Abstract.
In recent years, immune checkpoint inhibitor therapy has attracted a great deal of attention in the field of cancer treatment. In the clinical setting, antibodies targeting programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have been successfully used to treat adult patients with various types of intractable cancer. However, in a substantial number of patients, ICI therapy is associated with autoimmune toxicities known as immune-related adverse events (IRAEs). Endocrinopathies, such as hypophysitis or autoimmune thyroid disease, may occur and can present unique clinical features that have not been documented with traditional chemotherapies. A Japanese clinical trial evaluating the anti-PD-1 antibody nivolumab for the treatment of pediatric patients with refractory malignant solid tumors and Hodgkin lymphoma has been ongoing since 2017. Moreover, tumors associated with Lynch syndrome, a hereditary form of mismatch repair deficiency, are being focused and represent the next target for ICI therapy in Japan. For the safe management of pediatric cancer patients treated with ICIs, pediatric endocrinologists must be aware of the risk of autoimmune endocrinopathies and perform relevant screening tests at appropriate stages of growth and development.
Keywords: immune checkpoint inhibitor, pediatric cancer, immune-related adverse event, programmed cell death-1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)
Introduction
Oncology researchers have recognized the importance of reducing the immunosuppressive microenvironment around tumors to enhance the antitumor immune reaction (1, 2). Basic animal studies have established that malignant tumors can escape immune surveillance by inhibiting the immune response via checkpoint ligands. Therefore, immune checkpoint inhibitors (ICIs) are currently attracting a great deal of attention in the field of cancer treatment and are viewed as a type of immunotherapy that can target the Achilles heel of cancer (3, 4). In the clinical setting, treatment protocols using anti-programmed cell death-1 (PD-1) or anti-cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) antibodies have been successfully applied as ICI therapies for metastatic melanoma (3, 5), non-small-cell lung cancer (6), Hodgkin lymphoma (7), bladder cancer (8), and head and neck cancer (9). However, clinical trials evaluating ICIs for the treatment of childhood cancers remain limited (10,11,12,13).
Moreover, autoimmune toxicities known as immune-related adverse events (IRAEs) have been observed in a substantial number of patients and can arise in association with any ICI therapy (14, 15). In particular, endocrinopathies such as hypophysitis or autoimmune thyroid disease may occur, and they can present unique clinical features (16, 17). This review provides an overview of the characteristic manifestations and management of autoimmune endocrinopathies associated with ICI therapy and discusses the future clinical applications of ICIs for the treatment of pediatric cancers in Japan.
The Immune Checkpoint: PD-1 and CTLA-4 Pathways
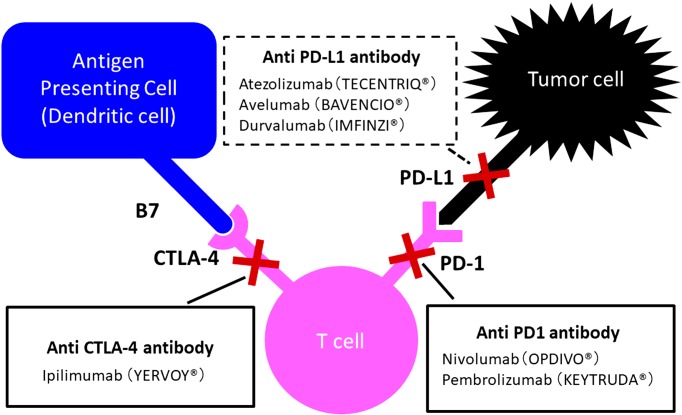
PD-1 is a receptor expressed on the surface of CD8+ and CD4+ T cells, B cells, dendritic cells, natural killer cells, and monocytes (18). PD-1 functions as a regulatory molecule that inhibits the immune response by interacting with either of two structurally related ligands, PD-L1 and PD-L2, located on the surface of normal tissues and tumor cells (19). As part of a distinct pathway, tumors can attenuate T cells activity via the CTLA-4 inhibitory receptor that prevents autoimmunity mediated by regulatory and memory T cells (20, 21). Indeed, T cell receptor (TCR) engagement results in an upregulation of the expression of CTLA-4, which competes with CD28. Consequently, the TCR and CD28 costimulation declines, which restrains T cells activity. Therefore, certain types of tumor can use the CTLA-4 signaling pathway to escape T cells antitumor activity.
