Abstract
Background and Purpose
It is unknown whether blood pressure (BP) reduction influences secondary brain injury in spontaneous intracerebral hemorrhage (ICH). We tested the hypothesis that intensive BP reduction is associated with decreased perihematomal edema expansion rate (PHER) in deep ICH.
Methods
We performed an exploratory analysis of the Antihypertensive Treatment of Acute Cerebral Hemorrhage-2 (ATACH-2) randomized trial. Patients with deep, supratentorial ICH were included. PHER is calculated as the difference in perihematomal edema volume between baseline and 24-hour CT divided by hours between scans. We used regression analyses to determine whether intensive BP reduction was associated with PHER and if PHER was associated with poor outcome (3-month modified Rankin Scale [mRS] score 4–6). We then used interaction analyses to test whether specific deep location (basal ganglia vs thalamus) modified these associations.
Results
Among 1000 patients enrolled in ATACH-2, 870 (87%) had supratentorial, deep ICH. Of these, 780 (90%) had neuroimaging data (336 thalamic and 444 basal ganglia hemorrhages). Baseline characteristics of the treatment groups remained balanced (p>0.2). Intensive BP reduction was associated with a decrease in PHER in univariable (β −0.15; 95% CI −0.26 to −0.05; p=0.007) and multivariable (β −0.12; 95% CI −0.21 to −0.02; p=0.03) analyses. PHER was not independently associated with outcome in all deep ICH (OR 1.14; 95% CI 0.93–1.41; p=0.20), but this association was modified by specific deep location (multivariable interaction p=0.02); in adjusted analyses, PHER was associated with poor outcome in basal ganglia (OR 1.42; 1.05–1.97; p=0.03) but not thalamic (OR 1.02; 95%CI 0.74–1.40; p=0.89) ICH.
Conclusions
Intensive BP reduction was associated with decreased 24-hour PHER in deep ICH. PHER was not independently associated with outcome in all deep ICH, but was associated with poor outcome in basal ganglia ICH. PHER may be a clinically relevant endpoint for clinical trials in basal ganglia ICH.
INTRODUCTION
Spontaneous intracerebral hemorrhage (ICH) remains a devastating disease with limited treatment options, as candidate therapies targeting primary injury have failed to improve outcomes.1,2 As such, secondary brain injury resulting from the hematoma has been proposed as a potential therapeutic target.3 Perihematomal edema (PHE), which appears as a perihematomal hypodensity on head CT, is a well-established neuroimaging biomarker of secondary brain injury and has been used as an endpoint in clinical trials targeting edema formation.4,5 Recent studies have shown that PHE expansion rate (PHER) is an accurate biomarker of underlying pathophysiological changes related to secondary brain injury and has been associated with poor outcome.6,7
Prior studies have shown that the association between PHE expansion and functional outcome varies by location, with the strongest associations seen in deep, small hemorrhages.8,9 However, it is unknown if interventions aimed at limiting primary injury, including intensive blood pressure (BP) reduction, are associated with decreased PHER in deep ICH and, furthermore, if specific deep location (thalamus versus basal ganglia) modifies these associations.
We performed an exploratory analysis of the Antihypertensive Treatment of Acute Cerebral Hemorrhage-2 (ATACH-2) trial, a large, multi-center study of intensive BP reduction with standardized neuroimaging timepoints, to determine if intensive BP reduction is associated with decreased PHER in deep ICH. Furthermore, we explored the association between PHER and outcome in this population and tested whether specific location (thalamus versus basal ganglia) modifies these associations. We hypothesize that intensive BP reduction decreases PHER in deep ICH and that clinical and anatomical differences between thalamic and basal ganglia ICH may contribute to differences in PHE formation and clinical outcome.
METHODS
Data Availability
Anonymized data from the ATACH-2 trial are publicly available by request through the National Institute of Neurological Disorders and Stroke (NINDS) Archive of Clinical Research Datasets and can be accessed at https://www.ninds.nih.gov.
