Abstract
Purpose:
To identify peri-operative risk factors and time to onset of pancreatic endocrine/exocrine insufficiency.
Methods:
We retrospectively analyzed a single institutional series of patients who underwent pancreaticoduodenectomy (PD) or distal pancreatectomy (DP) between 2000 and 2015. Endocrine/exocrine insufficiencies were defined as need for new pharmacologic intervention. Cox proportional modeling was used to identify peri-operative variables to determine their impact on post-operative pancreatic insufficiency.
Results:
1,717 patient records were analyzed (75.47% PD, 24.53% DP) at median follow-up 17.88 months. Average age was 62.62 years, 51.78% were male, and surgery was for malignancy in 74.35% of patients. Post-operative endocrine insufficiency was present in 20.15% (n=346). Male gender (p= 0.015), increased body mass index (BMI) (p<0.001), tobacco use (p=0.011), family history of diabetes (DM) (p<0.001), personal history of DM (p=<0.001), and DP (p=<0.001) were correlated with increased risk. Mean time to onset was 20.80 ± 33.60 (IQR: 0.49–28.37) months. Post-operative exocrine insufficiency was present in 36.23% (n=622). Race (p=0.014), lower BMI (p<0.001), family history of DM (p=0.007), steatorrhea (p<0.001), elevated pre-operative bilirubin (p=0.019), and PD (p=<0.001) were correlated with increased risk. Mean time to onset was 14.20 ± 26.90 (IQR: 0.89–12.69) months.
Conclusions:
In this large series of pancreatectomy patients, 20.15% and 36.23% of patients developed post-operative endocrine and exocrine insufficiency at a mean time to onset of 20.80 and 14.20 months, respectively. Patients should be educated regarding post-resection insufficiencies and providers should have heightened awareness long-term.
Keywords: Pancreaticoduodenectomy, distal pancreatectomy, pancreatic endocrine insufficiency, pancreatic exocrine insufficiency, pancreatic enzyme replacement
INTRODUCTION
Pancreatectomy is a common treatment modality for both benign and malignant pathologies. As a result of innovations both in surgical techniques and peri-operative patient care, mortality rate of pancreatectomy has been reduced drastically [1]. However, morbidity remains around 45% [1–3]. In addition to short-term post-operative morbidities, such as pancreatic fistula and delayed gastric emptying, long-term morbidities including endocrine and exocrine insufficiency are frequently encountered and can severely impact quality of life and overall health outcomes.
Pancreatogenetic diabetes mellitus (DM) or escalation of DM in the post-operative period can have lifelong implications with diabetic complications affecting multiple organ systems. The nutritional consequences of exocrine insufficiencies including fat malabsorption, micronutrient deficiencies, and altered bowel function must not be overlooked. Additionally, functional outcomes of pancreatic insufficiencies have a significant impact on quality of life [4].
Parenchymal-preserving resections, including enucleation and central pancreatectomy, were introduced with the aim of reducing the risk of post-operative endocrine and exocrine insufficiency. These procedures are used in select cases of benign and pre-malignant lesions and have been associated with increased post-operative morbidity, particularly pancreatic fistulas, but preserved endocrine and exocrine pancreatic function [5]. Pancreaticoduodenectomy (PD) and distal pancreatectomy (DP) remain the most common form of pancreatic resection compared to parenchymal-preserving procedures due to superior oncologic outcomes and decreased post-operative complications, specifically of pancreatic fistula [6–8].
Long-term pancreatic function after pancreatectomy is not well described but is important for both pre-operative risk assessment and counseling with both short- and long-term health consequences. The objective of this study was to identify risk factors for pancreatic endocrine and exocrine insufficiency after pancreatectomy and establish time to onset of clinically relevant symptoms to determine the appropriate follow-up period.
MATERIALS AND METHODS
We performed a retrospective review of a prospectively maintained database of patients who underwent PD or DP at a single high-volume tertiary-care center between January 2000 and December 2015. Patients were excluded if data were missing for post-operative endocrine or exocrine insufficiency, or in the case of in-hospital mortality. Patients who were receiving pancreatic enzyme replacement therapy pre-operatively and those who were diagnosed with diabetes within 3 months leading up to surgery were analyzed as separate groups. Approval for the study was obtained from the Washington University Institutional Review Board.
The demographic and peri-operative clinical variables of interest included: age, gender, race, body mass index (BMI) (analyzed as a continuous variable), history of tobacco use, heavy alcohol use (defined as more than 7 drinks/day by the North American Pancreatitis Study Group) [9], personal history DM (type 1-insulin dependent DM [IDDM], type 2- IDDM, and type 2-non-insulin dependent DM [NIDDM]), history of acute or chronic pancreatitis, family history of DM, pre-operative symptoms (including steatorrhea, jaundice, abdominal pain and weight loss), and laboratory data (including albumin and bilirubin analyzed as continuous variables), malignant or benign diseases, and surgical approach (PD or DP).
Clinical characteristics were summarized using descriptive statistics. Pancreatic endocrine insufficiency was defined as the need for new pharmacological intervention on discharge from hospital of oral hypoglycemic medications or insulin. More specifically, the definition of endocrine insufficiency was categorized into one of three scenarios: no DM to type 2 – NIDDM (requiring oral hypoglycemic treatment), no DM to type 2 – IDDM (requiring insulin therapy) or type 2 – NIDDM to type 2 – IDDM (transition from oral hypoglycemic treatment to insulin therapy). This was determined at outpatient follow-up, thus eliminating the peri-operative period where many patients require insulin short term while IV fluids and diet are being adjusted. We were unable to account for escalation of insulin dosing in patients who required insulin therapy pre-operatively, as records reviewed did not have this complete information. Institutional practice is in adherence with the American Diabetes Association and thus results should be universally applicable.
Pancreatic exocrine insufficiency was defined as the need for new pharmacological intervention in the form of supplemental pancreatic enzymes. This was based on clinical assessment of the patient at the time of hospital discharge or clinic evaluation based on gastrointestinal function and stool characteristics consistent with pancreatic exocrine insufficiency (excessive gas, bloating, steatorrhea).
Time to endocrine insufficiency was defined as days from operative procedure to clinical diagnosis and treatment of endocrine insufficiency. Patients without endocrine insufficiency were censored at the last follow-up. Similarly, time to exocrine insufficiency was defined as days from operative procedure to clinical diagnosis and treatment of exocrine insufficiency. Patients without exocrine insufficiency were censored at the last follow-up.
