Abstract.
Hashimoto’s thyroiditis (HT) is an autoimmune disease thought to involve a combination of genetic and environmental factors, but its detailed pathogenesis is unknown. We present a family with haploinsufficiency of the gene encoding tumor necrosis factor α-induced protein 3 (TNFAIP3, also known as A20) and show a link with HT in a three-generation pedigree. Currently, TNFAIP3 polymorphisms are associated with several autoimmune diseases, and haploinsufficiency of A20 was recently observed in families with an early-onset autoinflammatory disease resembling Behçet’s disease. However, HT has not been linked with TNFAIP3 variants. We analyzed TNFAIP3 and human leukocyte antigen (HLA) in the family showing HT as an autosomal dominant trait, and identified a novel heterozygous c.2209delC mutation of TNFAIP3 in the members with HT. The known HLA haplotypes linked to HT could not be identified. Based on our analysis of this pedigree, we consider HT as a possible phenotype of A20 haploinsufficiency.
Keywords: Hashimoto’s thyroiditis, hypothyroidism, TNFAIP3, A20, haploinsufficiency of A20
Introduction
Hashimoto’s thyroiditis (HT) is an autoimmune thyroid disease thought to develop through a combination of genetic and environmental factors; however, its detailed pathogenesis remains unknown. Recently Zhou et al. revealed that a familial early-onset autoinflammatory disease resembling Behçet’s disease (BD) inherited in an autosomal dominant manner was caused by haploinsufficiency of the gene encoding tumor necrosis factor α-induced protein 3 (TNFAIP3, also known as A20) (1). A20 is a negative regulator of multiple intracellular immune signaling pathways, including tumor necrosis factor-α (TNF-α) signaling. In this study, patients from a family with haploinsufficiency of A20 (HA20) who developed HT by autosomal dominant inheritance are reported.
Case Presentation
Patient 1 (P1) is the proband (Fig. 1) who suffered from recurring febrile episodes with severe abdominal pain, vomiting, and bloody stools from the age of 6 mo. Family histories show that individuals P2–P6 also experienced some episodes suggestive of an autoinflammatory disease, especially P2 who is P1’s mother. P2 had recurrent episodes of fever and cervical lymphadenitis diagnosed as subacute necrotizing lymphadenitis and chronic mild hepatitis in her childhood. At 21 and 22 yr of age, she suffered from two febrile episodes with diarrhea and elevated C-reactive protein levels, suggestive of inflammatory bowel disease. Colonoscopy revealed possible Crohn’s disease, but these symptoms were resolved spontaneously. Since childhood, P3, P1’s maternal grandmother, and P4, P1’s maternal uncle, have suffered from recurring episodes of stomatitis. At 21 yr of age, P4 was diagnosed with malignant lymphoma (Hodgkin’s disease) and at the age of 33 yr, he was diagnosed with a brain tumor (craniopharyngioma). P5, P1’s maternal cousin, experienced several episodes of fever from the age of 1 yr, whereas P6, P1’s maternal cousin, suffered four episodes of fever before the age of 1 yr. Moreover, P7, P1’s younger sister, had multiple perineal fistula possibly associated with inflammatory bowel disease at the age of 1 mo.
Fig. 1.
Pedigree of the patients.
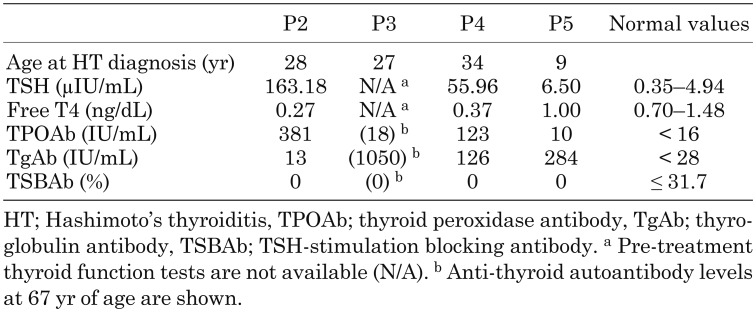
Among these patients, P2, P3, P4, and P5 were diagnosed with HT. P2 was diagnosed with severe HT at 28 yr of age (Table 1). Ultrasonography revealed a mild degree of diffuse swelling in her thyroid. She only exhibited increased weight (body mass index, 35.4 kg/m2) as a major clinical finding characteristic of hypothyroidism. P3 was diagnosed with HT at the age of 27 yr with symptoms such as fatigability. She has been treated with oral levothyroxine and her current anti-thyroid autoantibody levels are shown in Table 1. P4 was also diagnosed with HT during a hormone evaluation at his craniopharyngioma diagnosis. His levels of anti-thyroid autoantibodies at 34 yr of age are shown in Table 1. P5 had no clinical findings characteristic of hypothyroidism, but ultrasonography showed a mild degree of diffuse swelling in her thyroid and elevated thyrotropin (TSH) and anti-thyroglobulin antibody levels at the age of 9 yr (Table 1). P1, P6, and P7, currently 6-, 2-, and 1-yr-old, respectively, have normal thyroid functions and no anti-thyroid autoantibodies, but are too young for this to be apparent.