Study Design and Inclusion Criteria
We performed an exploratory analysis of the ATACH-2 trial, an international, randomized, multicenter, two-group, open-label trial.10 ATACH-2 randomized 1000 patients with primary ICH less than 60 mL and elevated systolic BP (SBP) (>180 mm Hg) presenting within 4.5 hours of onset to intensive (target SBP 110–139 mm Hg within 2 hours) or standard (target SBP 140–179 mm Hg within 2 hours) treatment using intravenous nicardipine. Patients with INR > 1.5 at presentation or ICH due to secondary causes were excluded. In the present analysis, enrolled patients with supratentorial deep ICH and available neuroimaging data were included. The study protocol was approved by an ethics committee at each site, and written informed consent was obtained from each participant or his or her legal surrogate. The ATACH-2 study is registered with ClinicalTrials.gov (NCT01176565) and study data are publicly available.
Neuroimaging and PHE Ascertainment
Head CT scans obtained at baseline and 24 hours as per the ATACH-2 study protocol and were sent to a core imaging analysis center for location determination and volumetric analysis. Trained readers blinded to treatment assignment, clinical status, and scan time point classified ICH location based on the location of its major component, defined as greater than or equal to 50% of total hematoma from visual evaluation, and measured ICH and PHE volumes via computerized analysis (Image-Pro Express; Media Cybernetics, Silver Spring, Maryland).11 Regions of ICH and PHE, identified as a rim of hypodensity surrounding the hemorrhage, were marked on each slice. The number of pixels constituting the area of hemorrhage with and without edema was determined on each slice and summed to obtain volume. The PHE volume was calculated by subtracting the hematoma volume from the hematoma plus PHE volumes. PHER was calculated as the difference in PHE volume between baseline and 24-hour CT scan divided by hours between scans.
Outcomes
The primary outcome measure was PHER. Our secondary outcome was poor outcome at 90 days, defined as a Modified Rankin Scale (mRS) of 4–6. The mRS was analyzed as a dichotomous variable as in the ATACH-2 trial.
Statistical Methods
Discrete variables are presented as counts (percentages [%]), and continuous variables are presented as means (standard deviation [SD]) or medians (interquartile range [IQR]). Unadjusted differences in baseline and imaging characteristics by location (thalamus versus basal ganglia) were evaluated using Fisher exact test (2‐tailed), Kruskal–Wallis, or unpaired t-test. Normality of variables was assessed by visually inspecting histogram plots, with confirmation of non-normal distributions using the Shapiro-Wilk test. PHER and ICH volume were modeled as continuous variables and natural-log transformed to remove skewness. The volume of hematoma expansion was modeled as a continuous variable. We used linear regression to evaluate the association between intensive BP reduction and PHER and logistic regression to evaluate the association between PHER and functional outcome. We tested for effect modification by the specific deep location involved (thalamus versus basal ganglia) by adding product terms to our regression models. We then implemented the analyses above stratified by specific deep location. We performed three sensitivity analyses; first, we tested the association between intensive treatment and PHER including hematoma expansion > 33% as a dichotomous variable as used in the ATACH-2 trial; second, we tested the association between 24-hour SBP and PHER by treatment group; finally, we tested for effect modification by intraventricular hemorrhage (IVH) on the association between PHER and poor outcome in basal ganglia and thalamic ICH. Models were built using forward selection of covariates with p<0.1 in univariable analyses followed by backwards elimination of covariates with p>0.1. Universal confounders (age and sex) were included in all models. Colinear covariates with a variance inflation factor >5 were identified, and one covariate was removed. A two-sided p-value of 0.05 was set as the significance threshold, and 95% confidence intervals [CI] were reported for all odds ratios. R (version 3.5.1) was used for all analyses.
