Abstract
Introduction
We estimated the age-specific duration of the preclinical, prodromal and dementia stages of AD, and the influence of sex, setting, APOE, and CSF tau on disease duration.
Methods
We performed multi-state modeling in a combined sample of 6 cohorts (n=3,268) with death as the end-stage, and estimated the preclinical, prodromal and dementia stage duration.
Results
The overall AD duration varied between 24 years (age 60) and 15 years (age 80). For individuals presenting with preclinical AD, age 70, the estimated preclinical AD duration was 10 years, prodromal AD 4 years, and dementia 6 years. Male sex, clinical setting, APOE ε4 genotype and abnormal CSF tau were associated with a shorter duration and these effects depended on disease stage.
Discussion
Estimates of AD disease duration become more accurate if age, sex, setting, APOE and CSF tau are taken into account. This will be relevant for clinical practice and trial design.
Keywords: Alzheimer disease, disease duration, preclinical, prodromal, dementia, APOE, clinical setting, progression, multi-state model
1. INTRODUCTION
Alzheimer disease (AD) is highly prevalent, and a major cause of dementia and death in elderly individuals [1-3]. Accumulation of amyloid in the brain is believed to be the first sign of the disease and can precede a clinical diagnosis of dementia by up to 20 years [1, 4, 5]. Based on the degree of cognitive impairment, AD is often divided into three stages: the preclinical stage, characterized by normal cognitive ability, the prodromal stage, characterized by mild cognitive impairment (MCI), and the dementia stage, with functional impairment [6-9], but it is unclear how long individuals with amyloid pathology spend in each stage. A better understanding of the stage-specific duration of AD is needed to inform patients, caregivers, and clinicians. This information is also useful for the design of clinical studies, as well as to provide context for the interpretation of trial results, in particular the clinical trials that include individuals in pre-dementia stages and aim to slow down progression to AD dementia.
Attempts to quantify the duration of AD should be age-specific, because age imposes the greatest risk for both dementia and mortality, and take into account APOE genotype, sex, and cerebrospinal fluid (CSF) tau levels [4, 6, 10-12]. Setting is also important, as progression from MCI to dementia was longer in research settings than in clinical settings [13]. Previous studies on the length of the AD dementia stage reported a duration of 3 to 10 years [14, 15]. Younger age, female sex and lower CSF total tau (t-tau) were found to be associated with a longer duration of the AD dementia stage, while the effect of APOE genotype was equivocal [14-17]. The median duration of prodromal AD was three years in a pooled memory clinic cohort study, but no age-specific estimates were provided and mortality was not taken into account [18]. The patients with prodromal AD and increased CSF t-tau levels tended to convert sooner to AD dementia [19, 20]. The duration of the preclinical AD stage has been estimated in combination with the prodromal AD stage, which was 17 years, based on extrapolations of change in positron emission tomography (PET) amyloid load over time [21]. We estimated disease duration by applying a multi-state modeling approach, which has been previously used in AD research [22-25], and can offer an estimate of disease duration based on stage progression and mortality rates in the absence of very long follow-up duration. The aim of this study was therefore to estimate the disease duration for preclinical, prodromal and AD dementia stage according to age, setting (clinical versus research), sex, APOE genotype, and baseline CSF t-tau levels.
2. METHODS
2.1. Participants
Six longitudinal cohort studies, including three memory clinic cohorts (Amsterdam Dementia cohort (ADC), DESCRIPA, and ICTUS), and three research cohorts (Alzheimer Disease Neuroimaging Initiative (ADNI), Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL) and Prospective Population Study of Women in Gothenburg H70 (Gothenburg H70)), provided data for the study (Supplement A for more cohort information) [26-31]. From these cohorts, we selected participants aged 50 years and older with evidence of amyloid accumulation, and with information on diagnosis and/or mortality at follow-up available. Evidence of amyloid pathology was an inclusion criterion for this study, defined by at least one abnormal marker of amyloid accumulation. The amyloid PET scans were visually rated or a published threshold was applied and for CSF amyloid-beta 1-42 (Aβ1-42) cohort-specific thresholds were applied (Supplement A). In absence of amyloid measures for the ICTUS cohort, only the patients with a clinical diagnosis of AD-type dementia were included and analyses repeated without this cohort. All studies were approved by an ethical review board and their participants gave informed consent.
2.2. AD stages
AD was categorized into four clinical stages: preclinical AD, prodromal AD, mild AD dementia, and moderate to severe AD dementia (from here on shortened to moderate AD dementia). Preclinical AD was defined by amyloid accumulation and normal cognition (Supplement A). Prodromal AD was in this study defined by amyloid accumulation and a diagnosis of MCI, amnestic and non-amnestic [9, 32, 33]. AD dementia was diagnosed according to the NINCDS-ADRDA criteria, and if an amyloid evaluation was available this had to be confirmative [7]. AD dementia was subdivided in mild AD dementia (Clinical Dementia Rating (CDR) below 2, or CDR sum of boxes (CDR-SOB) <10, or (if no CDR was available) MMSE>20), and moderate AD dementia (CDR>1, CDR-SOB>9, or (if no CDR was available) MMSE<21) [34, 35].
2.3. Mortality assessment
The ADC cohort mortality data were obtained from the Dutch population register, while the other studies provided mortality data recorded during the study. In AIBL the exact mortality date of those who died was unknown (n=19) and therefore set at the next planned visit, which is 1.5 years after the last follow-up. In others cases of a missing mortality date (n=4), the date was set 2 years after last follow-up.