Probabilities of endocrine insufficiency and exocrine insufficiency were calculated using cumulative incidence curves[10]. Differences between strata were determined by log-rank tests [11]. Cox proportional-hazards models were used to evaluate the relationship of select variables including pre-op and intra-op factors for endocrine insufficiency and exocrine insufficiency analysis, respectively [12]. The proportionality assumption was tested by adding a time-dependent covariate for each variable. The variables with p<0.20 from univariate models were considered in the multivariate model. The final multivariate model was built using the backward stepwise selection approach to identify all significant risk factors [13]. Factors significant at a 10% level were kept in the final model. All statistical tests were two-sided using an α = 0.05 level of significance. SAS Version 9.4 (Cary, NC) was used to perform all statistical analyses.
RESULTS
1,866 patients underwent pancreatic resection from January 2000 to December 2015. From this total, 149 patients were excluded for: pre-operative pancreatic enzyme replacement (n=60, analyzed as a separate group), missing post-operative endocrine or exocrine insufficiency data (n=22), and post-operative 30-day mortality (n=67). 1,717 patients remained and made up the study cohort: 75.47% (n=1,296) PD, 24.53% (n=421) DP. The average age was 62.62 years (SD = 12.79), 51.78% were male, and surgery was for malignancy in 74.35% of patients. The median follow-up was 17.88 months (range: 0.12–192.72 months). Baseline patient characteristics are summarized in Table 1.
Table 1:
Demographic and peri-operative clinical variables of 1,717 patients who underwent distal pancreatectomy (DP) or pancreaticoduodenectomy (PD), at a single tertiary-care center between 2000 and 2015. (IDDM = insulin-dependent diabetes mellitus, NIDDM = non-insulin dependent diabetes mellitus)
| DP (n=421, 24.53%) |
PD (n=1296, 75.47%) |
Overall | |
|---|---|---|---|
| Age (mean ± SD) | 59.11±14.37 | 63.76 ±12.02 | 62.62 ± 12.79 |
| Male | 190 (45.13) | 699 (53.94) | 889 (51.78%) |
| Caucasian | 354 (84.09) | 1160 (89.51) | 1514 (88.18%) |
| BMI (mean ± SD) | 404, 29.20±6.60 | 1289,_ 27.09±5.58 | 27.59 ± 5.90 |
| Tobacco Use | 411, 105 (25.55) | 1294, 325 (25.12) | 1705, 430 (25.04%) |
| Alcohol Abuse | 405, 7 (1.73) | 1293, 59 (4.56) | 1688, 66 (3.89%) |
| Family History Diabetes | 402, 148 (36.82) | 1265, 348 (27.51) | 1667, 496 (28.89%) |
| Pathology | 414 | 1290 | 1704 |
| Malignant | 217 (52.42) | 1050 (81.40) | 1267 (74.35%) |
| Benign | 197 (47.58) | 240 (18.60) | 437 (25.65%) |
| Pancreatitis | 419, 55 (13.13) | 1296, 219 (16.90) | 1715, 274 (15.98%) |
| Steatorrhea | 386, 9 (2.33) | 1290, 412 (31.94) | 1676, 421 (25.12%) |
| Jaundice | 406. 3 (0.74) | 1293, 759 (58.70) | 1696, 762 (44.85%) |
| Abdominal Pain | 403, 241 (59.80) | 1290, 796 (61.71) | 1693, 1037 (61.25%) |
| Weight Loss | 393, 145 (36.90) | 1255, 717 (57.13) | 1648, 862 (52.31%) |
| Albumin (mean ± SD) | 384,_ 4.16±0.55 | 1274,_ 3.94±0.53 | 1658, 3.99 ± 0.54 |
| Bilirubin (mean ± SD) | 385,_ 0.43±0.32 | 1275,_ 3.44±5.74 | 1660, 2.74 ± 5.19 |
| Hemoglobin A1c (mean ± SD) | 76,_ 7.23±1.49 | 182,_ 7.23±1.57 | 258, 7.23 ± 1.54 |
| Pre-operative DM | |||
| No DM | 315 (74.82) | 955 (73.69) | 1270 (73.97%) |
| Type 1 – IDDM | 5 (1.19) | 20 (1.54) | 25 (1.46%) |
| Type 2 – IDDM | 26 (6.18) | 144 (11.11) | 170 (9.90%) |
