Abstract
Several Boston Scientific pacemaker models have a known issue with intermittent oversensing of the minute ventilation sensor when paired with non-Boston Scientific leads. Several of our patients with these hybrid systems have had transient out of range impedances and oversensing after safety switching which we suspected may be related. A retrospective analysis of 390 patients who had pacemakers implanted between 2015–2017 found that transient out of range impedances with safety switching was present in 9% of Boston Scientific pacemakers paired with Abbott or Medtronic leads compared to 0% in other device-lead combinations (p=0.0089). We postulate that the root cause of the minute ventilation oversensing and transient high impedance issue is the same, a header-lead interaction from low-level incompatibility. Recognizing this issue is critical to prevent unnecessary lead revisions or extractions as it can be prevented with a simple reprogramming of lead pace/sense configuration.
Keywords: Lead fracture, impedance, header, safety switch, minute ventilation
Introduction:
We have recognized a new critical issue affecting a large family of Boston Scientific pacemakers paired with non-Boston Scientific leads. Specifically, a header-lead interaction has led to a high incidence of safety switch alerts, which we believe are erroneous and may led to inappropriate lead revision procedures.
Recently, Boston Scientific (St. Paul, MN) issued a physician advisory regarding intermittent oversensing of the minute ventilation (MV) sensor in some of its pacemakers enabled for rate adaptive pacing.1 Intermittent high impedance causes the 20 Hz MV sensor signal to be altered such that it is visible on device electrograms and appears as oversensing on atrial or ventricular channels. This phenomenon was found to occur at a particularly elevated rate when Medtronic (Minneapolis, MN) and Abbott (formerly St Jude Medical, St Paul, MN) pacing leads were connected to pacemakers of the Valitude, Accolade, Essentio, Visionist, Proponent, and Altrua 2 pacemaker lines as part of a “hybrid” pacing system. The incidence of transthoracic impedance measurement oversensing was 1.8% in a single center study.2 In December 2017, Boston Scientific recommended turning off the MV sensor in pacemaker-dependent patients to prevent inappropriate pacing inhibition from oversensing MV pulses.1 In addition to the MV oversensing issue we have identified a separate issue with these affected Boston Scientific pacemakers. Several patients at our institution have demonstrated spurious daily impedance measurements >2000 ohms triggering lead Safety Switch, an ON/OFF programmable feature that results in automatic polarity switching to unipolar pacing and sensing when a single daily lead impedance measurement is out of range. Since these patients were pacemaker dependent, we were concerned that these findings represented lead fracture and following discussions with Boston Scientific we extracted and replaced the affected leads. Given the higher incidence of suspected lead fracture than historical norms, our investigation determined that these patients all had Boston Scientific pacemakers paired with mostly Abbott leads. Notably, none of them had MV oversensing issues. However, a “deep dive” into the erroneous daily impedance measurements revealed a common root cause to the MV sensor: transient increase in electrical resistance of sub-threshold pulses at the terminal ring connector within the pacemaker header. We report our three initial cases as well as our subsequent investigation into the mechanism.
Case reports:
CASE 1
A 76-year-old man with severe aortic stenosis, coronary artery disease and permanent atrial fibrillation underwent single chamber pacemaker implantation for complete heart block complicating transcutaneous aortic valve replacement. An Abbott model 1688TC was implanted in the right ventricle (RV) in conjunction with a Boston Scientific Essentio SR model L100 pacemaker generator. At implantation, the RV lead impedance was 615 Ohms and had adequate sensing value and pacing threshold. Seventeen months after implant, routine device surveillance demonstrated a transiently high impedance of >2000 Ohms with safety switch to unipolar sensing and pacing, concerning for potential lead fracture. However, in-office evaluation showed normal lead testing including a bipolar lead impedance of 589 ohms (Table 1). The lead was inspected under fluoroscopy and there were no signs of lead fracture or loose set-screw. The device was switched back to bipolar lead sensing and pacing configuration and home monitoring was instituted. Three months later, remote alerts were received for high impedance values >2000 Ohms with repeat safety switching to unipolar and subsequent oversensing on the RV lead. Because of suspected lead fracture and pacemaker-dependency of the patient, the lead was extracted and a new St Jude Tendril model 1688TC was implanted and connected to the existing Boston Scientific Essentio generator. The procedure was uncomplicated and the patient has had no further events during subsequent 13-month follow-up.
