Abstract
Objectives.
Knee osteoarthritis (OA) is more common in women, and may be related to reproductive or hormonal factors. We evaluated these factors with the risk of total knee replacement (TKR) for severe knee OA among women.
Methods.
The Singapore Chinese Health Study recruited 63,257 Chinese aged 45–74 years from 1993 to 1998, and among them, 35,298 were women. Information on height, weight, lifestyle factors, number of biological children, ages at menarche and menopause, and use of hormonal therapies was collected through interviews. Incident cases of TKR were identified via linkage with nationwide database.
Results.
There were 1,645 women with TKR after mean follow-up of 14.8 years. Higher parity was associated with increased TKR risk in a stepwise manner (P for trend <0.001). Compared to nulliparous women, those with ≥ 5 children had the highest risk [hazard ratio (HR) 2.01, 95% confidence interval (CI) 1.50–2.70]. The effect of parity on TKR risk was significantly stronger among lean women compared to heavier women; HRs (95% CIs) for highest parity was 4.86 (2.22–10.63) for women with BMI <23 kg/m2 and 1.57 (1.14–2.14) for those ≥23 kg/m2 (p for interaction = 0.001). Earlier age at menarche and use of oral contraceptives were significantly associated with TKR in a stepwise manner (P for trend ≤0.002). Age at menopause and use of hormonal therapy were not associated with TKR risk.
Conclusion.
Higher parity, earlier age of menarche and use of oral contraceptives were associated with increased risk of TKR for severe knee OA among women.
Keywords: Knee osteoarthritis, cohort study, reproductive factors, parity
Introduction
Although knee osteoarthritis (OA) is one of the leading causes of disability [1], treatment options are limited partly due to incomplete understanding of its pathogenesis [2]. Interestingly, meta-analysis of studies mainly from Western populations has shown that the greatest disparity in incidence and prevalence of OA between men and women is in the knee joint, with women experiencing higher rates and also a greater severity of disease compared to men, particularly after menopausal age [3, 4]. These observations have led investigators to hypothesize that reproductive and hormonal factors, either endogenous or exogenous, may play a role in the development of knee OA among women [3].
Hitherto, epidemiologic studies on the association between reproductive and hormonal factors, and risk of knee OA have been conflicting. While earlier cross-sectional and case-control studies failed to find any association between parity and knee OA [5–7], a large cohort study involving 1.3 million women from the United Kingdom (UK) has shown positive associations of early age at menarche and increasing parity with incidence of total knee replacement (TKR) [8]. Another Danish cohort with 4.5 million participants demonstrated an increased risk of first hospitalization for OA with higher number of children in both men and women, but the association was most notable for knee OA in women (relative risk 1.10 per child) [9]. A recent study from Norway involving over 3 million women reported that the risk of TKR decreased with older age at menarche but was not associated with parity [10].
In the aforementioned studies, it was unclear whether the risk of TKR associated with parity could be mediated by an increase in body weight and change in body habitus related to childbirth. As shown in our previous cohort study [11] and other studies [12, 13], body mass index (BMI) is a strong risk factor for severe knee OA. Therefore, epidemiological studies in populations with low BMI are needed to minimize the potential confounding effect of obesity on the association between reproductive factors and knee OA in women. In this current study, we evaluated the effects of parity, age at menarche, age at menopause and hormonal therapies among women in the Singapore Chinese Health Study, a cohort in which participants were relatively leaner than those in Western studies.
Study participants and Methods
Study population
The Singapore Chinese Health Study is a prospective cohort study of 63,257 Chinese (27,959 men and 35,298 women) aged 45–74 years, and enrolled from 1993 to 1998 in Singapore [14]. All subjects were recruited from public housing estates where 86% of the Singapore population resided at the time of recruitment.
Consent and ethical approval
This study was approved by the Institutional Review Board at the National University of Singapore. All participants signed informed consents prior to participation.
Baseline exposure assessment
Participants were interviewed in-person using a structured questionnaire at recruitment. Information collected included education level, habitual diet, cigarette smoking, alcohol consumption, comorbidities, habitual physical activity, number of biological children, as well as reproductive history for women. For both genders, participants were asked “How many children have you had? (include only and all biological children)”. Women were asked to report their age at menarche (<11, 11–12, 13–14, 15–16 years, 17+ years) and their age at first live birth, if applicable (<15, 15–17, 18–20, 21–25, 26–30, 31–35, 36+ years). Women were also asked whether they ever had hysterectomy (yes or no). For those who did not have a hysterectomy, they were asked about their age at menopause with these questions: “Have your menstrual periods stopped permanently?” (yes or no); if yes, “how old were you when this happened?” (<40, 40–44, 45–49, 50–54, 55+ years). For the use of hormonal therapies, women were asked if they had ever taken oral birth control pills (yes or no) or hormone replacement therapy (HRT) (no or yes, currently taking, or yes, but I no longer take them), and the duration of use for each therapy (<1, 1–2, 3–5, 6–9, 10–14, 15–19, 20+ years).
Body weight and height were self-reported and BMI was computed by weight in kilograms divided by height in meters squared (kg/m2). A total of 10,349 cohort subjects (16%), which included 6,375 women (18% of women), did not report either weight and/or height, and their BMI was imputed from weight and/or height obtained from the linear regression equation: weight = y-intercept + gradient × height, where values for the y-intercept and gradient were derived from gender-specific weight-height regression lines obtained from all subjects with known heights and weights [15]. Participants were also asked about the history of physician-diagnosed hypertension, diabetes, coronary artery disease and stroke in separate questions.