| Type 2 – NIDDM | 75 (17.81) | 177 (13.66) | 252 (14.68%) |
| Adjuvant Therapy (% of total, % of malignant) | 419, 149 (35.56, ) | 1274, 620 (48.67, ) | 1693, 769 (45.42%, 60.69%) |
| Chemotherapy | 420, 146 (34.76, ) | 1272, 619 (48.66, ) | 1692, 765 (45.21%, 60.38%) |
| Radiation | 50 (11.90, ) | 314 (25.20, ) | 1666, 364 (21.85%, 28.73%) |
|
Follow-up, months
(median, range) |
20.64 (0.12–160.44) | 16.68 (0.12–192.72) | 17.88 (0.12 – 192.72) |
| Endocrine Insufficiency at Last Follow-up | 127 (30.17) | 219 (16.90) | 346 (20.15%) |
| Time from Surgery to Endocrine Onset in Months (mean ± SD (interquartile range)) |
22.46+24.91
(2.99–35.90) |
28.74+ 37.66
(1.81–41.11) |
27.20 ± 35.06 (2.14–39.35) |
| Diabetes Status at Last Follow-up | 192 | 472 | 664 |
| Type 1 – IDDM | 5 (2.60) | 20 (4.24) | 25 (3.77%) |
| Type 2 – IDDM | 120 (62.50) | 317 (67.16) | 437 (65.81%) |
| Type 2 – NIDDM | 67 (34.90) | 135 (28.60) | 202 (30.42%) |
| Pre-operative DM with No Change in Management | 64 (33.33) | 252 (53.39) | 316 (47.59%) |
| Type 1 – IDDM to Type 1 – IDDM | 5 (2.60) | 20 (4.24) | 25 (3.77%) |
| Type 2 – NIDDM to Type 2 – NIDDM | 33 (17.19) | 88 (18.64) | 121 (18.22%) |
| Type 2 – IDDM to Type 2 – IDDM | 26 (13.54) | 144 (30.51) | 170 (25.60%) |
| Pre-operative DM with Escalation of Regimen (Type 2 – NIDDM to Type 2 – IDDM) | 42 (21.88) | 89 (18.86) | 131 (19.73%) |
| No pre-operative DM to DM Post-operative | 86 (44.79) | 131 (27.76) | 217 (32.68%) |
| No DM to Type 2 – IDDM | 52 (27.08) | 84 (17.80) | 136 (20.48%) |
| No DM to Type 2 – NIDDM | 34 (17.71) | 47 (9.96) | 81 (12.20%) |
| Exocrine Insufficiency at Last Follow-up | 85 (20.19) | 537 (41.44) | 622 (36.23%) |
| Time from Surgery to Exocrine Onset in Months (mean ± SD (interquartile range)) |
24.26+26.31
(2.40–41.39) |
22.39 +35.54
(1.12–26.87) |
22.85 ± 33.52 (1.25-29.98) |
Pancreatic Endocrine Insufficiency
Three-hundred and forty-six (20.15%) patients developed post-operative pancreatic endocrine insufficiency requiring introduction or escalation of pharmacologic intervention. Of these, 217 developed de novo DM, with 136 patients (62.67%) requiring insulin (Table 1). One hundred and thirty-one (19.73%) patients with pre-operative DM required escalation of their prior regimen (Table 1). The mean time from surgery to insufficiency was 20.80 ± 33.60 (IQR: 0.49–28.37) months with the cumulative incidence demonstrated in Figure 1. As shown in Table 2, univariate analysis revealed that increasing BMI (p<0.001), family history of DM (p<0.001), presence of pre-operative DM (p<0.001), and DP compared with PD (p<0.001) were significant risk factors for post-operative pancreatic endocrine insufficiency. In multivariate Cox proportional-hazards models, male sex (p=0.015), increasing BMI (p<0.001), history of tobacco abuse (p=0.011), family history of DM (p<0.001), presence of pre-operative DM (p<0.001), and DP (p<0.001) were identified as independent predictive factors for endocrine insufficiency (Table 2). We did not find a difference in endocrine insufficiency between standard and pylorus-preserving PD, respectively (40.4 v. 42.3%; p = 0.61) or between surgery for benign or malignant disease (19.1 v. 21.7%; p = 0.77).
Figure 1:
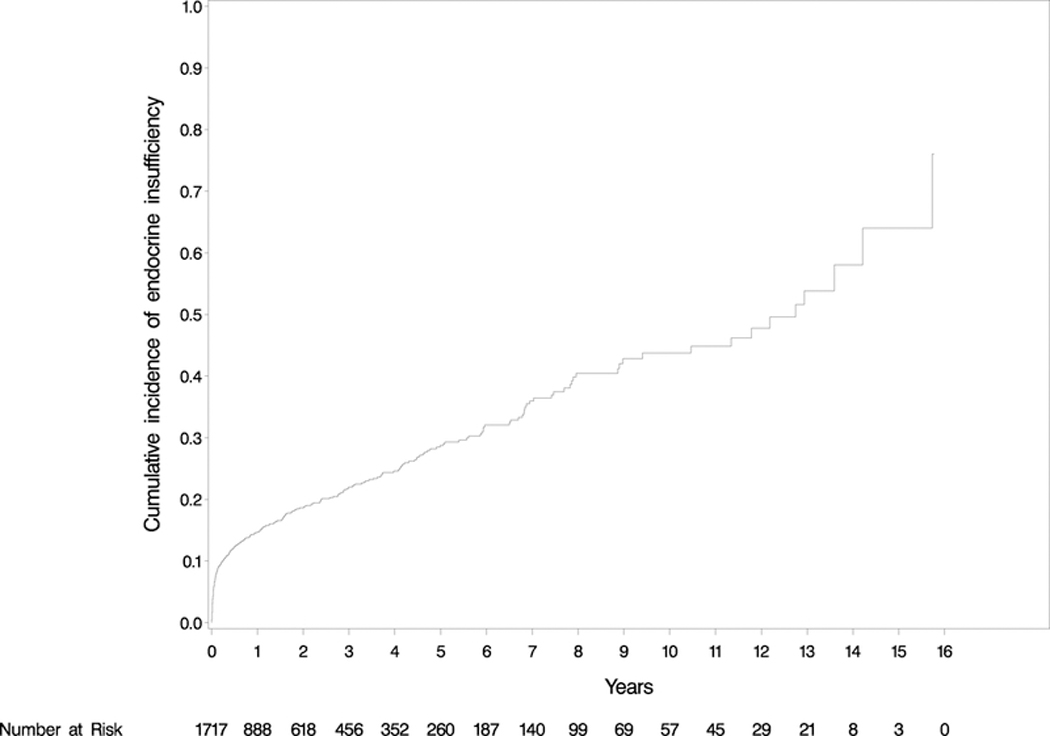
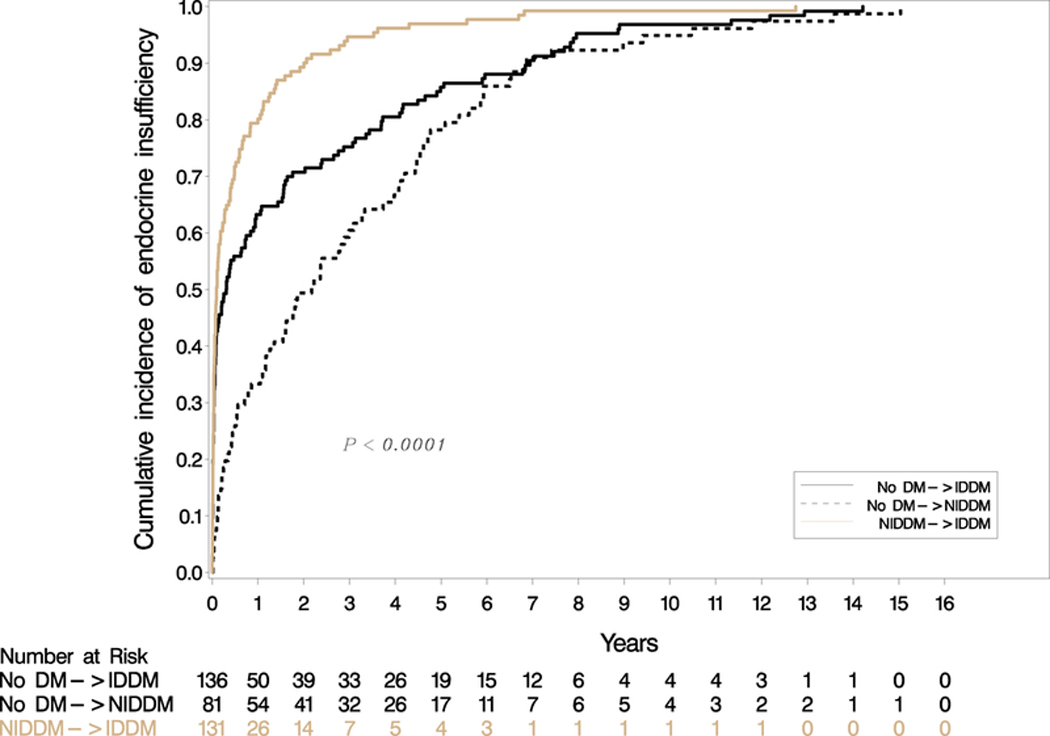
Figure 1a: Cumulative incidence of endocrine insufficiency. 20.15% of patients developed endocrine insufficiency at mean 20.80 months follow-up.