Table 1:
In-office lead measurements of the 3 case-study patients who ultimately underwent lead extraction due to transient elevated RV lead impedance. Lead impedance, sensing, and capture thresholds are shown in both bipolar as well as unipolar configurations after safety switch event occurred.
| In-Office Lead Assessment after High Impedance Event | |||||||
|---|---|---|---|---|---|---|---|
| Bipolar Configuration | Unipolar Configuration | ||||||
| Case # | Impedance (ohms) |
Sensing (mV) |
Capture threshold |
Presence of 0versensing |
Impedance (ohms) |
Sensing (mV) |
Capture threshold |
| 1 | 589 | 7.8 | 0.7V/0.4msec | No | 263 | 8.1 | 0.5V/0.5msec |
| 2 | 684 | 16 | 0.8V/0.5msec | No | 611 | 16 | 0.7V/0.5msec |
| 3 | 561 | 25 | 0.8V/0.5msec | No | 351 | 22.6 | Not available |
CASE 2
A 60-year-old woman with paroxysmal atrial fibrillation and tachy-brady syndrome underwent dual-chamber pacemaker implantation for sinus node dysfunction. Abbott model 1688TC leads were implanted in the RV and RA and connected to a Boston Scientific Essentio model L101 pacemaker generator. At implant, the RV and RA lead impedances were 1246 and 625 ohms. Sensing and capture thresholds for both leads were adequate. On routine device surveillance 6 months later, she was noted to have had a high RV lead impedance, (>2000 Ohms) leading to safety switch to unipolar configuration and electronic noise detected on the RV lead. In-office evaluation showed normal lead parameters including, a bipolar lead impedance of 684 ohms (Table 1). Furthermore, noise was not inducible with provocative maneuvers in the bipolar sensing configuration. The device was switched back to bipolar sensing and pacing but one month later a repeat safety switch occurred because of another high lead impedance > 2000 ohms measurement. Again in-office interrogation showed normal bipolar impedance and negative provocative testing. The device was switched back to bipolar sensing and pacing but after a third safety switch event, a decision was made to extract the lead for suspected impending RV lead fracture one- year after initial implant. A new Abbott Isoflex model 1948TC RV lead was implanted and connected to the existing Boston Scientific Essentio generator. The procedure was uncomplicated and the patient has had no further events during subsequent 15-month follow-up.
CASE 3
A 58 year-old woman with hypertrophic cardiomyopathy underwent dual-chamber pacemaker implantation for high-degree AV block two years following septal myectomy. Abbott model 1688TC pacemaker leads were implanted in the RV and RA and connected to a Boston Scientific Essentio model L101 pacemaker generator. At implant, the RV lead impedance was 620 Ohms and it had adequate values for sensing and capture threshold. Fourteen months later, she developed intermittent RV lead impedance of >2000 Ohms leading to safety switch to unipolar with subsequent noise detection on the RV lead. In office testing showed otherwise normal lead parameters (Table 1). At two years post-implant and after three safety-switch events, she underwent extraction of the RV lead because of suspected lead fracture. A new Abbott 1688TC RV lead was implanted and connected to a new Boston Scientific Essentio model L131 pacemaker generator. The procedure was uncomplicated and the patient has had no further events during subsequent 12-month follow-up.