2.4. Predictor variables
For all participants, age, sex and setting were available. The setting was classified as clinical for ADC, DESCRIPA and ICTUS and research for ADNI, AIBL and Gothenburg H70. APOE genotype was dichotomized according to the presence or absence of the AD-associated ε4 allele of APOE and was available in all cohorts except ICTUS. Baseline CSF t-tau was classified as normal or abnormal by applying the cohort-specific cut-off and available for the ADC, DESCRIPA, ADNI and Gothenburg H70 studies (Supplement A).
2.5. Statistical analyses
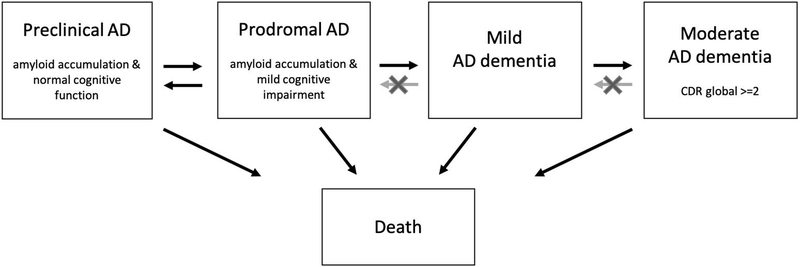
Baseline characteristics between diagnostic groups were compared using Chi-square, Kruskal-Wallis or ANOVA tests with Tukey post-hoc, where appropriate. To estimate the disease duration, a multi-state model (MSM) with the four stages of AD and death as the end-stage was fitted [36]. All transition rates between stages were incorporated in one model (Figure 1). Reversions from prodromal to preclinical AD were also included in the model. Reversion in the dementia stages were fitted using misclassification (see Supplement B for additional methods and specifications of multi-state model analysis).
Figure 1. Multi-state Model.
Arrows indicate fitted progression and reversion rates between stages in the multi-state model. Moderate to severe AD dementia is shortened to moderate AD dementia for readability.
Multi-state models with different numbers of covariates were fitted to the data. Age was a time-dependent covariate, and centered at age 70. For each covariate a hazard ratio was calculated for each transition. As most covariate effects on mortality were not estimable; a restricted model was applied. The first model included only age as covariate, then, the second model also included setting, and the third model had age, setting, and sex. The fourth model included age, setting, and APOE, while the fifth model had age, setting, and tau as covariates, and the sixth model included all five covariates. As not all covariates were available for all participants, the number of participants varied between models. The resulting transition rates and hazard ratios are based on every observation of every participant in combination with the time in between the observations.
In a second step, using the MSM maximum likelihood estimate as input, the duration for every stage was estimated. Confidence intervals of 95% were derived by simulation using the asymptotic properties of the maximum likelihood estimation, which allowed comparison between age-specific estimates for the different covariates. R-packages msm for the multi-state transition model and ELECT version 0.3 (Estimating Life-Expectancies for interval censored data) were used to estimate the duration estimates and confidence intervals [36, 37]. Sensitivity analyses included, aside of fitting all covariates in one model, sequentially removing cohorts from the analysis to ensure results were not driven by a single cohort. We also reran all models in the subset with data on all covariates (n=1518).
3. RESULTS
A total of 3,268 participants were included in the analyses across the six cohorts combined. The mean (SD) age at baseline was 73 (8) years with a range of 50 to 96 years. The mean (SD) number of follow-up years was 2.8 (1.9) with a range of 0.3 to 20 years, and a median (IQR) number of 4 (3-5) visits. Progression to at least one consecutive stage was apparent in 981 (32% of 3,034) participants. Table 1 shows how participants in the baseline stages differed in sex, APOE ε4 genotype, abnormal CSF t-tau, follow-up length and mortality (Suppl. table B.5 for subgroups with data on APOE and CSF t-tau available).
Table 1.
