Abstract
We studied 232 consecutive children transplanted between 1990 and 2011 with relapse after 1st hematopoietic cell transplant (HCT). Kaplan-Meier survival and hazard ratios for mortality were calculated for factors known at time of relapse using Cox proportional hazards models. The median (range) age at time of 1st HCT was 10.9 (0.5–20.9) years, time to relapse was 6.1 (0.2–89.5) months after HCT, and age at relapse was 11.7 (0.7–23.6) yrs. The 3-year overall survival (OS) after relapse was 13% (95% Confidence Interval (CI): 9%, 18%).The median (range) follow-up for the 18 surviving patients was 7.2 (3.0–24.4) years after relapse. The remaining 214 died after a median of 3 months (0.02–190.4). OS was not significantly different for patients with ALL as compared to AML. Fifty-one patients proceeded to 2nd transplant of whom 9 survive. Factors associated with improved survival included late relapse (greater than 12 months), ALL in first CR at the time of first transplant and chemotherapy-based first conditioning regimens. These results can be used to counsel patients at the time of relapse after first transplant and as a baseline for comparison as to the effectiveness of newer therapies which are greatly needed for treatment of post-transplant relapse.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) often offers the best and often only chance for cure for patients with very high-risk leukemia. While current risk-adapted chemotherapy regimens cure most children with acute lymphoblastic leukemia (ALL) and many with acute myeloid leukemia (AML), there remains a subset of patients as for whom cure is unlikely without allogeneic HCT. Historically, allogeneic HCT was indicated for patients with early relapse of ALL or AML, as well as those with high risk features at diagnosis or persistent minimal residual disease (MRD). [1–9] For these subsets of very high-risk patients, HCT after remission induction increases the likelihood of leukemia-free survival (LFS).
LFS following allogeneic HCT depends upon a number of factors, foremost the disease status at time of HCT.[10–16] However, the proportion of patients with “good-risk” disease at time of HCT appears to be shrinking as risk-adapted therapy has narrowed the group of children thought to benefit from HCT to those with extremely high-risk leukemias.[6–8,11–13,15,17–21] For example, in the past decade 1 in 3 patients referred to our center with ALL in remission were MRD-positive at time of HCT, leading to a >3-fold higher risk of relapse compared to those without MRD (p=0.0001).[21] Fortunately, the toxicity and mortality associated with HCT has substantially decreased over the last 20 years.[22] This great improvement in safety means that relapse is now the biggest barrier to improving survival after HCT. [6,10–13,15,17,23]
Our aim in this study was to determine factors associated with outcome in a historical group of patients who relapsed after allogeneic HCT, in order to define the baseline prognosis from which to compare future treatment strategies. We anticipate going forward that outcomes after relapse will improve in patients for whom therapies such as CD 19 chimeric antigen receptor (CAR) T cell therapies, monoclonal antibody-based bispecific T cell engagers, and antibody drug conjugates are available. However, until advanced targeted therapeutics become broadly applicable to all patients in relapse, our results are also important for understanding which patients might benefit from additional treatments or a second HCT.
Patients and Methods
Records from all patients who were <21 years of age at time of allogeneic HCT for acute leukemia or myelodysplastic syndrome (MDS) between January 1990 and December 2011 at Fred Hutchinson Cancer Research Center (FHCRC) were reviewed retrospectively for development of post-HCT relapse. The primary diagnosis of the hematologic malignancy was made at the referring institution and confirmed at FHCRC by review of diagnostic bone marrow samples. Remission status was determined within two weeks before HCT by histopathologic and cytogenetic analyses of marrow and cerebral spinal fluid. Patients were considered to be in remission if they had received chemotherapy and achieved a complete response in bone marrow (<5% blasts and normal marrow cellularity), while those given HCT before marrow recovery or with ≥5% marrow blasts were considered to be in relapse. MRD was defined as any level <5% of leukemic blasts detected by available technology, including histopathology, cytogenetics, molecular analysis, or flow cytometry. Disease phase was defined by the number of medullary remission or relapse events before HCT, but isolated extramedullary relapse was not considered as a separate relapse event. Patients were treated on standard treatment plans or research protocols for which informed consent was obtained using the consent forms approved by the FHCRC Institutional Review Board (IRB). Post-HCT relapse was defined as any morphologic, cytogenetic, or flow cytometric evidence of disease at any detectable level in the bone marrow or extramedullary site. A second hematologic malignancy without evidence of the original leukemia was not counted as a relapse.
Statistical Methods
Patient characteristics were summarized and reported using standard methods for categorical and continuous variables. Estimates of survival after relapse were calculated by the method of Kaplan and Meier, with time from relapse to the time of death or censoring at last contact. [24] Cox proportional hazards regression models were fit to evaluate risk factors associated with mortality.[25] Candidate variables examined included patient sex, year of HCT, time from HCT to relapse, donor type, diagnosis/phase at HCT, conditioning regimen, MRD status at HCT, time from diagnosis to HCT, age at HCT and age at relapse. Factors were retained in a final multivariable model if p-value was <0.10 or if their removal markedly modified the effect of another variable. In a separate model, we examined the hazard ratio of treatment assignment at relapse to a potentially curative treatment vs. no treatment or palliative care. All p-values are two-sided and considered significant at the 0.05 level. No adjustments were made for multiple comparisons. Data were frozen for analysis as of September, 2017.