RESULTS
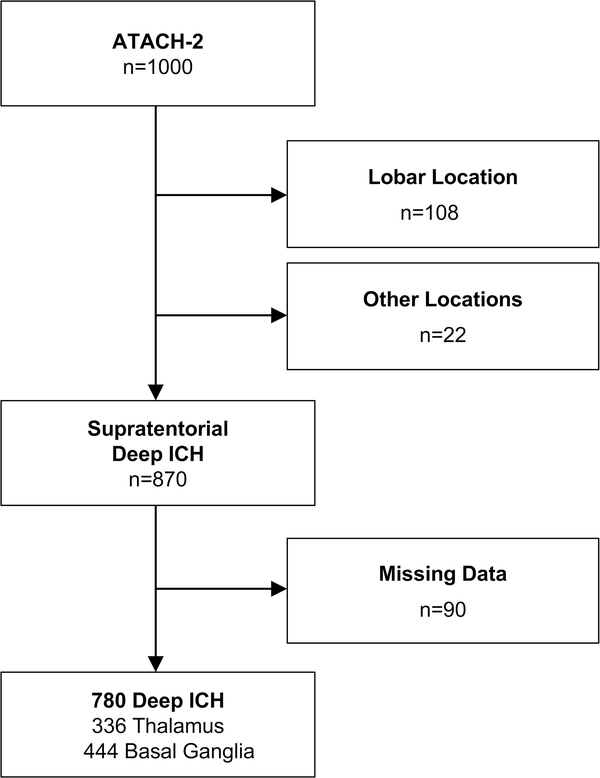
Among 1,000 patients enrolled in ATACH-2, 870 (87%) had ICH in supratentorial, deep locations. Of these, 780 (90%) had complete neuroimaging data and were included in this post-hoc analysis (Figure 1). Patients excluded due to missing neuroimaging data were more likely to be on antihypertensive medication prior to enrollment (57 [63%] versus 47 [47%], p=0.004), had larger baseline ICH volumes (median 11.5 mL [IQR 7–18 mL] versus 9.1 mL [5–17 mL], p=0.02), were more likely to have IVH extension (39 [43%] versus 216 [28%], p=0.003) and were more likely to have died (10 [12%] versus 41 [5%], p=0.03) (Supplemental Table 1). Among included patients, the ICH was located in the thalamus in 336 (43%) patients and the basal ganglia in 444 (57%) patients. The mean (SD) age was 62 (13) years, 489 (63%) were male, and 405 (52%) were randomized to intensive treatment. After stratification by deep location, baseline characteristics remained balanced between the intensive and standard treatment groups as in the original study (all p>0.2) (Table 1).
Figure 1.
Patient Inclusion Criteria
Legend: Abbreviations: ICH = intracerebral hemorrhage. Flowchart of patient inclusion and exclusion criteria. Included patients from ATACH-2 had supratentorial deep ICH and complete neuroimaging and outcome data.
Table 1.
Baseline Demographic and Clinical Characteristics of Included Patients, by Treatment Group.
| Variable, n (%) | Standard Treatment (n=375) | Intensive Treatment (n=405) | p |
|---|---|---|---|
| Age years, mean (SD) | 62 (13) | 62 (13) | 0.88 |
| Male sex | 245/375 (65) | 246/405 (61) | 0.21 |
| Black | 31/375 (8) | 56/405 (14) | 0.02 |
| White | 91/375 (24) | 103/405 (25) | 0.77 |
| Hispanic | 25/375 (7) | 32/405 (8) | 0.80 |
| Hypertension | 278/362 (77) | 328/393 (84) | 0.03 |
| Diabetes | 53/369 (14) | 76/397 (19) | 0.10 |
| Hyperlipidemia | 81/359 (23) | 97/381 (26) | 0.40 |
| Congestive heart failure | 8/372 (2) | 12/400 (3) | 0.61 |
| Atrial fibrillation | 10/374 (3) | 13/399 (3) | 0.71 |
| Prior ischemic stroke | 65/374 (17) | 62/402 (15) | 0.52 |
| Smoker | 183/375 (49) | 166/405 (41) | 0.03 |
| Cocaine use | 7/375 (2) | 10/405 (3) | 0.74 |
| On antihypertensive medication | 157/373 (42) | 204/402 (51) | 0.02 |
| Admission GCS, median (IQR) | 15 (13–15) | 15 (13–15) | 0.70 |
| Admission systolic BP mmHg, mean (SD) | 175 (25) | 176 (26) | 0.44 |
| Admission diastolic BP mmHg, mean (SD) | 111 (20) | 113 (21) | 0.39 |
| Admission INR | 1 (0.1) | 1 (0.1) | 0.23 |
Abbreviations: SD = standard deviation, GCS = Glasgow coma scale, BP = blood pressure, ICH = intracerebral hemorrhage, IQR = interquartile range
Intensive BP Reduction and PHE Expansion Rate