Baseline characteristics according to diagnosis
| Preclinical AD (n = 438) |
Prodromal AD (n = 729) |
Mild AD dementia (n = 1867) |
Moderate to severe AD dementia (n = 234) |
p-value overall group difference |
|
|---|---|---|---|---|---|
| Age (years) | 73 (7) | 72 (7) | 73 (9) | 75 (10) | <0.01a |
| Male (n) | 204 (47%) | 417 (57%) | 781 (42%) | 74 (33%) | <0.01 |
| MMSE (0-30, median (IQR)) (n=3252) | 29 (28-30) | 27 (26-29) | 22 (19-24) | 16 (13,19) | <0.01b |
| APOE ε4 genotype* (n) (n=1984) | 210 (49%) | 466 (66%) | 554 (71%) | 35 (51%) | <0.01 |
| Abnormal CSF total tau* (n) (n=1563) | 87 (38%) | 346 (57%) | 535 (80%) | 47 (82%) | <0.01 |
| Follow-up years (median (IQR)) | 3.8 (2-4.5) | 3.9 (2.5-4.8) | 2.0 (1.5-2.5) | 2.0 (1.2-2.3) | <0.01c |
| Progression to next clinical disease stage (n) | 87 (20%) | 325 (45%) | 569 (30%) | NA | NA |
| Death at follow-up (n) | 12 (3%) | 76 (10%) | 215 (12%) | 54 (23%) | NA |
| Participants by cohort (n ADC /ADNI /AIBL /DESCRIPA /Gothenburg /ICTUS) | 40/180/191 /23 /4 /0 | 140 /449 /73 /49/18 / 0 | 507 /224 /69 /0/1 / 1066 | 64 /1/3 /0/0 / 166 | NA |
Mean (SD), unless otherwise specified. In Tukey posthoc:
Moderate to severe AD dementia older than the MCI and Mild AD dementia group;
All groups significantly different from each other;
Normal cognition and MCI longer follow-up than dementia groups
Available in subset of cohorts, APOE not for ICTUS
3.1. Transition rates
In the model that included age, sex and setting, all transition rates to subsequent disease were significantly influenced by age, except mortality in the preclinical AD stage and progression from prodromal AD to mild AD dementia (suppl. table B.2 for all estimates of the models). Compared to data collected in a research setting, data from clinical settings was associated with a higher progression rate (HR=4.40 [95% CI, 2.80-6.94]) and reversion rate (HR=1.98 [95% CI, 1.15-3.39]) between preclinical and prodromal AD. Additionally, in the clinical setting the progression rates from the prodromal AD to the mild AD dementia stage (HR=1.48 [95% CI, 1.34-1.92]) and from the mild AD to the moderate AD dementia stage (HR=1.41 [95% CI,1.16-1.72]) were higher. Females had a higher progression rate from mild AD to moderate AD dementia, compared to males (HR=1.24 [95% CI, 1.04-1.47]), while their mortality risk in moderate AD dementia was lower (HR=0.60 [95% CI, 0.46-0.80]).
3.2. AD stage duration according to age, sex, and setting
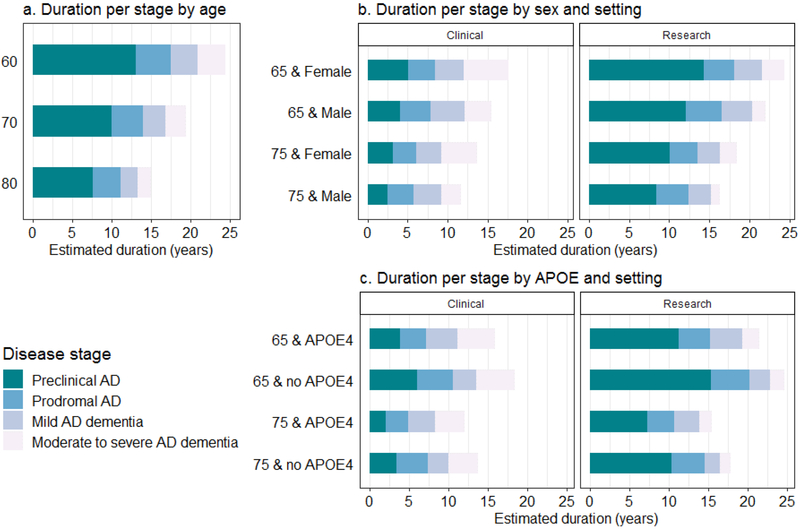
The predicted total disease duration, based on the model with age, for an individual with preclinical AD at age 70 was 20 years (95% CI, 17-21), consisting of a preclinical stage of 10 years (95% CI, 8-11), followed by a prodromal stage of 4 years (95% CI, 3-5), mild AD dementia for 3 years (95% CI, 2-3), and moderate AD dementia for 3 years (95% CI, 2-3, Table 2). Figure 2A shows for those with preclinical AD a lower predicted overall disease duration at older age, which ranged from 24 years (95% CI, 22-25) at age 60 to 15 years (95% CI, 11-17) at age 80. The duration of preclinical AD at age 70 was shorter in a clinical setting (4 years [95% CI, 3-5]) than in a research setting (11 years [95% CI, 9-13]). In the clinical setting, for individuals with prodromal AD, the stage duration of prodromal AD was also shorter, and while the dementia stage duration for these individuals was equal between settings, more time was spent in the moderate AD stage (Suppl. table B.7a and b). The estimated total duration with starting stage preclinical AD ranged in the clinical setting 19 years (95% CI, 17-20) at age 60 to 11 years (95% CI, 10-12) at age 80 and in the research setting from 26 years (95% CI, 23-28) at age 60 to 15 years (95% CI, 12-17) at age 80. In females the moderate AD dementia stage duration was longer than in males (e.g. 2.1 years (95% CI, 1.1-3.2, p<0.0001 at age 70 in a clinical setting; Figure 2B, suppl. table B.3).
Table 2.
