Abstract
Objective
Growth hormone (GH) has been reported to enhance the intestinal barrier; as such, recombinant GH has been administered for several intestinal diseases. However, excess GH action has been implicated in increasing the risk of intestinal dysfunction. The goal of this study was to examine the direct effects of GH on the small and large intestines to clarify the role GH plays in intestinal function through the use of a mouse model.
Design
An intestinal epithelial-specific GH receptor (GHR) knockout (IntGHRKO) mouse line was generated using Cre-lox with the villin promoter driving Cre expression. The generated mice were characterized with respect to growth and intestinal phenotypes.
Results
IntGHRKO mice showed no significant changes in body length, weight, or composition compared to floxed controls. Male IntGHRKO mice had significantly shorter large intestines at 4 and 12 months of age. Intestinal barrier function was assessed by measuring the expression of tight junction related genes, as well as levels of serum endotoxin and fecal albumin. Results showed sex differences as males had an increase in occludin levels but normal serum endotoxin and fecal albumin; while, females had changes in fecal albumin levels with normal occludin and serum endotoxin. Evaluation of glucose tolerance and fat absorption also showed sex differences as females were glucose intolerant, while males had impaired fat absorption. Histopathology revealed a trend towards decreased villus height in males, which could explain the sex difference in glucose homeostasis.
Conclusions
Overall, the data demonstrate that disruption of GH on the intestinal epithelial cells modestly affects the intestinal gross anatomy, morphology, and function in a sex-specific manner.
Keywords: Growth hormone, growth hormone receptor, intestine
INTRODUCTION
Growth hormone (GH) is a metabolic hormone with numerous activities beyond longitudinal bone growth. Since most tissues express the GH receptor (GHR), GH is thought to play an important role in cellular metabolism throughout the body. The intestines are one of the organs that expresses GHR. [1] This organ is crucial for the absorption of nutrients from food and thus, helps modulate blood glucose levels. The importance of GH action on intestine is not readily apparent, but several studies have demonstrated that GH plays an important role in this tissue. A prominent link between GH and the intestine is exemplified by the FDA-approved indication for use of recombinant human GH (rhGH) in the treatment of short bowel syndrome (SBS), where it improves absorption of nutrients such as carbohydrates, amino acids and fats [2-4]. Treatment with rhGH has also reportedly been used in the treatment of inflammatory bowel disease (IBD) in both adult [5] and pediatric [6] patients. Accordingly, GH [7] or its downstream effector signal transducer and activator of transcription 5 (STAT5) [8,9] have been shown to maintain gut barrier function in an inflammatory state. Despite excess GH action appearing to be beneficial in the context of certain intestinal diseases, individuals with acromegaly have an increased gut transit time [10,11] and increased risk of colon polyps [12], colon cancer [13-16], and colonic diverticula [17], demonstrating the complexity of GH signaling in the intestine.
To investigate GH’s direct effects on the intestine, we generated and characterized an intestinal epithelial cell-specific GHR knockout (IntGHRKO) mouse. These mice utilize a villin promoter/enhancer-driven Cre recombinase [18] that deletes exon 4 of the GHR gene in intestinal epithelial cells. Although the villin promoter/enhancer is widely used to direct intestine-specific gene disruption [8,19,20], it is important to note that the proximal convoluted tubular cells of the kidney also express villin [18].
Due to the growth promoting effects of GH both in the intestines [21,22] and in other tissues, it was hypothesized that IntGHRKO mice would have decreased intestinal length, as well as decreased villus height. As GH has been shown previously to enhance the intestinal barrier, improve absorption of nutrients and increase intestinal length, we also hypothesized that IntGHRKO mice would have defects in the intestinal barrier, impaired absorption and decreased body weight and changes to their body composition due to decreased intestinal length. Modest effects on intestinal gross anatomy, morphology, and function in a sex-specific manner were found that help explain the role of GH on intestinal structure and function.
MATERIALS AND METHODS
Mouse generation and care
IntGHRKO mice were developed using the Cre-lox system with villin promoter/enhancer-driven Cre expression, which expresses Cre recombinase in intestinal epithelial cells as well as in the proximal convoluted tubule of the kidney. B6.Cg-Tg(Vil1-cre)997Gum/J mice (Jackson Laboratory Stock #004586) were crossed with mice with exon 4 of the GHR gene flanked by LoxP sites. Because Cre recombinase excises any DNA sequence between two LoxP sites, mice with the GHR gene disrupted in all intestinal epithelial cells (IECs) [18] (hereafter referred to as IntGHRKO) were generated by this cross. along with control mice with only the flanked exon 4 (hereafter referred to as floxed). In the “floxed” mice that lack Cre recombinase, the GHR gene remains intact and is able to produce functional GHR. Mouse numbers, ages, and sexes are listed in the methods for each experiment. All mice were housed in the Ohio University animal facility on a 14 hour light, 10-hour dark cycle. Mice were given access to chow and water ad libitum, except where otherwise noted. All animal procedures were approved by the Ohio University Institutional Animal Care and Use Committee. All mice used were bred onto a C57Bl/6J background, with at least eight rounds of backcrossing.
Confirmation of GHR gene disruption
Disruption of the GHR gene at the DNA level was confirmed using polymerase chain reaction (PCR) of the GHR gene locus. DNA was isolated from mouse tail samples by incubation in 100 μl of 25mM NaOH, 2mM EDTA solution for 1 hour at 95°C followed by the addition of μl of 40mM Tris-HCl. PCR was carried out using GoTaq Hot Start Polymerase (Promega) according to manufacturer’s instructions. Primers were designed as described previously [23-25] to bind in the introns on either side of exon 4 of the GHR gene.
In order to detect this IEC-specific disruption, IECs were isolated as previously described [26]. Briefly, the intestines were cut into approximately 5mm slices and rinsed in PBS. The intestine pieces were then transferred to a tube containing PBS/3mM EDTA and incubated for 30 minutes on ice with light shaking. Next, the PBS/EDTA was replaced with PBS, and the tube was vortexed, releasing epithelial cells into the supernatant. The supernatant cells were pelleted, and DNA was extracted as described earlier for use in PCR.
In cells without Cre recombinase activity (most non-IEC cells), the exon is not excised, leaving a longer PCR product. In these cells, functional GHR protein may be produced. The primer sequences used to target the full length GHR gene were; Forward primer: 5’-TCAGAACGTGGAACATCTTCAG, Reverse primer: 5’-CGGACATTGCATCTGTGATT.
Measurement of RNA expression with quantitative reverse transcription PCR (qRT-PCR)
Intestinal tissue (25 mg; n = 7) was homogenized using a Precellys 24 homogenizer and Cryolys cooling system (Bertin Technologies) [27]. RNA was extracted from 25mg of snap-frozen tissue using the GeneJET RNA Purification Kit (Thermo Scientific) according to manufacturer’s instructions. Total RNA (1ug) was reverse transcribed to cDNA using the Maxima first strand cDNA synthesis kit (Thermo Scientific), and cDNA samples were diluted 1:20 for use in quantitative PCR (qPCR). The qPCR reaction was carried out using the Bullseye EvaGreen Mastermix-ROX kit (MidSci) according to the manufacturer’s instructions on a StepOne Plus thermocycler, using the manufacturer’s recommended consumables. The RNA expression was determined using qBasePLUS 2.3 software with two reference genes for each tissue [28]. The reference genes used for small intestine and kidney were Hprt and Ppib [29], and for liver were Actb and Hprt. Primer sequences for the 8 genes assayed are listed in Table 1.