Median (IQR) PHE volumes at baseline and 24 hours were 1.9 mL (0.6–2.3 mL) and 3.0 mL (0.9–3.7 mL), respectively (Table 2). The median PHER was 0.02 mL/hr (IQR 0.00 – 0.07 mL/hr). In patients with deep ICH, intensive BP reduction was associated with decreased PHER in univariable analysis (β −0.15; 95% CI −0.26 to −0.05; p=0.007). This association remained significant after adjustment for age, sex, time to baseline scan, baseline ICH volume, presence of IVH, and volume of hematoma expansion (β −0.12; 95% CI −0.21 to −0.02; p=0.03). Among those with basal ganglia ICH, intensive treatment was associated with decreased PHER in univariable (β −0.20; 95% CI −0.36 to −0.04; p=0.02) but not multivariable analysis (β −0.12; 95% CI −0.27 to 0.02; p=0.09). In patients with thalamic ICH, intensive BP reduction was not associated with PHER in univariable or multivariable analysis (all p>0.2) (Table 3). In a sensitivity analysis with hematoma expansion > 33% modeled as a dichotomous variable, the association between intensive BP reduction and PHER in basal ganglia ICH did not reach significance in multivariable analysis (β −0.13; 95% CI −0.29 to 0.02; p=0.07). In an analysis using 24-hour BP as the exposure, we found that 24-hour SBP was associated with increased PHER, and this association was modified by the treatment group (interaction p=0.02). In multivariable analysis, 24-hour SBP was associated with increased PHER in the intensive treatment group (β 0.01; 95% CI 0.00–0.015; p=0.04) but not in the standard treatment group (β −0.002; 95% CI −0.007–0.002; p=0.20) (Supplemental Figure 1).
Table 2.
Neuroimaging Characteristics of Included Patients by Treatment Group.
| Variable | Standard Treatment (n=375) | Intensive Treatment (n=405) | p |
|---|---|---|---|
| Time from symptom onset to baseline scan hours, mean (SD) | 1.6 (0.8) | 1.7 (0.9) | 0.36 |
| Baseline ICH volume mL, median (IQR) | 9.4 (4.6–17.1) | 8.9 (4.8–16.4) | 0.50 |
| Baseline PHE volume mL, median (IQR) | 1.3 (0.7–2.4) | 1.4 (0.6–2.3) | 0.91 |
| IVH | 111/375 (30) | 105/405 (26) | 0.29 |
| Time to 24-hour CT scan hours, mean (SD) | 22.9 (2.6) | 22.9 (2.6) | 0.95 |
| 24-hour ICH volume mL, median (IQR) | 10.5 (5.3–21.2) | 9.6 (4.7–18.7) | 0.16 |
| 24-hour PHE volume mL, median (IQR) | 2.1 (0.9–4.1) | 1.6 (0.8–3.3) | 0.02 |
| Volume of hematoma expansion mL, mean (SD) | 4.0 (9.1) | 3.0 (8.7) | 0.24 |
| Volume of PHE expansion mL, mean (SD) | 1.3 (3.3) | 0.9 (3.4) | 0.09 |
| PHER mL/hr, median (IQR) | 0.02 (0.0–0.08) | 0.01 (−0.01–0.05) | 0.009 |
Abbreviations: SD = standard deviation, ICH = intracerebral hemorrhage, IVH = intraventricular hemorrhage, PHE = perihematomal edema, PHER = perihematomal edema expansion rate
Table 3.
Association Between Intensive BP Reduction and PHER, by Location.
| Model | Overall (n=780) | Basal Ganglia (n=444) | Thalamus (n=336) | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Intensive BP Treatment, unadjusted | −0.15 (−0.26 to −0.05) | 0.007 | −0.20 (−0.36 to −0.04) | 0.02 | −0.08 (−0.22 to 0.06) | 0.23 |
| Intensive BP Treatment, adjusted† | −0.12 (−0.21 to −0.02) | 0.03 | −0.12 (−0.27 to 0.02) | 0.09 | −0.10 (−0.23 to 0.03) | 0.13 |
| Location*Intensive BP Treatment Interaction Term, adjusted† | −0.02 (−0.22 to 0.19) | 0.90 | - | - | - | - |
Model adjusted for age, sex, time to baseline scan, admission ICH volume (natural log transformed), presence of intraventricular hemorrhage, and volume of hematoma expansion.