Estimated stage-specific duration of Alzheimer Disease
| Starting stage |
Duration, time in years (95% CI) |
Age 60 | Age 70 | Age 80 |
|---|---|---|---|---|
| Preclinical AD | Preclinical AD | 13 (10.4, 14.9) † | 9.9 (8.4, 11.5) | 7.6 (5.6, 9.7) † |
| Prodromal AD | 4.4 (3.7, 4.8) | 4.0 (3.3, 4.7) | 3.5 (2.3, 4.5) * | |
| Mild AD dementia | 3.5 (3, 3.8) § | 2.9 (2.4, 3.3) | 2.1 (1.4, 2.5) § | |
| Moderate AD dementia | 3.5 (2.8, 4.1) § | 2.6 (2.1, 3.3) | 1.7 (1.1, 2.4) § | |
| Total duration | 24.1 (21.8, 25.4) | 19.5 (17.3, 20.8) | 15.0 (11.0, 16.9) | |
| Preclinical AD | 3.2 (2.2, 4.3) ‡ | 1.6 (1.1, 2.1) | 0.7 (0.4, 1.2) § | |
| Prodromal AD | Prodromal AD | 4.6 (4.0, 5.3) | 4.4 (3.9, 4.8) | 4.0 (3.4, 4.7) |
| Mild AD dementia | 4.5 (4.0, 4.9) ‡ | 3.9 (3.5, 4.2) | 3.0 (2.5, 3.4) § | |
| Moderate AD dementia | 4.9 (4.2, 5.5) § | 3.9 (3.3, 4.5) | 2.7 (2.2, 3.5) § | |
| Total duration | 17.2 (15.8, 18.3) | 13.6 (12.7, 14.5) | 10.3 (9.3, 11.5) | |
| Mild AD dementia | Mild AD dementia | 5.0 (4.3, 5.7)† | 4.3 (4.0, 4.7) | 3.6 (3.2, 3.9) § |
| Moderate AD dementia | 6.0 (5.1, 6.7) ‡ | 4.8 (4.2, 5.5) | 3.6 (3.0, 4.5) § | |
| Total duration | 10.9 (10.1, 11.8) | 9.0 (8.4, 9.7) | 7.1 (6.4, 7.9) | |
| Moderate AD dementia | Moderate AD dementia | 6.5 (5.4, 7.5) ‡ | 5.2 (4.0, 6.0) | 4.1 (3.5, 5.1) ‡ |
Estimates based on model including age as covariate (Model 1 in suppl. table B.2). Moderate AD dementia = Moderate to severe AD dementia. Stage estimates significantly different from estimates at age 70:
p<0.05
p<0.01;
p<0.001;
p<0.0001.
Figure 2. Estimated Stage-specific Duration for Starting Stage Preclinical AD.
The panels show the predicted time spend in each stage stacked and stratified for (a) age (model 1); for (b) age, sex, and setting (model 3); and for (c) age, APOE genotype, and setting (model 4). Models include age as continues, and (b) sex or (c) APOE, and setting as dichotomous covariates.
The age refers to the starting stage with preclinical AD and the estimated duration the predicted duration in the subsequent stages in years.
The 95% confidence intervals and p-values for estimate comparison can be found for (a) in table 2, for panel (b) in suppl. table B.3, and for panel (c) in suppl. table B.4)
3.3. APOE effect
APOE ε4 carriers had, compared to non-carriers, an increased rate of progression from the preclinical AD to prodromal AD stage (HR=1.63 [95% CI, 1.11-2.41]) and from the prodromal AD to mild AD dementia stage (HR=1.50 [95% CI, 1.18-1.90]), and a trend for slower decline from the mild to the moderate AD dementia stage (HR 0.77 [95% CI, 0.60-1.00]). When compared to a non-carrier, an APOE ε4 carrier aged 70 in the clinical setting had a 1.6 years (95% CI, 0.4-3.3; p=0.0295) shorter estimated preclinical AD stage duration, and 1.1 years (95% CI, 0.3-2.1; p=0.0110) shorter prodromal AD stage duration, but 1.0 year (95% CI, 0.3-1.8; p=0.0050) longer mild dementia stage duration (suppl. table B.4). Figure 2C shows how the total predicted disease duration ranged from 12 to 25 years depending on APOE ε4 genotype, age and setting.
3.4. Tau effect
As normal CSF t-tau level may become abnormal over time only the estimated duration of the starting stages are presented in Table 3. Individuals with preclinical AD and abnormal CSF t-tau showed a trend for an increased progression rate from preclinical to prodromal AD (HR=1.49 [95% CI, 0.95-2.35]). In prodromal AD, abnormal tau associated with a decreased reversion rate to preclinical AD stage (HR=0.41 [95% CI, 0.23-0.71]) and increased progression rate to the mild AD dementia stage (HR=1.91 [95% CI, 1.48-2.48]). The estimated preclinical AD stage was shortened by around 3 years and the prodromal AD stage by around 2.5 years (Table 3). There was no association of baseline abnormal t-tau with the duration of the dementia stages.
Table 3.
Estimated stage-specific duration stratified for baseline CSF total tau by setting at age 70
| Clinical setting | Research setting | ||||||
|---|---|---|---|---|---|---|---|
| Starting stage |
Duration, in years (95% CI) |
Tau normal |
Tau abnormal |
Difference (95% CI; p-value) |
Tau normal |
Tau abnormal |
Difference (95% CI; p-value) |
| Preclinical AD | Preclinical AD | 5.6 (3.7, 8.9) |
3 (1.9, 4.3) |
2.6 (0.7, 5.5; p=0.034) |
11.6 (8.3, 14.3) |
7.7 (5.6, 9.9) |
3.7 (0.4, 7.3; p=0.033) |
| Prodromal AD | Prodromal AD | 5.4 (4.0, 7.0) |
3 (2.3, 3.7) |
2.4 (1.2, 3.7; p=0.0002) |
6.8 (5.5, 8.1) |
3.9 (3.3, 4.6) |
2.9 (1.4, 4.2; p=0.0001) |
| Mild AD dementia | Mild AD dementia | 4.4 (3.2, 5.9) |
3.6 (2.9, 4.4) |
0.8 (−0.4, 2.2; p=0.230) |
6.4 (4.7, 7.9) |
5.4 (4.2, 6.5) |
1.1 (−0.5, 2.7; p=0.197) |
| Moderate AD dementia | Moderate AD dementia | 4.9 (3.1, 7.7) |
5.9 (4.1, 8.7) |
−0.9 (−3.0,1.6; p=0.439) |
2.8 (1.8, 4.1) |
3.5 (2.5, 4.7) |
−0.6 (−2.0, 1.0; p=0.438) |
Tau = baseline CSF total tau. Abbreviations: Moderate AD = moderate to severe AD. Estimates based on model including age as continues and baseline CSF t-tau and setting as dichotomous covariates (Model 5 in suppl. table B.2).