Table 1.
qPCR primer sequences
| Gene | Direction | Sequence (5'→3') |
|---|---|---|
| Actb | F | CAGCTTCTTTGCAGCTCCTT |
| Actb | R | CACGATGGAGGGGAATACAG |
| Ghr | F | GCCTGGGGACAAGTTCTTCTGGA |
| Ghr | R | TGCAGCTTGTCGTTGGCTTTCCC |
| Hprt | F | ATCAGTCAACGGGGGACATA |
| Hprt | R | AGAGGTCCTTTTCACCAGCA |
| Ocln | F | GGAGGACTGGGTCAGGGAATA |
| Ocln | R | TGACGTCGTCTAGTTCTGCC |
| Ppib | F | GCTACAGGAGAGAAAGGATTTGGC |
| Ppib | R | TGGGAAGCGCTCACCATAGA |
| Tjp1 | F | CTGATAGAAAGGTCTAAAGGC |
| Tjp1 | R | TGAAATGTCATCTCTTTCCG |
| Tjp2 | F | CAAATGAAGAGTATGGGCTC |
| Tjp2 | R | GATCTTGAGAATTATTGTCCCC |
| Tjp3 | F | CTTTATCTTACAGATCAACGGG |
| Tjp3 | R | GTCTGAGATGTCTTCCATTAG |
Gross morphology measurements
Mice were sacrificed via cervical dislocation following anesthesia with CO2.Mice (n=10) were dissected at 4 and 12 months of age, and tissues, including visceral organs, white and brown adipose tissue, brain, and leg muscles (quadriceps, gastrocnemius, and soleus), were weighed. Nasal-anal body length was determined (n=10) in euthanized mice just prior to dissection. The intestines were further processed using the following method. The abdominal cavity was opened, and the gastrointestinal tract was cut at the pylorus and the anus, and the intestines were removed. The pancreas and mesenteric fat were removed from the intestines, and the small and large intestines were divided at the ileocecal valve. The intestines were cut longitudinally, rinsed in ice cold PBS to remove the contents and then straightened without tension. The length was measured in centimeters for both the small and large intestine separately. Excess PBS was removed, and the intestines were weighed.
Microscopic morphology measurements
For histological analysis, intestines were prepared using the intestinal bundling technique as previously described in the literature [30]. After 24 hour fixation, the intestines were cut into short pieces, formed into bundles using surgical tape, and sliced into thin bundles and stored in 70% ethanol until embedding. Sections of 5μm thickness were transferred to slides and stained with hematoxylin and eosin (H&E) according to manufacturer’s instructions (Leica ST Infinity H&E Staining System).
For villus height and crypt depth measurements, images were taken at 100X magnification with Nikon Eclipse E600 microscope. Well-oriented crypts and villi were measured using the Fiji distribution of ImageJ. The intestines were measured on three mice per group, at least 3 images per mouse, with at least 10 well-oriented crypts or villi measured for each sample [31].
Body composition
Body composition was determined over time starting at 2 months (n≥42/sex/genotype) until 18 months of age (n≥13/sex/genotype) using a Bruker Minispec mq NMR analyzer (The Woodlands, TX, USA) as previously described [24,27,32].
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
GTTs were performed at 7 months of age (n=5-6/sex/genotype) [24,27,32,33]. Mice were fasted for 12 hours before commencement of the experiment. Each mouse received an intraperitoneal (IP) injection of 10% glucose at a dose of 1 g/kg body weight. Blood glucose was measured before the glucose injection and at 15, 30, 45, 60, and 90 minutes after injection. ITTs were performed at 7.25 months of age in a fed state (n= 5-6). Recombinant human insulin (Humulin-R; Eli Lilly & Co, Indianapolis, Indiana) was prepared by diluting Humulin-R (100 U/ml) to 0.075 U/mL in sterile saline (0.9%). Each mouse received an IP injection of the 0.075 U/ml insulin solution at a dose of 0.75 U/kg body weight. Blood glucose measurements were performed before the insulin injection and at 15, 30, 45, and 60 minutes after injection.
Intestinal barrier function: serum endotoxin and fecal albumin measurement
Serum was collected at dissection. Endotoxin levels in the serum were measured using a Pierce LAL Chromogenic Endotoxin Quantitation Kit according to manufacturer’s instructions (n=6-10/sex/genotype). For fecal albumin measurements, fecal pellets were collected at 24 months of age (n=7) and suspended in deionized water to a concentration of 100 mg/ml. The pellet suspension was vortexed until homogenized and centrifuged at 12 000 RCF for 30 seconds to pellet solids, and the supernatant was used in a bromocresol green assay (Sigma #MAK124). The manufacturer’s instructions were followed with the exception of using 100μl of diluted fecal sample in order to adjust the albumin concentration into the detectable range [34].
Intestinal fat absorption assay
Mice (aged 26 months; n=3) had their food replaced with a semi-synthetic chow with a fat component of 95% absorbable fats and 5% sucrose polybehenate (a non-absorbable food additive) for 3 days. Fecal pellets were collected from the cages of each mouse on days 3 and 4, sent to University of Cincinnati Mouse Metabolic Phenotyping Center, and analyzed using gas chromatography. The ratio of behenate to other fatty acids in the feces was compared to the ratio in the chow to determine the amount of fat absorbed in the intestines [35].
Statistics
For each experiment, data were analyzed using Student’s t-test to compare each group with their littermate controls. Data analysis were carried out using SPSS version 22. Results were deemed significant if the p < 0.05.
RESULTS
GHR gene disruption in the intestinal epithelium
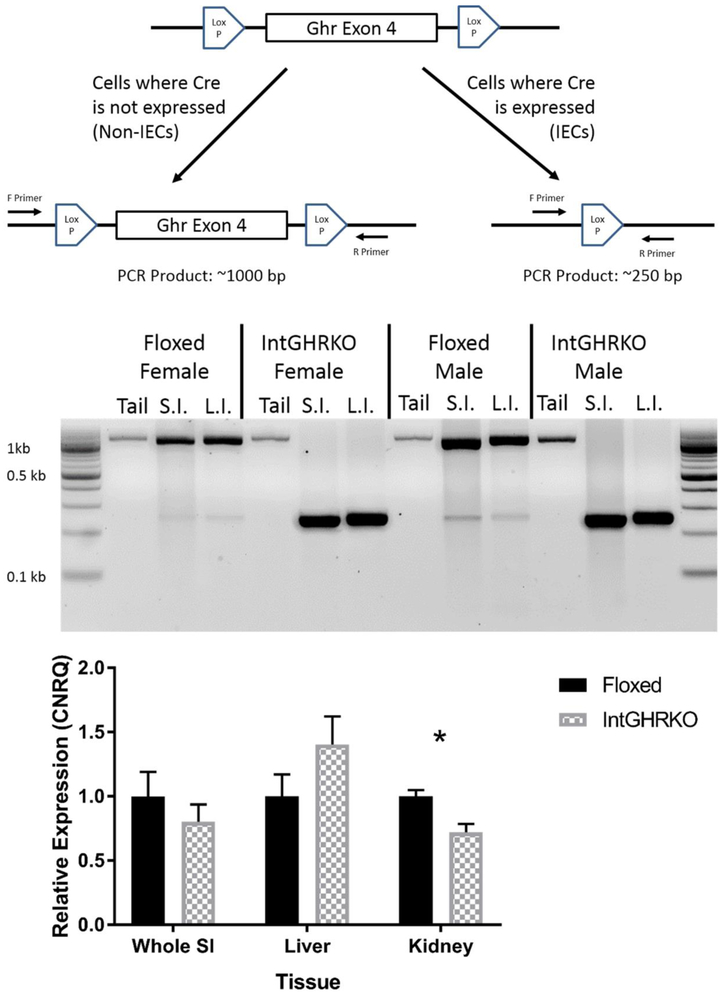
Mice with an intestinal epithelial cell-specific knockout of the GHR gene were generated by crossing villin-Cre transgenic mice with mice that have exon 4 of the GHR gene flanked by LoxP sit (Ghrflox/flox) (Figure 1, top) to produce IntGHRKO mice (CxFF) along with littermate floxed controls (xxFF). GHR gene knockout was confirmed using PCR of the GHR gene with primers that bind in the introns on either side of exon 4. Thus, successful disruption of the GHR gene results in different length DNA fragments following PCR. In isolated IECs of IntGHRKO mice, exon 4 of the GHR gene was deleted. The GHR gene remained intact in tail samples from the IntGHRKO mice and isolated IECs and tail samples from floxed controls (Figure 1, middle).