Identification of incident cases of TKR
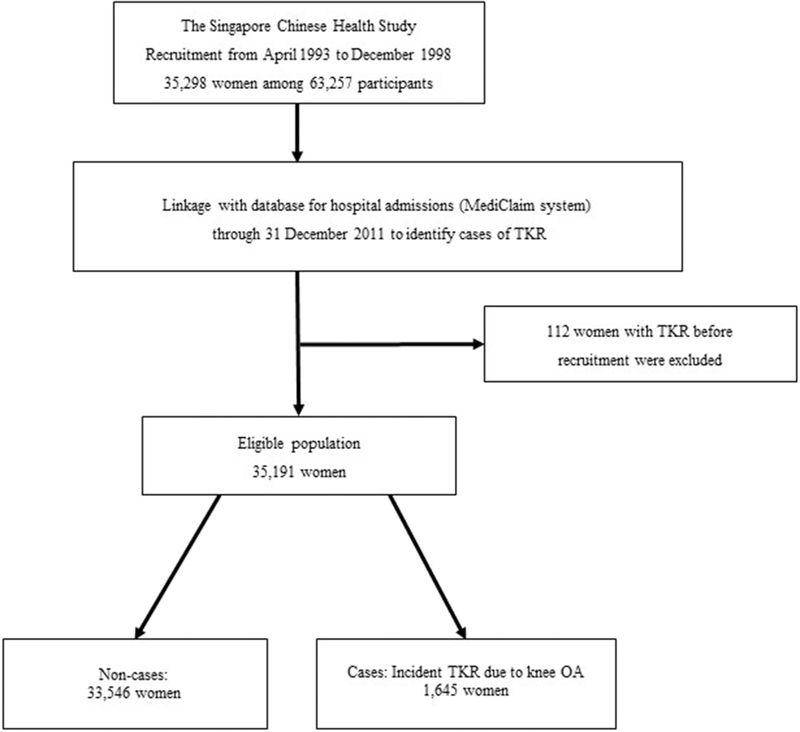
We identified participants who underwent TKR due to severe knee OA via record linkage with the nationwide MediClaim System hospital discharge database through 31 December 2011. The nationwide MediClaim system has captured information about all hospitalizations, including in-hospital surgical procedures, in Singapore since 1990, and collects up to three discharge diagnoses per patient. Although this database is used for consequent financial claims, and hence its name, it captures comprehensive information on all hospitalizations in both public and private hospitals in Singapore, regardless whether financial claims are made subsequently. The Singapore Ministry of Health (MOH) conducts regular check on the MediClaims Database to ensure its completeness [16, 17]. Non-compliance with timely submission of information on surgical procedures or discharge diagnoses can lead to suspension of the approval granted to any accredited hospital under the MOH’s demerit points framework [18]. Our case selection process began with identifying cases of TKR with the operation codes SB010K (unilateral right TKR), SB012K (unilateral left TKR), and SB013K (bilateral TKR), that were captured in the database for the first time. We excluded 128 prevalent cases of TKR that had occurred prior to subject enrolment into the cohort. Discharge diagnoses were captured based on the International Classification of Disease Codes Version 9 (ICD-9). We included only incident TKR cases with a diagnosis of OA (ICD-9 code 715), and excluded other TKR cases due to other diagnoses (n=89), such as septic arthritis, osteomyelitis, villonodular synovitis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and other inflammatory arthritis, or secondary causes of knee OA such as avascular or aseptic necrosis of joint, meniscus or ligament injuries, and other congenital or acquired deformities of the knee (Figure 1). In Singapore, a study in a public hospital has reviewed over 1,600 medical records and reported that primary knee OA accounted for 96% of the TKR cases [19]. Hence, although it was not specified for many cases in the database if the knee OA was primary or secondary in nature, we believe that almost all the TKR cases in this middle-aged and elderly cohort would be primary OA. We identified death of participants through record linkage with the Singapore Registry of Births and Deaths. As of December 2011, only 47 subjects were known to be lost to follow-up due to migration or other reasons, suggesting the ascertainment of vital status for cohort subjects was virtually complete.
Figure 1.
Flow diagram showing inclusion criteria for analysis and case selection in the Singapore Chinese Health Study.
TKR = total knee replacement surgery; OA = osteoarthritis.
Statistical analysis
The analyses of current study were performed on 35,191 women, and included 1,645 incident cases of TKR for severe knee OA. Number of person-years for each participant was calculated from date of recruitment until time of death, lost-to-follow-up, TKR, or December 31, 2011, whichever came first. We used Cox proportional hazards regression to estimate hazard ratios (HRs) and respective 95% confidence intervals (CIs) for the association of TKR risk with number of biological children, age at first birth, age at menarche, age at menopause, use of oral contraceptives and HRT. Proportional hazard assumption was tested using Schoenfeld residuals test, and no violation was observed for these variables. Tests for trend were carried out by including ordinal values of categorial exposures of interest as continuous variables in the regression models.
In multivariable model 1, we adjusted for age at interview (years), dialect (Hokkien, Cantonese), year of interview (1993–1995, 1996–1998), educational level (none, primary, secondary and more), smoking status (never, past, current), BMI (kg/m2), total physical activity (hours/week), sleep (hours/day) and sitting (hours/day) duration, baseline history of self-reported, diabetes, hypertension, coronary artery disease, and stroke. In multivariable model 2, we further adjusted for number of children (0, 1, 2, 3, 4, 5+), age at menarche (<13, 13–14, 15–16, 17+ years), oral contraceptive use (never, ever), HRT use (never, ever), and age at menopause (still menstruating, <40, 40–44, 45–49, 50–54, 55+ years). We conducted separate analysis for the associations with parity and age at first birth only among women who had ever given birth, and adjusted for both factors in the same model.
To avoid over-adjustment, we performed sensitivity analyses by removing all covariates that did not have p-values <0.20 for their risk estimates in order to create a parsimonious model. To minimize bias from possible mediators or colliders affecting the total effect of reproductive factors on risk of TKR, we determined a minimally sufficient set of covariates using directed acyclic graphs (DAG) [20] that provided visual representations of possible causal assumptions (DAGitty v3.0) [21], and repeated statistical analyses using this set of covariates in the Cox model. To evaluate the role of BMI as an interaction factor, we added cross-product terms between our exposures of interest and BMI categories as covariates into the Cox models. For stratified analysis by BMI categories, we used the cut-point recommended by the World Health Organization (WHO) to create underweight/normal (<23 kg/m2) and overweight/obese (≥23 kg/m2) categories that define cardiovascular risk in Asians [22]. We also conducted sensitivity analysis by excluding all subjects with imputed BMI due to missing weight and/or height (6,375 women) for the interaction with BMI on TKR risk.