3.5. Sensitivity analyses
Consecutively removing each of the cohorts did not affect the estimates (Suppl. table B.6). When all variables were combined in one model, most estimates remained unchanged. In the additional analysis of the same models in the subset of individuals with all covariates (n=1518, see Suppl. Table B.8), the effects were similar. Varying the mortality assumptions for unknown mortality dates of those who died, did not change the results.
4. DISCUSSION
We estimated the duration of the preclinical, prodromal, mild dementia, and moderate dementia stages of AD using a multi-state model. Depending on age, sex, APOE genotype, baseline CSF t-tau and setting, the total disease duration varied between 12 and 25 years, the preclinical stage between 2 and 15, the prodromal stage between 3 to 7, mild AD dementia stage between 2 and 6 and moderate AD dementia stage between 1 and 7 years.
4.1. Effect of age
Age had the strongest effect on the duration of the preclinical and dementia stages, which could be explained by higher progression and mortality rates. The decrease of disease duration of the preclinical AD stage could also be due to a reduction in resilience to AD pathology at higher age, for example due to co-morbid brain disorders, resulting in a faster clinical progression [38]. Alternatively, older individuals may have spent a longer period in the preclinical AD stage before inclusion in the study. Our estimated duration of the combined preclinical and prodromal stage for a 70-year-old (17 years) was very similar to the estimated duration of 17 years pre-dementia AD based on differential equation modeling of the amyloid accumulation rate in individuals aged 72 years on average [21].
4.2. Effect of setting
The shorter duration of the preclinical and prodromal stage in the clinical compared to the research setting could be explained by the fact that individuals who present in a clinical setting are in a more advanced stage of the disease. An alternative explanation is that individuals who present in a clinical setting have a more aggressive disease form of the disease, whereas those with a slower progressive variant would be picked up in the research setting [39]. The estimated differences between settings may be underestimated in the current study, as part of the individuals from the AIBL and ADNI research cohorts were recruited in memory clinics. The effects of setting on disease progression are consistent with other AD studies [40, 41].
4.3. Effect of APOE genotype
The shorter age-specific duration of the preclinical stage in APOE ε4 carriers is consistent with the observed earlier onset of dementia due to AD in epidemiological studies and the faster cognitive decline of APOE ε4 carriers with preclinical AD in research studies [11, 42-44]. While the prodromal stage was shorter in APOE ε4 carriers, the dementia stage was longer which would imply that the total symptomatic disease duration is similar, but differently divided over the stages. These findings are important for clinical trials. For example, exclusion of ε4 carriers during a trial, what happened in the high-dose group of the BAN2401 trial, may affect rate of progression and possibly the power of the study [45].
4.4. Effect of sex
The dementia stage duration was longer in women, which was driven by lower mortality in this group. The study did not reveal significant sex differences in the duration of preclinical and prodromal AD stages.
4.5. Effect of tau
The presence of increased CSF t-tau was associated with a shorter pre-dementia disease duration, which confirms that increased tau is associated with faster disease progression. Unlike previous studies, no effect of tau on mortality and duration of the AD dementia stage were found, which may be explained by dichotomization of CSF t-tau in our analysis [16, 17].
4.6. Duration and mortality
The estimation of total disease duration estimates were in some cases longer than the residual life expectancies of population data [46]. For example, the residual life expectancy at age 80 was reported to be 8-10 years in the USA and Australia (data from 2010-2012), while in our study this ranged from 4 years for those with moderate AD to 15 years for individuals with preclinical AD. One explanation for the longer duration is that we may have overestimated disease duration because mortality had not been checked systematically in all studies. On the other hand, mortality rates in our study cohorts may also be lower because both volunteers participating in studies and memory clinic patients may be healthier at study entry than individuals not participating in research or attending memory clinics.
4.7. Strengths and limitations
A strength of the study is the large sample of participants with amyloid accumulation. The multi-state model approach is another strength, because it enabled the incorporation of multiple clinical stages, including fluctuations between stage, and the mortality risk in a data driven manner. A limitation of the modeling approach is the underlying assumption that progression risk is independent on the previous time spend in a stage, while progression risk may actually change after being in a stage for a longer period of time. This was addressed by taking age as the time-dependent covariate, which has been applied before to overcome this issue [22, 47]. To estimate the disease duration, we had to combine data of multiple cohorts across the disease spectrum. As such, the sample consisted of over 3000 individuals, still not all the effects were estimable. Combining cohort data leads to heterogeneity, i.e. due to different application of diagnostic criteria, cognitive testing and amyloid status. Another limitation was that amyloid status and APOE genotype were unknown for AD-type dementia patients of the ICTUS study, but the sensitivity analysis without the ICTUS, yielded very similar results. Additionally, we used the old criteria for the preclinical AD definition, while the recent research criteria also require tau positivity [8]. Finally, our sample is not representative of the general population, but may be representative of the patients who physicians need to inform, and volunteers that participate in clinical trials.