Figure 1. Gene construct and knockout confirmation.
Top: GHR gene locus in GHR floxed mice, in which exon 4 of the GHR gene is flanked by LoxP sites. In cells with expressed Cre recombinase, exon 4 is excised from the genome, while cells that lack Cre can express functional GHR protein. Middle: PCR results showing GHR gene disruption. Cells that have exon 4 intact show ~1 kilobase bands, while cells with exon 4 excised show ~0.25 kilobase bands. Bottom: qPCR results showing GHR gene expression in different tissues. n=7, *signifies p<0.05. Abbreviations- Tail: tail sample, S.I.: isolated IECs from small intestines, L.I.: isolated IECs from large intestines.
RNA expression of the GHR gene (Figure 1, bottom) was used to further confirm the gene disruption and validate its tissue specificity. However, GHR expression was unchanged in the small intestine and liver, while the kidneys of InGHRKO mice had decreased GHR expression compared to the kidneys from control mice.
Body weight and composition
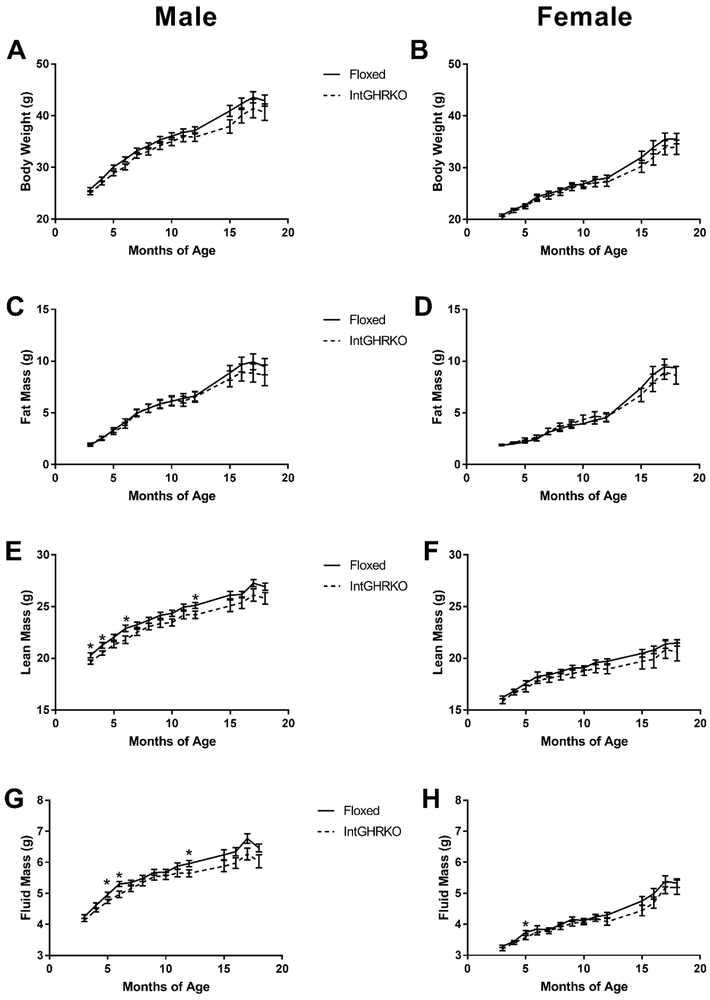
IntGHRKO mice, both males and females, showed no persistent change in body weight, fat, lean, or fluid mass (Figure 2). At a few isolated time points, male IntGHRKO mice showed a significant decrease in lean (3, 4, 6, and 12 months) and fluid (5, 6, and 12 months) mass, but these changes did not persist over time.
Figure 2. Body composition of IntGHRKO mice.
A-B: Body weights of IntGHRKO males (A) and females (B) and controls. C-D: Fat mass of IntGHRKO males (C) and females (D) and controls. E-F: Lean mass of IntGHRKO males (E) and females (F) and controls. G-H: Fluid mass of IntGHRKO males (G) and females (H) and controls. n≥42/sex/genotype at 2 months, n≥ 13/sex/genotype at 18 months, * indicates p<0.05.
Tissue size
Dissected organ weights (Tables 2 and 4) were unchanged in both male and female IntGHRKO mice at two ages, 4 months and 12 months. The organs measured included liver, kidney, heart, lung, brain, five different fat depots (perigonadal, subcutaneous, retroperitoneal, and mesenteric white adipose tissue and intrascapular brown adipose tissue), and three different muscles (gastrocnemius, soleus, and quadriceps) (Table 2, Table 4). Weights of the small and large intestine were also unchanged; however, the large intestinal length was decreased in IntGHRKO males at both ages, while the small intestine length remained unchanged. IntGHRKO females showed no change in length or weight of large or small intestine at either age (Table 3, Table 5).
Table 2.
Tissue weights of 4-month-old IntGHRKO mice and controls (expressed in grams ± S.E.M., n=10)
| Tissue | Male Floxed | Male IntGHRKO | p Value | Female Floxed | Female IntGHRKO | p Value | |
|---|---|---|---|---|---|---|---|
| Body Weight | 26.32±2.95 | 25.43±2.97 | 0.51 | 21.36±1.06 | 21.82±1.10 | 0.353 | |
| SI Weight | 0.97±0.14 | 0.91±0.09 | 0.29 | 0.91±0.10 | 0.98±0.14 | 0.253 | |
| LI Weight | 0.32±0.03 | 0.30±0.03 | 0.19 | 0.30±0.03 | 0.31±0.03 | 0.274 | |
| Liver | 0.93±0.13 | 0.97±0.06 | 0.41 | 0.84±0.06 | 0.82±0.04 | 0.287 | |
| Kidney | 0.33±0.05 | 0.30±0.02 | 0.16 | 0.25±0.02 | 0.26±0.02 | 0.274 | |
| Heart | 0.17±0.03 | 0.17±0.02 | 0.36 | 0.14±0.02 | 0.14±0.03 | 0.697 | |
| Gastrocnemius | 0.32±0.06 | 0.28±0.06 | 0.21 | 0.24±0.02 | 0.21±0.05 | 0.088 | |
| Soleus | 0.02±0.03 | 0.02±0.02 | 0.73 | 0.01±0.00 | 0.01±0.01 | 0.753 | |
| Quadriceps | 0.60±0.11 | 0.59±0.05 | 0.77 | 0.51±0.06 | 0.52±0.06 | 0.673 | |
| Subcutaneous Fat | 0.12±0.05 | 0.16±0.06 | 0.13 | 0.12±0.04 | 0.14±0.05 | 0.289 | |
| Perigonadal Fat | 0.23±0.10 | 0.30±0.12 | 0.16 | 0.15±0.07 | 0.18±0.09 | 0.386 | |
| Retroperitoneal Fat | 0.05±0.04 | 0.08±0.05 | 0.18 | 0.02±0.01 | 0.04±0.02 | 0.109 | |
| Mesenteric Fat | 0.13±0.06 | 0.13±0.09 | 0.82 | 0.07±0.03 | 0.09±0.03 | 0.118 | |
| Brown Fat | 0.06±0.01 | 0.06±0.02 | 0.44 | 0.05±0.01 | 0.05±0.02 | 0.939 | |
| Lung | 0.22±0.05 | 0.21±0.03 | 0.67 | 0.24±0.06 | 0.21±0.01 | 0.251 | |
| Brain | 0.45±0.01 | 0.45±0.02 | 0.77 | 0.46±0.02 | 0.46±0.03 | 0.812 |
Table 4.