Abbreviations: BP = blood pressure, CI = confidence interval
Association Between PHE Expansion Rate and Outcome
In patients with deep ICH, PHER was associated with poor outcome in univariable (OR 1.43; 95% CI 1.24–1.67; p<0.001) but not multivariable analysis (OR 1.14; 95% CI 0.93–1.41; p=0.20) (Table 4). However, this association was modified by the specific deep brain nuclei involved (multivariable interaction p=0.02); in the basal ganglia, PHER was greater in those with a poor outcome versus those with a good outcome (0.05 mL/hr [IQR 0.0–0.15 mL/hr] versus 0.02 mL/hr [IQR −0.01–0.07 mL/hr]; unadjusted p<0.001), but in the thalamus, there was no difference in PHER between those with a poor versus good outcome (0.1 mL/hr [IQR 0.0–0.03 mL/hr ] versus 0.1 mL/hr [IQR −0.01–0.07 mL/hr]; unadjusted p=0.64). PHER remained associated with poor outcomes in the basal ganglia (OR 1.42; 95% CI 1.05–1.97; p=0.03) but not in the thalamus (OR 1.02; 0.74–1.40; p=0.89) after adjustment for age, sex, admission GCS, baseline ICH volume, presence of IVH, volume of hematoma expansion, and treatment group (Table 5). The association between PHER and outcome was not modified by IVH (interaction p>0.2).
Table 4.
Demographic, Clinical and Radiographic Characteristics of Included Patients, by Outcome.
| Variable, n (%) | Good Outcome (n=469) | Poor Outcome (n=286) | p |
|---|---|---|---|
| Age years, mean (SD) | 59 (12) | 65 (13) | <0.001 |
| Male Sex | 315/469 (67) | 159/286 (56) | 0.003 |
| Black | 43/469 (9) | 35/286 (12) | 0.20 |
| White | 98/469 (21) | 85/286 (30) | 0.008 |
| Hispanic | 34/469 (7) | 20/286 (7) | 0.76 |
| Hypertension | 357/456 (78) | 232/276 (84) | 0.01 |
| Diabetes | 70/462 (15) | 55/279 (20) | 0.34 |
| Hyperlipidemia | 103/446 (23) | 67/269 (25) | 0.92 |
| Congestive heart failure | 8/465 (2) | 12/282 (5) | 0.05 |
| Atrial fibrillation | 9/468 (2) | 14/280 (5) | 0.01 |
| Prior ischemic stroke | 65/468 (14) | 56/283 (19) | 0.05 |
| Smoker | 210/469 (45) | 129/286 (45) | 0.67 |
| On antihypertensive medication | 193/466 (41) | 158/284 (56) | <0.001 |
| Admission GCS, median (IQR) | 15 (14–15) | 14 (11–15) | <0.001 |
| Admission systolic BP mmHg, mean (SD) | 176 (24) | 175 (29) | 0.84 |
| Admission diastolic BP mmHg, mean (SD) | 114 (20) | 109 (21) | 0.005 |
| Admission INR | 0.98 (0.09) | 1.01 (0.14) | <0.001 |
| Thalamic location | 180/469 (38) | 144/286 (50) | <0.001 |
| ICH Volume mL, median (IQR) | 7.2 (3.7–12.8) | 13.7 (7.8–22.6) | <0.001 |
| PHE Volume mL, median (IQR) | 1.1 (0.6–2.1) | 1.7 (0.9–3.0) | <0.001 |
| IVH | 77/469 (16) | 130/286 (46) | <0.001 |
| Hematoma Expansion mL, mean (SD) | 1.5 (7.7) | 4.0 (14.0) | 0.001 |
| PHER mL/hr, median (IQR) | 0.01 (0.0–0.05) | 0.03 (0.0–0.1) | <0.001 |
Abbreviations: SD = standard deviation, GCS = Glasgow coma scale, BP = blood pressure, ICH = intracerebral hemorrhage, IQR = interquartile range
Table 5.
Association between PHER and Poor Outcome at 3 Months, by Location.
| Model | Overall (n=754) | Basal Ganglia (n=431) | Thalamus (n=323) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| PHER, unadjusted | 1.43 (1.24–1.67) | <0.001 | 2.05 (1.60–2.68) | <0.001 | 1.21 (0.85–1.73) | 0.30 |
| PHER, adjusted† | 1.14 (0.93–1.41) | 0.20 | 1.42 (1.05–1.97) | 0.03 | 1.02 (0.74–1.40) | 0.89 |
| Location*PHER Interaction Term, adjusted† | 1.54 (1.15– 3.18) | 0.01 | - | - | - | - |
Model adjusted for age, sex, admission GCS, admission ICH volume (natural log transformed), presence of intraventricular hemorrhage, volume of hematoma expansion, and treatment group.