Results
Patient Characteristics
Between January 1990 and December 2011, 760 consecutive children <21 years of age with a hematologic malignancy underwent allogeneic HCT for treatment of ALL (n= 396), AML (n= 285) or MDS (n= 79). Of these, we identified 232 patients in whom relapse of the original disease was diagnosed after HCT. Characteristics of these patients at the time of their initial HCT are shown in Table 1. Supplementary Table 1 compares these characteristics for those treated with intent to cure versus palliative/end of life therapies. Among the patients classified as having leukemia in complete remission at time of first HCT, MRD was not assessed for 36 patients. The median time to relapse after HCT was 6.1 (range 0.2–89.5) months and the median age at relapse was 11.7 years (range 0.7–23.6).
Table 1:
Patient and Transplant Characteristics at time of 1st transplant
| Total N= 232 | |
|---|---|
| Characteristics at time of first HCT | |
| Age, median (range) in months | 10.9 (0.5–20.9) |
| Interval from diagnosis to HCT(Months), median (range) | 11 (0.5–141.6) |
| N (%) | |
| Gender Male:Female | 98 (42.2%) : 134 (57.8%) |
| Diagnosis | |
| ALL | 121 (52.2%) |
| AML | 98 (42.2%) |
| MDS | 13 (5.6%) |
| Disease Phase | |
| ALL CR1 | 15 (6.5%) |
| ALL CR2 | 52 (22.4%) |
| ALL advanced | 54 (23.3%) |
| AML CR1 | 34 (14.7%) |
| AML CR2 | 14 (6.0%) |
| AML advanced | 49 (21.1%) |
| MDS | 14 (6.0%) |
| Leukemia burden at HCT | |
| Pre-MRD era | 36 (15.5%) |
| Blasts>=25% | 50 (21.6%) |
| Blasts 0–24% | 80 (34.5%) |
| MRD positive | 17 (7.3%) |
| MRD negative | 47 (20.3%) |
| EMD only | 2 (0.9%) |
| Transplant Characteristics | |
| Decade of HCT | |
| 1990–2000 | 140 (60.3%) |
| 2001–2011 | 92 (39.7%) |
| Donor Type | |
| Matched Related | 79 (34.1%) |
| Unrelated Marrow/PB | 105 (45.3%) |
| Cord/Other Donor | 48 (20.7%) |
| Conditioning Regimen | |
| RIC | 11 (4.7%) |
| Chemo-based myeloablative | 23 (9.9%) |
| TBI-based | 198 (85.3%) |
| TBI dose (Gy) | |
| 2 | 2 (1.0%) |
| 12 | 31 (15.3%) |
| 13.2 | 72 (35.6%) |
| 14.4 | 74 (36.6%) |
| 15.75 | 23 (11.4%) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BU, busulfan; CR, complete remission; EMD, extra-medullary disease; MDS, myelodysplastic syndrome; MRD, minimal residual disease; PB, peripheral blood; RIC, reduced intensity conditioning; TBI, total body irradiation.
Survival
The 3-year overall survival after relapse was 13% (95% CI: 9%, 18%) (Figure 1). Among the 18 patients surviving at last contact, median follow-up was 7.2 years (range 3–24.3) after relapse. The remaining 214 died after a median of 3 months (range 0.02–190.4). Sixty patients declined further aggressive treatment and were given palliative or end-of-life therapies only, culminating in 59 deaths. One untreated patient had spontaneous resolution of cytogenetic relapse of ALL and survives. When outcome is confined to the 172 patients treated with intent to cure, OS was 16% (95% CI:11%, 22%) (Supplementary Figure 1). There was no significant difference in outcome between patients with ALL versus AML (p=0.27). Specifically, OS at 3 years for ALL was 15% (95% CI: 9%. 22%) and for AML was 9% (95% CI: 5%, 16%). Among the 13 patients with MDS, 3 survive long term without relapse.
Figure 1.
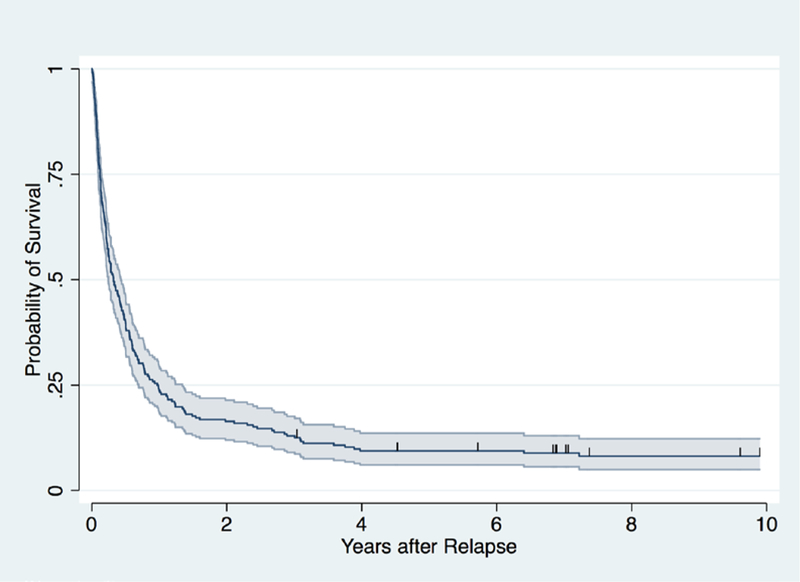
Probability of overall survival for 232 patients after relapse of leukemia following allogeneic hematopoietic cell transplant (HCT). 95% confidence interval band is shaded.