Tissue weights of 12-month-old IntGHRKO mice and controls (expressed in grams ± S.E.M., n=10)
| Tissue | Male Floxed | Male IntGHRKO | p Value | Female Floxed | Female IntGHRKO | p Value | |
|---|---|---|---|---|---|---|---|
| Body Weight | 35.19±0.86 | 35.71±1.31 | 0.22 | 26.8±0.86 | 26.21±1.16 | 0.438 | |
| SI Weight | 0.978±0.03 | 0.917±0.05 | 0.17 | 0.967±0.05 | 0.869±0.06 | 0.213 | |
| LI Weight | 0.367±0.01 | 0.343±0.01 | 0.37 | 0.351±0.01 | 0.337±0.01 | 0.178 | |
| Liver | 1.119±0.04 | 1.206±0.05 | 0.81 | 0.836±0.08 | 0.913±0.03 | 0.73 | |
| Kidney | 0.3881±0.01 | 0.3538±0.01 | 0.26 | 0.3106±0.01 | 0.3059±0.01 | 0.569 | |
| Heart | 0.129±0.00 | 0.126±0.01 | 0.76 | 0.113±0.00 | 0.11±0.00 | 0.558 | |
| Gastrocnemius | 0.2916±0.01 | 0.2807±0.01 | 0.33 | 0.22422±0.01 | 0.2289±0.01 | 0.5 | |
| Soleus | 0.018±0.00 | 0.016±0.00 | 0.95 | 0.015±0.00 | 0.016±0.00 | 0.413 | |
| Quadriceps | 0.9013±0.04 | 0.7765±0.02 | 0.89 | 0.6959±0.02 | 0.6801±0.02 | 0.489 | |
| Subcutaneous Fat | 0.4±0.06 | 0.555±0.08 | 0.15 | 0.245±0.05 | 0.297±0.08 | 0.959 | |
| Perigonadal Fat | 0.9699±0.12 | 1.122±0.15 | 0.43 | 0.4043±0.09 | 0.4526±0.14 | 0.773 | |
| Retroperitoneal Fat | 0.356±0.06 | 0.372±0.05 | 0.83 | 0.108±0.04 | 0.134±0.05 | 0.847 | |
| Mesenteric Fat | 0.364±0.05 | 0.389±0.06 | 0.22 | 0.197±0.04 | 0.185±0.05 | 0.724 | |
| Brown Fat | 0.108±0.01 | 0.109±0.01 | 0.58 | 0.065±0.01 | 0.066±0.01 | 0.435 | |
| Lung | 0.165±0.01 | 0.159±0.01 | 0.79 | 0.158±0.00 | 0.157±0.01 | 0.991 | |
| Brain | 0.461±0.01 | 0.454±0.01 | 0.84 | 0.47±0.00 | 0.467±0.00 | 0.681 |
Table 3.
Tissue lengths of 4-month-old IntGHRKO mice and controls (expressed in centimeters ± S.E.M., n=10)
| Tissue | Male Floxed | Male IntGHRKO | p Value | Female Floxed | Female IntGHRKO | p Value | |
|---|---|---|---|---|---|---|---|
| Body Length | 9.19±0.39 | 9.38±0.35 | 0.27 | 8.87±0.29 | 8.82±0.26 | 0.693 | |
| SI Length | 36.70±2.21 | 35.60±2.41 | 0.30 | 35.30±2.54 | 36.67±1.32 | 0.171 | |
| LI Length | 9.70±0.82 | 8.80±0.92 | 0.03 | 9.40±0.84 | 9.33±0.71 | 0.773 |
Table 5.
Tissue lengths of 12-month-old IntGHRKO mice and controls (expressed in centimeters ± S.E.M., n=10)
| Tissue | Male Floxed | Male IntGHRKO | p Value | Female Floxed | Female IntGHRKO | p Value | |
|---|---|---|---|---|---|---|---|
| Body Length | 10.1±0.07 | 10.06±0.06 | 0.15 | 9.75±0.07 | 9.81±0.08 | 0.832 | |
| SI Length | 36.2±0.42 | 33.1±1.05 | 0.16 | 35.9±0.87 | 32.9±0.89 | 0.713 | |
| LI Length | 10±0.21 | 9.5±0.17 | 0.048 | 9.6±0.22 | 9.5±0.27 | 0.535 |
Villus height and crypt depth
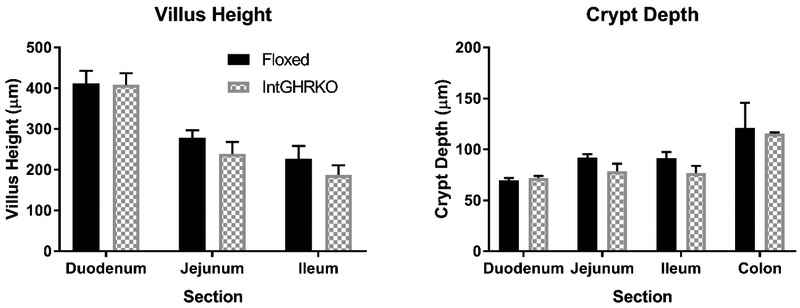
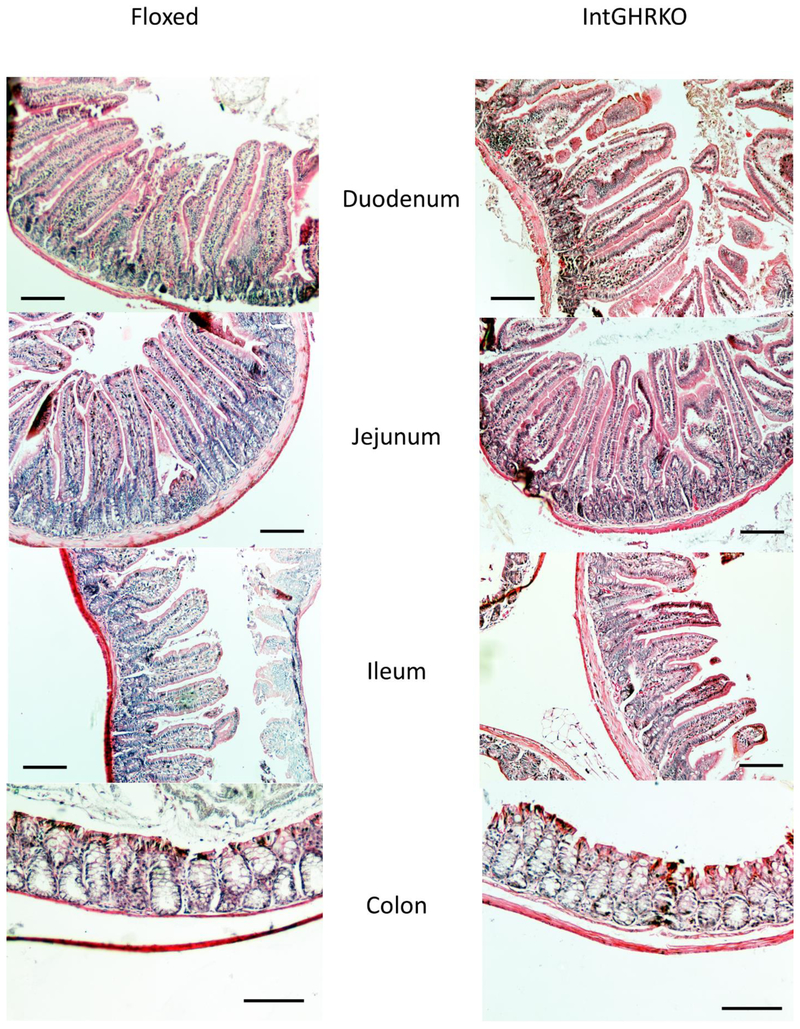
Villus height (in the small intestine) and crypt depth (in the small and large intestines) were used as measurements of mucosal morphology. Measurements were made in four regions of the intestines: duodenum, jejunum, ileum, and colon. IntGHRKO males had no significant changes to villus height in the duodenum, jejunum, or ileum, but there was a trend towards shorter villi in the IntGHRKO mice (Fig. 2 and 3). Crypt depth followed a similar pattern, with no significant changes in crypt depth in the duodenum, jejunum, ileum, or colon, but once again, a trend towards decreased crypt depth in the IntGHRKO mice.