Abbreviations: PHER = perihematomal edema expansion rate, OR = odds ratio, CI = confidence interval
DISCUSSION
In this exploratory analysis of the ATACH-2 trial, we tested the hypothesis that intensive BP reduction decreases PHER in spontaneous, deep ICH. We found that intensive BP reduction was associated with decreased 24-hour PHER in this population. While PHER was not independently associated with poor outcome in all deep ICH, this association was modified by the specific deep location of the hemorrhage: PHER was independently associated with poor functional outcome in basal ganglia, but not thalamic, ICH.
Our results provide evidence for an association between intensive BP reduction and 24-hour PHER in deep ICH. Although the mechanisms underlying the association between BP reduction and early PHE formation are unknown, our observations are consistent with the proposed pathophysiological basis of edema; early PHE formation is thought to result from clot retraction and cytotoxic edema caused by mass effect of the primary injury, whereas later stages of PHE may be driven by blood breakdown and downstream inflammatory pathways.4 Therefore, interventions aimed at limiting the extent of primary injury, through reduction in hematoma volume or hematoma expansion, may result in less early PHE formation and downstream secondary injury. This proposed mechanism is consistent with the results of our sensitivity analysis, which showed attenuation of the relationship between intensive BP treatment and PHER in basal ganglia ICH with hematoma expansion modeled dichotomously. Furthermore, this proposed mechanism is consistent with reports of less PHE growth in the intensive treatment group in a pooled analysis of the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT) studies8 and reduced 72-hour edema growth in the phase IIb trial of recombinant activated factor VII for limiting hematoma growth.12
Our results extend the findings of prior studies that demonstrated independent associations between PHE expansion and functional outcome. While early studies yielded conflicting results,13–15 more recent studies have shown independent associations between various measures of PHE16 and functional outcome in single-center6,17,18 and multi-center observational studies.7–9 A recent study of PHE in the VISTA-ICH collaboration reported that the association between 72-hour PHE growth and outcome is dependent on both location and volume, with the strongest associations seen in deep locations and small-to-moderate sized hemorrhages.9 Our results further clarify the clinical significance of PHE and add that the association between PHE expansion and outcome is not uniform among ICH in deep locations. We identified an important subset of ICH patients - those with basal ganglia ICH - in which 24-hour PHER is independently associated with outcomes after adjustment for age, sex, GCS, admission hematoma volume, the volume of hematoma expansion, and IVH. These findings could reflect unknown differences in underlying biology or could be the result of differential effects of hematoma volume, IVH, and other neuroanatomical factors in the deep brain nuclei. In this regard, higher rates of IVH in thalamic locations could impact our ability to detect the impact of PHE on outcomes, even in multivariable analysis. However, we did not find effect modification by IVH on the association between PHER and outcome in secondary analyses.
Our location-specific findings may have important implications for future trials targeting secondary injury in ICH. While deep ICH has been grouped as a single phenotype in prior and ongoing studies of secondary injury,5,19 our results provide evidence of clinically significant differences in PHER and functional outcome in the thalamus and basal ganglia. The population with basal ganglia ICH, which may be less affected by factors such as IVH, is ideal for proof-of-concept studies to demonstrate the intended effects of candidate therapies on PHE formation. Such a personalized therapeutic approach has shown success in several recent ischemic stroke trials20–22 and seems to be the logical next step in ICH research, where many negative trials have enrolled patients without regard to the underlying biology of ICH.
