Abstract.
Congenital central hypothyroidism (C-CH) is caused by defects in the secretion of thyrotropin-releasing hormone (TRH) and/or TSH, leading to an impairment in the release of hormones from the thyroid. The causes of C-CH include congenital anomalies of the hypothalamic-pituitary regions and several genetic defects. In terms of endocrinology, C-CH is divided into two categories: (1) accompanied by another pituitary hormone deficiency and called combined pituitary hormone deficiency, and (2) isolated C-CH, showing mainly TSH deficiency. For isolated C-CH, a mutation in the TSH gene (TSHB) encoding the β-subunit of the protein was first found in 1990 by Japanese researchers, and thereafter several mutations in TSHB have been reported. Mutations in the thyrotropin-releasing hormone receptor gene (TRHR), as well as genetic defects in immunoglobulin superfamily 1 (IGSF1), have also been identified. It was recently found that isolated C-CH is caused by mutations in transducin β-like 1 X-linked and insulin receptor substrate 4. It is noted that all patients with TSHB deficiency and some with IGSF1 deficiency show severe hypothyroidism soon after birth. Among the causes of C-CH, high frequency of mutations in IGSF1 is the most prevalent. This review focuses on recent findings on isolated C-CH.
Keywords: congenital central hypothyroidism (C-CH), immunoglobulin superfamily 1 (IGSF1), TRHR, TBL1X
Introduction
Congenital central hypothyroidism (C-CH) is defined as hypothyroidism due to a lack of stimulation of the normal thyroid gland (1, 2). C-CH is caused by anatomical or functional impairments in neurons that secrete hypothalamic-thyrotropin releasing hormone (TRH) and/or pituitary thyrotropes that secrete TSH. C-CH is more frequently recognized as a part of a combined pituitary hormone deficiency (CPHD), but a small proportion of C-CH cases demonstrate isolated C-CH where mainly TSH secretion is impaired.
The frequency of C-CH among the general population has been reported as 1/16,000 to 1/30,000 (2–4). In Japan, newborn screening for congenital hypothyroidism has been performed since 1979. While the main purpose of such screening is to detect primary hypothyroidism (thyroid origin), some regions in the country employ simultaneous determination of blood TSH and free T4 (FT4) levels to diagnose C-CH in addition to primary hypothyroidism (2,3,4). Nagasaki et al. reported that C-CH occurs in about 10% of patients with congenital hypothyroidism in Japan (5).
In 1971, Miyai et al. (6) first reported a family with isolated TSH deficiency, and in 1989, a mutation in the TSH gene (TSHB) encoding the β-subunit of the protein was eventually discovered (7). Thereafter, over the following three decades, several genetic defects associated with isolated C-CH have been identified (Table 1) (1, 2, 8, 9). This review summarizes the current knowledge regarding the genetic causes and clinical features of isolated C-CH.
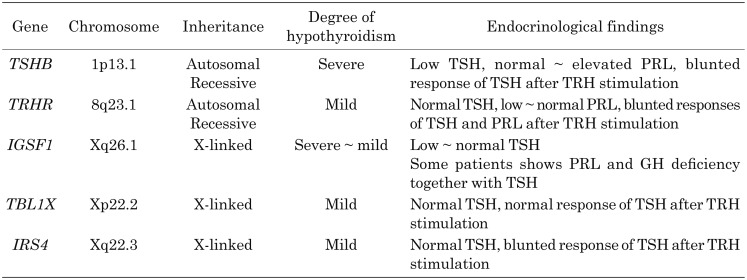
Table 1. Genetic causes, severity of hypothyroidism, and endocrine findings of isolated-Cent-H.
TSHB Deficiency
As mentioned, a family in Japan showing TSH deficiency was first reported by Miyai et al. (6), and eventually a mutation in TSHB was discovered in 1989 (7). In the first report, patients showed symptoms of severe congenital hypothyroidism, such as failure to thrive, prolonged jaundice, and developmental delay. Despite very low levels of serum T4 and T3, TSH was not measurable, indicating C-CH. A TRH stimulation test did not increase TSH level at all, but showed a normal PRL response. Finally, their group identified a homozygous mutation p.Gly29Arg in TSHB. Miyai et al. (1) further found several Japanese patients with TSHB deficiency. Genetic analysis showed that all patients had p.Gly29Arg, suggesting a founder effect (1).
