of Core with ADC ≤620 μm2/sec at 24-hours, median [IQR] | 48% [14–86] | 33% [8–60] | 92% [62–99] | <0.001 |
| ADC intensity value of Core lesion at 24-hours, median [IQR] | 640 [468–732] | 709 [600–790] | 437 [361–560] | <0.001 |
| Significant core lesion reversal by 24-hours (%) | 26 (52%) | 22 (69%) | 4 (22%) | 0.002 |
| DWI lesion volume (mL) at 24-hours, median [IQR] | 40 [14–134] | 29 [11–66] | 79 [27–184] | 0.102 |
| Early Neurologic Improvement (ENI) at 24-hours (%) | 19/49 (38%) | 17/31 (55%) | 2 (11%) | 0.003 |
| NIHSS at Discharge | 4 [0–13] | 4 [0–10] | 8 [0–26] | 0.074 |
| DWI Lesion Volume at 5 days, median [IQR] | 43 [18–107] | 39 [15–67] | 93 [23–204] | 0.048 |
| Good clinical outcome (GCO), mRS 0–2 at 90 days | 16/35 (46%) | 15/24 (63%) | 1/11 (9%) | 0.003 |
IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; MCA, middle cerebral artery; tPA, tissue plasminogen activator; TICI, Thrombolysis in Cerebral Infarction; mRS, modified Rankin Scale
Two patients, one from each stratified group, did not have visible large vessel occlusions on cerebral angiogram or COW MRA
Twenty-five patients (50%) received IVT while the other 25 patients received EVT (50%) with or without IVT first. Overall 80% of study patients received IVT. Patients were dichotomized by revascularization at 24-hours [Table 1]. Thirty-two patients (64%) revascularized with 69% receiving EVT. Eighteen patients (36%) did not revascularize by 24-hours with only 17% receiving EVT. There were no differences in age, sex, transfer from outside hospital rates, baseline NIHSS, or IVT treatment rates. Time from onset to start of IVT did not differ between revascularization subgroups. However, EVT rates, 69% versus 17% (p<0.001), and complete recanalization solely based on TICI scores of 2b/3, 73% versus 0%, (p<0.001), were significantly higher in the patients achieving revascularization.
Large vessel occlusion location was M1 in 75% of the revascularization patients versus 47% in the non-revascularization patients. Fifty-three percent of the occlusion locations in the non-revascularization patients were intracranial ICA, extracranial ICA or M2. As noted in Table 1, there is one IVT patient who did not have a visible LVO on COW MRA. The matching EVT patient had a LVO on COW MRA but did not have a visible LVO on subsequent baseline angiogram after receiving IV tPA. There were no patients with carotid artery stenosis or intracranial stenosis based on COW MRA.
Core lesion volumes were smaller in the revascularization patients, 9mL versus 35mL, but this result did not reach significance (p=0.115). The baseline median ADC values of these core lesions were significantly different, 496 versus 466 μm2/sec (p=0.041). The baseline perfusion Tmax lesions volumes were comparable, 212mL versus 128mL (p=0.236) with a trend of more baseline mismatch, defined as Tmax volume >core lesion volume, in the revascularization patients.
Imaging Findings
At 24-hours, significant increase in median ADC value was demonstrated in the revascularization patients, see Figure 1, with median ADC of the core lesion reaching 709 μm2/sec while a further reduction in ADC was seen, 437 from 466 μm2/sec, in non-revascularization patients. The significance of the 24-hour ADC value difference (p<0.001) remained after adjustment by baseline median ADC value. Figure 2 demonstrates the difference in ADC reversal at 24-hours between patients with revascularization, via EVT or IVT, versus no revascularization. Furthermore, the percent of patients with significant core reversal at 24 hours was significantly higher in revascularization patients, 69% versus 22% (p=0.002). Revascularization patients, with their higher median ADC values and core reversal, also had less growth of their DWI lesion volumes at 24-hours, 29mL versus 79mL (p=0.102), compared to non-revascularization patients, though this difference did not reach significance. Median DWI lesion volume at 5-days was significantly smaller in the revascularization patients compared to non-revascularization patients, 39mL versus 93mL (p=0.048). The significance of the smaller DWI volume at 5-days (p=0.002) remained after adjustment by baseline median ADC value.