Figure 3. Epithelial measurements.
Villus height and crypt depth of IntGHRKO and control mice in each section of small intestine or large intestine. (n=3/genotype, ≥10 villi or crypts per mouse)
Glucose metabolism
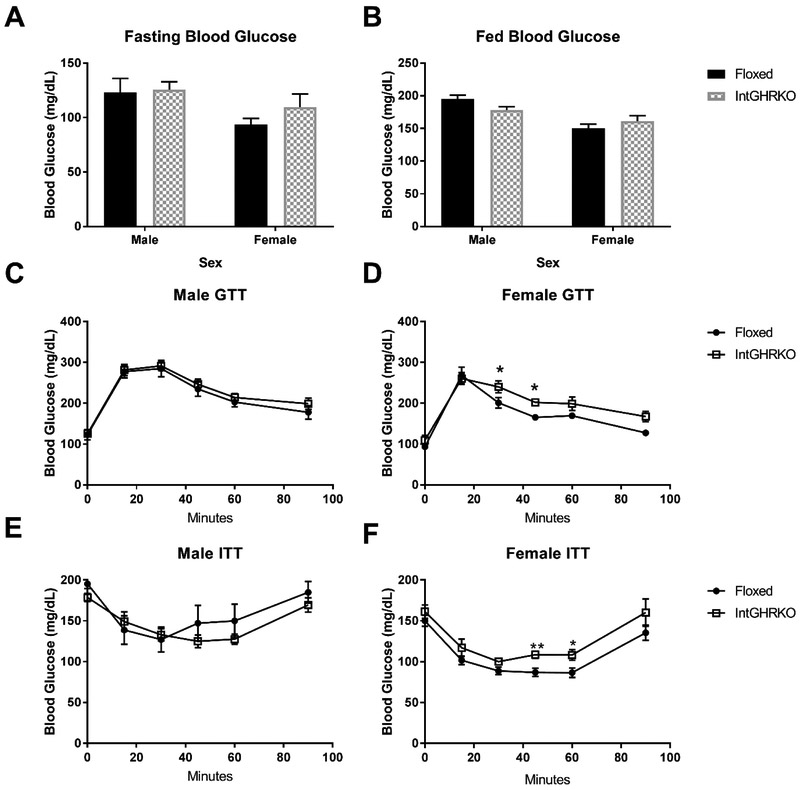
Male and female IntGHRKO mice were tested for glucose tolerance at seven months of age. When compared to floxed littermate controls, male mice had no significant change in glucose tolerance compared to controls (AUC 20716±1167 in IntGHRKO vs. 25259±1657 in Floxed, p=0.49) (Fig. 5 C-D). However, female IntGHRKO mice were glucose intolerant (area under the curve [AUC] 23403±786 in IntGHRKO vs. 19688±413 in Floxed, p<0.006).
Figure 5. Glucose metabolism.
A and B: Blood glucose measurements in the fasting and fed state. C and D: Male (C) and female (D) intraperitoneal glucose tolerance tests. E and F: Male (e) and female (f) insulin tolerance tests. (n=5-6, *indicates p<0.05)
IntGHRKO males showed no significant change in insulin tolerance at any time point. IntGHRKO females, on the other hand, showed a significant insulin resistance at the 45 and 60-minute time points (Fig. 5 E-F); however, when normalized to basal glucose levels to account for differences in basal glucose, no significant difference was detected. No difference in fasting or fed blood glucose levels was observed in either sex (Fig. 5 A-B).
Gut barrier
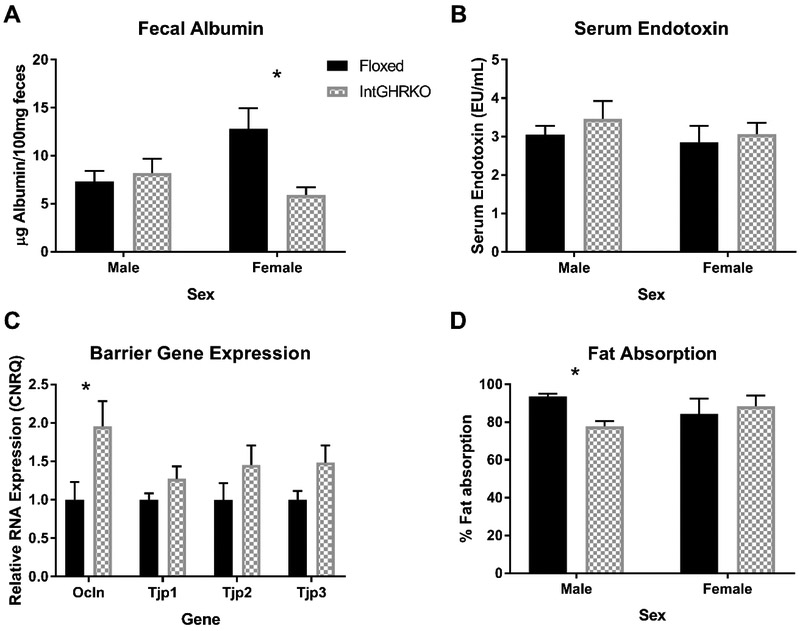
Gut barrier function was measured in three ways: 1) RNA expression of the tight junction-related genes occludin (gene symbol Ocln) and zona occludens 1, 2 and 3 (gene symbols Tjp1, Tjp2, and Tjp3), 2) fecal albumin, a marker for blood entering the lumen and 3) serum endotoxin, which serves as a marker for luminal contents entering the blood.
The qRT-PCR results of small intestine tissue in male IntGHRKO mice showed a significant increase in occludin expression when compared to controls, while no significant change was observed in the expression of the zonula occludens genes (Fig. 6 C). Despite the increase in occludin expression, no change was observed in males in the fecal albumin or serum endotoxin tests (Fig. 6 A and B). Female IntGHRKO mice showed decreased fecal albumin when compared to controls, while no change is observed in the serum endotoxin test.