Since the first report, several patients with TSHB mutations have been reported worldwide (10,11,12,13,14,15,16,17,18,19,20). In all cases, the inheritance of TSHB defects is autosomal recessive. Almost all patients with a TSHB defect develop severe symptoms of congenital hypothyroidism after birth. A delay in diagnosis and thyroid hormone replacement therapy results in poorer psychomotor development.
Regarding mutations, nonsense mutations (p.Glu32Ter and p.Gln69Ter), frameshift mutations (p.Cys125ValfsTer10), missense mutations (p.Met1?, p.Glu32Lys, p.Gly49Arg, p.Cys108Tyr, and p.Cys105Arg), and a splice site mutation (c.1625+5G>A) have been identified. One mutation (c.162G>A) did not cause an amino acid change, but it was located at the 5′ donor splice site of exon/intron2, leading to the skipping of exon 2. In addition, a homozygous deletion of TSHB was also reported (16). Among these mutations and deletions, p.Cys125ValfsTer10 has been most frequently reported in Europe, Argentina, and the USA (10, 12, 18,19,20). This mutation has been found in various unrelated families, but several studies using haplotype analysis in Germany, the UK, and Ireland showed that this mutation was derived from common ancestors (18,19,20).
TRHR Deficiency
To date, three homozygous mutations in the thyrotropin-releasing hormone receptor (TRHR) gene of five individuals from three unrelated families and a compound heterozygous mutation in a case from another family have been reported (21,22,23,24). In contrast to cases with TSHB deficiency, cases with TRHR deficiency do not show severe symptoms of congenital hypothyroidism or developmental delay despite a delay in the start of thyroxine replacement.
The first case with a TRHR mutation was identified through clinical evaluation because of the observation of a short stature at 8.9 yr of age (21). The patient showed low serum T4 and normal TSH levels. Neither TSH nor PRL responded upon TRH stimulation. Genetic analysis revealed that the patient was a compound heterozygote for p. Arg17Ter and p.Ser115-Tyr117del;Ala118Thr. Furthermore, two missense mutations in two unrelated families were reported. One patient who was homozygous for p.Pro81Arg, a recently identified TRHR missense mutation, showed prolonged jaundice; a subsequent endocrine examination was performed and low free T4 and normal TSH levels at 18 d of age were found (23). Thyroid hormone replacement therapy was initiated at 2 mo of age. The patient showed normal growth and development at 4 yr of age. A functional study of p.Pro81Arg revealed that mutant TRHR was normally expressed in the cell membrane, but showed reduced capacities for binding TRH and signal transduction through Gqα, a member of the G alpha q family of GTP-binding proteins.
The other homozygous TRHR missense mutation, p.Ile131Thr, was reported in an 8-yr-old boy (24). He showed mildly low free T4 and normal TSH levels and was overweight (body mass index (BMI): 20.4 kg/m2). This mutation was also associated with decreased TRH binding affinity. In addition, two cases with a homozygous nonsense mutation (p.Arg17Ter) have been reported (22). A boy was medically evaluated because of short stature and easy fatigue at 11 yr of age. His neurological development was normal. Endocrinological evaluation showed low free T4 and normal TSH levels, but TRH stimulation did not increase either serum TSH or PRL level. Thyroxine replacement improved the boy’s symptoms. Interestingly, his 33-yr-old sister was also found to be homozygous for p.Arg17Ter in a family screen. She showed normal growth and neurological development. She had two deliveries and subsequent lactation was normal. This finding indicates that, while TRHR is expressed in lactotropes as well as thyrotropes, TRH-stimulated PRL is not necessary for human pregnancy and lactation. In this case, thyroid hormone replacement therapy was reported to improve the sister’s quality of life (8).