Treatment of Relapsed ALL
Among the 121 patients with relapsed ALL, 97 were treated with intent to cure (Figure 2). Of these, 7 survive for a median of 7 (range 4.5–12.3) years. Treatment modalities included withdrawal of immune suppression (IS) (n=15), chemotherapy (+/− IS withdrawal and/or local radiation, n=71), donor lymphocyte infusion (DLI) +/− chemotherapy (n=6), and tyrosine kinase inhibitor treatment (n=5). Figure 2 depicts outcomes in these treatment groups including those who proceeded to second HCT. Overall, 4 patients treated with intent-to-cure without second transplant survive.
Figure 2. Post-relapse treatment plan and outcomes.
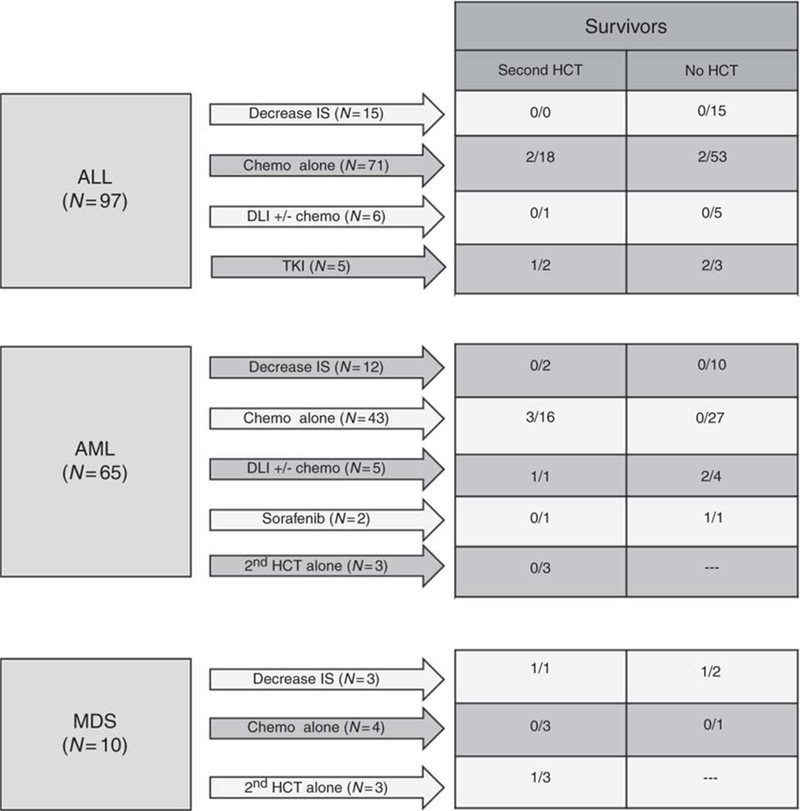
Patients with relapse after 1st transplant are grouped by disease (acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS)). Arrows represent different treatment choices with the number (N) of patients receiving selected treatment. The first column to the right of arrows indicates patients who went onto second transplant for a given treatment plan reflected as # survivors/total # patients proceeding to 2nd transplant. The final column on the right indicates patients who did not elect to proceed to 2nd transplant reflected as # survivors/total # patients who did not proceed to 2nd transplant. * Single patient with cytogenetic relapse and subsequent spontaneous resolution
Twenty-one patients with relapsed ALL proceeded to second HCT (Table 2). Second HCT regimens included myeloablative conditioning (MAC) in 8 cases, reduced intensity conditioning (RIC) in 9, and nonmyeloablative conditioning (NMC) in 4. None of the patients with persistent disease at time of second HCT survived long term. Three patients survive among those in CR at time of 2nd HCT, 2 of these without subsequent relapse. Two out of 4 patients receiving CB transplants survive.