Strengths of this study include the standardized follow-up imaging and PHE measurements in a large, multi-site trial, as most studies of PHE have been limited by observational cohorts with variable time to follow-up. However, there are several limitations in our study that arise from the use of the ATACH-2 trial data. First, as with all post-hoc subgroup analyses, our findings may be due to chance, although our analysis was prespecified and informed by an a priori biological rationale and the baseline characteristics between treatment groups remained well-balanced as in the original study. Second, we are limited by the use of a trial population with mild to moderate ICH severity and small hematomas (<60 mL), and thus, our findings may not be generalizable. Patients excluded due to missing follow-up neuroimaging data also had larger hemorrhages at baseline, so it is possible that severe cases more likely to die before the 24-hour CT scan were excluded. Our results will require validation in a cohort of patients with larger hematomas. However, our population is relevant for proof-of-concept studies targeting secondary injury and PHE formation. Third, with stratification by specific deep location, there is limited power to detect differences between groups, which may contribute to the lack of association between PHER and outcome in thalamic ICH. Fourth, we are limited by the lack of data on ICH involving multiple locations. It is possible that larger hemorrhages may involve both the thalamus and the basal ganglia. However, ATACH-2 enrolled patients with smaller hemorrhages, so misclassification of location, if present, is likely random and non-differential and would introduce bias towards the null. We are also limited by lack of data on other modifiable factors that may influence PHER, such as temperature, osmotherapy, antiplatelet agents, and statins. Fifth, there is no follow-up imaging at other time points with which to study later edema formation. Prior studies have evaluated PHER at 72 hours to account for the continued growth of edema during the days following the primary injury.15 However, PHER has been shown to be the fastest in the first few hours after ICH,23,24 so 24 hours may be an optimal window in which to study PHER. Further studies of PHER at later time points with standardized scan follow-up times are needed to determine the clinical significance of continued edema growth. Finally, this study does not address balancing the risks of intensive BP reduction with the potential benefits of decreased PHER.25
In conclusion, we demonstrate that intensive BP reduction is associated with decreased PHER in ATACH-2 patients with deep ICH. While PHER was not associated with outcome in all deep ICH, we found that PHER is independently associated with functional outcome in basal ganglia ICH. These results suggest that PHER may be a clinically relevant endpoint in early phase trials targeting secondary injury in patients with basal ganglia ICH. Further evaluation of PHER as an intermediate endpoint for trials of growth-limiting interventions in deep ICH - including BP reduction - is warranted.
Supplementary Material
Acknowledgments
Funding Sources
ACL is supported by the NIH (T35HL007649) and the American Heart Association Student Scholarship in Cerebrovascular Diseases and Stroke. AIQ reports no disclosures. SBM is supported by the NIH (K23NS105948). HK is supported by the NIH (R01NS097443, K23NS082367, U01NS095869). JNG is supported by the NIH (U24NS10065). KBW reports no disclosures. DW reports no disclosures. FS reports no disclosures. HBH reports no disclosures. WCZ is supported by the NIH (R01 NS102583, U01NS080824). DFH is supported by the NIH (U01NS080824, U24TR001609). CCM reports no disclosures. LHS is supported by the NIH (R01NS095993, R01NS097728). GJF is supported by the NIH (K76AG059992), the American Heart Association (18IDDG34280056), the Yale Pepper Scholar Award (P30AG021342) and the Neurocritical Care Society Research Fellowship. KNS is supported by the NIH (U24NS107136, U24NS107215, R01NR018335, U01NS106513) and the American Heart Association (18TPA34170180 and 17CSA33550004). The funding entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Disclosures
Dr. Goldstein reports consulting fees from CSL Behring and Octapharma and research grants from Pfizer and Portola. Dr. Ziai reports consulting fees from Bard. Dr. Sansing reports research support from Genentech. Dr. Sheth is supported by Biogen, Bard and Novartis in his role as the co-lead or lead investigator of the CHARM, INTREPID, AND S1P ICH clinical trials.