As mentioned previously, in contrast to cases with TSHB deficiency, cases with TRHR deficiency did not show severe symptoms of congenital hypothyroidism and had normal neurological developmental. The T4 levels in cases with TRHR deficiency were reduced by approximately 50–80% for the lower limit range, but were at higher levels than those in cases with TSHB deficiency. These findings indicate that, without TRHR-mediated TRH stimulation, thyrotropes can secret bioactive TSH, leading to the secretion of thyroid hormone to achieve normal neurological development.
IGSF1 Deficiency
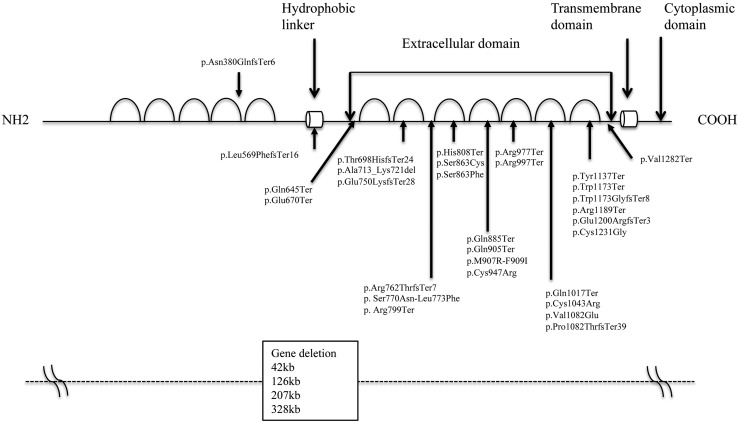
The immunoglobulin superfamily 1 (IGSF1) gene was cloned from the X chromosome in 1998 and found to encode a transmembrane glycoprotein containing 12 extracellular immune loops, a single transmembrane domain, and a short cytoplasmic domain (25) (Fig. 1). The protein is cleaved by peptidase between immune loops 5 and 6, and only the C-terminal region moves to and is expressed in the cell membrane (25). It was suggested that IGSF1 has a role as a coreceptor of inhibin; however, Igsf1 knockout mice showed normal fertility and FSH levels, indicating that IGSF1 does not function as a coreceptor of inhibin (26). In 2012, Sun et al. (27) reported that mutations and deletions of IGSF1 were causal to C-CH. In Japan in 2013, our group reported four mutations in IGSF1 in patients with C-CH (28, 29). Thereafter, to date, several reports of IGSF1 deficiency have been made (30,31,32,33,34,35,36,37,38).
Fig. 1.
Schema of IGSF1 and mutations/deletions. Immunoglobulin superfamily 1 (IGSF1) has 12 immune loops. The protein is cleaved at N- and C-terminal domains between immunoglobulin loops 5 and 7. Whereas the C-terminal domain moves to the cell membrane, the N-terminal domain remains in the endoplasmic reticulum. Deletions, nonsense, frameshift, missense, and splicing mutations reported are summarized.
Regarding genetic defects of IGSF1, deletion, nonsense, missense, and splicing mutations have been identified, and all mutations except one (p.Asn380GlnfsTer6) (30) were found to be located in the C-terminal region of IGSF1 (Fig. 1). Regarding missense mutations, since an in vitro study demonstrated that mutant proteins could not traffic to the cell membrane, these were thought to be loss-of-function mutations (27, 29, 34, 37).