Figure 1b: Cumulative incidence of endocrine insufficiency by diabetes type (IDDM = insulin-dependent diabetes mellitus, NIDDM = non-insulin dependent diabetes mellitus)
Table 2:
Univariate and multivariate analysis of endocrine function post pancreaticoduodenectomy (PD) and distal pancreatectomy (DP).(IDDM = insulin-dependent diabetes mellitus, NIDDM = non-insulin dependent diabetes mellitus)
| Univariate | Multivariable | ||
|---|---|---|---|
| P value | P value | HR (95% CI) | |
| Age | 0.51 | ||
| Gender | 0.11 | p=0.015 | 1.33 (1.06–1.66) |
| Race (Caucasian vs. Other) | 0.37 | ||
| Body Mass Index (BMI)† | <.0001 | p=0.0002 | 1.03 (1.02–1.05) |
| Tobacco Use (Yes vs. No/In past) | 0.12 | p=0.011 | |
| Smoker vs. Non-smoker | 1.28 (0.96–1.70) | ||
| Ex-smoker vs. Non-smoker | 1.48 (1.14–1.93) | ||
| Alcohol Abuse (No vs. Yes) | 0.31 | ||
| Family History Diabetes | <.0001 | p=0.0002 | |
| Yes vs. No | 1.71 (1.29-2.28) | ||
| Unknown vs. No | 1.12 (0.83–1.52) | ||
|
Tumor Type (Malignant vs. Benign) |
p=0.47 | ||
| Pancreatitis | p=0.56 | ||
| Steatorrhea | p=0.082 | ||
| Jaundice | p=0.057 | ||
| Weight Loss | p=0.36 | ||
| Albumin† | p=0.30 | ||
| Bilirubin† | p=0.13 | ||
| Pre-operative Diabetes | p<.0001 | p<.0001 | |
| None vs. Type 2 NIDDM | 0.27 (0.21–0.34) | ||
| Type 1 IDDM vs. Type 2 NIDDM | 0.00 (0.00-.) | ||
| Type 2 IDDM vs. Type 2 NIDDM | 0.00 (0.00-1.2E242) | ||
| Operation (DP vs. PD) | p<.0001 | p<.0001 | 1.99 (1.53-2.60) |
Analyzed as a continuous variables
Pancreatic Exocrine Insufficiency
Six-hundred and twenty-two (36.23%) patients developed post-operative pancreatic exocrine insufficiency. The mean time from surgery to insufficiency was 14.20 ± 26.90 (IQR: 0.89–12.69) months with the cumulative incidence demonstrated in Figure 2. As shown in Table 3, univariate analysis revealed that older age (p=0.0084), increasing BMI (p<0.001), history of tobacco use (p=0.012), family history of DM (p=0.012), malignant diseases (p<0.001), steatorrhea (p<0.001), jaundice (p<0.001), weight loss (p<0.001), high pre-operative bilirubin (p<0.001), and PD compared with DP (p<0.001) were significant risk factors for post-operative pancreatic exocrine insufficiency. In multivariate Cox proportional-hazards models, race (p=0.014), increasing BMI (p<0.001), family history of DM (p=0.0072), steatorrhea (p<0.001), high pre-operative bilirubin (p=0.019) and PD (p<0.001) were identified as independent prognostic factors for exocrine insufficiency (Table 3). Although not analyzed in the overall study cohort, of the 60 patients that were on pancreatic enzyme supplementation prior to surgery, 11 (18.33%) stopped enzymes after resection. We did not find a difference in exocrine insufficiency between standard and pylorus-preserving PD, respectively (16.1% v. 18.9%; p = 0.32) or between surgery for benign or malignant disease (34.5 v. 37.2; p = 0.52). Patients on pancreatic enzyme replacement therapy pre-operatively were excluded from further analysis due to the inability to detect change with our definition, the need for new pharmacologic intervention.