All statistical analyses were conducted using SAS Version 9.4 (SAS Institute, Inc., Cary, North Carolina). All reported p values were two-sided, and p <0.05 was considered statistically significant.
Results
Table 1 shows characteristics of women in the extreme groups according to number of children (nulliparous versus 5+), age at menarche (≥17 versus <13 years), age at menopause (<40 versus ≥55 years) and use of oral contraceptives (no versus yes). Women who had more children were older and less educated, had higher BMI and were more likely to be ever smokers at recruitment. They were also more likely to have a history of hypertension, diabetes, coronary artery disease or stroke. Women with earlier age at menarche were younger at recruitment and had higher educational level; they were also more likely to have ever used oral contraceptives or HRT, but had lower smoking prevalence and fewer children. Women who had a history of oral contraceptive use for at least one year were younger, better educated, less likely to smoke and more likely to have used HRT. Among postmenopausal women, those with older age at menopause were older at recruitment, less educated, less likely to have ever smoked and more likely to have had more children. After a mean follow-up of 14.8 (standard deviation 4.0) years, we identified 1,645 cases of TKR. Compared to the rest of the women in the cohort, cases of TKR were older at recruitment, had higher BMI, had lower level of education, and were less likely to be current smokers. They were also more likely to have hypertension but less likely to have diabetes (see Supplementary Table 1).
Table 1.
Study population characteristics of women according to number of children, age at menarche, oral contraceptive use, and age at menopause
| Number of children | Age at menarche (years) | Oral contraceptives | Age at menopause (years) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5+ | ≥17 | <13 | No | Yes | <40 | ≥55 | |
| n | 2507 | 9756 | 4491 | 5034 | 25905 | 9280 | 894 | 1921 |
| Age at interview, years | 55.1 ± 8.1 | 62.1 ± 6.7 | 59.7 ± 7.7 | 52.8 ± 6.9 | 57.4 ± 8.3 | 53.3 ± 6.2 | 57.8 ± 8.0 | 62.5 ± 5.0 |
| Body mass index, kg/m2 | 22.5 ± 3.5 | 23.6 ± 3.3 | 23.1 ± 3.2 | 23.4 ± 3.5 | 23.2 ± 3.3 | 23.3 ± 3.3 | 23.4 ± 3.6 | 23.5 ± 3.3 |
| Dialect group, n (%) | ||||||||
| Hokkien | 1532 (61.1) | 3491 (35.8) | 1696 (37.8) | 3146 (62.5) | 12057 (46.5) | 4824 (52.0) | 400 (44.7) | 1038 (54.0) |
| Cantonese | 975 (38.9) | 6265 (64.2) | 2795 (62.2) | 1888 (37.5) | 13848 (53.5) | 4456 (48.0) | 494 (55.3) | 883 (46.0) |
| Education, n (%) | ||||||||
| None | 656 (26.2) | 6563 (67.3) | 2699 (60.1) | 1046 (20.8) | 11456 (44.2) | 2739 (29.5) | 426 (47.7) | 1032 (53.7) |
| Primary | 879 (35.1) | 2840 (29.1) | 1467 (32.7) | 1793 (35.6) | 9361 (36.1) | 4345 (46.8) | 297 (33.2) | 717 (37.3) |
| Secondary school or higher | 972 (38.8) | 353 (3.6) | 325 (7.2) | 2195 (43.6) | 5088 (19.6) | 2196 (23.7) | 171 (19.1) | 172 (9.0) |
| Smoking, n (%) | ||||||||
| Never | 2269 (90.5) | 8338 (85.5) | 3985 (88.7) | 4707 (93.5) | 23430 (90.4) | 8659 (93.3) | 774 (86.6) | 1714 (89.2) |
| Former | 88 (3.5) | 380 (3.9) | 153 (3.4) | 99 (2.0) | 714 (2.8) | 180 (1.9) | 32 (3.6) | 72 (3.8) |
| Current | 150 (6.0) | 1038 (10.6) | 353 (7.9) | 228 (4.5) | 1761 (6.8) | 441 (4.8) | 88 (9.8) | 135 (7.0) |
| Hypertension, n (%) | 507 (20.2) | 2969 (30.4) | 1081 (24.1) | 1201 (23.9) | 6246 (24.1) | 2194 (23.6) | 243 (27.2) | 663 (34.5) |
| Diabetes, n (%) | 164 (6.5) | 1445 (14.8) | 462 (10.3) | 434 (8.6) | 2503 (9.7) | 746 (8.0) | 120 (13.4) | 263 (13.7) |
| Coronary artery disease, n (%) | 65 (2.6) | 560 (5.7) | 189 (4.2) | 147 (2.9) | 970 (3.7) | 252 (2.7) | 64 (7.2) | 96 (5.0) |
| Stroke, n (%) | 164 (6.5) | 1445 (14.8) | 462 (10.3) | 434 (8.6) | 2503 (9.7) | 746 (8.0) | 120 (13.4) | 263 (13.7) |
| Physical activity, h/week | 1.1 ± 3.3 | 0.9 ± 2.8 | 1.0 ± 3.0 | 1.1 ± 3.0 | 1.0 ± 3.0 | 1.0 ± 2.9 | 1.0 ± 3.2 | 1.0 ± 2.9 |
| Sitting, hours/day | 7.3 ± 3.8 | 5.1 ± 2.6 | 5.4 ± 2.8 | 6.7 ± 3.4 | 5.8 ± 3.1 | 6.2 ± 3.1 | 5.9 ± 3.0 | 5.6 ± 2.7 |
| Sleep, h/day | 7.1 ± 1.1 | 7.0 ± 1.2 | 7.0 ± 1.2 | 7.0 ± 1.1 | 7.0 ± 1.1 | 7.0 ± 1.1 | 7.0 ± 1.2 | 7.0 ± 1.2 |
| Age at menarche, years | 14.0 ± 1.8 | 14.9 ± 1.7 | 17.5 ± 0.0 | 11.5 ± 0.2 | 14.5 ± 1.8 | 14.2 ± 1.8 | 14.4 ± 1.9 | 15.0 ± 1.7 |
| Oral contraceptives, n (%) | 44 (1.8) | 1901 (19.5) | 938 (20.9) | 1592 (31.6) | 0 (0.0) | 9280 (100.0) | 210 (23.5) | 417 (21.7) |
| Age at menopause, years | 48.3 ± 4.7 | 49.9 ± 4.1 | 49.7 ± 4.4 | 48.2 ± 4.5 | 49.4 ± 4.3 | 49.3 ± 4.3 | 37.0 ± 0.0 | 56.0 ± 0.0 |
| Hormone replacement therapy, n (%) | 195 (7.8) | 204 (2.1) | 130 (2.9) | 479 (9.5) | 1206 (4.7) | 808 (8.7) | 126 (14.1) | 71 (3.7) |
| Number of children | 0.0 ± 0.0 | 6.6 ± 1.7 | 4.3 ± 2.5 | 2.7 ± 1.9 | 3.6 ± 2.5 | 3.5 ± 1.7 | 3.4 ± 2.4 | 4.5 ± 2.5 |
The data are expressed as n (%) or mean ± standard deviation.