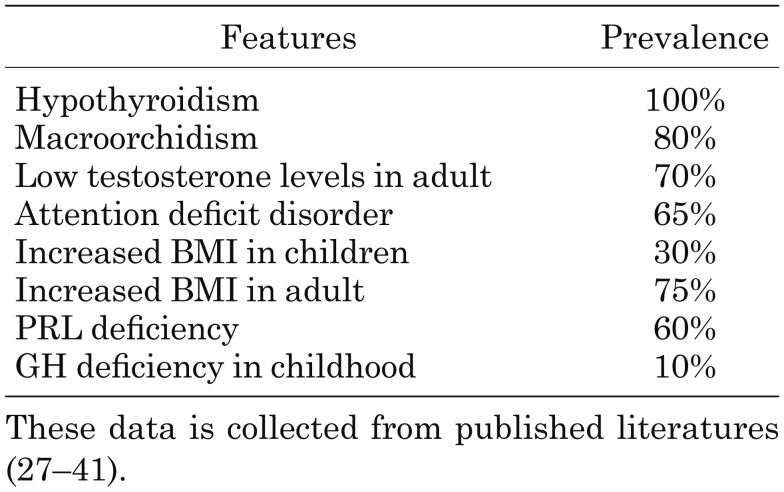
Clinical features of IGSF1 deficiency and the frequency of these features are summarized in Table 2 (27–40). Almost all male individuals with IGSF1 deficiency had C-CH, but the severity of C-CH varied widely. Whereas some individuals showed symptoms of hypothyroidism such as failure to thrive, constipation, icterus, and growth failure from early childhood, family screening of thyroid function accidentally detected several asymptomatic individuals when affected family members were first diagnosed.
Table 2. Clinical features of male patients with IGSF1 deficiency.
It is notable that one of the characteristic features of IGSF1 deficiency is macroorchidism. In the study by Joustra et al., approximately 80% of adult cases showed macroorchidism (40). Some patients also showed a delayed rise in LH and FSH levels during puberty. In addition, serum testosterone levels were low in adult male patients, but male fertility was likely to be normal. Furthermore, about 50% of adult patients developed obesity despite appropriate thyroxine replacement. It was also reported that 65% of patients showed attention deficit disorder. Attention deficit disorder has been found in patients despite early initiation of thyroxine treatment (41). Since IGSF1 is expressed in the brain, any neurological deficits may be due to a direct effect of IGSF1 deficiency.
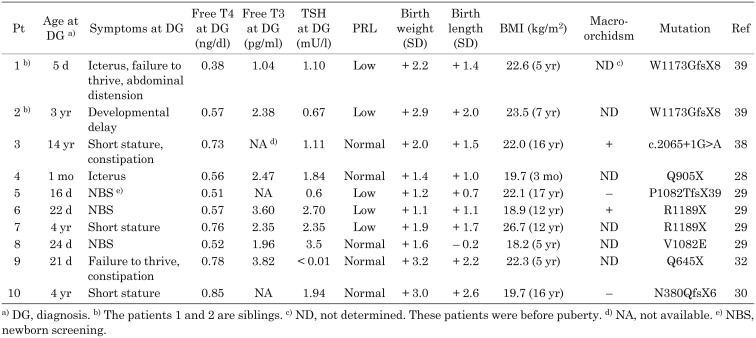
To date, in Japan, 10 patients described in six publications within the medical literature have been identified (28,29,30, 32, 38, 39). Clinical features and endocrine findings are summarized in Table 3. Patients 1 and 2 were siblings and their detailed clinical courses have been reported (38). In brief, patient 1 developed a failure to thrive, marked abdominal distention, and icterus at 5 d of age; a subsequent examination of thyroid function showed marked low FT4 and free T3 levels (Table 3). To our knowledge, this patient presented the most severe case to have been reported so far in the literature. However, patient 2, who was an older brother of patient 1, did not develop severe symptoms of hypothyroidism, but showed a mild psychomotor developmental delay at 1 yr of age and was followed regularly. After his younger brother was diagnosed with C-CH, he was evaluated for thyroid function and diagnosed with C-CH. Thyroid hormone replacement was started at 3 yr of age, but his psychomotor development was mildly delayed.
Table 3. Biochemical, clinical features and mutations of Japanese patients with IGSF1 deficiency.
Asakura et al. (30) pointed out that patients with IGSF1 deficiency are born larger than the mean for a specific gestational age. Furthermore, Japanese cases reported after the publication of the study by Asakura et al. (30) also described a heavy birth weight (Table 3). The BMI data of 10 reported Japanese patients are summarized in Table 3. Most patients showed an increased BMI, concordant with a study from Europe (40). Interestingly, the patient described by Asakura et al. (30) showed hypoplastic anterior and ectopic posterior lobes in the pituitary by magnetic resonance imaging (MRI), accompanying a growth hormone deficiency. So far, hypoplasia of the corpus callosum in one patient and in the small stalk region in another as shown by MRI have been identified (27). Whether these anatomical findings observed through MRI occurred by accident or were a direct effect of IGSF1 deficiency is still unknown.