ICIs and Their Clinical Applications in the Treatment of Cancers
(Fig. 1
Fig. 1.
CTLA-4 and PD-1/PD-L1 immune checkpoints. The figure was modified and adapted from the figure in (59).
)
The ICI nivolumab is a human IgG4 anti-PD-1 monoclonal antibody and was approved for clinical use in 2014. Clinical trials conducted in Japan have shown that nivolumab improves the survival rate of adults with metastatic melanoma. Subsequent trials have evaluated nivolumab for the treatment of renal cell carcinoma, non-small cell lung cancer, and other types of cancer worldwide (6, 9). Furthermore, pembrolizumab, a high affinity humanized IgG4 anti-PD-1 monoclonal antibody (mAb), showed convincing results for the treatment of metastatic non-small cell lung cancer (5). ICIs targeting CTLA-4 have also been evaluated in clinical trials. While ipilimumab, a human IgG4 anti-CTLA-4 mAb, has been shown to increase the survival rate in adults with metastatic melanoma and other malignancies (22), the human IgG2 anti-CTLA-4 mAb tremelimumab was successfully used to treat metastatic melanoma and other cancers (23). Importantly, antibodies targeting PD-L1, such as atezolizumab, durvalumab, and avelumab, have been shown to inhibit the binding of PD-L1 to PD-1, without interfering with the interaction between PD-L2 and PD-1 (24). These anti-PD-L1 mAbs have demonstrated promising antitumor effects with acceptable toxicity, not only against melanoma and renal cell carcinoma, but also against less immunogenic epithelial cancers such as non-small cell lung, colorectal, gastric, cervical, and bladder cancers. Moreover, ICIs blocking the PD-1/PD-L1 pathway have been associated with considerably milder toxicity profiles, compared to adverse events related to ICIs targeting the CTLA-4 pathway (16). Remarkably, a therapy combining PD-1 and CTLA-4 blockade resulted in a higher response rate in patients with metastatic melanoma, compared to either agent used in monotherapy (25).
Clinical Applications of ICIs for the Treatment of Pediatric Cancers
Significant progress has been made in the development of ICI therapies for the treatment of cancers in adult patients. However, despite ongoing clinical trials and the existence of FDA-approved protocols, the treatment of pediatric cancers with ICIs has been far less successful (11, 13). The first report on the use of ipilimumab for the treatment of advanced solid tumors in pediatric patients noted that some tumor regression was durable (10). Following the first phase I study evaluating anti-PD-1 antibodies for the treatment of pediatric patients with advanced soft tissue and bone sarcomas, the Sarcoma Alliance for Research through Collaboration concluded that, except for undifferentiated pleomorphic sarcoma, anti-PD-1 antibodies did not exhibit antitumor effects (26). In March 2017, the FDA approved the anti-PD-1 antibody pembrolizumab for the treatment of both adults and children with refractory classic Hodgkin lymphoma or those who relapsed after three or more prior treatments (27). Altogether, except for Hodgkin lymphoma, the outcome of ICI monotherapies in pediatric clinical trials has been disappointing.
While both PD-1 and CTLA-4 function as immune checkpoint proteins, they affect different stages of the immune response. Indeed, PD-1 primarily regulates the proliferation of cytotoxic T lymphocytes, whereas CTLA-4 has a unique role in inhibiting memory T cell activity. Therefore, combination therapy may enhance the response and result in substantial effects. Accordingly, an ongoing trial is testing a combination of PD-1 and CTLA-4 blockade therapies for the treatment of recurrent/refractory pediatric cancers (28).
The mutational load or lack thereof can partly explain the differences in the response rates of certain cancers observed between adults and children. Meanwhile, ICIs can be used to stimulate the T cell-mediated antitumor response to neoantigens presented by tumor cells via the major histocompatibility complex. Indeed, high mutational burden (nonsynonymous mutations), high frequency of tumor neoantigens, and mutations in DNA repair pathways showed a strong association with the therapeutic benefits of ICIs (29,30,31,32,33). Remarkably, pediatric cancers do not usually exhibit high rates of mutations (34), except for children with Lynch syndrome, a hereditary form of mismatch repair deficiency (35). Lynch syndrome is known to cause childhood cancers and is associated with rates of mutations that are even higher than in most adult cancers. In this context, the FDA has approved the PD-1 antibody pembrolizumab for the treatment of children aged 12 yr and older with hypermutated malignancies due to a mismatch repair deficiency (13).