Figure 6. Gut barrier function and Fat absorption.
A: Measurement of fecal albumin concentrations using bromocresol green assay (n=7). B: Serum endotoxin measurement using a LAL assay (n=6-10). C: RNA expression of gut barrier-related genes measured with quantitative PCR (n=7). D: A specialized diet using sucrose polybehenate was used to determine efficiency of fat absorption in the intestines. (n=3/sex/genotype). *indicates p<0.05
Intestinal fat absorption assay
The ability of the IntGHRKO mice to absorb fat during digestion was measured using a sucrose polybehenate assay. IntGHRKO males had significantly decreased fat absorption compared to floxed controls, while females had no difference (Fig. 6 D). Both groups of females had intermediate values compared to the extreme values in males, and the female mice had a much higher variation between individuals within each group, which might account for the lack of difference between the female groups.
DISCUSSION
Overall, the differences between IntGHRKO mice compared to controls were modest and sex specific. The only morphological change (gross or microscopic) observed in IntGHRKO mice was a male-specific decrease in large intestinal length, as the other intestinal lengths and weights and villus heights and crypt depths were unchanged compared to controls. Female IntGHRKO mice showed impaired glucose metabolism, while males had decreased fat absorption. Unexpectedly, IntGHRKO mice showed a weak improvement to gut barrier function. IntGHRKO mice show no change in body weight or fat mass, and no persistent change in fluid mass or lean mass.
Disruption of the GHR gene was validated using PCR of the GHR gene in the genomic DNA and qPCR of the GHR mRNA. Although the DNA and RNA results seem contradictory, the DNA extraction was performed on isolated IECs, while the RNA extraction was performed on the whole small intestine, without an IEC isolation. This difference was due to the length of the IEC isolation process, as RNA from isolated IECs was not of sufficient quality to perform qPCR. The unchanged small intestine GHR RNA expression could be explained by the presence of non-IECs in the intestine that retain an intact GHR gene.
Global disruption of the GHR gene results in dwarf mice that are highly insulin sensitive and serve as a model of Laron syndrome, which is the human disease characterized by GH insensitivity due to a GHR mutation. GHR has also been knocked out specifically in tissues such as liver [23], fat [25,36], muscle [24], and heart [33], as well as cell types, including pancreatic beta cells [37] and macrophages [38]. When GHR is disrupted individually in the major insulinsensitive tissues (liver, fat, and muscle), changes in the body composition are observed. For example, liver GHRKOs have decreased body weight [23] but also decreased fat mass, which is also seen in muscle GHRKO mice [24]. Meanwhile, fat specific knockouts (FaGHRKO and AdGHRKO) have increased fat mass, but only FaGHRKO mice have increased body weight. [27,36].
IntGHRKO mice, like other cell type-specific knockouts, showed no persistent change in body weight or fat mass. However, changes are seen in fluid mass. This change may be due to the reduced GHR expression in the kidneys, which presumably is a result of the expression of villin (and therefore, Cre recombinase in IntGHRKO mice) in the proximal tubule of the kidney [18]. Despite the changes in fluid mass observed in IntGHRKO mice, GH alterations in the proximal tubule should not cause alterations in water retention according to current knowledge about GH’s effects on the kidney. [39] GH’s effects on the nephron are either IGF-1 mediated (calcium and phosphate homeostasis) or limited to the distal tubule (sodium and water homeostasis). One exception to this is kidney gluconeogenesis, which will be discussed below.
Other results from this study support the weakness of the kidney phenotype in IntGHRKO mice. Although kidney GHR expression was reduced, no change in kidney weight was observed. One of the main GH-responsive genes in the proximal tubule of the kidney [40], 1-a-hydroxylase, showed no change in RNA expression in IntGHRKO mice compared to controls (0.44±0.5 in IntGHRKO vs 1±0.8 in floxed, p=0.18). These data indicate that the phenotype in the kidneys is mild; however, the possibility of the kidney GHR disruption affecting the other experimental results cannot be completely excluded.
Nearly all GHR gene disruptions, global or tissue specific, result in changes in glucose metabolism. Global GHRKO mice have improved glucose metabolism [41], as do the muscle specific GHR knockout animals [24]. In contrast, conditional knockouts in the liver, heart, pancreatic beta cells, and macrophages resulted in an impaired glucose homeostasis phenotype. Since the intestinal epithelium is crucial for nutrient absorption and, therefore, nutrient balance, it makes sense that alterations in the IECs could affect glucose homeostasis. In the current study, IntGHRKO mice had a range of glucose metabolism from normal (male mice) to impaired (females), which aligns the weak phenotype of the other cell-type specific (macrophage and beta cell) knockout lines, as both required a high fat diet for their changes in glucose metabolism to be observed [37,38]. Renal gluconeogenesis is a major source of circulating glucose in fasting conditions, but has minimal effect in a fed state.[39] Although the glucose tolerance tests in this study were conducted in fasted mice, the altered glucose metabolism in females is also observed in the insulin tolerance tests, which occurred in a fed state. If alterations to renal gluconeogenesis were occurring in the IntGHRKO mice, a change in fasting glucose would be expected; however, none was observed, indicating that the alterations to glucose metabolism seen in IntGHRKO females were kidney-independent.
It has previously been reported [21] that excess GH not only increases small intestinal length, but also the mucosal mass, including increased villus height and crypt depth. As a result, the intestinal length and weight, as well as the villus height and crypt depth of IntGHRKO mice were compared to floxed littermate controls. The small intestine length of IntGHRKO mice were unchanged compared to controls, while large intestine length was decreased in IntGHRKO males. Since IntGHRKO mice only have intestinal GHR disruption in the epithelial cells, this indicates that the effects of GH on intestinal length are primarily due to GH actions on the subepithelial layers of the intestine, or that GH is not directly involved. Despite the GHR disruption in the epithelial cells, no significant change was seen in the measures of mucosal morphology, the villus height, and crypt depth. This lack of difference contrasts to what is seen in transgenic mice with excess GH (bGH mice), which show increased villus height and crypt depth (unpublished data). This supports the notion that GH action in the subepithelial layers of the intestine is the major determinant of GH responses in intestinal morphology.
When STAT5, a downstream effector of GHR, is knocked out in the IECs, a gut barrier defect is the result [8,42]. Therefore, the gut barrier function of IntGHRKO mice was examined with two physiological tests and measurement of the RNA expression of gut barrier genes. In our study, the expression of tight junction genes occludin (Ocln), as well as Zonula occludens 1, 2, and 3 (Tjp1, Tjp2, and Tjp3) were measured at the RNA level. IntGHRKO male mice have a significant increase in occludin expression in the small intestine, indicating that they may have a capacity for improved barrier function, although the other tight junction genes were unchanged. When the barrier function was measured with physiological tests including fecal albumin and serum endotoxin, no difference was seen in males in either test, while females had decreased fecal albumin, indicating a possible negative effect of GH on this aspect of the intestinal barrier.
The IntGHRKO mice have male-specific impaired intestinal fat absorption. This impairment occurs despite having no change in small intestinal length, which is notable considering intestinal fat absorption is heavily influenced by intestinal length, as demonstrated by the steatorrhea in short bowel syndrome [43]. IntGHRKO mice also have no change in intestinal weight, villus height, or crypt depth, which in combination with the intestine length results indicates that the IntGHRKO mice may have similar epithelial surface area to controls. Despite the decreased fat absorption, no change is seen in body mass, fat mass, or the mass of individual fat depots. This may be due to the nature of the assay used to measure fat absorption: the assay can only measure the amount of fat that has left the intestinal lumen, but it does not distinguish between different fates of the fat, whether it accumulates in the enterocytes, is processed into chylomicrons, or follows some other pathway.