Retrospective analysis:
All three patients had Boston Scientific pacemakers paired with Abbott leads. We surmised that the results were unusual in our experience over an extended period of time with this lead. We realized that the MV sensor and daily lead impedance measurements shared a common element; both use subthreshold pulses to measure impedance and we hypothesized they may be related. In order to test our hypothesis we compiled a list of all patients at our institution who underwent implantation of Boston Scientific Essentio (L100, L101, L110, 121, 131), Accolade (L301, L311), and Valitude CRT-P (U125, U128) pacemakers between September 2015 and December 2017 (Table 2). A total of 255 patients were identified as having one of these Boston Scientific generators. Among these, 200 patients had Abbott pacing leads, 37 patients had Boston Scientific pacing leads, and 18 patients had Medtronic pacing leads. Routine follow-up data was available on 173 of the 255 patients with the affected Boston Scientific generators and 15 patients were identified as having elevated impedance issue on either the RA or RV leads. Twelve of the 15 patients had Abbott 1688TC leads. One patient had an Abbott 1388TC lead while another had an Abbott 1488 lead. One patient had a Medtronic 5076 lead. Twelve patients had transient out of range impedances (3 on the RA lead, 9 on the RV lead) triggering safety switch and resulting over-sensing (Figure 1) but normal in-office bipolar lead integrity testing. Of these twelve patients, three underwent lead extraction with revision, seven were switched to unipolar pacing / bipolar sensing and one patient was left in unipolar pacing/sensing. The remaining patient has missed follow up. Two of the 15 identified patients with elevated impedance issue had loss of RV bipolar capture and persistently elevated bipolar lead impedance during in-office evaluation. These patients were clinically diagnosed with true lead fracture. One of the 15 identified patients with elevated impedance issue had electronic noise and intermittent high impedances on both RA and RV leads. Safety switch events and crosstalk was observed. This patient was found to have fractures of both leads during lead explantation. One patient out of the 173 patients had MV oversensing but did not have intermittent high lead impedance issues or safety switch events. None of the patients with affected Boston Scientific pacemaker generators and Boston Scientific leads (models 7732, 7741, 7742) had intermittent high lead impedance measurements.
Table 2:
All pacemaker implants at our center between September 2015 and December 2017. At total of 395 devices were implanted. Only Boston Scientific pacemakers with incongruent (Medtronic or Abbott) leads had high impedance readings. Twelve were adjudicated to be from a lead-header issue and 3 were from a lead fracture. Three patients with the identified lead-header issue underwent unnecessary lead extraction and revision procedure.
| Device/Lead(s) | Total Patients | Number of Patients with Follow Up |
Intermittent high lead Impedance attributed to lead- header interaction |
High Impedance attributed to lead fracture |
|---|---|---|---|---|
| Boston Scientific Device/Boston Scientific Lead(s) |
37 | 31 | 0 | 0 |
| Boston Scientific Device/Abbott Medical Lead(s) |
200 | 131 | 11 (3 extracted and 7 changed to unipolar pacing/bipolar sensing)* |
3 |
| Boston Scientific Device/Medtronic Lead(s) |
18 | 13 | 1 (left in unipolar pacing/sensing) |
0 |
| Abbott Device/Abbott Lead(s) |
22 | 14 | 0 | 0 |
| Medtronic Device/Abbott Lead(s) |
118 | 87 | 0 | 0 |
One patient lost to follow up
Figure 1:
Representative example of transient out of range high impedances on atrial lead (upper panel) with noise seen on atrial channel (lower panel) after safety switch to unipolar sensing.
As a control, we looked at our patients with Abbott Tendril (1688TC, 1388TC, 1888ST) leads who had Medtronic Sensia (SEDRO1, SESR01), Adapta (ADDR01), Versa (VEDRO1), or Syncra (C2TR01) pacemaker generators (118 patients) and Abbott Assurity (PM2240, PM1240), Verity (DR5356) pacemaker generators (22 patients) implanted during the same time frame (Table 2). Overall, 9% of patients with Boston Scientific pacemaker generators with Abbott or Medtronic leads had transient out-of-range high impedance measurements and no other lead abnormalities (11 out of 12 were Abbott leads). In comparison, no patients with Medtronic or Abbott pacemaker generators and Abbott Tendril leads and no patients with Boston Scientific pacemaker generators and Boston Scientific leads had intermittent high lead impedance issues (p=0.0089 with 2-tailed Fisher’s exact test).