Number of children was associated with a higher risk of TKR in stepwise manner (P for trend <0.001). After adjusting for all other variables including BMI and other reproductive factors (model 2), compared to nulliparous women (referent), HR (95% CI) was 1.21 (0.84–1.73) for one child, 1.39 (1.03–1.88) for two children, 1.91 (1.43–2.56) for three children, 1.99 (1.48–2.68) for four children and 2.01 (1.50–2.70) for five or more children (P for trend <0.001). We further stratified women by BMI categories and observed a significantly stronger association between higher parity and TKR risk in lean women with BMI <23 kg/m2 than in heavier women. Among lean women, compared to those with no children, the risk of TKR in those with five or more children was higher by almost five folds (HR 4.86; 95% CI (2.22–10.63). Correspondingly, among heavier women, this risk was only increased to about 1.6 folds (HR 1.57; 95% CI 1.14–2.14) (Table 2). The HR for TKR was 1.24 (95% CI 1.14–1.36) per child birth among lean women, and 1.10 (95% CI 1.05–1.15) per child birth among heavier women (P for interaction=0.001).
Table 2.
Hazard ratio (95% confidence interval) of total knee replacement according to number of children
| Model 1† | Model 2†† | BMI | |||||
|---|---|---|---|---|---|---|---|
| < 23 kg/m2†† | ≥ 23 kg/m2†† | ||||||
| Cases | HR (95% CI) | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | |
| Number of children | |||||||
| Nulliparous | 53 | 1.00 | 1.00 | 7 | 1.00 | 46 | 1.00 |
| 1 | 69 | 1.24 (0.86–1.77) | 1.21 (0.84–1.73) | 17 | 2.27 (0.94–5.48) | 52 | 1.02 (0.68–1.51) |
| 2 | 240 | 1.47 (1.09–1.98) | 1.39 (1.03–1.88) | 60 | 2.81 (1.28–6.19) | 180 | 1.15 (0.83–1.59) |
| 3 | 389 | 2.03 (1.52–2.72) | 1.91 (1.43–2.56) | 90 | 3.91 (1.80–8.51) | 299 | 1.56 (1.14–2.14) |
| 4 | 304 | 2.15 (1.60–2.89) | 1.99 (1.48–2.68) | 66 | 4.22 (1.91–9.32) | 238 | 1.60 (1.16–2.21) |
| ≥5 | 590 | 2.13 (1.60–2.85) | 2.01 (1.50–2.70) | 124 | 4.86 (2.22–10.63) | 466 | 1.57 (1.14–2.14) |
| P for trend§ | <0.001 | <0.001 | <0.001 | <0.001 | |||
| HR per child birth | 1.15 (1.10–1.19) | 1.14 (1.09–1.18) | 1.24 (1.14–1.36) | 1.10 (1.05–1.15) | |||
| P for interaction (BMI category and number of children) | 0.001 | ||||||
Multivariable model 1: adjusted for age at interview, dialect, year of interview, educational level, smoking status, body mass index, total physical activity duration, sleep duration and sitting duration, baseline history of self-reported, diabetes, hypertension, coronary artery disease, and stroke;
Multivariable model 2: further adjusted for age at menarche, oral contraceptive use, hormone replacement therapy use and age at menopause.
Linear trend was tested by treating the categories as a continuous variable.
Women who had more children were more likely to have younger age at first birth than women with fewer children. Hence, these two factors may confound each other in their associations with TKR risk. We excluded 2,507 nulliparous women in the analysis for age at first birth and parity. Among women who had ever given birth, higher parity was associated with increased risk of TKR, and the association remained even after adjusting for age at first birth (Table 3). However, although age at first birth was inversely associated with risk of TKR, the association was attenuated and no longer significant after adjusting for parity (Table 3).
Table 3.
Hazard ratio (95% confidence interval) of total knee replacement according to number of children and age at first birth among women who had ever given birth
| Model 1† | Model 2†† | ||
|---|---|---|---|
| Cases | HR (95% CI) | HR (95% CI) | |
| Number of children | |||
| 1 | 69 | 1.00 | 1.00 |
| 2 | 240 | 1.16 (0.88–1.51) | 1.14 (0.87–1.49) |
| 3 | 389 | 1.59 (1.23–2.06) | 1.52 (1.17–1.98) |
| 4 | 304 | 1.66 (1.27–2.17) | 1.58 (1.20–2.07) |
| ≥5 | 590 | 1.69 (1.30–2.19) | 1.58 (1.20–2.07) |
| P for trend§ | <0.001 | <0.001 | |
| Age at first birth | |||
| <20 years | 69 | 1.00 | 1.00 |
| 21–25 years | 240 | 0.93 (0.82–1.06) | 0.97 (0.85–1.09) |
| 26–30 years | 389 | 0.81 (0.70–0.94) | 0.90 (0.77–1.05) |
| ≥31 years | 304 | 0.73 (0.59–0.90) | 0.87 (0.69–1.09) |
| P for trend§ | <0.001 | 0.12 |
Multivariable model 1: adjusted for age at interview, dialect, year of interview, educational level, smoking status, body mass index, total physical activity duration, sleep duration and sitting duration, baseline history of self-reported, diabetes, hypertension, coronary artery disease and stroke, age at menarche, oral contraceptive use, hormone replacement therapy use and age at menopause;
Multivariable model 2: further adjusted for number of children or age at first birth.