As a whole, IntGHRKO mice are similar to some of the other cell-type specific (macrophage and beta cell) GHR knockout mouse lines in that a relatively mild phenotype is seen. The changes that are seen in IntGHRKO mice generally agree with the results expected based on previous studies in this area, with effects seen on intestinal growth and the intestinal barrier. Future work is needed to better understand the difficult to explain results, namely the discrepancy between the gut barrier gene expression and the physiological test of barrier function, as well the counterintuitive changes seen in the fat absorption assay. Further experiments also would include challenging the IntGHRKO mice with a high fat diet, which has been successful in amplifying the phenotype in other cell type-specific GHR knockout lines. [37,38]
Figure 4. Intestinal morphology.
Micrographs (100x magnification) of H&E stained sections of intestines from 24 month old male IntGHRKO mice and littermate controls. Scale bars signify 100μm.
HIGHLIGHTS.
IntGHRKO mice had normal gross morphology in most tissues
Sex-specific changes in the gut barrier were observed in IntGHRKO mice
Glucose metabolism was impaired in IntGHRKO females
IntGHRKO males had decreased fat absorption
IntGHRKO mice had similar microscopic morphology to controls
ACKNOWLEDGEMENTS
This work was supported by NIH grant #AG059779, by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, by AMVETS, and by the Diabetes Institute at Ohio University
ABBREVIATIONS
- GH
Growth hormone
- GHR
Growth hormone receptor
- rhGH
Recombinant human GH
- IBD
Inflammatory bowel disease
- STAT5
Signal transducer and activator of transcription 5
- IntGHRKO
Intestinal epithelial cell-specific GHR knockout mice
- IEC
Intestinal epithelial cell
- GTT
Glucose tolerance test
- ITT
Insulin tolerance test
- IP
Intraperitoneal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
REFERENCES
- [1].Delehaye-Zervas MC, Mertani H, Martini JE, Nihoul-Feketé C, Morel G, Postel-Vinay MC, Expression of the growth hormone receptor gene in human digestive tissue., J. Clin. Endocrinol. Metab. 78 (1994) 1473–1480. doi: 10.1210/jcem.78.6.8200952. [DOI] [PubMed] [Google Scholar]
- [2].Byrne TA, Wilmore DW, Iyer K, Dibaise J, Clancy K, Robinson MK, Chang P, Gertner JM, Lautz D, Growth Hormone, Glutamine, and an Optimal Diet Reduces Parenteral Nutrition in Patients With Short Bowel Syndrome, Ann. Surg. 242 (2005) 655–661. doi: 10.1097/01.sla.0000186479.53295.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Seguy D, Vahedi K, Kapel N, Souberbielle JC, Messlng B Low-dose growth hormone in adult home parenteral nutrition-dependent short bowel syndrome patients: A positive study, Gastroenterology. 124(2003) 293–302. doi: 10.1053/gast.2003.50057. [DOI] [PubMed] [Google Scholar]
- [4].Tavakkolizadeh A, Shen KR, Jasleen J, Soybel DI, Jacobs DO, Zinner MJ, Ashley SW, Whang EE Effect of Growth Hormone on Intestinal Na+/Glucose Cotransporter Activity, J. Parenter. Enter. Nutr 25 (2001) 18–22. doi: 10.1177/014860710102500118. [DOI] [PubMed] [Google Scholar]
- [5].Slonim AEE, Bulone L, Damore MBB, Goldberg T, Wingertzahn MAA, McKinley MJJ, A preliminary study of growth hormone therapy for Crohn’s disease, NEngl J Med. 342 (2000) 1633–1637. doi: 10.1056/NEJM200006013422203. [DOI] [PubMed] [Google Scholar]
- [6].Denson LA, Kim M-O, Bezold R, Carey R, Osuntokun B, Nylund C, Willson T, Bonkowski E, Li D, Ballard E, A Randomized Controlled Trial of Growth Hormone in Active Pediatric Crohn’s Disease, J. Pediatr. Gastroenterol. Nutr. 51 (2010) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yue C, Wang W Tian WL, Huang Q Zhao RS, Zhao YZ, Li QR, Li JS, Lipopolysaccharide-induced failure of the gut barrier is site-specific and inhibitable by growth hormone, Inflamm. Res. 62 (2013) 407–415. doi: 10.1007/s00011-013-0593-4. [DOI] [PubMed] [Google Scholar]
- [8].Gilbert S, Zhang R, Denson LA, Moriggl R, Steinbrecher K, Shroyer NF, Lin J, Han X, Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation, EMBO Mol. Med. 4 (2012) 109–124. doi: 10.1002/emmm.201100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gilbert S, Nivarthi H, Mayhew CN, Lo Y-H, Noah TK, Vallance J, Rülicke T, Müller M, Jegga AG, Tang W, Zhang D, Helmrath M, Shroyer NF, Moriggl R, Han X, Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration, Stem Cell Reports. 4 (2015)209–225. doi: 10.1016/j.stemcr.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Resmini E, Parodi A, Savarino V, Greco A, Rebora A, Minuto F, Ferone D, Evidence of Prolonged Orocecal Transit Time and Small Intestinal Bacterial Overgrowth in Acromegalic Patients, J. Clin. Endocrinol. Metab. 92(2007) 2119–2124. doi: 10.1210/jc.2006-2509. [DOI] [PubMed] [Google Scholar]
- [11].Thomas LA, Veysey MJ, Murphy GM, Russell-Jones D, French GL, Wass JAH, Dowling RH, Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly., Gut. 54 (2005) 630–5. doi: 10.1136/gut.2003.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Renehan AG, Brennan BM, Acromegaly, growth hormone and cancer risk, Best Pract. Res. Clin. Endocrinol. Metab. 22 (2008) 639–657. doi: 10.1016/j.beem.2008.08.011. [DOI] [PubMed] [Google Scholar]
- [13].Rokkas T, Pistiolas D, Sechopoulos P, Margantinis G, Koukoulis G, Risk of colorectal neoplasm in patients with acromegaly: A meta-analysis, World J. Gastroenterol. 14 (2008) 3484–3489. doi: 10.3748/wjg.14.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chesnokova V, Zonis S, Zhou C, Recouvreux MV, Ben-Shlomo A, Araki T, Barrett R, Workman M, Wawrowsky K, Ljubimov VA, Uhart M, Melmed S, Growth hormone is permissive for neoplastic colon growth, Proc. Natl. Acad. Sci. 113 (2016) 201600561. doi: 10.1073/pnas.1600561113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gordon MB, Nakhle S, Ludlam WH, Patients with Acromegaly Presenting with Colon Cancer: A Case Series, Case Rep. Endocrinol 2016(2016) 1–4. doi: 10.1155/2016/5156295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yamamoto M, Fukuoka H, Iguchi G, Matsumoto R, Takahashi M, Nishizawa H, Suda K, Bando H, Takahashi Y, The prevalence and associated factors of colorectal neoplasms in acromegaly: a single center based study, Pituitary. 18 (2015) 343–351. doi: 10.1007/s11102-014-0580-y. [DOI] [PubMed] [Google Scholar]
- [17].Wassenaar MJE, Cazemier M, R Biermasz N, Pereira AM, Roelfsema F, Smit JWA, Hommes DW, Felt-Bersma RJF, Romijn JA, Acromegaly is associated with an increased prevalence of colonic diverticula: A case-control study, J. Clin. Endocrinol. Metab. 95 (2010) 2073–2079. doi: 10.1210/jc.2009-1714. [DOI] [PubMed] [Google Scholar]