Nine of the 12 affected patients did not undergo lead revision as we increasingly recognized that the transient high lead impedance measurements were likely not due to lead fracture. The lead configuration was switched to unipolar pacing/bipolar sensing configuration in 7 out of these 9 patients. After a mean follow-up of 4 months, there have been no clinical events, high lead impedance, or safety switch events. We suspect that none of the 3 patients who underwent lead extraction for the safety switch issue had recurrent events despite extended follow-up (13–16 months) because the odds of this problem reoccurring is low (9%). Accordingly, we did not prophylactically reprogram these three patients or any other patient with a Boston Scientific hybrid pacing system to unipolar pacing/bipolar sensing. There is also a small risk of pectoral stimulation with unipolar pacing which we would have subjected a large number of otherwise asymptomatic patients to.
Discussion:
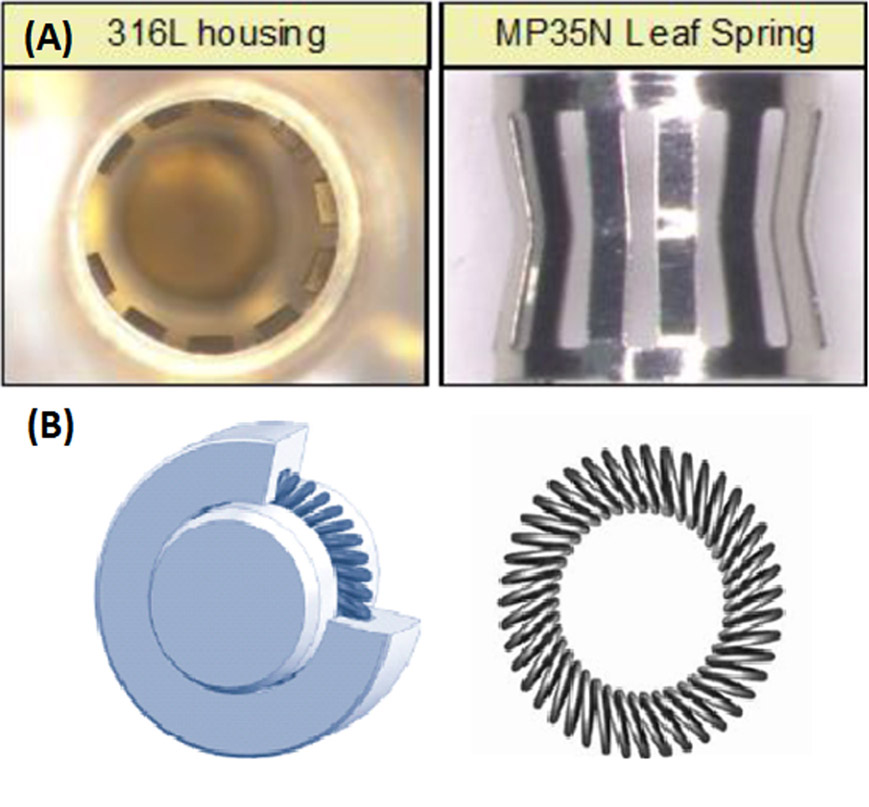
We attribute the high incidence of intermittent high lead impedance measurements in hybrid pacemaker systems involving Boston Scientific generators to the “setscrew-less” multipoint terminal ring connector used in contemporary pacemakers and implantable cardioverter defibrillators (ICD). The multipoint terminal ring connector has been implicated in the cause of the intermittent oversensing of the MV sensor. Older Boston Scientific devices and current Medtronic devices use a multibeam leaf-spring composed of MP35N® nickel alloy which have 10 points of contact with the IS-1 terminal ring, which is composed of 316L stainless steel (figure 2a). Newer Boston Scientific and Abbott devices use a multipoint canted spring (also composed of MP35N® nickel alloy) and this spring has up to 70 points of contact (figure 2b). Small axial or radial movements of the terminal ring lead to microscarring and accumulation of oxidative particles. 3 This can affect the electrical connectivity between the spring and the terminal ring and is the likely cause of our finding of intermittent high impedance measurements, recurrent safety switching to unipolar configuration, and lead oversensing. Microscarring and oxidation could be amplified with the newer canted spring configuration, which has 5 times the number of contact points as the older generation leaf spring. Moreover, the interaction has been found to be higher with non-Boston Scientific leads and attributed to a greater microscopic surface roughness of the terminal ring as well as greater axial/radial movement on Medtronic and Abbott leads as compared to Boston Scientific leads.1 Of note, neither microscopic surface specifications nor axial/radial movement are described within the IS1 standard.4
Figure 2:
Types of multipoint terminal ring connector used in contemporary pacemakers. (a) Older leaf-spring connector made of MP35N® nickel alloy with 316L stainless steel housing which has 10 points of contact with the IS-1 terminal ring. (b) Newer canted spring composed of MP35N® nickel alloy which has up to 70 points of contact with the IS-1 terminal ring.