Table 1. Results of pre-treatment thyroid function tests and anti-thyroid autoantibodies.
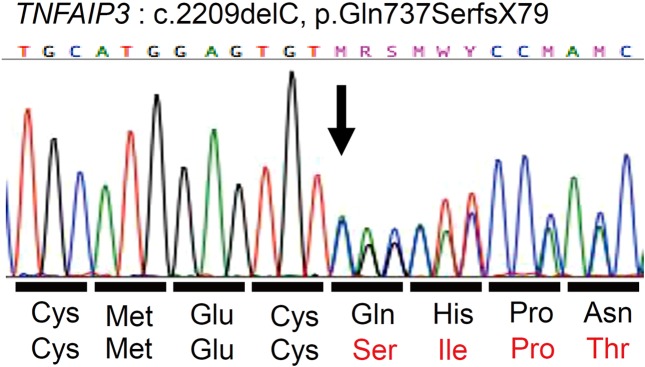
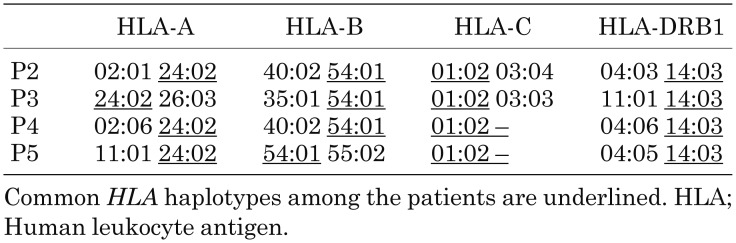
We analyzed TNFAIP3 in the present family and identified a novel heterozygous c.2209delC mutation that was present in all the HT members tested (Figs. 1 and 2) (RefSeq transcript NM_006290.2). We also analyzed human leukocyte antigen (HLA) alleles in P2, P3, P4, and P5 who were diagnosed with HT (Table 2). All HT members appeared to have an A*24:02-B*54:01-C*01:02-DRB1*14:03 haplotype.
Fig. 2.
Results of TNFAIP3 analysis.
Table 2. Results of HLA allele typing.
A brief case report mentioning this family was previously described by Kadowaki et al. as part of a study examining HA20 patients in nine independent Japanese families (2).
Discussion
A20 is encoded by TNFAIP3 and inhibits the tumor necrosis factor-induced transmission pathway for nuclear factor-κB (NF-κB) signals. TNFAIP3 polymorphisms are associated with several autoimmune diseases including Graves’ disease (GD) (3). However, the pathogenic contribution of TNFAIP3 variants to HT has not been revealed. The major clinical presentations of HA20 patients include some BD-like symptoms such as mucous membrane lesions, including recurrent stomatitis, ophthalmic lesions, dermal lesions, and articular lesions (1, 2, 4, 5). Moreover, these reports also showed clinical heterogeneity among the members of the same family. We, therefore, analyzed TNFAIP3 in the present family (P1–P7) and identified a heterozygous c.2209delC mutation that was present in all members tested (Figs. 1 and 2). In vitro functional analysis of this variant was previously reported as a pathogenic mutation (2). Hence, P1–P7 were all diagnosed with HA20.