ICIs and IRAEs
ICIs can induce particular side effects known as IRAEs (36), and autoimmunity has been suggested as the underlying mechanism for this type of toxicity (37). IRAEs mainly include colitis/diarrhea, dermatitis, hepatitis, and endocrinopathies. In the case of endocrinopathies related to the anti-CTLA-4 antibody ipilimumab, symptoms usually appear after six or seven wk of treatment, with a median time to onset of seven to twenty wk (15, 38). Interestingly, endocrinopathies associated with the anti-PD-1 antibodies pembrolizumab and nivolumab exhibit a similar median time to onset (10 and 11 wk, respectively) (15). Among the endocrinopathies related to ICIs, thyroid dysfunction is the most frequent, while hypophysitis is typically associated with ipilimumab and can cause a secondary, life-threatening adrenal insufficiency. The incidence, timing, and clinical presentation of these endocrinopathies are heterogeneous, and there is no method currently available to prevent their occurrence. The Japan Endocrine Society has recently issued guidelines for the diagnosis of endocrinopathies related to ICIs (https://doi.org/10.1507/endocrine.94.S.November_1 [in Japanese]).
Self-reactive T cells can cause autoimmune diseases and are normally eliminated by negative selection in the thymus or the induction of peripheral tolerance mechanisms. CTLA-4 and PD-1/PD-L1 play significant roles in the prevention of autoimmunity and respectively act as a negative regulator of T cell activation and a physiologic brake on unrestrained cytotoxic T effector function. Therefore, ICIs may cause endocrinopathies by sustaining self-reactive T cells. In support of this idea, there is an established causal link between mutations in human CTLA-4 and various clinical autoimmune syndromes, including autoimmune thyroiditis (39). Furthermore, genetic variations in the CTLA-4 gene have been associated with multiple autoimmune endocrinopathies, while reduced levels of expression of CTLA-4 have been associated with Graves’ disease and Hashimoto’s thyroiditis (40). Besides, the CTLA-4 protein was found to be ectopically expressed in normal pituitary cells, which could explain the high occurrence of hypophysitis associated with anti-CTLA-4 treatment (41).
Moreover, a disruption of the immune tolerance via the PD-1/PD-L1 axis has been involved in the pathogenesis of autoimmune endocrinopathies. Genetic variations in the PD-1 and PD-L1 genes have been associated with Addison’s and autoimmune thyroid diseases (42), while PD-1 or PD-L1 blockade has been shown to trigger diabetes in prediabetic female nonobese diabetic mice via a T cell-mediated pathway (43). These observations strongly suggest that the interactions between ICIs and genetic susceptibilities or environmental factors may lead to the development of endocrinopathies (17).
Thyroid disorders
The incidence of anti-CTLA-4 mAb-induced thyropathy varies but remains relatively low. For example, the incidence of thyropathy in patients treated with tremelimumab and ipilimumab is 0–4% and 0–2%, respectively (14). In contrast, trials for anti-PD-1 antibodies have reported higher rates of thyropathy in patients treated with pembrolizumab (0–19.2%) and nivolumab (26%). In most cases, the thyropathy runs a subclinical course or is transient, which is consistent with silent autoimmune thyroiditis. Occasionally, ICI-induced hypothyroidism can be preceded by a period of destructive thyroiditis and transient thyrotoxicosis, with a release of thyroid antigen and the subsequent production of secondary antibodies (17). In comparison, hyperthyroidism was less common (0–2.8%), while Graves’ ophthalmopathy has also been reported with an elevation of TSH receptor antibodies under a normal thyroid function (44).