Linear trend was tested by treating the categories as a continuous variable.
We also observed that the risk of TKR increased with earlier age at menarche in a dose-dependent manner. Compared to women whose age at menarche was ≥17 years, those with menarche at age <13 years had the highest risk (Table 4). Women who had ever used oral contraceptives had higher risk than those who had never used them, and this increased risk was in those who had used oral contraceptives for at least one year (P for trend = 0.002). BMI did not significantly modify these associations (P for interaction >0.4; Table 4).
Table 4.
Hazard ratio (95% confidence interval) of total knee replacement according to age at menarche and use of oral contraceptives.
| Model 1† | Model 2†† | BMI | |||||
|---|---|---|---|---|---|---|---|
| < 23 kg/m2†† | ≥23 kg/m2†† | ||||||
| Cases | HR (95% CI) | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | |
| Age at menarche | |||||||
| ≥17 years | 187 | 1.00 | 1.00 | 38 | 1.00 | 149 | 1.00 |
| 15–16 years | 568 | 1.20 (1.02–1.42) | 1.19 (1.01–1.40) | 134 | 1.45 (1.01–2.09) | 434 | 1.13 (0.94–1.36) |
| 13–14 years | 671 | 1.36 (1.16–1.61) | 1.37 (1.16–1.62) | 146 | 1.67 (1.16–2.40) | 525 | 1.28 (1.07–1.55) |
| <13 years | 219 | 1.35 (1.10–1.65) | 1.38 (1.13–1.70) | 46 | 1.77 (1.12–2.77) | 173 | 1.28 (1.02–1.62) |
| P for trend§ | <0.001 | <0.001 | 0.006 | 0.006 | |||
| P for interaction (BMI category and age at menarche) | 0.68 | ||||||
| Use of oral contraceptives | |||||||
| Never | 1163 | 1.00 | 1.00 | 253 | 1.00 | 910 | 1.00 |
| Ever | 482 | 1.27 (1.14–1.42) | 1.18 (1.05–1.32) | 111 | 1.21 (0.96–1.53) | 371 | 1.16 (1.02–1.31) |
| P for interaction (BMI category and use of oral contraceptives) | 0.77 | ||||||
| Never | 1163 | 1.00 | 1.00 | 253 | 1.00 | 910 | 1.00 |
| <1 year | 82 | 1.09 (0.87–1.37) | 1.03 (0.82–1.29) | 14 | 0.74 (0.43–1.27) | 68 | 1.10 (0.8–1.41) |
| 1–2 years | 140 | 1.31 (1.1–1.57) | 1.21 (1.01–1.45) | 34 | 1.30 (0.90–1.87) | 106 | 1.17 (0.95–1.43) |
| 3 or more years | 260 | 1.31 (1.14–1.51) | 1.22 (1.06–1.40) | 63 | 1.35 (1.02–1.79) | 197 | 1.17 (1.00–1.37) |
| P for trend§ | <0.001 | 0.002 | 0.025 | 0.026 | |||
| P for interaction (BMI category and use of oral contraceptives) | 0.41 | ||||||
Multivariable model 1: adjusted for age at interview, dialect, year of interview, educational level, smoking status, body mass index, total physical activity duration, sleep duration and sitting duration, baseline history of self-reported, diabetes, hypertension, coronary artery disease, and stroke;
Multivariable model 2: further adjusted for number of children, age at menarche (model for use of oral contraceptives), oral contraceptive use (model for age at menarche), hormone replacement therapy use and age at menopause.
Linear trend was tested by treating the categories as a continuous variable.
Among the study population, 25,435 (72.3%) of women were post-menopausal at the time of recruitment. Among these post-menopausal women, we did not find a significant association between the age at menopause and risk of TKR. The use of HRT was also not significantly associated with TKR risk (Table 5).
Table 5.
Hazard ratio (95% confidence interval) of total knee replacement according to age at menopause and use of hormone replacement therapy among postmenopausal women (n=25,435).
| Model 1† | Model 2†† | BMI < 23 kg/m2†† | BMI ≥23 kg/m2†† | ||||
|---|---|---|---|---|---|---|---|
| Cases | HR (95% CI) | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | |
| Age at menopause | |||||||
| <40 years | 47 | 1.00 | 1.00 | 11 | 1.00 | 36 | 1.00 |
| 40–44 years | 91 | 0.75 (0.53–1.07) | 0.74 (0.52–1.05) | 17 | 0.55 (0.26–1.17) | 74 | 0.79 (0.53–1.18) |
| 45–49 years | 401 | 0.90 (0.67–1.22) | 0.87 (0.64–1.18) | 102 | 0.85 (0.46–1.59) | 299 | 0.89 (0.63–1.27) |
| 50–54 years | 726 | 1.03 (0.77–1.39) | 0.99 (0.73–1.33) | 165 | 0.88 (0.48–1.63) | 561 | 1.03 (0.73–1.44) |
| ≥55 years | 101 | 0.92 (0.65–1.30) | 0.88 (0.62–1.25) | 18 | 0.64 (0.30–1.36) | 83 | 0.95 (0.64–1.41) |
| P for trend§ | 0.051 | 0.11 | 0.83 | 0.11 | |||
| P for interaction (BMI category and age at menopause) | 0.83 | ||||||
| Use of hormone replacement therapy | |||||||
| Never | 1277 | 1.00 | 1.00 | 285 | 1.00 | 992 | 1.00 |
| Ever | 89 | 1.07 (0.86–1.34) | 1.08 (0.87–1.36) | 28 | 1.21 (0.80–1.82) | 61 | 1.06 (0.81–1.38) |
| P for interaction (BMI category and use of hormone therapy) | 0.79 | ||||||
Multivariable model 1: adjusted for age at interview, dialect, year of interview, educational level, smoking status, body mass index, total physical activity duration, sleep duration and sitting duration, baseline history of self-reported, diabetes, hypertension, coronary artery disease, and stroke;
Multivariable model 2: further adjusted for number of children, age at menarche, oral contraceptive use (model for age at menarche), hormone replacement therapy use (model for age at menopause) and age at menopause (model for use of hormone replacement therapy).