Figure 2:
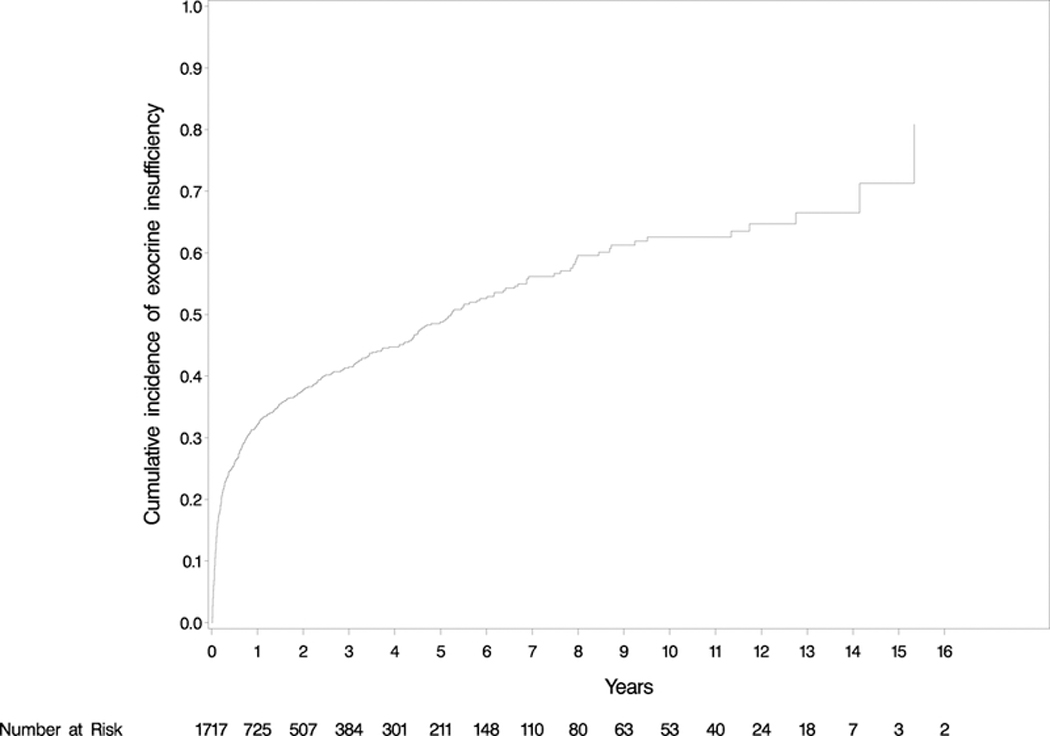
Cumulative incidence of exocrine insufficiency. 36.23% of patients developed exocrine insufficiency at mean 14.20 months follow-up.
Table 3:
Univariate and multivariate analysis of exocrine function post pancreaticoduodenectomy (PD) and distal pancreatectomy (DP). (IDDM = insulin-dependent diabetes mellitus, NIDDM = non-insulin dependent diabetes mellitus)
| Univariate | Multivariable | ||
|---|---|---|---|
| P value | P value | HR (95% CI) | |
| Age | 0.0084 | ||
| Gender | 0.52 | ||
| Race (Caucasian vs. Other) | 0.01 | 0.014 | 1.42 (1.07–1.87) |
| Body Mass Index (BMI)† | <.0001 | 0.0003 | 0.97 (0.96–0.99) |
| Tobacco Use (Yes vs. No/In past) | 0.0038 | ||
| Smoker vs. Non-smoker | |||
| Ex-smoker vs. Non-smoker | |||
| Alcohol Abuse (No vs. Yes) | 0.34 | ||
| Family History Diabetes | 0.12 | 0.0072 | |
| Yes vs. No | 1.09 (0.89–1.34) | ||
| Unknown vs. No | 0.80 (0.66–0.98) | ||
|
Tumor Type (Malignant vs. Benign) |
<.0001 | ||
| Pancreatitis | 0.18 | ||
| Steatorrhea | <.0001 | 0.0007 | 1.38 (1.14–1.66) |
| Jaundice | <.0001 | ||
| Weight Loss | <.0001 | ||
| Albumin† | 0.20 | ||
| Bilirubin† | <.0001 | p=0.019 | 1.02 (1.00–1.03) |
| Pre-operative Diabetes | 0.64 | ||
| None vs. Type 2 NIDDM | |||
| Type 1 IDDM vs. Type 2 NIDDM | |||
| Type 2 IDDM vs. Type 2 NIDDM | |||
| Operation (PD vs. DP) | <.0001 | <.0001 | 1.99 (1.53–2.60) |
Analyzed as a continuous variables
DISCUSSION
To our knowledge, this analysis represents the largest series describing the incidence of both endocrine (20.15%) and exocrine (36.23%) insufficiency after pancreatectomy, with mean time to onset of diagnosis and pharmacologic intervention of 20.80 and 14.20 months, respectively. These results were dependent on the clinical diagnosis of endocrine and exocrine insufficiency and may be underestimates, particularly for exocrine insufficiency of which mild and moderate symptoms often go undetected and untreated. In fact, we observed a steady increase in both endocrine and exocrine insufficiency (Figures 1 and 2), highlighting the importance of pancreatic insufficiency awareness during continued follow-up after pancreatectomy as subclinical symptoms may manifest in additional patients over time. This will only become more relevant as the treatment of pancreatic cancer improves and patients survive longer post-pancreatectomy.
Without strict screening protocols, symptoms may take time to declare themselves and providers should be cognizant of post-pancreatectomy functional insufficiencies at subsequent follow-up visits. Our results are congruent with a recent retrospective study that observed documenting only 30-day outcomes misses 78% of pancreatic insufficiency diagnoses after pancreatic resection and recommended at least 90-day follow up of functional outcomes[14]. Based on our results, post-operative strategies should include regular follow-up with ongoing awareness and a high degree of suspicion for impaired endocrine and exocrine pancreatic function long-term.
Factors such as etiology of disease, extent of resection, nature of disease (benign versus malignant), functional capacity of remnant pancreatic tissue, and reconstruction methods altering intestinal physiology have been described as influential in the development of functional insufficiencies [15–16]. In the present study, we demonstrated that male gender, increasing BMI, tobacco use, family history of DM, pre-operative DM, and DP correlated with an increased risk of post-operative endocrine insufficiency. Diabetes after pancreatectomy, or de novo DM, was present in 217 patients. One hundred and thirty-six (62.67%) of newly diagnosed diabetics, were insulin dependent. Understanding the incidence of post-operative pancreatic endocrine insufficiency is important because a new diagnosis of DM has important health and quality of life related outcomes with a significantly higher risk for stroke, coronary artery disease, and peripheral vascular disease than the non-diabetic population [4,17]. Early detection and management may reduce the burden of DM and its complications.
The American Diabetes Association recommends screening the general population at 3-year intervals beginning at the age of 45 using fasting plasma glucose or hemoglobin A1C [18]. Post-pancreatectomy patients should be considered high-risk for the development of DM and screened more frequently. Patients should be educated about the symptoms of hyperglycemia including polyuria, polydyspia, weight loss, and blurred vision and post-pancreatectomy care should be coordinated with primary care physicians. Of patients with pre-operative type 2-NIDDM, 51.98% experienced worsening of diabetic control, requiring insulin at 1-year follow-up (Table 1). According to previous reports, 18–60% of pre-operative diabetic patients developed post-operative worsening of endocrine function [19–21]. This is not unexpected, pre-operative DM likely reflects a vulnerable pancreas, as the gland was not working at full capacity prior to resection. Patients who are diabetic prior to pancreatic resection should be educated of the importance of close glucose monitoring and potential for worsening glycemic control.