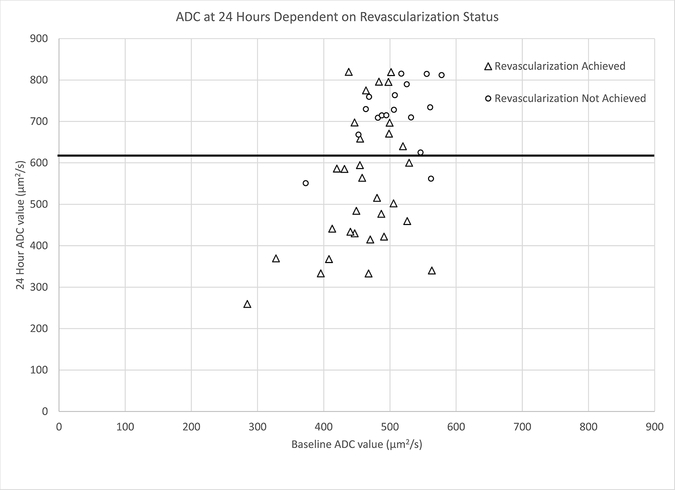
Figure 1.
Plot of baseline ADC value (μm2/s) versus 24-hour ADC value (μm2/s) - ADC at 24 hours is dependent on revascularization status.
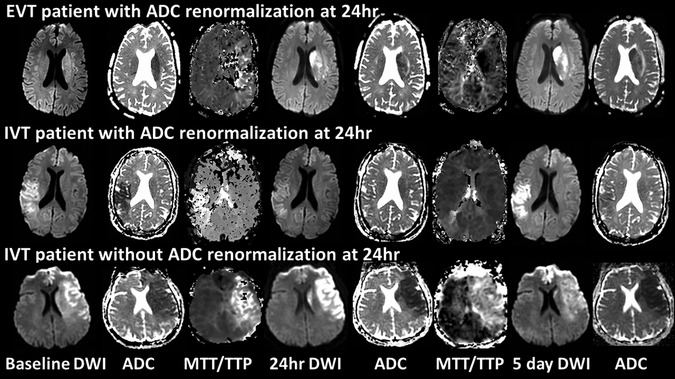
Figure 2.
Top panel: EVT patient, 54-year-old male presenting 2 hours from onset, baseline NIHSS 23, baseline DWI volume 26mL and ADC positive, treated with IV tPA within 60 minutes from triage at outside hospital, transferred and received EVT within 90 minutes of triage at hub hospital. TICI score of 3, complete reperfusion at 24hr, renormalization of 24hr ADC, ENI with 24hr NIHSS 8, and 24hr and 5-day DWI volumes 30mL and 34mL with 5-day ADC.
Middle panel: IVT patient, 45-year-old female presenting 2 hours from onset, baseline NIHSS 17, baseline DWI volume 46mL and ADC positive, treated with standard IV tPA 70 minutes from triage at the hub hospital, complete reperfusion at 24hr and renormalization of 24hr ADC, ENI with 24hr NIHSS 8, and 24hr and 5-day DWI volumes 28mL and 44mL with 5-day ADC.
Bottom panel: IVT patient, 59-year-old female presenting 39 minutes from onset, baseline NIHSS 22, baseline DWI volume 143mL and ADC positive, treated with IV tPA 69 minutes from triage at the hub hospital, no reperfusion or renormalization of 24hr ADC, no ENI with 24hr NIHSS 22, and 24hr and 5-day DWI volumes 135mL and 111mL with 5-day ADC.
Clinical Outcomes
ENI at 24-hours was seen in 55% of revascularization patients versus 11% of non-revascularization patients (p=0.003). Discharge NIHSS was also lower, 4 versus 8, in the revascularization patients (p=0.074). Patients with ENI at 24-hours compared to those who did not achieve ENI, had smaller DWI lesion volumes at 5-days, 31mL versus 93mL (p=0.001). Revascularization patients had good clinical outcome at 90-days at a significantly higher rate, 63% versus 9% (p=0.003). However only 35 of the 50 patients had available 90-day clinical outcome data.
Grouping patients based on significant core lesion reversal (Supplementary Table I), ENI at 24-hours was achieved in 64% with significant core reversal versus 13% with no reversal (p<0.001). Patients with significant core reversal had lower Discharge NIHSS, 3 versus 9, than those without (p=0.113). Median DWI lesion volume at 5-days was significantly smaller in those with significant core reversal compared to those without, 35mL versus 63mL (p=0.038). The significance of the smaller DWI volume at 5-days (p=0.002) remained after adjustment by baseline median ADC value. Patients with significant core lesion reversal also had more frequent good clinical outcome, 65% vs. 20% (p=0.009).