Linear trend was tested by treating the categories as a continuous variable.
In sensitivity analysis, results from the parsimonious model and the model with minimally sufficient set of covariates determined by DAG remained virtually unchanged compared to the fuller Model 2 (data not shown). We also conducted sensitivity analysis by excluding those with imputed BMI, and the results of all analyses remained materially similar. Among the 28,844 women with reported weight and height for BMI computation, the HR for TKR was 1.24 (95% CI 1.13–1.35) per child birth among lean women, and 1.08 (95% CI 1.03–1.14) per child birth among heavier women (P for interaction=0.002).
Discussion
In this prospective population-based cohort, higher parity was associated with the risk of TKR for severe knee OA in a stepwise manner among women. This positive association with parity was stronger among lean women compared to their heavier counterparts. Earlier age at menarche and having ever used oral contraceptives for at least one year were associated with increased TRK risk. Conversely, among postmenopausal women, age at menopause and having ever used HRT were not associated with the risk.
A major strength of our study is that the cohort participants were relatively lean, and the variation in BMI among the different reproductive factors was fairly small; this would help minimize the confounding effect of BMI on the observed associations between the reproductive factors investigated in this study and the risk of TKR. We also had sufficient number of women in the lean category to enable us to detect a significantly higher relative risk in lean women compared to heavier women. In addition, our study participants had a wide range in the number of biological children for meaningful analysis. Other strengths included the prospective population-based cohort design with long follow-up time and a large number of TKR cases identified through linkage with a comprehensive nationwide database that had essentially captured all TKR surgeries in Singapore from both private and public hospitals [16].
There are some limitations in our current study. We used TKR as a surrogate outcome for severe knee OA, and did not collect information on preceding history of knee OA. Hence, we were unable to differentiate between risk factors for initiation and progression of knee OA. The participants were asked to recall age at menarche during recruitment, and erroneous reports could result in non-differential misclassification and possible underestimation of the risk estimates. However, a previous study has reported that women were fairly good in reporting original age at menarche [23]. We did not collect the specific type of oral contraceptives and hormonal therapies participants had used. We also did not collect the relevant data and therefore could not adjust for change of BMI before and after reproductive period, history of past knee injuries, or occupational exposure to kneeling or squatting tasks as risk factors for knee OA. The body weight and height were self-reported in this study. Nevertheless, correlations between self-reported and measured height and weight have been generally high, and self-reported weight has been shown to be valid across populations [24, 25]. A systematic review of 64 studies have suggested trends of underestimation of self-reported weight and overestimation of self-reported height [24], and those with higher measured BMI tend to have greater underestimation [26]. As 94% of our study participants had BMI<30 kg/m2, we therefore speculated that the underestimation of BMI from self-reported weight and height could be less problematic compared to cohorts with higher BMI [25]. Finally, we also could not rule out the potential for residual confounding by unmeasured socioeconomic or lifestyle factors related to reproductive factors. In our cohort, women who were younger at time of recruitment had earlier age at menarche, higher level of education and fewer children. This observation could be explained by the rapid industrialization and socio-economic advancement in Singapore starting in the 1960s, leading to improvement in nutritional status and earlier age of menarche among younger women compared to older women. Younger women were more likely to have fewer children due to a government advocacy of two-child family norm introduced in Singapore in the 1970s [27].
The finding that parity was positively associated with the risk of severe knee OA in our study concurs with several cohort studies. A UK study of 1.3 million women has shown that relative risk for increasing parity with total joint replacement was 2% and 8% per birth for hip and knee respectively [8], suggesting that the risk attributed by parity may be different by joint site. Another large cohort of 4.5 million participants in Denmark also demonstrated a positive association between number of children and risk of first hospitalization for OA at different sites for both genders, and the risk was most prominent for knee OA in women [9]. A cross-sectional study using magnetic resonance imaging of knee showed that increasing parity was associated with decreasing cartilage volume and greater cartilage defects [28]. In the National Institutes of Health (NIH)-funded Multicenter Osteoarthritis Study (MOST), there was a relative risk of 2.6 times for incident knee OA among women with high parity (5–12 children) compared to those with one child [29]. However, it was uncertain whether the risk was related to increase in body weight or habitus related to childbirth, or other physical activities related to parenthood.
Apart from showing a stepwise relationship between number of biological children and risk of TKR in women, we demonstrated, for the first time, that the effect of parity was more evident in lean women compared to heavier women. This difference was mainly due to the remarkably low risk of TKR for lean nulliparous women. A weaker association between parity and TKR risk in women with BMI ≥23 kg/m2 in the present study population may explain a null association between parity and knee OA in previous studies conducted in women with higher mean BMI ranging from 25.6–28.5 kg/m2 [10, 30]. Biologically, experimental studies in animal models have shown that high serum estradiol levels during the third trimester of pregnancy correlate with increased anterior cruciate ligament laxity [31], and either downregulation and (or) desensitization of alpha1 and beta2-adrenoceptors in the ligament, which could disrupt normal joint homeostasis [32]. Laxity of ligaments and soft tissue structures around the joints may explain the biological plausibility for the association between parity and development of severe knee OA. We also postulate that leaner women may be more susceptible to the detrimental stress that the increase in weight bearing and kinetic changes in gait during pregnancy may impose on the knee joints [33]. However, our novel finding of such an interaction between BMI and parity on the risk of severe knee OA should be validated by further studies.
In this study, we also found early age at menarche to be a risk factor for knee OA. This is consistent with two large prospective cohorts in the UK [8] and Norway [10]. This finding is interesting as it suggests that an earlier age of exposure or a longer exposure to cyclic hormonal changes during menstrual cycle due to a younger age at menarche may have detrimental effects on the knees. Early menarche is linked to weight gain at young age [34], and this may also explain higher subsequent risk of OA [35–37]. However, two previous cross-sectional studies showed early age at menarche [38, 39] and longer menstrual period [38] to be independent factors for risk of hand OA, a condition in joints less influenced by body weight. Estrogen receptors are expressed not only in the cartilage of joints, but also in various periarticular tissues including subchondral bone, synovium, peri-articular ligaments and muscles [40]. Hence, changes in concentration of sex hormone across the menstrual cycle could be of sufficient magnitude to influence collagen metabolism, and may indirectly influence knee structure and function [41]. Sex hormones also have pleiotropic effects in bone metabolism and in maintaining chondrocyte hemostasis, and it has been postulated that while estrogen increases bone mass and density, the latter consequence could also increase mechanical loading and promote onset of OA in joints [42].