Hypophysitis
The reported incidence of hypophysitis in patients treated with ipilimumab was 0–17.4% (14), and a clear dose-dependent relationship was observed (45). Unlike sporadic hypophysitis, which mostly affects women, ICI-induced hypophysitis is more frequent in men (46). In comparison, tremelimumab is associated with a lower incidence of hypophysitis (0–2%), while anti-PD-1 antibodies rarely induce the condition (< 1%) (17). Hypophysitis usually develops after five to 36 wk of anti-CTLA-4 antibody treatment. However, late occurrence at 19 mo of treatment has also been reported (16, 47). The clinical manifestations related to sellar compression (as the pituitary gland enlarges) include headache or visual defects, while those caused by hormonal disturbance (due to autoimmune inflammation of the pituitary gland) include hypotension, nausea, abdominal pain, anorexia, weight loss, temperature intolerance, loss of libido, polyuria, and polydipsia. Many of these symptoms are nonspecific and could be attributed to either pituitary dysfunction or the underlying illness. Moreover, anterior hypopituitarism was found to have a higher incidence than diabetes insipidus, while ACTH and/or TSH deficiencies were the most common complications (16, 47). Hypogonadotropic hypogonadism and low levels of insulin-like growth factor 1 (IGF-1) have also been reported. It is, therefore, essential to perform a baseline assessment of TSH, fT4, ACTH, morning cortisol, IGF-1, electrolytes, and glucose before initiating ICI therapy (16). When a patient presents symptoms suggestive of hypophysitis (e.g., headache, nausea, weakness, or fatigue), systematic hormonal evaluation is mandatory before each treatment cycle, taking into consideration the fact that cancer and hypopituitarism complications may give rise to similar symptoms and laboratory results. In the event of sellar compression symptoms (e.g., headache or visual defects), the patient should undergo MRI of the pituitary gland and an endocrine workup, including measurement of FSH and LH, estradiol and testosterone, IGF-1, PRL, TSH, fT4, morning cortisol, and ACTH. The differential diagnosis should also exclude the occurrence of brain metastasis by MRI. Importantly, an MRI scan showing a normal pituitary gland does not rule out the presence of subclinical hypophysitis or metastasis (46, 47).
Type 1 diabetes mellitus (T1DM)
T1DM is a rare complication associated with anti-PD-1, anti-PD-L1, and anti-CTLA-4 mAbs (16). Autoantibodies against islet cell antigens have only been detected in a small number of cases, and the time to onset ranged from less than a month to one yr (48, 49).
Primary adrenal insufficiency (adrenalitis)
One review on the ICI-induced endocrinopathies reported two cases (0.8%) of adrenalitis in patients treated with ipilimumab (47), while adrenal insufficiencies of unspecified cause have also been reported in patients treated with tremelimumab, nivolumab, or a combination of anti-CTLA-4 and anti-PD-1 mAbs (50). When adrenal enlargement is observed by echography in patients receiving ICIs, the adrenal function should be assessed (via measurement of the ACTH and cortisol levels) to rule out adrenalitis (16). Given that the incidence of ACTH deficiency due to hypophysitis is higher than the incidence of adrenalitis caused by ICIs targeting CTLA-4, a CRH or insulin test should be considered when clinical symptoms suggestive of adrenal failure are observed.
Combination of CTLA-4 and PD-1/PD-L1 blockade therapies
Previous observations have suggested that blocking both CTLA-4 and PD-1/PD-L1 pathways has synergistic antitumor effects and results in high response rates. However, the IRAEs associated with combination therapy present a parallel exponential increase in incidence and severity of the toxicities. Endocrinopathies have been reported to affect 14–50% of the patients treated with this combination of drugs. Among IRAEs, thyroid-related adverse events were the most frequent (7–28%), followed by hypophysitis (0–12.8%), while grade 3/4 events occurred in 1–20% of the cases (15).
Management of Endocrinopathies Related to the Use of ICIs as Antitumor Drugs
The National Cancer Institute has recommended grading adverse events in patients receiving cancer chemotherapy according to the Common Terminology Criteria for Adverse Events version 4.03 (U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda; MD; USA). Although the published guidelines do not specifically comment on hypophysitis, the toxicity grading system has been applied to hypothyroidism, hyperthyroidism, adrenal insufficiency, and hypophysitis (4, 14).
The management of hypophysitis primarily involves hormonal therapy for the deficient pituitary hormones, while discontinuation of the ICI therapy and/or the use of high-dose (immunosuppressive) steroids should be taken under consideration. For patients with mild (grade 1) hypophysitis, a continuation of immunotherapy and close observation is recommended. However, if the symptoms do not resolve within a week, steroid treatment (e.g., prednisolone 0–5 mg/kg/d or equivalent) should be initiated. For patients with grade ≥ 2 toxicities, the guidelines recommend withholding and not resuming the ICI therapy (e.g., ipilimumab) until the adverse events resolve to grade ≤ 1. Alternatively, continuation of immunotherapy alongside hormonal therapy may be considered.