In our cohort, DP was correlated with increased risk of developing endocrine insufficiency compared to PD. A retrospective study reported that the incidence rates of post-operative endocrine insufficiency were different for different operative procedures, 31%, 4%, and 19% for DP, PD, and pylorus preserving PD respectively[22]. The observed higher incidence of endocrine insufficiency after DP may be explained in part by the heterogeneity of islet distribution, with a higher islet volume density in the tail than in the head and body of the pancreas[23–24].
Race, increasing BMI, family history of DM, steatorrhea, higher bilirubin, and PD were correlated with an increased risk of post-operative exocrine insufficiency. Post-operative exocrine insufficiency induces mal-digestion and malabsorption of nutrients, patients experience symptoms of steatorrhea, abdominal pain, flatulence, weight loss and malnutrition. Pancreatic enzyme replacement therapy has been the standard of care for post-operative exocrine insufficiency[16]. It is possible that pre-operative steatorrhea and low BMI, two of our predictive factors, reflect patients with undiagnosed pre-operative exocrine insufficiency thus the increased likelihood of post-operative insufficiency is expected.
As presented in Table 3, post-operative exocrine insufficiency was observed more frequently in the PD group than the DP group. Kachare et al. also identified a significant difference in post-operative exocrine insufficiency with the rates of 32% and 17% for PD and DP, respectively [25]. Pancreatic head tumors with ductal obstruction often result in atrophy and fibrosis of the remaining pancreas [14]. Our group has previously shown that pre-operative jaundice is a poor prognostic factor in adenocarcinoma of the pancreas [26]. For exocrine insufficiency, documented pre-operative clinical jaundice was identified as a statistically significant risk factor on univariate analysis and elevated bilirubin on multivariate analysis. These values likely give us similar information in different ways, both indicating some degree of ductal obstruction with the potential for gland atrophy and fibrosis.
This difference between operative procedures may be due to the resection and subsequent reconstruction. In addition to resection of pancreatic parenchyma, the cumulative loss of duodenum, accelerated gastric emptying, and intestinal transit of biliary and pancreatic fluids inherent in PD likely contribute to altered pancreatic exocrine function [15]. The duodenum is the principal region for fat digestion and absorption by exogenous enzymes, which are modulated by duodenum-producing substances such as cholecystokinin, secretin and motilin [27]. In addition, modifications of gastrointestinal anatomy and asynchrony between gastric emptying of nutrients and pancreatic enzyme secretion play a major role in the establishment of post-operative maldigestion [28]. Furthermore, patency of the pancreatic anastomosis, which is only an issue post-PD, has been hypothesized as an important factor in pancreatic exocrine function [29].
In univariate analysis, malignant pathology was determined to be a significant risk factor for development of exocrine insufficiency. Tumor obstruction and administration of chemo-radiotherapy increase fibrosis and pancreatic atrophy, thus decreasing function [14,26,27,30]. In those patients with malignant pathology, 769 (60.69%) received adjuvant therapy. Of these, most received chemotherapy (n=765, 60.38%) and 364 patients (28.73%) received adjuvant chemo-radiation. These treatment effects did not reach significance in the cumulative group; however, adjuvant therapy may play a role in pancreatic burnout and functionality. It is possible that neoadjuvant treatment may have a more significant effect, specifically radiation [14,31–34]. During the cumulative study period, a low number of patients received neoadjuvant treatment regimens at our institution; however, the increasing use of neoadjuvant protocols at our center and worldwide warrants further investigation as to the effect of various chemotherapy, targeted therapy, immunotherapy, and chemo-radiation regimens effect on long-term pancreatic function.
Pancreatic insufficiency is a common clinical manifestation after pancreatectomy, yet long-term risks of pancreatic insufficiencies are not well understood. The incidence of post-operative endocrine insufficiency varies widely in the literature, reported between 3% and 40% (Table 4) [5,6,14,22,34–37]. This has limited our ability to precisely define the risk and provide adequate pre-operative patient counseling on the risks of long term pancreatic endocrine and exocrine insufficiency. Likewise, post-operative exocrine insufficiency has been reported to range from 12 to 86% (Table 3) [5,6,14,17,36,37]. A recent meta-analysis summarized published data of 1,295 patients reporting a 22% incidence of endocrine insufficiencies and 34% incidence of exocrine insufficiencies, emphasizing the importance of analyzing functional outcomes post-pancreatectomy and the impact of metabolic long-term risks [38]. Our series of 1,717 patients represents the largest reported experience on this topic in a modern cohort extracted from a prospectively maintained database with a low incidence of missing data. We also report important time to incidence, which is valuable in guiding awareness and follow-up recommendations for providers.