While it is now clear that IGSF1 deficiency causes C-CH, the physiological role of IGSF1 in humans has not yet been determined. The expression of IGSF1 and murine Igsf1 mRNAs were observed in Rathke’s pouch and the adult pituitary gland (27), suggesting that IGSF1 is probably involved in the regulation of TRH and/or the secretion of TSH. In this regard, a study using Igsf1 knockout mice may be useful. Turgeon et al. (42) recently reported about Igsf1 knockout mice that showed a loss-of-function mutation in the C-terminal domain of Igsf1. In this study, the TSH content of the pituitary was reduced compared with that of wild-type mice. In addition, mRNA levels of Tshb and Trhr in thyrotropes were decreased. A low iodine diet together with propylthiouracil-induced hypothyroidism in knockout mice lead to a TSH elevation in response to hypothyroidism that was less than that of wild-type mice. Furthermore, TRH-stimulated TSH release was blunted compared with that of wild-type mice. These findings suggest that TRH stimulated signaling through TRHR in thyrotropes is impaired by IGSF1 deficiency. In vitro, Garcia et al. (35) analyzed the effect of IGSF1 on TSHR transcription. According to their study, transforming growth factor (TGF) β suppressed Trhr gene expression in rat pituitary GH4C1 cells and somato-lactrope cell lines, but transfection of IGSF1 into GH4C1 cells cancelled the repression of transcription of Trhr induced by TGFβ. Therefore, it is speculated that, in IGSF1 deficiency, the transcription of TRHR is constantly suppressed by TGFβ, resulting in reduced TRHR numbers in the cell membrane and eventually leading to decreased TSH secretion from thyrotropes. Since it was just an in vitro study, further studies on IGSF1 function in the hypothalamic-pituitary thyroid axis are necessary. Furthermore, the mechanisms involved in macroorchidism, heavy birth weight, and increased BMI are still unknown. Elucidation of the physiological role of IGSF1 in the future will yield answers to these questions in addition to the development of C-CH.
TBL1X Deficiency
In 2016, Heinen et al. reported that mutations in the transducing β-like protein, X-linked gene (TBL1X) caused C-CH (43). TBL1X is one of components of the nuclear receptor repressor (NCoR) and is a silencing mediator of retinoid and thyroid receptors (SMRT). Its expression is observed in hypothalamic nuclei, including the paraventricular nucleus, and in the pituitary gland (44). In Heinen’s study, five missense mutations (p.Asn365Tyr, p. His453Tyr, p.Ala366Thr, p.Tyr458Cys, and p.Trp369Arg) and one splice site mutation (c.1312-1G>A) were identified in eight patients from six families. Further analysis identified mutations in 11 other asymptomatic individuals. Previously, partial deletion of TBL1X was identified in two unrelated patients with hearing loss (45). Indeed, 12 of 19 individuals harboring mutations in TBL1X had mild to moderate hearing loss. Garcia et al. (46) also reported a patient with p.Arg433Ter in TBL1X. In addition to low FT4, the patient had encopresis, constipation, and attention deficit/hyperactivity disorder. Patients thus far reported with mutations in TBL1X have shown lower limit of normal to normal concentrations of FT4, and thus whether these patients show true hypothyroidism at the organ level is not clear.