Table 2:
Second hematopoietic transplants
| HCT#1 | HCT #2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Original Transplant Indication |
Regimen (Gy) |
Donor | Time to Relapse (days) |
Phase of Disease at 2nd HCT |
Regimen | Donor | Alive | Cause of death |
| ALL | ||||||||
| CR1 | CYTBI 12.0 | MSD | 91 | CR | M-CH | MSD | N | Relapse |
| CR1 MRD(−) | TBICY 12.0 | URD | 96 | CR MRD(−) | NM | URD | N | Relapse |
| CR1 MRD(−) | CYTBI 13.2 | MSD | 216 | CR MRD(−) | RIC | CB | N | Relapse |
| CR1 MRD(−) | TBICY 13.2 | MSD | 309 | CR MRD(+) | RIC | URD | N | Relapse |
| CR1 MRD(−) | TBICY 13.2 | MSD | 323 | CR MRD(+) | RIC | CB | N | Relapse |
| CR1 MRD(+) | TBICY 13.2 | MSD | 191 | CR MRD(+) | RIC | URD | N | TRM |
| CR1 MRD(+) | TBICY 13.2 | URD | 343 | CR MRD (−) | NM | URD | Y* | |
| CR1 MRD(+) | CYTBI 14.4 | URD | 348 | PD | M-CH | URD | N | TRM |
| CR2 | CYTBI 15.75 | MSD | 239 | PD | M-CH | MSD | N | Relapse |
| CR2 | CYTBI 15.75 | MSD | 315 | PD | M-CH | MSD | N | TRM |
| CR2 | CYTBI 15.75 | MSD | 361 | PD | M-CH | MSD | N | Relapse |
| CR2 | TBICY 13.2 | MSD | 1009 | CR MRD(−) | RIC | CB | Y | |
| CR2 MRD(−) | TBICY 13.2 | URD | 1319 | CR MRD(−) | NM | URD | N | Relapse |
| CR2 MRD(+) | TBICY 13.2 | MSD | 80 | CR MRD(+) | RIC | Haplo | N | Relapse |
| CR2 MRD(+) | TBICY 13.2 | URD | 96 | PD | M-CH | URD | N | TRM |
| CR2 MRD(+) | TBICY 13.2 | MSD | 395 | CR MRD(−) | RIC | URD | N | TRM |
| CR2 MRD(+) | CYTBI 12.0 | URD | 439 | CR MRD(−) | NM | URD | N | Relapse |
| CR3 | TBICY13.2 | URD | 517 | CR MRD(+) | RIC | CB | Y | |
| CR3 | TTCYTBI 12.0 | MSD | 2756 | CR MRD(+) | M-CH | MSD | N | TRM |
| CR3 MRD(+) | TBICY14.4 | URD | 1230 | UN | RIC | URD | N | Relapse |
| PrimRef | CYTBI 15.75 | MSD | 178 | PD | M-CH | MSD | N | Relapse |
| AML | ||||||||
| CR1 MRD(−) | BUCY | MSD | 75 | CR MRD(+) | M-TBI | URD | N | TRM |
| CR1 | BUCY | Haplo | 189 | PD | M-TBI | Haplo | N | TRM |
| CR1 | BUCYTBI | MSD | 307 | PD | M-TBI | MSD | N | TRM |
| CR1 | BUCY | MSD | 691 | CR | M-TBI | MSD | Y | |
| CR1 MRD(−) | CYTBI 14.4 | Haplo | 648 | PD | M-CH | Haplo | N | Relapse |
| CR1 MRD(−) | BUCY | MSD | 2493 | CR MRD(−) | M-TBI | URD | N | TRM |
| CR1 MRD(+) | FluCYTBI13.2 | CB | 219 | CR MRD(−) | RIC | CB | N | TRM |
| CR1 MRD(+) | BUCY | MSD | 231 | CR MRD(−) | M-TBI | MSD | N | TRM |
| CR1 MRD(+) | BUCY | Haplo | 257 | CR MRD(−) | M-TBI | URD | N | TRM |
| CR1 MRD(+) | BUCY | MSD | 320 | CR MRD(−) | M-TBI | MSD | Y | |
| CR1 MRD(+) | CYTBI 14.4 | URD | 333 | CR | M-CH | MSD | N | Relapse |
| CR1 MRD(+) | 131IBUCY | MSD | 408 | CR | M-TBI | MSD | N | Relapse |
| CR2 | BUCY | MSD | 2215 | CR | M-TBI | MSD | N | TRM |
| PrimRef | TBICY 13.2 | URD | 78 | CR MRD- | RIC | CB | Y | |
| PrimRef | CYTBI 14.4 | URD | 144 | CR | M-CH | URD | Y | |
| PrimRef | TBICY 13.2 | MSD | 214 | CR MRD- | NM | MSD | N | Relapse |
| PrimRef | BUCYTBI 12 | MSD | 229 | PD | M-CH | MSD | N | Relapse |
| PrimRef | CYTBI 14.4 | Hap | 391 | PD | M-CH | Hap | N | Relapse |
| PrimRef | CYTBI 14.4 | URD | 416 | CR MRD- | M-CH | URD | N | TRM |
| PrimRef | CYTBI 15.75 | Hap | 452 | PD | M-CH | Hap | N | TRM |
| REL | BUCY | MSD | 55 | PD | M-TBI | MSD | N | Relapse |
| REL | TBICY 13.2 | HAP | 75 | PD | M-CH | HAP | N | Relapse |
| REL | TBICY 13.2 | URD | 126 | CR MRD+ | NM | URD | N* | Trauma |
| MDS/MPS | ||||||||
| MDS EB | CYTBI 14.4 | MSD | 82 | PD | RIC | HAP | Y | |
| MDS EB | BUCY | URD | 128 | CR MRD- | M-TBI | URD | N | Relapse |
| MDS EB | BUCY | MSD | 132 | PD | M-TBI | MSD | N | Relapse |
| MDS EB | BUCY | MSD | 132 | PD | M-TBI | MSD | N | TRM |
| MDS EB | BUCY | URD | 247 | PD | M-TBI | URD | Y | |
| MDScEB | CYTBI 12 | MSD | 961 | PD | M-CH | MSD | N | Relapse |
| MPS EB | FluCY 13.2 | CB | 307 | PD | RIC | URD | N | Relapse |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BU, busulfan; CB, cord blood; CH, chemotherapy; CR, complete remission; CY, cyclophosphamide; D/C IS, discontinue immune suppression; DLI, donor lymphocyte infusion; EB, excess blasts; FLU, fludarabine; HAP, HLA-haploidentical donor; HCT, hematopoietic cell transplant; 131I, iodine-131; MDS, myelodysplastic syndrome; M-CH, chemotherapy-based myeloablative conditioning; MPS, myeoproliferative syndrome; MRD (−), no minimal residual disease; MRD (+), minimal residual disease; MSD, matched sibling donor; M-TBI, TBI- based myeloablative conditioning; N, no; NM, nonmeyloablative conditioning; PD, disease not in remission; PrimRef, primary refractory; REL, refractory relapse; RIC, reduced intensity conditioning; Rx, treatment for relapse; SOR, sorafenib; TBI, total body irradiation; TKI, tyrosine kinase inhibitor; TT, thiotepa; UN, unknown; URD, unrelated donor; Y, yes.