Table 4:
Literature review of post-pancreatectomy endocrine and exocrine insufficiency
| Study | n = | Study Type |
Type of pancreatectomy |
Endocrine Insufficiency |
Exocrine Insufficiency |
|---|---|---|---|---|---|
| Cherif et al.5 | 118 | Prospective, controlled cohort |
PD = 28 DP = 27 PSP = 63 |
6 (21.4%) 1 (3.7%) 3 (4.8%) |
16 (57.1%) 2 (7.4%) 2 (3.2%) |
| Falconi et al.6 | 135 | Prospective, controlled cohort |
PD = 51 DP = 50 AR = 34 |
9 (17.6%) 7 (14.0%) 1 (2.9%) |
17 (33.3%) 9 (18.0%) 0 (0%) |
| Lim et al.13 | 227 | Prospective, case series |
PD = 159 DP = 63 Enucleation = 5 |
28/178 without pre- operative endocrine insufficiency (15.7%) |
94/214 without pre- operative exocrine insufficiency (43.9%) |
| Halloran et al.16 | 40 | Prospective, cohort |
PD = 37 DP = 3 |
- | 30/39 at 6 weeks (76.9%) 19/22 at 1 year (86.4%) |
| Hirata et al.18 | 167 | Retrospective, cohort |
PD = 100 DP = 67 |
68 (40.7%) | - |
| Kwon et al.21 | 229 | Retrospective, cohort |
PD = 94 DP = 118 CP = 17 |
52 (22.7%) | - |
| Wu et al.33 | 3914 | Retrospective, population based |
PD = 3914 | 632 (16.1%) | - |
| Ferrara et al.34 | 564 | Retrospective, cohort |
PD = 564 | 22 (3.9%) | - |
| Orfanidis et al.35 | 41 | Retrospective, cohort (prospective interviews) |
PD = 41 | 8 (19.5%) | 5 (12.2%) |
| Elliott et al.36 | 1165 | Retrospective, population based |
PD = 692 DP = 473 |
274/678 without pre- operative endocrine insufficiency (40.4%) |
235/678 without pre- operative endocrine insufficiency (34.7%) |
PD – pancreaticoduodenectomy, DP– distal pancreatectomy, PSP – pancreas sparing pancreatectomy (enucleation and central pancreatectomy), CP – central pancreatectomy, AR – atypical resection
This study has several limitations. This was a retrospective cohort study in a single institution with all associated risks of unintended bias. Larger prospective studies with standardized protocols are warranted to confirm the validity of these results, including a post hoc analysis of completed prospective studies. Strict functional tests to evaluate pancreatic insufficiency after surgery were not performed and the clinical diagnosis of pancreatic exocrine insufficiency can be quite subjective and dependent on questions asked during hospital discharge and follow-up. For example, BT-PABA-test, fecal elastase test, coefficient of fat absorption, and C- mixed triglyceride breath test are objective methods that have been widely accepted to assess fat malabsorption [14,17,28]. Relying on clinical diagnosis potentially underestimated incidence as it is not unusual for mild and moderate symptoms to go undetected when compared to objective tests [39]. However, this reflects the common clinical practice of most pancreatic surgeons. Similarly, not every patient was screened pre-operatively for DM. In this study, including data collected over fifteen years, practices in pre-operative DM screening and assessment have also changed over time. Importantly, the complex relationship between development of diabetes an pancreatic cancer represents an active area of research in endocrinology and oncology. There may be uncharacterized dynamics that affect the development of diabetes, often years before the detection of cancer, that could affect these results and our conclusions.
Pancreatic functional insufficiencies are common and may develop over time following pancreatic resections. Our data supports close long-term follow-up with clinical screening for signs and symptoms of endocrine and exocrine insufficiency, as well as biochemical work-up including fasting plasma glucose or hemoglobin A1C for DM. Post-pancreatectomy patients are at high-risk for the development of DM and should be screened at least yearly following surgery. Objective testing for exocrine insufficiency can be performed as well, if clinically indicated. Use of fecal testing may increase diagnostic yield, as mild and moderate symptoms are often undiagnosed and untreated. Educating patients and primary care physicians may improve capture and help to prevent these patients from going undiagnosed in the long-term.
CONCLUSION
In summary, our analysis is the largest report of the cumulative incidence of pancreatic endocrine and exocrine insufficiency after pancreatectomy. We show incidence rates of 20.15% endocrine and 36.23% exocrine insufficiency following pancreatic resection, with mean time to onset of diagnosis and pharmacologic intervention of 20.80 and 14.20 months, respectively. Patients should be educated regarding post-resection insufficiencies and providers should have heightened awareness with long-term follow-up required.
Acknowledgments
Grant Support and Other Assistance
G.A.W. and J.L. supported by the SPORE grant 5P50CA196510–02. REDCap Supported by Clinical and Translational Science Award (CTSA) Grant [UL1 TR000448] and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Footnote
Presented at the American Hepato-Pancreato-Biliary Association Annual Meeting, Miami Beach, FL, March-April 2018.
REFERENCES
- 1.Cameron JL. He J Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, Wargo JA, Ferrone CR, Thayer SP, Lillemoe KD, Warshaw AL. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery. 2012;152:S56–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: A systematic review and meta-analysis. Ann Surg. 2012;255:1048–1059. [DOI] [PubMed] [Google Scholar]
- 4.Huang JJ, Yeo CJ, Sohn TA, Lillemoe KD, Sauter PK, Coleman J, Hruban RH, Cameron JH. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg. 2000;231:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherif R, Gaujoux S, Couvelard A, Dokmak S, Vuillerme MP, Ruszniewski P, Belghiti J, Sauvanet A. Parenchyma-sparing resections for pancreatic neuroendocrine tumors. J Gastrointest Surg. 2012;16:2045–2055. [DOI] [PubMed] [Google Scholar]
- 6.Falconi M, Mantovani W, Crippa S, Masetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumors. Br J Surg. 2008;95:85–91. [DOI] [PubMed] [Google Scholar]
- 7.Crippa S, Bassi C, Warshaw AL, Falconi M, Stefano P, Thayer SP, Pederzoli P, Castillo CFD. Middle pancreatectomy. Ann Surg. 2007;246:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falconi M, Zerbi A, Crippa S, Balzano G, Boninsegna L, Capitanio V, Bassi C, Di Carlo V, Pederzoli P. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17:1621–1627. [DOI] [PubMed] [Google Scholar]