Little is known about the exact pathogenesis of HT. However, considering that certain families are more susceptible to this disease than others, it is likely that genetic factors are involved. Previous reports suggest the possible association of HT with polymorphisms of HLA, cytotoxic T-lymphocyte-associated protein 4 (CTLA4), protein tyrosine phosphatase non-receptor type 22 (PTPN22), or thyroglobulin (Tg) genes (6). In particular, the HLA gene is associated with a number of autoimmune diseases. An HA20 patient demonstrating very early-onset psoriatic arthritis, spondylitis, and aortic insufficiency was reported. This patient also had another possible genetic factor, HLA-B27, which is well known to cause psoriatic arthritis and ankylosing spondylitis (2). Thus, we considered that HA20 might drive other genetic factors associated with autoimmune disorders such as HLA. HT is linked to particular HLA antigens or HLA alleles such as A2 (7), A*02:07 (8), DR3 (9), DRB1*03:01 (10), DR4 (DR53, DRB4) (7-9), DR5 (11), and DRB1*11:04 (12). Furthermore, because both the TNFAIP3 and HLA are located on chromosome 6, we speculate that an association exists between TNFAIP3 and HLA. As a result, P2, P3, P4, and P5 all have the DRB1*14:03 allele, which has been demonstrated as the susceptible HLA allele for GD in the Japanese population, but not for HT (13). To our knowledge, the identified common HLA allele is not reported to be associated with HT. Hence, in the present family, it is considered that TNFAIP3 dominantly contributes to the pathogenesis of HT without any relation to HLA. Nonetheless, this study is limited because we cannot exclude other genetic effects such as CTLA4.
Moreover, some HA20 patients have been reported to show systemic as well as endocrinological autoimmune disorders, including HT, GD, and insulin-dependent diabetes (14). Additionally, a case showing GD in the previously summarized cohort of Japanese HA20 had a TSH-receptor antibody (TRAb) as well as a thyroid peroxidase antibody (TPOAb) and a thyroglobulin antibody (TgAb) (2). Furthermore, an HA20 patient accompanied with HT was reported recently (15). Excessive differentiation of T helper 17 (Th17) cells was observed in HA20 patients (1, 2). Takagi et al. reported an HA20 patient who presented symptoms similar to the autoimmune lymphoproliferative syndrome and whose double-negative T (DNT) cell counts were increased (4). Th17 and DNT are well known subsets of T lymphocytes that play a role in autoimmunity (16, 17). Thus, it is possible that HA20 patients have autoimmune diseases as well as autoinflammatory diseases. In addition, particular immune dysregulation disorders in primary immunodeficiency syndromes (for example, signal transducer and activator of transcription 3 (STAT3) gain-of-function mutations or CTLA4 deficiency) are known to complicate various autoimmune diseases including autoimmune thyroid disease (AITD) (18, 19). As mentioned above, A20 is one of the immunoregulators, and disruption of these regulators may readily cause AITD. Therefore, it is possible that TNFAIP3 mutation contributes to the onset of AITD much more than other genetic effects such as HLA or CTLA4 polymorphisms, considering the existence of this family case and other sporadic cases with AITD and the etiology. For all these reasons, HT can be considered as one of the many symptoms to be found in HA20.
This is the first familial case report of HA20 associated with HT. We propose that if HT patients demonstrate an autosomal dominant inheritance pattern and have BD-like clinical presentations such as recurrent stomatitis, a diagnosis of HA20 should be considered.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This study was performed according to the Helsinki Declaration. All subjects provided informed consent to participate in the study. We thank the patients and their families for participating in this study.
This work was supported by MEXT KAKENHI Grant Number JP18K07840 and by Health and Labour Science Research Grants for Research on Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (Grant Number 17933688 and 17933299).