Why is this issue not seen with other pacemaker manufacturers? While Boston Scientific pacemakers use a sub-threshold pulse of 80 μA (0.04 V, assuming an impedance of 500 Ω) to test pacing impedances, Abbott pacemakers (which also have the multipoint canted spring connector) use a pulse of 2 or 4 V which can more easily overcome elevated resistance from terminal ring oxidation. Medtronic Sensia/Versa/Adapta pacemakers use a pulse of 5V to test pacing impedances. Newer Medtronic pacemakers on the ICD platform (Advisa/Revo/Azur/Percepta) use a sub-threshold pulse but the company would not disclose the amplitude in our discussion. Nonetheless, Medtronic devices are far less likely to have the high impedance issue as they use the multibeam spring connector. Whereas Boston Scientific pacemakers will safety switch after only one elevated impedance reading, Abbott pacemakers will deliver up to four more pulses to confirm an out of range impedance before safety switching. (Personal communication, Boston Scientific, Medtronic, and Abbott technical services).
This problem has also been recently reported to affect conventional implantable cardioverter defibrillators (ICDs) which use multipoint canted springs. Abbott ICDs use a sub-threshold triphasic pulse of 187.5 to 750 μA5 (0.094 to 0.375 V, assuming an impedance of 500 Ω) and similar lead – header issues leading to periodic high impedance readings have been reported in Abbott Fortify ICDs paired with Medtronic 6935 and 6947 leads due to oxidation on the 316L stainless lead tip conductor affecting contact with the titanium set screw.6 Boston Scientific ICDs use a sub-threshold pulse of 80 μA to test pacing impedances. MV oversensing has been reported in a Boston Scientific ICD paired with an Abbott 1688 atrial lead; although impedance measurements were normal.7 Tanawuttiwat et al recently published a case series of patients with Medtronic Sprint Quattro Secure ICD leads paired with Boston Scientific ICDs with intermittent high impedance alerts.3
The differential diagnosis for high impedance readings includes lead fractures or lead-header connection issues. Swerdlow et al8 proposed an algorithm to differentiate between the two based on analysis of 91 Medtronic ICDs with high impedance issues. Extremely high maximum impedance (>10,000 Ω) or noise oversensing with a normal impedance trend were indicative of fracture. Onset of high impedance or noise oversensing within 30 days of implant, high impedance measurement with return to baseline >45 days, or high impedance returning to baseline < 45 days in a device within 200 days of implant were indicative of a connection issue. One limitation of the algorithm is that Medtronic devices use a multibeam spring terminal ring connector and not a canted spring as do Boston Scientific devices, the former being less likely to cause terminal ring oxidation. Our three patients who underwent lead extraction had high impedance recordings 6, 14, and 17 months post implant and had return to baseline impedance in <45 days. Based on the algorithm, a lead fracture would have been diagnosed in 2 out of the 3 cases and highlights the difficulty of diagnosing this lead-header issue using a conventional approach.