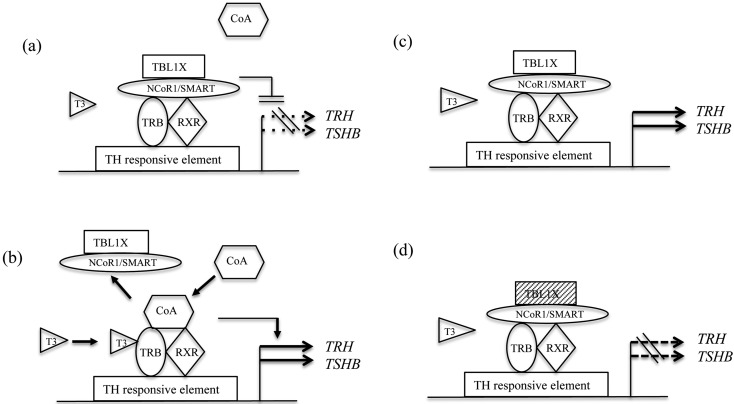
Usually in the absence of T3, NCoR/SMRT complexes bind thyroid hormone receptor β (TRB) with retinoid X receptor (as a heterodimer; Fig. 2a). These complexes bind to a thyroid hormone (TH) response element in the promoter region of TH targeting genes to repress their transcription (Fig. 2a). When T3 binds TRBs, the dissociation of the repressor complex and the recruitment of a coactivator complex occur, thus activating the transcription of TH target genes (Fig. 2b). However, several in vitro studies showed that cotransfection of NCoR/SMRT with TRBs conversely stimulates the transcription of TSHB and TRH in the absence of T3 (47, 48). Recently, Takamizawa et al. (49) have demonstrated that the ligand-independent thyroid hormone receptor-mediated stimulation of TSHB and TRH promoters was enhanced by TBL1X alone or by cotransfection with TBL1X and NCoR (Fig. 2c); however, the stimulating effect was attenuated by cotransfection with TBL1X mutants (p.Asn365Tyr and p.Tyr458Cys) and NCoR (Fig. 2d). The authors speculated that, despite low thyroid hormone levels, TSHB and TRH transcription is not up-regulated by TBL1X mutations, resulting in C-CH.
Fig. 2.
Mechanism of C-CH: Mutations in TBL1X. (a) In the absence of T3, NCoR/SMRT complexes bind thyroid hormone receptor β (TRB) with RXR (as a heterodimer). These complexes bind to the thyroid hormone response element in the promoter region of thyroid hormone (TH) targeting genes and repress the transcription of these genes. (b) When T3 binds TRBs, the dissociation of the repressor complex and the recruitment of coactivator (CoA) occur, thus activating the transcription of TH target genes. (c) Several in vitro studies showed that cotransfection of NCoR/SMRT with TRBs conversely stimulates the transcription of TSHB and TRH in the absence of T3. The ligand-independent thyroid hormone receptor-mediated stimulation of TSHB and TRH promoters was enhanced by TBL1X. (d) TBL1X (shaded box) mutants of N365Y and Y458C impaired the stimulating effect. TBL1X, transducin β-like 1 X-linked; NCoR, nuclear receptor repressor; SMRT silencing mediator of retinoid and thyroid receptors; TRB, thyroid hormone receptor β; RXR, retinoid X receptor; CoA, coactivator; TH, thyroid hormone.
IRS4 Deficiency
Most recently, four different mutations (one nonsense and three frameshift) from five families of insulin receptor substrate 4 (IRS4) have been reported (9). These patients showed 72 to 92.5% of the lower limit of the normal range of FT4 concentrations, but normal T3 levels. The expression of IRS4 mRNA was observed in the hypothalamus, pituitary gland, thyroid, and ovaries, but the mechanism for developing low FT4 is unknown.
Conclusion
It is noteworthy that IGSF1 deficiency is the most prevalent cause of C-CH, with some patients developing severe hypothyroidism soon after birth, similar to that seen with TSHB deficiency. In Japan, although TSH-based newborn screening is usually performed for congenital hypothyroidism, except in some regional areas, pediatricians must bear in mind that the screen may overlook C-CH. This highlights the importance of suspecting severe to moderate C-CH based on the clinical symptoms of hypothyroidism during infancy and childhood, with a prompt diagnosis and subsequent initiation of thyroid hormone replacement in severe cases.