REFERENCES
- 1.Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N. Engl. J. Med 2016;375:1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N. Engl. J. Med 2013;368:2355–2365. [DOI] [PubMed] [Google Scholar]
- 3.Selim M, Sheth KN. Perihematoma edema: a potential translational target in intracerebral hemorrhage? Transl. Stroke Res 2015;6:104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urday S, Kimberly WT, Beslow LA, Vortmeyer AO, Selim MH, Rosand J, et al. Targeting secondary injury in intracerebral haemorrhage--perihaematomal oedema. Nat. Rev. Neurol 2015;11:111–122. [DOI] [PubMed] [Google Scholar]
- 5.Leasure A, Kimberly WT, Sansing LH, Kahle KT, Kronenberg G, Kunte H, et al. Treatment of Edema Associated With Intracerebral Hemorrhage. Curr. Treat. Options Neurol 2016;18:9. [DOI] [PubMed] [Google Scholar]
- 6.Grunwald Z, Beslow LA, Urday S, Vashkevich A, Ayres A, Greenberg SM, et al. Perihematomal Edema Expansion Rates and Patient Outcomes in Deep and Lobar Intracerebral Hemorrhage. Neurocrit. Care 2017;26:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy SB, Urday S, Beslow LA, Jesse Dawson, Lees K, Kimberly WT, et al. Rate of perihaematomal oedema expansion is associated with poor clinical outcomes in intracerebral haemorrhage. J. Neurol. Neurosurg. Psychiatry 2016;87:1169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Arima H, Wu G, Heeley E, Delcourt C, Zhou J, et al. Prognostic significance of perihematomal edema in acute intracerebral hemorrhage: pooled analysis from the intensive blood pressure reduction in acute cerebral hemorrhage trial studies. Stroke. 2015;46:1009–1013. [DOI] [PubMed] [Google Scholar]
- 9.Murthy SB, Moradiya Y, Dawson J, Lees KR, Hanley DF, Ziai WC, et al. Perihematomal Edema and Functional Outcomes in Intracerebral Hemorrhage: Influence of Hematoma Volume and Location. Stroke. 2015;46:3088–3092. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit. Care 2011;15:559–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi AI, Palesch YY, Martin R, Novitzke J, Cruz-Flores S, Ehtisham A, et al. Effect of Systolic Blood Pressure Reduction on Hematoma Expansion, Perihematomal Edema, and 3-Month Outcome Among Patients With Intracerebral Hemorrhage. Arch. Neurol 2010;67:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diringer MN, Davalos A, Mayer SA, Brun NC, Begtrup K, Broderick J, et al. Effects of Recombinant Activated Factor VII on Perilesional Edema in Patients with Acute Intracerebral Hemorrhage801. Neurosurgery. 2005;57:395–395. [Google Scholar]
- 13.Appelboom G, Bruce SS, Hickman ZL, Zacharia BE, Carpenter AM, Vaughan KA, et al. Volume-dependent effect of perihaematomal oedema on outcome for spontaneous intracerebral haemorrhages. J. Neurol. Neurosurg. Psychiatry 2013;84:488–493. [DOI] [PubMed] [Google Scholar]
- 14.Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, et al. Significance of perihematomal edema in acute intracerebral hemorrhage: the INTERACT trial. Neurology. 2009;73:1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staykov D, Wagner I, Volbers B, Hauer E-M, Doerfler A, Schwab S, et al. Natural course of perihemorrhagic edema after intracerebral hemorrhage. Stroke. 2011;42:2625–2629. [DOI] [PubMed] [Google Scholar]
- 16.Volbers B, Giede-Jeppe A, Gerner ST, Sembill JA, Kuramatsu JB, Lang S, et al. Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology. 2018;90:e1005–e1012. [DOI] [PubMed] [Google Scholar]
- 17.Urday S, Beslow LA, Dai F, Zhang F, Battey TWK, Vashkevich A, et al. Rate of Perihematomal Edema Expansion Predicts Outcome After Intracerebral Hemorrhage. Crit. Care Med 2016;44:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volbers B, Willfarth W, Kuramatsu JB, Struffert T, Dörfler A, Huttner HB, et al. Impact of Perihemorrhagic Edema on Short-Term Outcome After Intracerebral Hemorrhage. Neurocrit. Care 2016;24:404–412. [DOI] [PubMed] [Google Scholar]
- 19.Fu Y, Hao J, Zhang N, Ren L, Sun N, Li Y-J, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71:1092–1101. [DOI] [PubMed] [Google Scholar]
- 20.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N. Engl. J. Med 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 22.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 23.Wu TY, Sharma G, Strbian D, Putaala J, Desmond PM, Tatlisumak T, et al. Natural History of Perihematomal Edema and Impact on Outcome After Intracerebral Hemorrhage. Stroke. 2017;48:873–879. [DOI] [PubMed] [Google Scholar]
- 24.Gebel JM, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2631–2635. [DOI] [PubMed] [Google Scholar]
- 25.Toyoda K, Koga M, Yamamoto H, Foster L, Palesch YY, Wang Y, et al. Clinical Outcomes Depending on Acute Blood Pressure After Cerebral Hemorrhage. Ann. Neurol 2019;85:105–113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data from the ATACH-2 trial are publicly available by request through the National Institute of Neurological Disorders and Stroke (NINDS) Archive of Clinical Research Datasets and can be accessed at https://www.ninds.nih.gov.