alive after 3rd transplant
died in remission from car accident
Treatment of Relapsed AML or MDS
Among the 98 patients with relapsed AML, 65 were treated with intent to cure (Figure 2) and 7 survive without subsequent relapse for a median of 14.1 (range 4.5 to 24.3) years. Treatment modalities included withdrawal of IS (n=12), chemotherapy (+/− withdrawal of IS and/or local radiation, n=43), DLI (+/− chemotherapy, n=5), tyrosine kinase inhibitor (n=2) or 2nd transplant alone (n=3). Patient outcomes for each treatment group are indicated in Figure 2.
Second HCT was performed for 23 of the patients with relapsed AML (Table 2). Second HCT regimens included MAC in 19 cases, RIC in 2, and NMC in 2. None of the patients with detectable disease at time of second HCT survived long term. Among the patients in MRD-negative CR at time of HCT, 4 survive, including 1 given RIC and 3 given MAC HCT.
Ten of the 13 patients with relapsed MDS/MPL were treated with curative intent, and 3 survive long term (Figure 2). Treatment modalities included withdrawal of immune suppression (n=3), chemotherapy alone (n=4), or direct second HCT (n=3). Second transplant was performed for 7 of the 10 patients (Table 2). Second HCT regimens were all myeloablative and all but one patient had progressive disease at the time of transplant. Two patients survive without subsequent relapse after second HCT.
Cause of Death
Cause of death after first HCT relapse was classified as either disease or treatment (including GVHD) related. Among the patients with relapsed ALL, death from progressive disease was the only cause of death for patients given no therapy, palliative therapy, or withdrawal of immune suppression. Death after chemotherapy or second HCT was caused by progressive ALL in 75% and treatment complications in 25%. Among the patients with relapsed AML or MDS, death from progressive disease was the only cause of death for patients given no therapy, palliative therapy, withdrawal of immune suppression, or DLI. Death after chemotherapy or second HCT was caused by progressive AML/MDS in 69% and by complications of therapy in 29%. Other causes of death included 1 patient with AML in remission after second HCT who died in a car accident and 1 patient with ALL who died of a secondary malignancy.
Factors Associated with Mortality
We sought to determine whether there were characteristics at the time of post-HCT relapse that could be used to determine future prognosis (Table 3). Despite the limited number of survivors, several factors were found in multivariable analysis to be significantly associated with the risk of mortality. The time interval between HCT to relapse was strongly associated with the hazard of mortality (Figure 3, left panel). Compared to patients who experienced relapse more than one year after HCT, mortality was significantly higher for those with a relapse <6 months after HCT (HR 3.2; 95% CI: 2.2, 4.7) or between 6 months and 1 year after HCT (HR 2.1; 95% CI: 1.4, 3.2). Patients whose initial HCT regimens were chemotherapy-based MAC had a higher probability of surviving; in comparison, those conditioned with either high dose TBI or RIC had 1.8-fold (95% CI 1.00, 3.1) and 4.1-fold (95% CI 1.9, 9.1) higher hazards of death, respectively. Finally, disease status at the time of first HCT predicted outcome after relapse (Figure 3, right panel). Patients with ALL who underwent first HCT while in CR1 were more likely to survive long term compared to those who had more advanced disease at the time of first HCT or those with myeloid leukemia in any phase of relapse. Other factors examined for potential inclusion in the multivariable Cox regression model included sex, age, decade of HCT, donor type, MRD status among patients in CR, time from diagnosis to HCT, and age at relapse.
Table 3:
Factors associated with mortality
| Multivariable Cox Regression Model for Mortality | ||||
|---|---|---|---|---|
| N | HR | 95% CI | p-value | |
| Diagnosis/Phase | ||||
| ALL CR1* | 15 | 1.0 | -- | -- |
| ALL CR2/Advanced | 106 | 2.6 | (1.4, 4.9) | 0.002 |
| AML CR1/MDS | 48 | 2.2 | (1.1, 4.3) | 0.028 |
| AML CR2/AML Advanced | 63 | 2.9 | (1.5, 5.4) | 0.001 |
| 1st HCT Preparative Regimen | ||||
| TBI-based | 198 | 1.8 | (1.0, 3.1) | 0.052 |
| Chemo-based* | 23 | 1.0 | -- | -- |
| RIC | 11 | 4.1 | (1.9, 9.1) | <0.001 |
| 1st HCT to relapse (months) | ||||
| 0–6 | 114 | 3.2 | (2.2, 4.7) | <0.001 |
| >6–12 | 66 | 2.1 | (1.4, 3.2) | <0.001 |
| >12+ | 52 | 1.0 | -- | -- |
Reference group
Note: Variables examined for potential inclusion in model were: Sex, Year of HCT, Time from HCT to relapse, Donor type, Diagnosis/Phase at HCT, Conditioning regimen, MRD status at relapse, Time from diagnosis to HCT, Age at HCT, Age at relapse.