Conclusions:
We have found an unexpectedly high incidence (9%) of transient high lead impedance measurements leading to safety switching in patients with Boston Scientific Essentio pacemakers paired with Abbott or Medtronic leads. In a joint effort with Boston Scientific, we have attributed it to lead-header interaction causing terminal ring oxidation and altering electrical connectivity. We contend that this problem is seen only in hybrid systems that use sub-threshold pulses to test impedance, which are unable to surmount ring oxidation. While the issue with oversensing caused by the MV sensor shares the same root cause, the intermittent high lead impedance issue is not fixed by turning off the MV sensor. Rather this lead problem can be avoided by using Boston Scientific pacemakers with Boston Scientific leads, which are less prone to this oxidative effect. We have found that for existing Boston Scientific pacemakers paired with Medtronic or Abbott leads, inappropriate safety-switching to unipolar pace/sense polarity can be avoided by programming the lead configuration to unipolar pacing/bipolar sensing. This removes the ring from the pacing circuit and prevents safety-switching, which can lead to myopotential sensing and inhibition of pacing. Appropriate recognition of this lead-header interaction and cause for intermittent high impedance measures is important to avoid unnecessary lead revision procedures. Finally, based on our findings, we recommend caution and careful consideration before implanting hybrid pacing systems, which may lead to unpredictable and unforeseen issues. It is critical that the IS-1 and DF-1 standards are updated to bring uniformity across manufacturers and to eliminate low-level incompatibility.
Acknowledgements:
We wish to thank Boston Scientific, Medtronic, and Abbott technical services for their assistance, and especially David Casavant, MS (Clinical engineer, Boston Scientific) and Timothy McIntyre, MS (Clinical engineer, Abbott).
We also wish to thank Howard Cabral, PhD, MPH, Boston University School of Public Health, Department of Biostatistics and Director, Biostatistics and Research Design Program, Boston University Clinical and Translational Science Institute for help with statistical analyses.
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through BU-CTSI Grant Number 1UL1TR001430. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations:
- MV
Minute ventilation
- RV
Right ventricle
- RA
Right atrium
- ICD
Implantable cardioverter defibrillator
Footnotes
Disclosure Statement:
The authors have no conflicts to disclose
References:
- 1.Boston Scientific. Important Medical Device Information. https://www.bostonscientific.com/content/dam/bostonscientific/quality/documents/Recent%20Product%20Advisories/2017Dec_MV_PhsLtr_Final11.pdf. Accessed 1/17/19.
- 2.Ryan JD, Tempel ND, Engle DD, Hayes DL, Cha YM, Asirvatham SJ. Oversensing of transthoracic excitation stimuli in contemporary pacemakers. Pacing Clin Electrophysiol. 2018;41(2):161–166. [DOI] [PubMed] [Google Scholar]
- 3.Tanawuttiwat T, Berger RD, Love CJ. Intermittent high impedance from the lead-device compatibility problem. Heart Rhythm. 2019. [DOI] [PubMed] [Google Scholar]
- 4.ISO 5841–3:2013, Implants for surgery -- Cardiac pacemakers -- Part 3: Low-profile connectors (IS-1) for implantable pacemakers. [Google Scholar]
- 5.Williams ES, Knapp MI, Patton KK. Inappropriate mode switching in a dual-chamber defibrillator system: what is the mechanism? Circ Arrhythm Electrophysiol. 2012;5(3):e67–68. [DOI] [PubMed] [Google Scholar]
- 6.Mourad AR, Kim MH, Kirk MM. A case series of anomalous high pacing lead impedances in normally functioning leads. HeartRhythm Case Rep. 2015;1(6):449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClelland I, Nayak HM, Tung R, Upadhyay GA. Respiratory rate trending as a cause for atrial lead noise: A first report in an implantable cardioverter-defibrillator patient. HeartRhythm Case Rep. 2018;4(11):545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swerdlow CD, Sachanandani H, Gunderson BD, Ousdigian KT, Hjelle M, Ellenbogen KA. Preventing overdiagnosis of implantable cardioverter-defibrillator lead fractures using device diagnostics. J Am Coll Cardiol. 2011;57(23):2330–2339. [DOI] [PubMed] [Google Scholar]