In our study, we did not find any association between age at menopause and the risk of TKR, which is consistent with all other studies on this topic [5, 7, 8, 10]. There is only one small cross-sectional study that has shown a possible association of later age at menopause with the presence and severity of hand OA, but not for knee OA [38]. Findings on whether the use of exogenous hormonal therapies could be related to OA have been controversial. Majority of the cross-sectional and case-control studies have demonstrated a protective effect of HRT on development of OA [43–45]. Out of the five cohort studies that evaluated the association between HRT and knee OA, two [8, 10] have shown increased risk while three [29, 46, 47] did not demonstrate any association. Data from the Woman Health Initiative, the largest randomized controlled trial of HRT, did not find significantly lower rates of TKR in women on either estrogen-alone or estrogen and progesterone combined therapy [48]. After censoring for non-adherence, estrogen-alone users had a significant reduction in risk of hip replacement, but not TKR [48]. In the current study, we noted an increased risk of TKR associated with use of oral contraceptives during the reproductive age but not with use of HRT after menopause. However, we would like to point out that the overall use of oral contraceptives (26.4%) and HRT (6.6%) in our study participants was very low, especially for HRT. Therefore, we could be limited in statistical power to uncover any possible association between exogenous hormone use and risk of TKR.
In conclusion, using data from a large prospective study among Singapore Chinese, we found higher parity, earlier age at menarche and use of oral contraceptives to be positively associated with the risk of TKR for severe knee OA. These findings implicate the important role of reproductive and hormonal factors in pathogenesis of knee OA.
Supplementary Material
Acknowledgments
We thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study. We thank the Ministry of Health in Singapore for assistance with the identification of TKR cases and mortality via database linkages.
Funding
This study was supported by the National Institutes of Health, USA (NIH R01 CA144034 and UM1 CA182876). YYL and WPK were supported by the National Medical Research Council, Singapore (NMRC/CSA-INV/0022/2017 and NMRC/CSA/0055/2013, respectively).
Role of the funding sources
The funding sources had no role in the study design, data collection, analysis, interpretation of data, manuscript writing, or decision to submit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
All authors (YYL, MT, LWA, JMY and WPK) have no financial disclosures or notable competing interests.
References
- 1.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 2.Mueller AJ, Peffers MJ, Proctor CJ, Clegg PD. Systems approaches in osteoarthritis: Identifying routes to novel diagnostic and therapeutic strategies. J Orthop Res 2017; 35: 1573–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 2005; 13: 769–781. [DOI] [PubMed] [Google Scholar]
- 4.Leung YY, Ma S, Noviani M, Wong SB, Lee CM, Soh IA, et al. Validation of screening questionnaires for evaluation of knee osteoarthritis prevalence in the general population of Singapore. Int J Rheum Dis 2018; 21: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol 1988; 128: 179–189. [DOI] [PubMed] [Google Scholar]
- 6.Dawson J, Juszczak E, Thorogood M, Marks SA, Dodd C, Fitzpatrick R. An investigation of risk factors for symptomatic osteoarthritis of the knee in women using a life course approach. J Epidemiol Community Health 2003; 57: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samanta A, Jones A, Regan M, Wilson S, Doherty M. Is osteoarthritis in women affected by hormonal changes or smoking? Br J Rheumatol 1993; 32: 366–370. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Balkwill A, Cooper C, Roddam A, Brown A, Beral V. Reproductive history, hormonal factors and the incidence of hip and knee replacement for osteoarthritis in middle-aged women. Ann Rheum Dis 2009; 68: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen KT, Pedersen BV, Nielsen NM, Hansen AV, Jacobsen S, Frisch M. Socio-demographic factors, reproductive history and risk of osteoarthritis in a cohort of 4.6 million Danish women and men. Osteoarthritis Cartilage 2011; 19: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 10.Hellevik AI, Nordsletten L, Johnsen MB, Fenstad AM, Furnes O, Storheim K, et al. Age of menarche is associated with knee joint replacement due to primary osteoarthritis (The HUNT Study and the Norwegian Arthroplasty Register). Osteoarthritis Cartilage 2017; 25: 1654–1662. [DOI] [PubMed] [Google Scholar]
- 11.Leung YY, Allen JC Jr., Noviani M, Ang LW, Wang R, Yuan JM, et al. Association between body mass index and risk of total knee replacement, the Singapore Chinese Health Study. Osteoarthritis Cartilage 2015; 23: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, et al. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine 2012; 79: 291–297. [DOI] [PubMed] [Google Scholar]
- 13.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2010; 18: 24–33. [DOI] [PubMed] [Google Scholar]
- 14.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 2001; 39: 187–195. [DOI] [PubMed] [Google Scholar]
- 15.Koh WP, Yuan JM, Wang R, Lee HP, Yu MC. Body mass index and smoking-related lung cancer risk in the Singapore Chinese Health Study. Br J Cancer 2010; 102: 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung YY, Ang LW, Allen JC Jr., Noviani M, Wang R, Yuan JM, et al. Response to Letters to the Editors: 1. More details on the database used by the study should be provided. 2. Mediclaim Hospital Discharge System and income levels of cohort. Osteoarthritis Cartilage 2015; 23: 499–500. [DOI] [PubMed] [Google Scholar]
- 17.Terms and conditions for medical institutions e MediClaim [online]. Available at: https://www.mediclaim.moh.gov.sg/mmae/OverviewRules.aspx?Tag=TCMedicalInstitution. Accessed 5 Feb 2019.
- 18.Health SMo. Demertit Point Framework for non-compliance with casemix data submission. [Online]. Available at: https://www.mediclaim.moh.gov.sg/mmae/Documents/Annex_C.pdf. Accessed 3 March 2019.