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CHEMO, chemotherapy; CI, confidence interval; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; HR, hazard ratio; IS, immune suppression; MDS, myelodysplastic syndrome; RIC, reduced intensity conditioning; TBI, total body irradiation.
Figure 3.
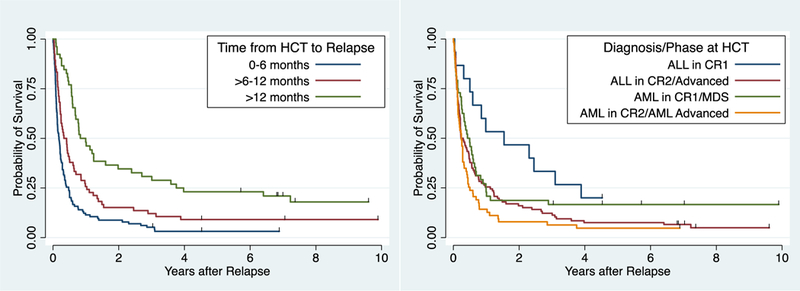
Probability of overall survival according after relapse following first allogeneic HCT by time from HCT to relapse (panel A, p<0.0001) or disease phase at time of HCT (panel, p=0.0078).
Among the 51 patients who received a second HCT, 7 died before donor cell engraftment. Of the remaining patients, acute or chronic GVHD developed in all 11 of the surviving patients compared to 23 of 33 (70%) who did not survive long term (p=0.046, Fisher’s Exact Test).
Discussion
The primary aim of this study was to ascertain the risk for mortality among pediatric patients with leukemic relapse after HCT in a relatively recent era. In addition, we sought to determine factors that could be used at the time of relapse to predict likelihood of successful treatment. Overall, we found that at approximately 85% of pediatric patients with post HCT relapse will not survive long-term, with leukemia accounting for approximately 4 out of every 5 deaths. These results can serve as a baseline for comparison as novel therapies emerge for treating relapse as well as help physicians set realistic expectations for patients for whom there are no available novel therapies.
Several studies have shown encouraging results with second HCT for treatment of relapse, with LFS ranging 20–30%.[26–28] Bajwa, et al, reported 2 year LFS of 35% for pediatric patients given second HCT, compared to 2% for those without [29]. However, it is difficult to generalize these data without knowing the overall number of patients treated with curative intent and the number who died before second HCT was feasible. In our cohort, 84% of patients treated with intent-to-cure died before second HCT was feasible, mainly from progressive disease. Therefore, in order to counsel patients at time of relapse, it is important to understand which patients are likely to survive with further therapy.
The current analysis helps to define subsets of pediatric patients that may have a realistic chance for long term OS with current therapies and suggests that successful strategies might differ between disease morphologies. In contrast to ALL, it was possible to achieve DFS in patients with early relapse AML/MDS, likely due in part to the difference in response to DLI which was able to bridge some AML/MDS patients to second HCT. Patients with AML/MDS also were less likely to have received a TBI-containing regimen as conditioning for the first HCT allowing a TBI-based second HCT regimen. A myeloablative TBI-based second transplant was associated with increased OS, consistent with our previous results, which showed <10% risk for TRM provided an interval of 6 months had elapsed between transplants.[14,30] Accordingly, patients with relapsed AML who did not receive myeloablative TBI in the first HCT can be counseled to consider remission induction and second HCT.
A second group with a higher chance for survival with intent-to-cure approaches were patients with ALL in CR1 at the time of first HCT and whose relapse occurred 6 months or more post HCT. In the absence of a molecular target, such as BCR/ABL, reinduction chemotherapy followed by a second HCT provided the best results. Since all the ALL patients were given myeloablative TBI as the first conditioning regimen, choice of the second regimen was limited. Although the numbers are small, a second myeloablative chemotherapy-based regimen was not found to be successful, consistent with previous reports.[27,31] The best outcome in the second HCT cohort was a RIC regimen followed by a single or double umbilical cord blood graft. There is emerging evidence that CB grafts are associated with a potent GVL effect, particularly in high risk or MRD positive leukemia, which may explain its benefit as the second allograft after RIC.[32] Regimen intensity likely still plays a role, as in this study DFS after a treosulfan-based RIC appeared to be superior to non-myeloablative conditioning.[33,34]
Previous studies of second allogeneic HCT indicate that the best outcome is found among patients with late relapse and disease in remission at time of second HCT.[26–28,30] Second myeloablative regimens have been associated with a high rate of sinusoidal obstruction syndrome (64%) and TRM (45%), particularly in adult patients, whereas second RIC regimens have been associated with much lower risk for toxicity.[28,35–37] In our pediatric patients who underwent second HCT, the overall TRM was less than observed in adult patients, and the main cause of death was progressive leukemia. Outcome relative to second HCT regimen intensity appeared to differ between disease subtypes, with outcome for relapsed ALL being better with RIC, whereas relapsed AML/MDS fared better with MAC. While the development of GVHD after second HCT appeared to be a factor associated with survival (observed in 100% of survivors compared to 73% of those who died, p=0.046), it also was the primary cause of death for three patients.