Discussion
We have demonstrated that the evolution of the ADC of the ischemic core is strongly influenced by early revascularization and is not just dependent on time. The ADC of ischemic core, identified as the region of tissue with ADC ≤620 μm2/s at baseline, continues to remain low out to 24 hours in patients who have a persistent occlusion, whereas the ADC of the core returns to a value seen in healthy tissue after recanalization and reperfusion. While this observation is neither novel nor unrecognized13, 16, 23, it remains underappreciated, confounded by differences in timing of imaging, and of debatable clinical significance.14, 16, 18, 21 Our study differs in that we were able to evaluate the baseline, pre-EVT ADC for comparison to post-treatment ADC, and we found a much greater absolute change in ADC between the baseline and 24-hour time points than what had been observed by An et al.13 We have shown this divergent behavior is evident on a per-patient basis through qualitative rater assessment of reversal, on a per-lesion basis based on the quantitative median value of the ADC in the ischemic core and on a per-voxel basis in the core based on the fraction of voxels that renormalize (Figure 1 and Table 1). We demonstrated that early revascularization and renormalization of the ADC are associated and occur frequently together. An et al demonstrated in a cohort of IV tPA-treated and untreated patients that ADC intensities vary with time after stroke onset and that, similar to our study, early change and increase in ADC intensity is not associated with initial ADC intensity but instead is related to reperfusion.13 Similar to our study, Roh et al demonstrated in 18 EVT patients with TICI 2b/3 recanalization who were able to have both pre-EVT and immediate post-EVT (performed mean 72 min post-recanalization) MRI a significant increase in ADC intensity in territorial infarction (as compared to watershed infarction) lesions from a mean ADC value of 436.09 μm2/sec at baseline to 666.45 μm2/sec immediately after EVT.28
The implication of an association between early revascularization and renormalization of the ADC is worth considering. A recent study evaluated DWI only and interpreted lack of lesion growth as evidence of lack of reperfusion injury in the setting of complete reperfusion.29 We found while lesions remained conspicuously bright on DWI, the ADC renormalized in patients who revascularized. This implies that the dominant factor contributing to the hyperintensity on DWI seen at 24 hours following recanalization is a change in T2, not ADC. It is surprising that historically the ischemic core, demarcated by the region of low ADC at baseline, was thought to predominantly contain irreversibly injured tissue, and presumed to remain low on ADC for days. Though reversal of the ADC in the core has been reported, debate continues about how frequently reversal occurs and whether or not reversal represents recovery of tissue or an irrelevant epiphenomenon in the progression of ischemia to infarction.22, 23, 28
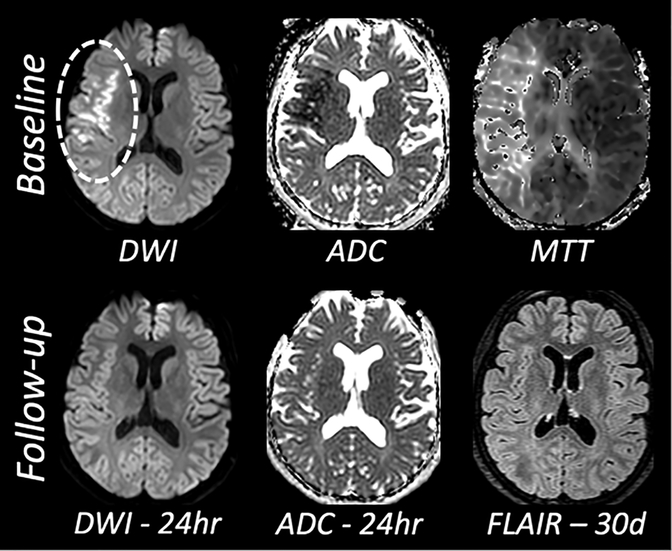
Two salient aspects of cerebral ischemia are generalized as i) cytotoxic edema characterized by loss of energy substrate, failure of ATP-dependent ion transport, cellular depolarization, accumulation of intracellular sodium and cell swelling and ii) vasogenic edema characterized by increased permeability of the endothelium, movement of water from the vasculature to the parenchyma, and concomitant swelling and mass effect. These two processes may occur concurrently; however, they are expected to have diametrically-opposed influence on the diffusion of water: cytotoxic edema decreases the apparent diffusion coefficient whereas vasogenic edema increases the apparent diffusion coefficient. It is widely appreciated that T2 is largely unaffected by cytotoxic edema whereas the ADC of water is decreased: DWI positive, ADC low, T2-FLAIR negative. As time progresses, the appearance of a lesion on T2 is expected and is frequently attributed to the development of vasogenic edema, contrary to the case shown in Figure 3. Much further out in time, i.e., days to weeks, the ADC of the most severely injured tissue renormalizes as vasogenic edema becomes dominant.