References
- 1.Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet 2016;48: 67–73. doi: 10.1038/ng.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadowaki T, Ohnishi H, Kawamoto N, Hori T, Nishimura K, Kobayashi C, et al. Haploinsufficiency of A20 causes autoinflammatory and autoimmune disorders. J Allergy Clin Immunol 2018;141: 1485–1488.e11. doi: 10.1016/j.jaci.2017.10.039 [DOI] [PubMed] [Google Scholar]
- 3.Song RH, Yu ZY, Wang Q, Muhali FS, Jiang WJ, Xiao L, et al. Polymorphisms of the TNFAIP3 region and Graves’ disease. Autoimmunity 2014;47: 459–65. doi: 10.3109/08916934.2014.914504 [DOI] [PubMed] [Google Scholar]
- 4.Takagi M, Ogata S, Ueno H, Yoshida K, Yeh T, Hoshino A, et al. Haploinsufficiency of TNFAIP3 (A20) by germline mutation is involved in autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol 2017;139: 1914–22. doi: 10.1016/j.jaci.2016.09.038 [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi H, Kawamoto N, Seishima M, Ohara O, Fukao T. A Japanese family case with juvenile onset Behçet’s disease caused by TNFAIP3 mutation. Allergol Int 2017;66: 146–8. doi: 10.1016/j.alit.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 6.Ajjan RA, Weetman AP. The pathogenesis of Hashimoto’s thyroiditis: further developments in our understanding. Horm Metab Res 2015;47: 702–10. doi: 10.1055/s-0035-1548832 [DOI] [PubMed] [Google Scholar]
- 7.Wan XL, Kimura A, Dong RP, Honda K, Tamai H, Sasazuki T. HLA-A and -DRB4 genes in controlling the susceptibility to Hashimoto’s thyroiditis. Hum Immunol 1995;42: 131–6. doi: 10.1016/0198-8859(94)00089-9 [DOI] [PubMed] [Google Scholar]
- 8.Sasazuki T, Inoko H, Morishima S, Morishima Y. Gene map of the HLA region, Graves’ disease and Hashimoto thyroiditis, and hematopoietic stem cell transplantation. Adv Immunol 2016;129: 175–249. doi: 10.1016/bs.ai.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 9.Zeitlin AA, Heward JM, Newby PR, Carr-Smith JD, Franklyn JA, Gough SC, et al. Analysis of HLA class II genes in Hashimoto’s thyroiditis reveals differences compared to Graves’ disease. Genes Immun 2008;9: 358–63. doi: 10.1038/gene.2008.26 [DOI] [PubMed] [Google Scholar]
- 10.Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, et al. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci USA 2008;105: 14034–9. doi: 10.1073/pnas.0806584105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissel M, Höfer R, Zasmeta H, Mayr WR. HLA-DR and Hashimoto’s thyroiditis. Tissue Antigens 1980;16: 256–7. doi: 10.1111/j.1399-0039.1980.tb00302.x [DOI] [PubMed] [Google Scholar]
- 12.Jabrocka-Hybel A, Skalniak A, Piątkowski J, Turek-Jabrocka R, Vyhouskaya P, Ludwig-Słomczyńska A, et al. How much of the predisposition to Hashimoto’s thyroiditis can be explained based on previously reported associations? J Endocrinol Invest 2018;41: 1409–16. doi: 10.1007/s40618-018-0910-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda S, Oryoji D, Yamamoto K, Noh JY, Okamura K, Noda M, et al. Identification of independent susceptible and protective HLA alleles in Japanese autoimmune thyroid disease and their epistasis. J Clin Endocrinol Metab 2014;99: E379–83. doi: 10.1210/jc.2013-2841 [DOI] [PubMed] [Google Scholar]
- 14.Duncan CJA, Dinnigan E, Theobald R, Grainger A, Skelton AJ, Hussain R, et al. Early-onset autoimmune disease due to a heterozygous loss-of-function mutation in TNFAIP3 (A20). Ann Rheum Dis 2018;77: 783–6. doi: 10.1136/annrheumdis-2016-210944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berteau F, Rouvière B, Nau A, Le Berre R, Sarrabay G, Touitou I, et al. Response to: ‘A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease’. Ann Rheum Dis 2018; pii: annrheumdis-2018-213347. [Epub ahead of print]. doi: 10.1136/annrheumdis-2018-213347 [DOI] [PubMed] [Google Scholar]
- 16.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014;14: 585–600. doi: 10.1038/nri3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieux-Laucat F. What’s up in the ALPS. Curr Opin Immunol 2017;49: 79–86. doi: 10.1016/j.coi.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 18.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Allen HL, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet 2014;46: 812–4. doi: 10.1038/ng.3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med 2014;20: 1410–6. doi: 10.1038/nm.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]