A small number of patients survived without undergoing second HCT, and these all had leukemias that could be treated with molecularly targeted agents. Both sorafenib treatment of FLT3-ITD+ AML and imatinib treatment of Ph+ ALL were effective as sole therapies. TKIs are reasonably well tolerated after HCT, and can be used as relapse prophylaxis, as we and others have shown.[38–40] For patients not receiving prophylaxis, administration of TKIs at the first detection of MRD is warranted. Patients who achieve MRD-negative response by molecular detection methods may not require second HCT.
We sought to develop a risk score based on the presence of observable characteristics at the time of relapse to determine futility of further treatment. However, the small proportion of successful outcomes precluded our ability to test and validate such a score. The multivariable analysis suggests that a patient with advanced AML at time of HCT or with ALL relapsed within 6 months after HCT will not benefit from further therapies with any of the modalities use in our study population. Fortunately, the landscape of therapies for patients with relapsed/refractory leukemias has advanced dramatically with the availability of new immunotherapies including antibody-drug conjugates, bi-specific t-cell engagers (BiTE) and CAR T-cells.[41] [42,43] [44] [45,46] The effectiveness of these emerging modalities for achieving a MRD negative remission in the post-HCT setting including prior to second HCT will need to be determined and compared to the outcomes reported here.
There are several limitations to this analysis. While the study includes a large number of pediatric patients, the small number of survivors limited the development of a relapse risk score. This important goal might be attainable by analyzing patients in one of the cooperative group registries. Due to the retrospective nature of the study, the decisions about using curative or palliative treatments made by physicians and parents may have introduced bias. Finally, these data are from a single transplant center that focuses on high risk patients.
Overall our analysis shows that even in the absence of these new therapies, durable OS is attainable for 10–15% of pediatric patients who experience relapse of leukemia after HCT. However, for patients who do not fall into favorable subgroups the outcome can be expected to be exceedingly poor. None of the treatment approaches used during the timeframe of this analysis can be expected to be successful in these high-risk malignancies and patient counseling should be congruent with these observations. Patients who seek curative treatment should enroll on studies of novel therapies, such as cellular or immunologic therapies, that might increase the likelihood of remission induction and second HCT.
Supplementary Material
Acknowledgments
Sources of funding: M.B. is supported through NHLBI RO1 HL121568–04, Alex’s Lemonade Stand Foundation Biotherapeutic Impact Grant, Leukemia and Lymphoma Society Translational Research Program 6519–17, Cookies for Kids Cancer and ACCR SU2C Innovative Research Grant 14–17. C.D. is supported by K23HL077446 and RC2HL101844. A.W. is supported by NHLBI P01 HL 122173–01 and NHLBI P01 HL 036444–30.
Footnotes
The authors have no conflicts of interest to disclose.
Supplementary information is available at Bone Marrow Transplantation’s website.
References
- 1.Shiba N, Ohki K, Kobayashi T, et al. High PRDM16 expression identifies a prognostic subgroup of pediatric acute myeloid leukaemia correlated to FLT3-ITD, KMT2A-PTD, and NUP98-NSD1: the results of the Japanese Paediatric Leukaemia/Lymphoma Study Group AML-05 trial. Br J Haematol. 2016;172:581–591. [DOI] [PubMed] [Google Scholar]
- 2.Chen IM, Harvey RC, Mullighan CG, et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119:3512–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borowitz MJ, Shuster J, Carroll AJ, et al. Prognostic significance of fluorescence intensity of surface marker expression in childhood B-precursor acute lymphoblastic leukemia. Blood. 1997;89:3960–3966. [PubMed] [Google Scholar]
- 4.Roberts KG, Mullighan CG. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nat Rev Clin Oncol. 2015;12:344–357. [DOI] [PubMed] [Google Scholar]
- 5.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood. 2007;109:926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Linden MH, Valsecchi MG, De Lorenzo P, et al. Outcome of congenital acute lymphoblastic leukemia treated on the Interfant-99 protocol. Blood. 2009;114:3764–3768. [DOI] [PubMed] [Google Scholar]
- 7.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncology. 2010;11:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolfrey AE, Anasetti C, Storer B, et al. Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukemia in children. Blood. 2002;99:2002–2008. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf U, Frangoul HA, Gooley TA, et al. Allogeneic bone marrow transplantation in children with myelodysplastic syndrome or juvenile myelomonocytic leukemia: the Seattle experience. Bone Marrow Transplantation. 2004;33:805–814. [DOI] [PubMed] [Google Scholar]
- 12.Nemecek ER, Gooley TA, Woolfrey AE, Carpenter PA, Matthews DC, Sanders JE. Outcome of allogeneic bone marrow transplantation for children with advanced acute myeloid leukemia. Bone Marrow Transplantation. 2004;34:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemecek ER, Ellis K, He W, et al. Outcome of myeloablative conditioning and unrelated donor hematopoietic cell transplantation for childhood acute lymphoblastic leukemia in third remission. Biology of Blood and Marrow Transplantation. 2011;17:1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolfrey AE, Gooley TA, Sievers EL, et al. Bone marrow transplantation for children less than 2 years of age with acute myelogenous leukemia or myelodysplastic syndrome. Blood. 1998;92:3546–3556. [PubMed] [Google Scholar]