4.8. Implications
Our estimates are of practical use to clinicians needing to provide prognostic information to research participants and patients. For instance, in a research study with disclosure of abnormal amyloid status, these estimates can give an indication of the prognosis, often asked for by the trial participants before joining the study. The estimates of AD duration are also useful to define target populations for trials. Furthermore, these estimates can be used to indicate how a preventive treatment in the early stage of the disease could impact total disease duration.
4.9. Conclusion
We provided age-specific disease estimates of the duration of AD, including the long pre-dementia stage, according to setting, sex, APOE genotype, and presence of tau pathology. Our findings will be useful to provide patients a prognosis, to inform clinical trial design, and can help to model how interventions in early stage AD may influence long-term outcome.
Supplementary Material
HIGHLIGHTS.
First age-specific estimates of the duration of AD, including pre-dementia stages
Overall AD duration ranged from 24 years at age 60 to 15 years at age 80.
Preclinical AD stage was much shorter in a clinical compared to a research setting.
Females had a longer dementia duration.
APOE ε4 and CSF tau abnormality shortened the preclinical and prodromal AD stages.
Research in context.
Systematic review: Articles on the duration of each part of the Alzheimer disease (AD) spectrum showed that the dementia stage was 3-10 years and the estimate of the preclinical and prodromal AD stage combined 17 years. Although several studies reported on the effect of age, sex, tau and APOE genotype on disease progression, this was not translated to subgroup- and age-specific disease duration estimates.
Interpretation: We improved previous estimates, by combining data from cognitive aging cohorts, to estimate the age-specific duration of preclinical, prodromal, and dementia stages of AD in a single multi-state model, taking mortality into account, as well as age, sex, APOE and/or tau abnormality.
Future directions: Our findings are useful prognostic information for the different stages of AD, can help to select individuals for clinical trials, and to model how interventions in early stage AD may influence long-term outcome. Long-term follow-up studies are needed to confirm our findings and are currently ongoing.
ACKNOWLEDGEMENTS
The authors are very thankful to all patients and participants in the studies included in the paper, as well as to everyone involved in the data collection and data sharing.
ICTUS study Group refers to: Vellas B., Reynish E., Ousset PJ., Andrieu S. (Toulouse), Burns A. (Manchester), Pasquier F. (Lille), Frisoni G. (Brescia), Salmon E. (Liège), Michel J.P., Zekry D.S. (Geneva), Boada M. (Barcelona), Dartigues J.F. (Bordeaux), Olde-Rikkert M.G.M. (Nijmegen), Rigaud A.S. (Paris), Winblad B. (Huddinge), Malick A., Sinclair A. (Warwick), Frölich L.(Mannheim), Scheltens P. (Amsterdam), Ribera C.(Madrid), Touchon J. (Montpellier), Robert P. (Nice), Salva A.(Barcelona), Waldemar G. (Copenhagen), Bullock R. (Swindon), Tsolaki M. (Thessaloniki), Rodriguez G. (Genoa), Spiru L. (Bucharest), Jones R.W. (Bath), Stiens G., Stoppe G. (Goettingen), Eriksdotter Jönhagen M. (Stockholm), Cherubini A. (Perugia), Lage P.M., Gomez-Isla T. (Pamplona), Camus V. (Tours), Agüera-Morales E., Lopez F. (Cordoba). DSA Group refers to: Andrieu S., Savy S., Cantet C., Coley N.
Disclosures personal: Kern, Wallin, Olde Rikkert, Ousset, Spiru and Freund-Levi, Tsolaki, Muniz-Terrera, vd Hout, report no disclosures. Vermunt, Sikkes, Visser and Handels report the following related to this study: grants from European Brain Council (VoT project; 2017); Dr Bos has received research support from the Innovative Medicines Initiatives Joint Undertaking under resources that are composed of financial contributions from EU FP7 (FP7/2007-2013) and in-kind EFPIA. Ron Handels reports grants from BIOMARKAPD (EU JPND; 2012-2016); grants from Actifcare (EU JPND; 2014-2017); grants from Dutch Flutemetamol Study (2012-2017); grants from ROADMAP (IMI2; 2016-2019); grants from SNAC (Sweden public funding; 2016-2018); grants from MIND-AD (EU JPND; 2017-2018); grants from Alzheimer association Nederland (NL fellowship; 2017-2019); grants from Economic and policy implications new treatment for AD (ARUK; 2017-2018); grants from various ZonMw projects (NL public funding; 2017-2022); grants from RECAGE (EU H2020; 2018-2022); personal fees from Piramal (advisory; 2016); personal fees from Roche (advisory; 2017). Research programs of Dr van der Flier have been funded by ZonMW, the Netherlands Organization of Scientific Research, Seventh European Framework Programme, Alzheimer Nederland, Cardiovascular Onderzoek Nederland, Stichting Dioraphte, Gieskes,Strijbis fonds, Boehringer Ingelheim, Piramal Imaging, Roche BV, Janssen Stellar, and Combinostics. All funding is paid to her institution. Skoog reports consultant for Takeda. Dr Scheltens has acquired grant support (for the institution) from GE Healthcare, Danone Research, Piramal, and Merck. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, GE Healthcare, Novartis, Sanofi, Nutricia, Probiodrug, Biogen, Roche, Avraham, and EIP Pharma. Paul Maruff is an employee of Cogstate Ltd . Frans RJ Verhey received grants from H2020 (Induct (2016-2020); Pride Alzheimer UK (2015-2020); Actifcare (EU JPND; 2014-2017); Gieskes-Strijbis (PRECODE 2018-2022); Noaber foundation (INPAD 2017-2021); Interreg (SFC, 2016-202) Hilkka Soininen reports advisory board member for ACImmune and MERCK. Kaj Blennow is advisor for Fujirebio Europe, IBL International, Roche Diagnostics and co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. Henrik Zetterberg is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. Dr. Visser reports grants from Innovative Medicine Initiative, during the conduct of the study; non-financial support from GE Healthcare, other from Eli-Lilly, other from Janssen Pharmaceutical, grants from Biogen, outside the submitted work.