References
- 1.Miyai K. Congenital thyrotropin deficiency--from discovery to molecular biology, postgenome and preventive medicine. Endocr J 2007;54: 191–203Review. doi: 10.1507/endocrj.KR-107 [DOI] [PubMed] [Google Scholar]
- 2.Tajima T, Nakamura A, Morikawa S, Ishizu K. Neonatal screening and a new cause of congenital central hypothyroidism. Ann Pediatr Endocrinol Metab 2014;19: 117–21Review. doi: 10.6065/apem.2014.19.3.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiwara F, Fujikura K, Okuhara K, Tsubaki J, Fukushi M, Fujita K, et al. Central congenital hypothyroidism detected by neonatal screening in sapporo, japan (2000-2004): it’s prevalence and clinical characteristics. Clin Pediatr Endocrinol 2008;17: 65–9. doi: 10.1297/cpe.17.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adachi M, Soneda A, Asakura Y, Muroya K, Yamagami Y, Hirahara F. Mass screening of newborns for congenital hypothyroidism of central origin by free thyroxine measurement of blood samples on filter paper. Eur J Endocrinol 2012;166: 829–38. doi: 10.1530/EJE-11-0653 [DOI] [PubMed] [Google Scholar]
- 5.Nagasaki K, Kubota T, Kobayashi H, Sawada H, Numakura C, Harada S, et al. Clinical characteristics of septo-optic dysplasia accompanied by congenital central hypothyroidism in Japan. Clin Pediatr Endocrinol 2017;26: 207–13. doi: 10.1297/cpe.26.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyai K, Azukizawa M, Kumahara Y. Familial isolated thyrotropin deficiency with cretinism. N Engl J Med 1971;285: 1043–8. doi: 10.1056/NEJM197111042851902 [DOI] [PubMed] [Google Scholar]
- 7.Hayashizaki Y, Hiraoka Y, Endo Y, Miyai K, Matsubara K. Thyroid-stimulating hormone (TSH) deficiency caused by a single base substitution in the CAGYC region of the beta-subunit. EMBO J 1989;8: 2291–6. doi: 10.1002/j.1460-2075.1989.tb08355.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persani L, Rurale G, de Filippis T, Galazzi E, Muzza M, Fugazzola L. Genetics and management of congenital hypothyroidism. Best Pract Res Clin Endocrinol Metab 2018;32: 387–96Review. doi: 10.1016/j.beem.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 9.Heinen CA, de Vries EM, Alders M, Bikker H, Zwaveling-Soonawala N, van den Akker ELT, et al. Mutations in IRS4 are associated with central hypothyroidism. J Med Genet 2018;55: 693–700. doi: 10.1136/jmedgenet-2017-105113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dacou-Voutetakis C, Feltquate DM, Drakopoulou M, Kourides IA, Dracopoli NC. Familial hypothyroidism caused by a nonsense mutation in the thyroid-stimulating hormone beta-subunit gene. Am J Hum Genet 1990;46: 988–93. [PMC free article] [PubMed] [Google Scholar]
- 11.Medeiros-Neto G, Herodotou DT, Rajan S, Kommareddi S, de Lacerda L, Sandrini R, et al. A circulating, biologically inactive thyrotropin caused by a mutation in the beta subunit gene. J Clin Invest 1996;97: 1250–6. doi: 10.1172/JCI118540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuissoz JM, Deladoëy J, Buyukgebiz A, Cemeroglu P, Gex G, Gallati S, et al. New autosomal recessive mutation of the TSH-beta subunit gene causing central isolated hypothyroidism. J Clin Endocrinol Metab 2001;86: 4468–71. [DOI] [PubMed] [Google Scholar]
- 13.Pohlenz J, Dumitrescu A, Aumann U, Koch G, Melchior R, Prawitt D, et al. Congenital secondary hypothyroidism caused by exon skipping due to a homozygous donor splice site mutation in the TSHbeta-subunit gene. J Clin Endocrinol Metab 2002;87: 336–9. [DOI] [PubMed] [Google Scholar]