- 15.Woodard P, Carpenter PA, Davies SM, et al. Unrelated donor bone marrow transplantation for myelodysplastic syndrome in children. Biology of Blood and Marrow Transplantation. 2011;17:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schechter T, Gassas A, Chen H, et al. The outcome of allogeneic hematopoietic cell transplantation for children with FMS-like tyrosine kinase 3 internal tandem duplication-positive acute myelogenous leukemia. Biol Blood Marrow Transplant. 2015;21:172–175. [DOI] [PubMed] [Google Scholar]
- 17.Sanders JE, Im HJ, Hoffmeister PA, et al. Allogeneic hematopoietic cell transplantation for infants with acute lymphoblastic leukemia. Blood. 2005;105:3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strahm B, Nollke P, Zecca M, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG-MDS 98 study. Leukemia. 2011;25:455–462. [DOI] [PubMed] [Google Scholar]
- 19.Loh ML, Zhang J, Harvey RC, et al. Tyrosine kinome sequencing of pediatric acute lymphoblastic leukemia: a report from the Children’s Oncology Group TARGET Project. Blood. 2013;121:485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann G, Attarbaschi A, Schrappe M, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL) -rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood. 2010;116:2644–2650. [DOI] [PubMed] [Google Scholar]
- 21.Bar M, Wood BL, Radich JP, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leukemia Research and Treatment. 2014;2014:421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. New England Journal of Medicine. 2010;363:2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. Journal of Clinical Oncology. 2009;27:377–384. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 25.Cox DR. Regression models and life tables (with discussion). Journal of the Royal Statistical Society,Series B. 1972;34:187–220. [Google Scholar]
- 26.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplantation. 2004;34:721–727. [DOI] [PubMed] [Google Scholar]
- 27.Radich JP, Sanders JE, Buckner CD, et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. Journal of Clinical Oncology. 1993;11:304–313. [DOI] [PubMed] [Google Scholar]
- 28.Shaw BE, Mufti GJ, Mackinnon S, et al. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone Marrow Transplantation. 2008;42:783–789. [DOI] [PubMed] [Google Scholar]
- 29.Bajwa R, Schechter T, Soni S, et al. Outcome of children who experience disease relapse following allogeneic hematopoietic SCT for hematologic malignancies. Bone Marrow Transplantation. 2013;48:661–665. [DOI] [PubMed] [Google Scholar]
- 30.Meshinchi S, Leisenring WM, Carpenter PA, et al. Survival after second hematopoietic stem cell transplantation for recurrent pediatric acute myeloid leukemia. Biology of Blood and Marrow Transplantation. 2003;9:706–713. [DOI] [PubMed] [Google Scholar]
- 31.Mielcarek M, Storer BE, Flowers MED, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2007;13:1160–1168. [DOI] [PubMed] [Google Scholar]
- 32.Milano F, Gooley T, Wood B, et al. Cord blood transplant in patients with minimal residual disease. New England Journal of Medicine. 2016;375:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salit RB, Milano F, Delaney C. Outcomes of cord blood transplantation as salvage therapy after graft failure or relapse after prior allogeneic transplantation. Biol Blood Marrow Transplant. 2016;22:339–343. [DOI] [PubMed] [Google Scholar]
- 34.Nemecek ER, Guthrie KA, Sorror ML, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biology of Blood and Marrow Transplantation. 2011;17:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill BT, Bolwell BJ, Rybicki L, et al. Nonmyeloablative second transplants are associated with lower nonrelapse mortality and superior survival than myeloablative second transplants. Biol Blood Marrow Transplant. 2010;16:1738–1746. [DOI] [PubMed] [Google Scholar]
- 36.Arfons LM, Tomblyn M, Rocha V, Lazarus HM. Second hematopoietic stem cell transplantation in myeloid malignancies. Curr Opin Hematol. 2009;16:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menon NN, Jenkins LM, Cui H, et al. Factors associated with improved outcomes after second allogeneic hematopoietic cell transplantation for relapsed pediatric leukemia. Ann Hematol. 2016;95:637–644. [DOI] [PubMed] [Google Scholar]
- 38.Tarlock K, Chang B, Cooper T, et al. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatr Blood Cancer. 2015;62:1048–1054. [DOI] [PubMed] [Google Scholar]
- 39.Antar A, Kharfan-Dabaja MA, Mahfouz R, Bazarbachi A. Sorafenib Maintenance Appears Safe and Improves Clinical Outcomes in FLT3-ITD Acute Myeloid Leukemia After Allogeneic Hematopoietic Cell Transplantation. Clin Lymphoma Myeloma Leuk. 2015;15:298–302. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter PA, Snyder DS, Flowers MED, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109:2791–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster JB, Maude SL. New developments in immunotherapy for pediatric leukemia. Curr Opin Pediatr. 2018;30:25–29. [DOI] [PubMed] [Google Scholar]
- 42.Rytting M, Triche L, Thomas D, O’Brien S, Kantarjian H. Initial experience with CMC-544 (inotuzumab ozogamicin) in pediatric patients with relapsed B-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. Journal of Clinical Oncology. 2016;34:3011–3011 (abstract). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