Figure 3.
Thirty-year old woman with right M1 middle cerebral artery occlusion, treated with IV tPA at 90 minutes from symptom onset and endovascular therapy with TICI score of 3; reperfusion achieved at 3 hours from symptom onset. Baseline NIHSS 7, 24-hour NIHSS 0, 90-day mRS 0. The 24-hour MRI was obtained at 21.7 hours relative to the baseline MRI. The 30-day FLAIR MRI was obtained at 37 days relative to the baseline MRI.
However, it is now apparent that the inflection point of ADC renormalization may depend on successful revascularization13 and may occur as early as 24 hours. There are at least three potential interpretations of reversal of the core. One, the core remains irreversibly damaged and revascularization simply facilitates a faster transition to vasogenic edema, thereby accelerating progression to infarction. Two, infarction may have been averted by revascularization, where the renormalization of the ADC suggests recovery of ATP-dependent ion transport and reversal of cytotoxic edema. And three, a combination of the two where the primary insult of ischemia is averted, however secondary damage as the result of revascularization becomes the dominant factor. It is this third possibility that is the most intriguing.
Acute therapies for ischemic stroke have, for many good reasons, focused on rapid and early restoration of blood flow. Other approaches, in the absence of revascularization have largely proven futile. With modern endovascular therapy, early and complete restoration of blood flow is achievable and will have the greatest impact on outcome. Concomitant to revascularization are secondary injury mechanisms which include oxidative stress, mitochondrial dysfunction, complement-mediated immune response, leukocyte infiltration, and blood brain barrier dysfunction. In a small, retrospective observational study such as this, it is not possible to establish causation or to explore the clinical relevance of these secondary processes to the imaging observation. Further we utilized revascularization measures at two different time points due to the two different treatment groups. However, the divergent behavior of the ADC of water dependency on revascularization may be a good starting point to detect and understand the relevant contribution of secondary injury to cell death and clinical outcome. Regardless of the biological interpretation, the observation of a DWI positive lesion without an ADC correlate should no longer be considered solely a result of time, but a product of revascularization as well.13
Summary
We have observed striking differences in the evolution of ADC in acute ischemic stroke patients with early complete revascularization, now more commonly seen with EVT, as compared to our traditional understanding and expectations in the context of the natural ischemic course or even with IVT. This early ADC normalization is associated with smaller ischemic lesion growth and improved clinical outcomes, though it may also reflect a component of secondary injury and serve as a potential imaging biomarker for the development of future adjunctive therapies.
Supplementary Material
Acknowledgements
The investigators thank the stroke programs at MedStar Washington Hospital Center and Suburban Hospital.
Sources of Funding
This research was supported by the Intramural Research Program of the NIH/National Institute of Neurological Disorders and Stroke.
Footnotes
Clinical Trial Registration Information: URL: https://www.clinicaltrials.gov. Unique identifier:.
Conflicts of Interest/Disclosures
All authors: None.
References
- 1.Baird AE, Warach S. Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab. 1998;18:583–609 [DOI] [PubMed] [Google Scholar]
- 2.Schuier FJ, Hossmann KA. Experimental brain infarcts in cats. Ii. Ischemic brain edema. Stroke. 1980;11:593–601 [DOI] [PubMed] [Google Scholar]
- 3.Moseley ME, Kucharczyk J, Mintorovitch J, Cohen Y, Kurhanewicz J, Derugin N, et al. Diffusion-weighted mr imaging of acute stroke: Correlation with t2-weighted and magnetic susceptibility-enhanced mr imaging in cats. AJNR Am J Neuroradiol. 1990;11:423–429 [PMC free article] [PubMed] [Google Scholar]
- 4.Norris DG, Niendorf T, Leibfritz D. Health and infarcted brain tissues studied at short diffusion times: The origins of apparent restriction and the reduction in apparent diffusion coefficient. NMR Biomed. 1994;7:304–310 [DOI] [PubMed] [Google Scholar]
- 5.Schlaug G, Benfield A, Baird AE, Siewert B, Lovblad KO, Parker RA, et al. The ischemic penumbra: Operationally defined by diffusion and perfusion mri. Neurology. 1999;53:1528–1537 [DOI] [PubMed] [Google Scholar]