Funding support: Funders had no role in study design, data analysis, data interpretation, or writing of the report. The work was supported by the IALSA (Integrative Analysis of Longitudinal Studies of Aging and Dementia) network, which received support by NIH grant P01AG043362; 2013-2018; from the Innovative Medicines Initiative Joint Undertaking EMIF grant agreement number 115372, EPAD grant agreement number 115736, resources and ROADMAP grant agreement number 116020 of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution; and the European Brian Council.
Funding of each of the studies: ADC: The VU University Medical Center (VUMC) Alzheimer Center is supported by Alzheimer Nederland and Stichting VUMC funds. This study was performed within the framework of the Dutch ABIDE project and was supported by a ZonMW-Memorabel grant (project No 733050201) in the context of the Dutch Deltaplan Dementie and through a grant of Piramal Imaging (positron emission tomography scan costs) to the Stichting Alzheimer & Neuropsychiatrie, Amsterdam. Research of the VUMC Alzheimer Center is part of the neurodegeneration research program of Amsterdam Neuroscience. The clinical database structure was developed with funding from Stichting Dioraphte.
ADNI: Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol,Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann,La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California
AIBL: Funding for the AIBL study was provided in part by the study partners [Australian Commonwealth Scientific Industrial and research Organization (CSIRO), Edith Cowan University (ECU), Mental Health Research Institute (MHRI), Alzheimer’s Australia (AA), National Ageing Research Institute (NARI), Austin Health, CogState Ltd., Hollywood Private Hospital, Sir Charles Gardner Hospital]. The study also received support from the National Health and Medical Research Council (NHMRC) and the Dementia Collaborative Research Centres program (DCRC2), as well as ongoing funding from the Science and Industry Endowment Fund (SIEF). The authors acknowledge the financial support of the Australian Government Cooperative Research Centre for Mental Health.
DESCRIPA: The project was funded by the European Commission as part of the 5th Framework Programme (QLK-6-CT-2002-02455). The centre in Bucharest received support from the Ana Aslan International foundation.
Gothenburg H70: The Swedish Research Council (2015-02830,2013-8717), Swedish Research Council for Health, Working Life and Wellfare (No 2013-2496, 2013-2300, 2010-0870, 2012-1138), Sahlgrenska University Hospital (ALF 716681), The Alzheimer's Association Zenith Award (ZEN-01-3151), The Alzheimer's Association Stephanie B. Overstreet Scholars (IIRG-00-2159), Alzheimerfonden, Hjärnfonden, Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse
ICTUS/DSA The ICTUS study was partially supported by a grant from the European Commission within the 5th framework programme (QLK6-CT-2002-02645) and partially from an unrestricted equal grant from each of Eisai, Janssen, Lundbeck, and Novartis pharmaceutical companies. The pharmaceutical companies had no role in study design, data collection, data analysis, data interpretation. Promotion of the ICTUS study was supported by the University Hospital Centre of Toulouse. The data sharing activity was supported by the “Association Monegasque pour la recherche sur la maladie d’Alzheimer”(AMPA) and the UMR 1027 Unit INSERM – University of Toulouse III.