In most cases of severe hypophysitis (grade 3/4), the patients were treated with high-dose systemic steroids (e.g., prednisolone 1 mg/kg/d or equivalent) with a gradual transition to physiological replacement doses of hydrocortisone or prednisolone. However, this treatment course did not appear to improve hormonal recovery, and there is some concern regarding the immunosuppressant effect of high-dose steroids and its negative impact on the antitumor efficacy of ICIs. Accordingly, high-dose steroids should be reserved for cases involving clinically significant illness, hyponatremia, severe headache, or marked pituitary enlargement that approaches the optic apparatus. Regardless of the severity of the toxicities, ICI therapy can resume once the patient’s condition improves with steroid treatment, taking into consideration the risks and benefits for the patient. Accordingly, hypopituitarism should be managed with the appropriate hormonal therapy for thyroxine, testosterone or estrogen, GH (which is contraindicated in active malignancy), cortisol, and antidiuretic hormone (4).
Primary hypothyroidism, primary hypoadrenalism, and autoimmune diabetes should be treated with conventional hormonal therapies following current guidelines. Hormonal recovery in patients treated for hormone insufficiency should be evaluated at three to six mo intervals. Similarly, hyperthyroidism should be managed with conventional antithyroid drugs following current guidelines (4).
Similarities between IRAEs Related Endocrinopathies and Autoimmune Polyglandular Ayndromes
The recent emergence of autoimmune endocrinopathies in cancer patients treated with ICIs has added a new dimension to considerations of autoimmune polyendocrinopathy syndromes. Autoimmune polyglandular syndromes are clusters of endocrine abnormalities that occur in patients with immune dysregulation and require hormonal therapy (51,52,53). Based on the underlying genetic cause, three major types of autoimmune polyendocrinopathy syndromes are recognized: APS-1, APS-2, and APS-3. APS-1 is characterized by a loss of endocrine and non-endocrine tissues through autoimmune destructive processes and is caused by recessive inactivating mutations in the autoimmune regulator AIRE gene, which plays a crucial role in the negative selection of self-reactive T cells and prevention of autoimmunity. APS-2 and APS-3 are polygenic disorders, and their pathogenesis involves mutations in the human leukocyte antigen-DQ and -DR genes, which regulate antigen presentation to TCRs. APS-2 is clinically characterized by the occurrence of T1DM, Addison’s disease, and hypothyroidism, whereas APS-3 essentially presents the same clinical manifestations, but without Addison’ disease. Despite differences in predisposition for the endocrine organs affected by IRAEs related endocrinopathies and APS, these conditions might share a common underlying pathophysiology.
ICIs for the Treatment of Pediatric Cancers in Japan
Although the final contribution of immunotherapy to the outcome of pediatric cancer patients remains uncertain, the face of cancer therapy is changing rapidly, and shortly, the treatments for childhood cancers will probably be entirely different from the traditional surgery, radiation, and chemotherapy. Various innovative immunotherapies, such as chimeric antigen receptor T cell therapy, have brought new hope for children with cancer (54, 55), by improving the survival rates and reducing the incidence of late adverse effects. In May 2017, the National Cancer Center Central Hospital started the first Japanese trial on ICIs for the treatment of pediatric cancers. This doctor-initiated clinical trial (NCCH 1606, abbreviated test: PENGUIN) is evaluating nivolumab for patients aged 1 to 24 yr with refractory malignant solid tumors or Hodgkin lymphoma. Additional clinical trials for pediatric cancer patients have not been initiated, and data from trials conducted in the US and European countries will help plan the next clinical trials conducted in Japan (11).
In December 2018, Keytruda (pembrolizumab) was approved by the Japanese Ministry of Health, Labor, and Welfare for the treatment of solid cancer in adults that have high-frequency microsatellite instability (MSI-High) tumors and exhibit exacerbated progression and recurrence after cancer chemotherapy. Around the same time, the MSI test was also approved as a companion diagnostic test for pembrolizumab. Therefore, following genetic testing, Keytruda treatment is now available nationwide under the national health insurance system in Japan.