- 19.Xu GG, Sathappan SS, Jaipaul J, Chan SP, Lai CH. A review of clinical pathway data of 1,663 total knee arthroplasties in a tertiary institution in Singapore. Ann Acad Med Singapore 2008; 37: 924–928. [PubMed] [Google Scholar]
- 20.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 2011; 22: 745. [DOI] [PubMed] [Google Scholar]
- 22.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 23.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 2002; 155: 672–679. [DOI] [PubMed] [Google Scholar]
- 24.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 2007; 8: 307–326. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB. Obesity Epidemiology, Oxford University Press 2008. [Google Scholar]
- 26.Ng SP, Korda R, Clements M, Latz I, Bauman A, Bambrick H, et al. Validity of self-reported height and weight and derived body mass index in middle-aged and elderly individuals in Australia. Aust N Z J Public Health 2011; 35: 557–563. [DOI] [PubMed] [Google Scholar]
- 27.National family planning campaign is launched. In: Government S Ed. Online. History SG, An online resource guide1972. [Online]. Available at: http://eresources.nlb.gov.sg/history/events/eea3d96d-93aa-455a-ac8a-1564d1b6d215. Accessed 5 Feb 2019.
- 28.Wei S, Venn A, Ding C, Martel-Pelletier J, Pelletier JP, Abram F, et al. The associations between parity, other reproductive factors and cartilage in women aged 50–80 years. Osteoarthritis Cartilage 2011; 19: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 29.Wise BL, Niu J, Zhang Y, Felson DT, Bradley LA, Segal N, et al. The association of parity with osteoarthritis and knee replacement in the multicenter osteoarthritis study. Osteoarthritis Cartilage 2013; 21: 1849–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu S, Heo M, Plankey M, Faith MS, Allison DB. Associations of body mass index and anthropometric indicators of fat mass and fat free mass with all-cause mortality among women in the first and second National Health and Nutrition Examination Surveys follow-up studies. Ann Epidemiol 2003; 13: 286–293. [DOI] [PubMed] [Google Scholar]
- 31.Charlton WP, Coslett-Charlton LM, Ciccotti MG. Correlation of estradiol in pregnancy and anterior cruciate ligament laxity. Clin Orthop Relat Res 2001: 165–170. [DOI] [PubMed] [Google Scholar]
- 32.McDougall JJ, Bray RC, Hart DA. Late gestational changes in sympathomimetic sensitivity in primagravid rabbit ligaments. Can J Physiol Pharmacol 2000; 78: 528–534. [PubMed] [Google Scholar]
- 33.Ogamba MI, Loverro KL, Laudicina NM, Gill SV, Lewis CL. Changes in Gait with Anteriorly Added Mass: A Pregnancy Simulation Study. J Appl Biomech 2016; 32: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond) 2013; 37: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 35.Apold H, Meyer HE, Nordsletten L, Furnes O, Baste V, Flugsrud GB. Weight gain and the risk of knee replacement due to primary osteoarthritis: a population based, prospective cohort study of 225,908 individuals. Osteoarthritis Cartilage 2014; 22: 652–658. [DOI] [PubMed] [Google Scholar]
- 36.Holliday KL, McWilliams DF, Maciewicz RA, Muir KR, Zhang W, Doherty M. Lifetime body mass index, other anthropometric measures of obesity and risk of knee or hip osteoarthritis in the GOAL case-control study. Osteoarthritis Cartilage 2011; 19: 37–43. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Wluka AE, Simpson JA, Giles GG, Graves SE, de Steiger RN, et al. Body weight at early and middle adulthood, weight gain and persistent overweight from early adulthood are predictors of the risk of total knee and hip replacement for osteoarthritis. Rheumatology (Oxford) 2013; 52: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 38.Cooley HM, Stankovich J, Jones G. The association between hormonal and reproductive factors and hand osteoarthritis. Maturitas 2003; 45: 257–265. [DOI] [PubMed] [Google Scholar]
- 39.Kalichman L, Kobyliansky E. Age, body composition, and reproductive indices as predictors of radiographic hand osteoarthritis in Chuvashian women. Scand J Rheumatol 2007; 36: 53–57. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Millan M, Castaneda S. Estrogens, osteoarthritis and inflammation. Joint Bone Spine 2013; 80: 368–373. [DOI] [PubMed] [Google Scholar]
- 41.Shultz SJ, Wideman L, Montgomery MM, Beasley KN, Nindl BC. Changes in serum collagen markers, IGF-I, and knee joint laxity across the menstrual cycle. J Orthop Res 2012; 30: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Im GI, Kim MK. The relationship between osteoarthritis and osteoporosis. J Bone Miner Metab 2014; 32: 101–109. [DOI] [PubMed] [Google Scholar]
- 43.Parazzini F, Progretto Menopausa Italia Study G. Menopausal status, hormone replacement therapy use and risk of self-reported physician-diagnosed osteoarthritis in women attending menopause clinics in Italy. Maturitas 2003; 46: 207–212. [DOI] [PubMed] [Google Scholar]
- 44.Spector TD, Nandra D, Hart DJ, Doyle DV. Is hormone replacement therapy protective for hand and knee osteoarthritis in women?: The Chingford Study. Ann Rheum Dis 1997; 56: 432–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wluka AE, Davis SR, Bailey M, Stuckey SL, Cicuttini FM. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann Rheum Dis 2001; 60: 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannan MT, Felson DT, Anderson JJ, Naimark A, Kannel WB. Estrogen use and radiographic osteoarthritis of the knee in women. The Framingham Osteoarthritis Study. Arthritis Rheum 1990; 33: 525–532. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, McAlindon TE, Hannan MT, Chaisson CE, Klein R, Wilson PW, et al. Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: the Framingham Study. Arthritis Rheum 1998; 41: 1867–1873. [DOI] [PubMed] [Google Scholar]
- 48.Cirillo DJ, Wallace RB, Wu L, Yood RA. Effect of hormone therapy on risk of hip and knee joint replacement in the Women’s Health Initiative. Arthritis Rheum 2006; 54: 3194–3204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.