ABREVIATIONS:
- AD
Alzheimer disease
- APOE
apolipoprotein
- CDR
clinic dementia rating scale
- CSF
cerebrospinal fluid
- MCI
mild cognitive impairment
- MMSE
mini-mental state examination
- PET
proton emission tomography
- t-tau
total tau
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Winblad B, et al. , Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol, 2016. 15(5): p. 455–532. [DOI] [PubMed] [Google Scholar]
- 2.Scheltens P, et al. , Alzheimer's disease. Lancet, 2016. 388(10043): p. 505–17. [DOI] [PubMed] [Google Scholar]
- 3.Fargo KN, et al. , 2014 Report on the Milestones for the US National Plan to Address Alzheimer's Disease. Alzheimers & Dementia, 2014. 10(5): p. S430–S452. [DOI] [PubMed] [Google Scholar]
- 4.Jansen WJ, et al. , Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA, 2015. 313(19): p. 1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR Jr., et al. , Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol, 2013. 12(2): p. 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR Jr., et al. , NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement, 2018. 14(4): p. 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKhann G, et al. , Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology, 1984. 34(7): p. 939–44. [DOI] [PubMed] [Google Scholar]
- 8.Sperling RA, et al. , Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 2011. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert MS, et al. , The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement, 2011. 7(3): p. 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neu SC, et al. , Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol, 2017. 74(10): p. 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim YY, et al. , Association of beta-Amyloid and Apolipoprotein E epsilon4 With Memory Decline in Preclinical Alzheimer Disease. JAMA Neurol, 2018. 75(4): p. 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vos SJ, et al. , Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol, 2013. 12(10): p. 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farias ST, et al. , Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol, 2009. 66(9): p. 1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodaty H, Seeher K, and Gibson L, Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr, 2012. 24(7): p. 1034–45. [DOI] [PubMed] [Google Scholar]
- 15.Wattmo C, Londos E, and Minthon L, Risk factors that affect life expectancy in Alzheimer's disease: a 15-year follow-up. Dement Geriatr Cogn Disord, 2014. 38(5-6): p. 286–99. [DOI] [PubMed] [Google Scholar]
- 16.Rhodius-Meester HFM, et al. , Disease-related determinants are associated with mortality in dementia due to Alzheimer's disease. Alzheimers Res Ther, 2018. 10(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degerman Gunnarsson M, et al. , High tau levels in cerebrospinal fluid predict nursing home placement and rapid progression in Alzheimer's disease. Alzheimers Res Ther, 2016. 8(1): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vos SJ, et al. , Prevalence and prognosis of Alzheimer's disease at the mild cognitive impairment stage. Brain, 2015. 138(Pt 5): p. 1327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rossum IA, et al. , Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology, 2012. 79(17): p. 1809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchhave P, et al. , Cerebrospinal fluid levels of beta-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry, 2012. 69(1): p. 98–106. [DOI] [PubMed] [Google Scholar]
- 21.Villemagne VL, et al. , Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol, 2013. 12. [DOI] [PubMed] [Google Scholar]
- 22.Jack CR Jr., et al. , Transition rates between amyloid and neurodegeneration biomarker states and to dementia: a population-based, longitudinal cohort study. Lancet Neurol, 2016. 15(1): p. 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robitaille A, et al. , Transitions across cognitive states and death among older adults in relation to education: A multistate survival model using data from six longitudinal studies. Alzheimers Dement, 2018. 14(4): p. 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coley N, et al. , A Longitudinal Study of Transitions Between Informal and Formal Care in Alzheimer Disease Using Multistate Models in the European ICTUS Cohort. J Am Med Dir Assoc, 2015. 16(12): p. 1104 e1–7. [DOI] [PubMed] [Google Scholar]
- 25.Brookmeyer R, et al. , Forecasting the prevalence of preclinical and clinical Alzheimer's disease in the United States. Alzheimers Dement, 2018. 14(2): p. 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiner MW, et al. , The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers Dement, 2010. 6(3): p. 202–11 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Flier WM, et al. , Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis, 2014. 41(1): p. 313–27. [DOI] [PubMed] [Google Scholar]
- 28.Reynish E, et al. , The ICTUS Study: A Prospective longitudinal observational study of 1,380 AD patients in Europe. Study design and baseline characteristics of the cohort. Neuroepidemiology, 2007. 29(1-2): p. 29–38. [DOI] [PubMed] [Google Scholar]
- 29.Rowe CC, et al. , Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging, 2010. 31. [DOI] [PubMed] [Google Scholar]
- 30.Gustafson DR, et al. , Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry, 2007. 78(5): p. 461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visser PJ, et al. , Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol, 2009. 8(7): p. 619–27. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC, et al. , Mild cognitive impairment: clinical characterization and outcome. Arch Neurol, 1999. 56(3): p. 303–8. [DOI] [PubMed] [Google Scholar]
- 33.Winblad B, et al. , Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med, 2004. 256(3): p. 240–6. [DOI] [PubMed] [Google Scholar]
- 34.O'Bryant SE, et al. , Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol, 2008. 65(8): p. 1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perneczky R, et al. , Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry, 2006. 14(2): p. 139–44. [DOI] [PubMed] [Google Scholar]
- 36.Jackson Ch L, Multi-State Models for Panel Data: The msm Package for R Journal of Statistical Software, 2011. 38 ((8)): p. 1–29. [Google Scholar]
- 37.Van den Hout A, Multi-State Survival Models for Interval-Censored Data. Boca Raton:CRC/Chapman & Hall. 2017. [Google Scholar]
- 38.Vemuri P, et al. , Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol, 2017. 82(5): p. 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Croswell JM, Ransohoff DF, and Kramer BS, Principles of cancer screening: lessons from history and study design issues. Semin Oncol, 2010. 37(3): p. 202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian J, et al. , APOE-related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts. PLoS Med, 2017. 14(3): p. e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snitz BE, et al. , Risk of progression from subjective cognitive decline to mild cognitive impairment: The role of study setting. Alzheimers Dement, 2018. 14(6): p. 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts RO, et al. , Prevalence and Outcomes of Amyloid Positivity Among Persons Without Dementia in a Longitudinal, Population-Based Setting. JAMA Neurol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Lee SJ, et al. , The effect of APOE and other common genetic variants on the onset of Alzheimer's disease and dementia: a community-based cohort study. Lancet Neurol, 2018. 17(5): p. 434–444. [DOI] [PubMed] [Google Scholar]
- 44.Mormino EC, et al. , Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology, 2014. 82(20): p. 1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.https://www.alzforum.org/news/conference-coverage/ban2401-removes-brain-amyloid-possibly-slows-cognitive-decline 20 February 2019].
- 46.www.lifetable.de (2010-2012. USA).
- 47.Brookmeyer R and Abdalla N, Estimation of lifetime risks of Alzheimer's disease dementia using biomarkers for preclinical disease. Alzheimers Dement, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.