MSI-High tumors are commonly found in patients with Lynch syndrome, also known as hereditary non-polyposis colorectal cancer. Lynch syndrome is the most common hereditary cancer syndrome (affecting an estimated 1 in 300 people) and is caused by germline mutations in mismatch repair genes, such as MLH1, MSH2, MSH6, and PMS2 (56). The condition predisposes patients to different types of cancer, including childhood brain and blood cancers (57). Therefore, ICI therapy should soon be applied to the treatment of any type of cancer in pediatric patients of all age with Lynch syndrome (58). When ICIs are used to treat patients with cancer, comprehensive management by a multidisciplinary team of health care professionals is recommended to provide optimal care. For the safe management of pediatric cancer patients treated with ICIs, pediatric endocrinologists must be aware of the risk of autoimmune endocrinopathies and perform relevant screening tests at appropriate stages of growth and development.
Conflict of Interest
The author declares no conflict of interest.
Acknowledgments
I thank Dr. Brian Quinn for his support.
References
- 1.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007;121: 1–14. doi: 10.1111/j.1365-2567.2007.02587.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart TJ, Smyth MJ. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev 2011;30: 125–40. doi: 10.1007/s10555-011-9280-5 [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33: 1974–82. doi: 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi K, Chain BM, Peggs KS, Quezada SA. The “Achilles’ heel” of cancer and its implications for the development of novel immunotherapeutic strategies. Cold Spring Harb Perspect Med 2018;8: 8. doi: 10.1101/cshperspect.a027086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363: 711–23. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373: 123–35. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015;372: 311–9. doi: 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powles T. Immune checkpoint inhibitors for urologic cancer: the tip of the iceberg? Eur Urol 2015;68: 280–2. doi: 10.1016/j.eururo.2015.03.022 [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366: 2443–54. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res 2016;22: 1364–70. doi: 10.1158/1078-0432.CCR-15-0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JA, Cheung NV. Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer Treat Rev 2017;58: 22–33. doi: 10.1016/j.ctrv.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabir TF, Chauhan A, Anthony L, Hildebrandt GC. Immune checkpoint inhibitors in pediatric solid tumors: status in 2018. Ochsner J 2018;18: 370–6. doi: 10.31486/toj.18.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedekind MF, Denton NL, Chen CY, Cripe TP. Pediatric cancer immunotherapy: opportunities and challenges. Paediatr Drugs 2018;20: 395–408. doi: 10.1007/s40272-018-0297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 2013;98: 1361–75. doi: 10.1210/jc.2012-4075 [DOI] [PubMed] [Google Scholar]
- 15.González-Rodríguez E, Rodríguez-Abreu D, Spanish Group for Cancer Immuno-Biotherapy (GETICA). Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist 2016;21: 804–16. doi: 10.1634/theoncologist.2015-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ntali G, Kassi E, Alevizaki M. Endocrine sequelae of immune checkpoint inhibitors. Hormones (Athens) 2017;16: 341–50. [DOI] [PubMed] [Google Scholar]
- 17.Girotra M, Hansen A, Farooki A, Byun DJ, Min L, Creelan BC, et al. Investigational Drug Steering Committee (IDSC) Immunotherapy Task Force collaboration. The current understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for management. JNCI Cancer Spectr 2018;2: pky021. doi: 10.1093/jncics/pky021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11: 3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol 2015;36: 265–76. doi: 10.1016/j.it.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci USA 2010;107: 1524–8. doi: 10.1073/pnas.0910341107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA 2011;108: 266–71. doi: 10.1073/pnas.1016791108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364: 2517–26. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 23.Comin-Anduix B, Escuin-Ordinas H, Ibarrondo FJ. Tremelimumab: research and clinical development. Onco Targets Ther 2016;9: 1767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26: 677–704. doi: 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373: 23–34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017;18: 1493–501. doi: 10.1016/S1470-2045(17)30624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. KEYNOTE-087Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017;35: 2125–32. doi: 10.1200/JCO.2016.72.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lettieri CK, Appel N, Labban N, Lussier DM, Blattman JN, Hingorani P. Progress and opportunities for immune therapeutics in osteosarcoma. Immunotherapy 2016;8: 1233–44. doi: 10.2217/imt-2016-0048 [DOI] [PubMed] [Google Scholar]