- 6.Rohl L, Ostergaard L, Simonsen CZ, Vestergaard-Poulsen P, Andersen G, Sakoh M, et al. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted mri and apparent diffusion coefficient. Stroke. 2001;32:1140–1146 [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Goyal M, Jahan R, Bonafe A, Diener HC, Levy EI, et al. Ischemic core and hypoperfusion volumes predict infarct size in swift prime. Ann Neurol. 2016;79:76–89 [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Ann Neurol. 2006;60:508–517 [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731 [DOI] [PubMed] [Google Scholar]
- 10.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2017;378:11–21 [DOI] [PubMed] [Google Scholar]
- 11.Schwamm LH, Wu O, Song SS, Latour LL, Ford AL, Hsia AW, et al. Intravenous thrombolysis in unwitnessed stroke onset: Mr witness trial results. Ann Neurol. 2018;83:980–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. Mri-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–622 [DOI] [PubMed] [Google Scholar]
- 13.An H, Ford AL, Vo K, Powers WJ, Lee JM, Lin W. Signal evolution and infarction risk for apparent diffusion coefficient lesions in acute ischemic stroke are both time- and perfusion-dependent. Stroke. 2011;42:1276–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell BC, Purushotham A, Christensen S, Desmond PM, Nagakane Y, Parsons MW, et al. The infarct core is well represented by the acute diffusion lesion: Sustained reversal is infrequent. J Cereb Blood Flow Metab. 2012;32:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa Y, Fisher M, Latour LL, Dardzinski BJ, Sotak CH. Mri diffusion mapping of reversible and irreversible ischemic injury in focal brain ischemia. Neurology. 1994;44:1484–1490 [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Mlynash M, Christensen S, Wheeler HM, Straka M, Tipirneni A, et al. Early diffusion-weighted imaging reversal after endovascular reperfusion is typically transient in patients imaged 3 to 6 hours after onset. Stroke. 2014;45:1024–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469 [PubMed] [Google Scholar]
- 18.Labeyrie MA, Turc G, Hess A, Hervo P, Mas JL, Meder JF, et al. Diffusion lesion reversal after thrombolysis: A mr correlate of early neurological improvement. Stroke. 2012;43:2986–2991 [DOI] [PubMed] [Google Scholar]
- 19.Minematsu K, Li L, Sotak CH, Davis MA, Fisher M. Reversible focal ischemic injury demonstrated by diffusion-weighted magnetic resonance imaging in rats. Stroke. 1992;23:1304–1310; discussion 1310–1301 [DOI] [PubMed] [Google Scholar]
- 20.Olivot JM, Mlynash M, Thijs VN, Purushotham A, Kemp S, Lansberg MG, et al. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in defuse: Insights from radar. Stroke. 2009;40:1692–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C, et al. Severe adc decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33:79–86 [DOI] [PubMed] [Google Scholar]
- 22.Fiehler J, Knudsen K, Kucinski T, Kidwell CS, Alger JR, Thomalla G, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35:514–519 [DOI] [PubMed] [Google Scholar]
- 23.Grant PE, He J, Halpern EF, Wu O, Schaefer PW, Schwamm LH, et al. Frequency and clinical context of decreased apparent diffusion coefficient reversal in the human brain. Radiology. 2001;221:43–50 [DOI] [PubMed] [Google Scholar]
- 24.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Mri profile and response to endovascular reperfusion after stroke (defuse 2): A prospective cohort study. Lancet Neurol. 2012;11:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kretzer L, Grassel D, Bokemeyer MA, Gunther A, Witte OW, Axer H, et al. Effect of intravenous thrombolysis on the time course of the apparent diffusion coefficient in acute middle cerebral artery infarction. J Neuroimaging. 2015;25:978–982 [DOI] [PubMed] [Google Scholar]
- 26.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T, et al. Revascularization end points in stroke interventional trials: Recanalization versus reperfusion in ims-i. Stroke. 2005;36:2400–2403 [DOI] [PubMed] [Google Scholar]
- 27.Purushotham A, Campbell BC, Straka M, Mlynash M, Olivot JM, Bammer R, et al. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke. 2015;10:348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roh J, Yeom JA, Kim YS, Yoon CH, Park M-G, Park K-P, et al. Change of apparent diffusion coefficient immediately after recanalization through intra-arterial revascularization therapy in acute ischemic stroke. Journal of the Korean Society of Radiology. 2016;74:245–253 [Google Scholar]
- 29.Gauberti M, Lapergue B, Martinez de Lizarrondo S, Vivien D, Richard S, Bracard S, et al. Ischemia-reperfusion injury after endovascular thrombectomy for ischemic stroke. Stroke. 2018;49:3071–3074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.