- 9.Whitcomb DC, Yadav D, Adam S, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, Disario J, Burton FR, Gardner TB, Amann ST, Gelrud A, Lo SK, DeMeo MT, Steinberg WM, Kockman ML, Etemad B, Forsmark CE, Elinoff B, Greer JB, O’Connell M, Lamb J, Barmada MM. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: The North American Pancreatitis Study 2 (NAPS2). Pancreatology. 2008; 8:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 11.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 13.Klein JP, Moeschberger ML. Semiparametric proportional hazards regression with fixed covariates In: Klein JP, Moeschberger ML, eds. Survival Analysis: Techniques for Censored and Truncated Data. New York: Springer; 1997:229–263. [Google Scholar]
- 14.Lim PW, Dinh KH, Sullican M, Wassef WY, Zivny J, Whalen GF, LaFemina J. Thirty-day outcomes underestimate endocrine and exocrine insufficiency after pancreatic resection. HPB. 2016;18:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips ME. Pancreatic exocrine insufficiency following pancreatic resection. Pancreatology. 2015;15:449–455. [DOI] [PubMed] [Google Scholar]
- 16.Seiler CM, Izbicki J, Vargas-Szabo L, Czak L, Fiok J, Sperti C, Lerch MM, Pezzilli R, Vasileva G, Pap A, Varga M, Friess H. Randomised clinical trial: A 1-week, double-blinded, placebo-controlled study of pancreatin 25 000 Ph. Eur minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment Pharmacol Ther. 2013;37:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halloran CM, Cox TF, Chauhan S, Raraty MG, Sutton R, Neoptolemos JP, Ghaneh P. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: A prospective study. Pancreatology. 2012;11:535–545. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes – 2018. Diabetes Care. 2018;41:S13. [DOI] [PubMed] [Google Scholar]
- 19.Hirata K, Nakata B, Amano R, Yamazoe S, Kimura K, Hirakawa K. Predictive factors for change of diabetes mellitus status after pancreatectomy in preoperative diabetic and nondiabetic patients. J Gastrointest Surg. 2014;18:1597–1603. [DOI] [PubMed] [Google Scholar]
- 20.White MA, Agle SC, Fuhr HM, Mehaffey JH, Waibel BH, Zervos EE. Impact of pancreatic cancer and subsequent resection on glycemic control in diabeteic and nondiabetic patients. Am Surg. 2011;77:1032–1037. [PubMed] [Google Scholar]
- 21.Dinorcia J, Ahmed L, Lee MK, Reavey PL, Yakaitis Ea, Lee JA, Schrope BA, Chabot JA, Allendorf JD. Better preservation of endocrine function after central versus distal pancreatectomy for mid-gland lesions. Surgery. 2010;148:1247–1254. [DOI] [PubMed] [Google Scholar]
- 22.Kwon JH, Kim SC, Shim IK, Song KB, Lee JH, Hwang DW, Park KM, Lee YJ. Factors affecting the development of diabetes mellitus after pancreatic resection. Pancreas. 2015;44:1296–1303. [DOI] [PubMed] [Google Scholar]
- 23.Gersell DJ, Gingerich RL, Greider MH. Regional distribution and concentration of pancreatic polypeptide in the human and canine pancreas. Diabetes. 1979;28:11–15. [PubMed] [Google Scholar]
- 24.Henquin JC, Ibrahim MM, Rahier J. Insulin, glucagon and somatostatin stores in the pancreas of subjects with type-2 diabetes and their lean and obese non-diabetic controls. Sci Rep. 2017;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kachare SD, Fitzgerald TL, Schuth O, Vohra NA, Zervos EE. The impact of pancreatic resection on exocrine homeostasis. Am Surg. 2014;80:704–709. [PubMed] [Google Scholar]
- 26.Strasberg SM, Gao F, Sanford D, Linehan DC, Hawkins WG, Fields RC, Carpenter DH, Brunt EM, Phillips C. Jaundice: an important, porrly recognized risk factor for diminished survival with adenocarcinoma of the head of the pancreas. HPB. 2014;16:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beger HG, Nakao A, Mayer B, Porch B. Duodenum-preserving total and partial pancreatic head resection for benign tumors – systematic review and meta-analysis. Pancreatology. 2015;15:167–178. [DOI] [PubMed] [Google Scholar]
- 28.Vujasinovic M, Valente R, Del Chiaro M, Permert J, Lohr JM. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients. 2017;9:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemaire E, O’Toole D, Sauvanet A, Hammel P, Belghiti J, Ruszniewski P. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br J Surg. 2000;87:434–438. [DOI] [PubMed] [Google Scholar]
- 30.Fong ZV, Tan WB, Lavu H, Kennedy EP, Mitchell DG, Koniaris LG, Sauter PK, Rosato EL, Yeo CJ, Winter JM. Preoperative imaging for resectable periampullary cancer: Clinicopathologic implications of reported radiographic findings. J Gastrointest Surg. 2013;17:1098–1106. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa O, Ohigashi H, Imaoka S, Teshima T, Inoue T, Sasaki Y, Iwanaga T, Nakaizumi A. Concomitant benefit of preoperative irradiation in preventing pancreas fistula formation after pancreatoduodenectomy. Arch Surg. 1991; 126:885–889. [DOI] [PubMed] [Google Scholar]
- 32.Wydmanski J, Polanowski P, Tukiendorf A, Maslyk B. Radiation. Radiation-induced injury of the exocrine pancreas after chemoradiotherapy for gastric cancer. Radiother Oncol. 2016; 118, 535–539. [DOI] [PubMed] [Google Scholar]
- 33.Gemici C, Sargin M, Uygur-Bayramicli O, Mayadagli A, Yaprak G, Dabak R, Kocak M. Risk of endocrine insufficiency in patients receiving adjuvant chemoradiation for resected gastric cancer. Radiother Oncol. 2013; 107, 195–199. [DOI] [PubMed] [Google Scholar]
- 34.Wu JM, Ho TW, Kuo TC, Yang CY, Lai HS, Chiang PY, Hsieh SH, Lai F, Tien YW. Glycemic change after pancreaticoduodenectomy: A population-based study. Medicine (Baltimore). 2015l94:e1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrara MJ, Lohse C, Kudva YC, Farnell MB, Que FG, Reid-Lombardo KM, Donohue JH, Nagorney DM, Chari ST, Vege SS, Kendrick ML. Immediate post-resection diabetes mellitus after pancreaticoduodenectomy: Incidence and risk factors. HPB. 2013;15:170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orfanidis NT, Loren DE, Santos C, Kennedy EP, Siddiqui AA, Lavu H, Yeo CJ, Kowalski TE. Extended follow-up and outcomes of patients undergoing pancreaticoduodenectomy for no-malignant disease. J Gastrointest Surg. 2012;16:80–88. [DOI] [PubMed] [Google Scholar]
- 37.Elliott IA, Epelboym I, Winner M, Allendorf JD, Haigh PI. Population-level incidence and predictors of surgically induced diabetes and exocrine insufficiency after partial pancreatic resection. Perm J. 2017; 21:16–095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beger HG, Poch B, Mayer B, Siech M. New onset of diabetes and pancreatic exocrine insufficiency after pancreaticoduodenectomy fo benign and malignant tumors: A systematic review and meta-analysis of long-term results. Ann Surg. 2018;267:259–270. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez-Munoz JE, Iglesias-Garcia J. Oral pancreatic enzyme substitution therapy in chronic pancreatitis: Is clinical response and appropriate marker for evaluation of therapeutic efficacy? JOP. 2010;11:158–162. [PubMed] [Google Scholar]