- 29.Champiat S, Ferté C, Lebel-Binay S, Eggermont A, Soria JC. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. OncoImmunology 2014;3: e27817. doi: 10.4161/onci.27817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515: 563–7. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371: 2189–99. doi: 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade. N Engl J Med 2015;372: 783. doi: 10.1056/NEJMc1415938 [DOI] [PubMed] [Google Scholar]
- 33.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348: 124–8. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499: 214–8. doi: 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shlien A, Campbell BB, de Borja R, Alexandrov LB, Merico D, Wedge D, et al. Biallelic Mismatch Repair Deficiency Consortium. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat Genet 2015;47: 257–62. doi: 10.1038/ng.3202 [DOI] [PubMed] [Google Scholar]
- 36.Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol 2010;37: 499–507. doi: 10.1053/j.seminoncol.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 37.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005;23: 6043–53. doi: 10.1200/JCO.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS, MDX010-20 Investigators. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013;119: 1675–82. doi: 10.1002/cncr.27969 [DOI] [PubMed] [Google Scholar]
- 39.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med 2014;20: 1410–6. doi: 10.1038/nm.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotsa K, Watson PF, Weetman APA. A CTLA-4 gene polymorphism is associated with both Graves disease and autoimmune hypothyroidism. Clin Endocrinol (Oxf) 1997;46: 551–4. doi: 10.1046/j.1365-2265.1997.1710996.x [DOI] [PubMed] [Google Scholar]
- 41.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014;6: 230ra45. doi: 10.1126/scitranslmed.3008002 [DOI] [PubMed] [Google Scholar]
- 42.Mitchell AL, Cordell HJ, Soemedi R, Owen K, Skinningsrud B, Wolff AB, et al. Programmed death ligand 1 (PD-L1) gene variants contribute to autoimmune Addison’s disease and Graves’ disease susceptibility. J Clin Endocrinol Metab 2009;94: 5139–45. doi: 10.1210/jc.2009-1404 [DOI] [PubMed] [Google Scholar]
- 43.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198: 63–9. doi: 10.1084/jem.20022125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol 2011;164: 303–7. doi: 10.1530/EJE-10-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torino F, Barnabei A, De Vecchis L, Salvatori R, Corsello SM. Hypophysitis induced by monoclonal antibodies to cytotoxic T lymphocyte antigen 4: challenges from a new cause of a rare disease. Oncologist 2012;17: 525–35. doi: 10.1634/theoncologist.2011-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary 2016;19: 82–92. doi: 10.1007/s11102-015-0671-4 [DOI] [PubMed] [Google Scholar]
- 47.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017;13: 195–207. doi: 10.1038/nrendo.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Masood A, Bari S, Yavuz S, Grosbach AB. Autoimmune diabetes and thyroiditis complicating treatment with nivolumab. Case Rep Oncol 2017;10: 230–4. doi: 10.1159/000456540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godwin JL, Jaggi S, Sirisena I, Sharda P, Rao AD, Mehra R, et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer 2017;5: 40. doi: 10.1186/s40425-017-0245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18: 31–41. doi: 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med 2004;350: 2068–79. doi: 10.1056/NEJMra030158 [DOI] [PubMed] [Google Scholar]
- 52.Kahaly GJ. Polyglandular autoimmune syndromes. Eur J Endocrinol 2009;161: 11–20. doi: 10.1530/EJE-09-0044 [DOI] [PubMed] [Google Scholar]
- 53.Kahaly GJ, Frommer L. Polyglandular autoimmune syndromes. J Endocrinol Invest 2018;41: 91–8. doi: 10.1007/s40618-017-0740-9 [DOI] [PubMed] [Google Scholar]
- 54.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371: 1507–17. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385: 517–28. doi: 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valle L, Vilar E, Tavtigian SV, Stoffel EM. Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J Pathol 2019;247: 574–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, et al. American Society of Clinical OncologyEuropean Society of Clinical Oncology. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol 2015;33: 209–17. doi: 10.1200/JCO.2014.58.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salman P, Panay S, Fernández R, Mahave M, Soza-Ried C. Evidence of response to pembrolizumab in a patient with Lynch syndrome-related metastatic colon cancer. Onco Targets Ther 2018;11: 7295–300. doi: 10.2147/OTT.S167645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inthagard J, Edwards J, Roseweir AK. Immunotherapy: enhancing the efficacy of this promising therapeutic in multiple cancers. Clinical Science 2019;133: 181–193. [DOI] [PubMed] [Google Scholar]