- [18].Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL, cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine, J. Biol. Chem. 277 (2002) 33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- [19].Rowland KJ, Trivedi S, Lee D, Wan K, Kulkami RN, Holzenberger M, Brubaker PL, Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion, Gastroenterology. 141(2011) 2166–2175.e7. doi: 10.1053/j.gastro.2011.09.014. [DOI] [PubMed] [Google Scholar]
- [20].Andres SF, Santoro MA, Mah AT, Keku JA, Bortvedt AE, RE. Blue, P.K.Lund, Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs, Am. J. Physiol. Gastrointest. Liver Physiol. 308 (2015) G100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ulshen MH, Dowling RH, Fuller CR, Zimmermann EM, Lund PK, Enhanced growth of small bowel in transgenic mice overexpressing bovine growth hormone, Gastroenterology. 104 (1993) 973–980. doi: 10.5555/uri:pii:001650859390263C. [DOI] [PubMed] [Google Scholar]
- [22].Jeschke MG, Herndon DN, Finnerty CC, Bolder U, Thompson JC, Mueller U, Wolf SE, Przkora R, The effect of growth hormone on gut mucosal homeostasis and cellular mediators after severe trauma, J. Surg. Res. 127 (2005) 183–189. doi: 10.1016/j.jss.2005.02.008. [DOI] [PubMed] [Google Scholar]
- [23].List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W Mastemak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ, Liver-Specific GH Receptor Gene-Disrupted (LiGHRKO) Mice Have Decreased Endocrine IGF-I, Increased Local IGF-I, and Altered Body Size, Body Composition, and Adipokine Profiles, Endocrinology. 155 (2014) 1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].List EO, Berryman DE, Ikeno Y, Hubbard GB, Funk K, Comisford R, Young JA Stout MB, Tchkonia T, Mastemak MM, Bartke A, Kirkland JL, Miller RA, Kopchick JJ, Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR−/− mice., Aging (Albany. NY). 7 (2015) 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H Vesel C, Junnila RK, Frank SJ, Mastemak MM, Bartke A, Kopchick JJ, The Role of GH in Adipose Tissue: Lessons from Adipose-Specific GH Receptor Gene-Disrupted Mice, Mol. Endocrinol. 27 (2013) 524–535. doi: 10.1210/me.2012-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zeineldin M, Cunningham J, McGuinness W, Alltizer P, Cowley B, Blanchat B, Xu W, Pinson D, Neufeld KL, A knock-in mouse model reveals roles for nuclear Apc in cell proliferation, Wnt signal inhibition and tumor suppression, Oncogene. 31 (2012) 2423–2437. doi: 10.1038/onc.2011.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Mastemak MM, Bartke A, Kopchick JJ, The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice., Mol. Endocrinol. 27(2013)524–35. doi: 10.1210/me.2012-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J, qBase relative quantification framework and software for management and automated analysis of realtime quantitative PCR data., Genome Biol 8 (2007)R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Young JA Jara A, Kopchick JJ, Selection of Stable Reference Genes for RT-qPCRin Mouse Intestinal Tissue, Endocr. Rev. 35 (2014). [Google Scholar]
- [30].Williams JM, Duckworth CA, Vowell K, Burkitt MD, Pritchard DM, Williams JM, Duckworth CA, Vowell K, Vowell K, Burkitt MD, Pritchard DM, Intestinal preparation techniques for histological analysis in the mouse Curr. Protoc. Mouse Biol, John Wiley & Sons, Inc, Hoboken, NJ, USA: 2016, pp. 148–168, 10.1002/cpmo.2 [DOI] [PubMed] [Google Scholar]
- [31].Williams KL, Fuller CR, Fagin J, Lund PK, Mesenchymal IGF-I overexpression: paracrine effects in the intestine, distinct from endocrine actions, Am. J. Physiol. Liver Physiol. 283 (2002) G875–G885. doi: 10.1152/ajpgi.00089.2002. [DOI] [PubMed] [Google Scholar]
- [32].List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Mastemak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ, Liver-Specific gh receptor gene-Disrupted (lighrko) mice have decreased endocrine igf-I, increased local igf-I, and altered body size, body composition, and adipokine profiles, Endocrinology. 155 (2014) 1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jara A, Liu X, Sim D, Benner CM, Duran-Ortiz S, Qian Y, List EO, Berryman DE, Kim JK, Kopchick JJ, Cardiac-specific disruption of GH receptor alters glucose homeostasis while maintaining normal cardiac performance in adult male mice, Endocrinology. (2016) en.2015–1686. doi: 10.1210/en.2015-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Doumas BT, Watson WA, Biggs HG, Albumin standards and the measurement of serum albumin with Bromocresol Green, Clin. Chim. Acta. 31 (1971) 87–96. doi: 10.1016/S0009-8981(96)06447-9. [DOI] [PubMed] [Google Scholar]
- [35].Jandacek RJ, Heubi JE, Tso P, A novel, noninvasive method for the measurement of intestinal fat absorption, Gastroenterology. 127 (2004) 139–144. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- [36].List EO, Berryman DE, Buchman M, Parker C, Funk K, Bell S, Duran-Ortiz S, Qian Y, Young JA, Wilson C, Slyby J, McKenna S, Jensen EA, Kopchick JJ, Adipocyte-specific GH receptor null (AdGHRKO) mice have enhanced insulin sensitivity with reduced liver triglycerides, Endocrinology. 160 (2019)68–80. doi: 10.1210/en.2018-00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu Y, Liu C, Sun H, Vijayakumar A, Giglou PR, Qiao R, Oppenheimer J, Yakar S, LeRoith D, Growth hormone receptor regulates beta cell hyperplasia and glucose-stimulated insulin secretion in obese mice, J. Clin. Invest. 121 (2011)2422–2426. doi: 10.1172/JCI45027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu C, Kumar PA, Sun J, Aggarwal A, Fan Y, Sperling MA, Lumeng CN Menon RK, Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity, J. Biol. Chem. 288 (2013) 15725–15735. doi: 10.1074/jbc.M113.460212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kamenický P, Mazziotti G, Lombès M, Giustina A, Chanson P, Growth Hormone, Insulin-Like Growth Factor-1, and the Kidney: Pathophysiological and Clinical Implications, Endocr. Rev. 35 (2014) 234–281. doi: 10.1210/er.2013-1071. [DOI] [PubMed] [Google Scholar]
- [40].Zoidis E, Gosteli-Peter M, Ghirlanda-Keller C, Meinel L, Zapf J, Schmid C, IGF-I and GH stimulate Phex mRNA expression in lungs and bones and 1,25-dihydroxyvitamin D(3) production in hypophysectomized rats., Eur. J. Endocrinol. 146 (2002) 97–105. [DOI] [PubMed] [Google Scholar]
- [41].List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J Cruz-Topete D, Kopchick JJ, Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse, Endocr. Rev. 32 (2011) 356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Han X, Ren X, Jurickova I, Groschwitz K, Pasternak BA, Xu H, Wilson TA, Hogan SP, Denson LA, Regulation of intestinal barrier function by signal transducer and activator of transcription 5b, Gut. 58 (2009) 49–58. doi: 10.1136/gut.2007.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seetharam P, Rodrigues G, Short bowel syndrome: a review of management options., Saudi J. Gastroenterol. 17 (2011) 229–35. doi: 10.4103/1319-3767.82573. [DOI] [PMC free article] [PubMed] [Google Scholar]