- 14.Borck G, Topaloglu AK, Korsch E, Martiné U, Wildhardt G, Onenli-Mungan N, et al. Four new cases of congenital secondary hypothyroidism due to a splice site mutation in the thyrotropin-beta gene. J Clin Endocrinol & Metab 2004;89: 4136–41. [DOI] [PubMed] [Google Scholar]
- 15.Baquedano MS, Ciaccio M, Dujovne N, Herzovich V, Longueira Y, Warman DM, et al. Two novel mutations of the TSH-beta subunit gene underlying congenital central hypothyroidism undetectable in neonatal TSH screening. J Clin Endocrinol Metab 2010;95: E98–103. doi: 10.1210/jc.2010-0223 [DOI] [PubMed] [Google Scholar]
- 16.Hermanns P, Couch R, Leonard N, Klotz C, Pohlenz J. A novel deletion in the thyrotropin Beta-subunit gene identified by array comparative genomic hybridization analysis causes central congenital hypothyroidism in a boy originating from Turkey. Horm Res Paediatr 2014;82: 201–5. doi: 10.1159/000362413 [DOI] [PubMed] [Google Scholar]
- 17.Özhan B, Boz Anlaş Ö, Sarıkepe B, Albuz B, Semerci Gündüz N. Congenital central hypothyroidism caused by a novel thyroid-stimulating hormone-beta subunit gene mutation in two siblings. J Clin Res Pediatr Endocrinol 2017;9: 278–82. doi: 10.4274/jcrpe.4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott MT, Haugen BR, Black JN, Wood WM, Gordon DF, Ridgway EC. Congenital isolated central hypothyroidism caused by a “hot spot” mutation in the thyrotropin-beta gene. Thyroid 2002;12: 1141–6. doi: 10.1089/105072502321085252 [DOI] [PubMed] [Google Scholar]
- 19.Deladoëy J, Vuissoz JM, Domené HM, Malik N, Gruneiro-Papendieck L, Chiesa A, et al. Congenital secondary hypothyroidism due to a mutation C105Vfs114X thyrotropin-beta mutation: genetic study of five unrelated families from Switzerland and Argentina. Thyroid 2003;13: 553–9. doi: 10.1089/105072503322238818 [DOI] [PubMed] [Google Scholar]
- 20.Domené HM, Gruñeiro-Papendieck L, Chiesa A, Iorcansky S, Herzovich VC, Papazian R, et al. The C105fs114X is the prevalent thyrotropin beta-subunit gene mutation in Argentinean patients with congenital central hypothyroidism. Horm Res 2004;61: 41–6. [DOI] [PubMed] [Google Scholar]
- 21.Collu R, Tang J, Castagné J, Lagacé G, Masson N, Huot C, et al. A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropin-releasing hormone receptor gene. J Clin Endocrinol Metab 1997;82: 1561–5. [DOI] [PubMed] [Google Scholar]
- 22.Bonomi M, Busnelli M, Beck-Peccoz P, Costanzo D, Antonica F, Dolci C, et al. A family with complete resistance to thyrotropin-releasing hormone. N Engl J Med 2009;360: 731–4. doi: 10.1056/NEJMc0808557 [DOI] [PubMed] [Google Scholar]
- 23.Koulouri O, Nicholas AK, Schoenmakers E, Mokrosinski J, Lane F, Cole T, et al. A novel thyrotropin-releasing hormone receptor missense mutation (P81R) in central congenital hypothyroidism. J Clin Endocrinol Metab 2016;101: 847–51. doi: 10.1210/jc.2015-3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García M, González de Buitrago J, Jiménez-Rosés M, Pardo L, Hinkle PM, Moreno JC. Central hypothyroidism due to a TRHR mutation causing impaired ligand affinity and transactivation of Gq. J Clin Endocrinol Metab 2017;102: 2433–42. doi: 10.1210/jc.2016-3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard DJ, Brûlé E, Smith CL, Joustra SD, Wit JM. From consternation to revelation: Discovery of a role for IGSF1 in pituitary control of thyroid function. J Endocr Soc 2018;2: 220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard DJ, Burns KH, Haupt B, Matzuk MM, Woodruff TK. Normal reproductive function in InhBP/p120-deficient mice. Mol Cell Biol 2003;23: 4882–91. doi: 10.1128/MCB.23.14.4882-4891.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Bak B, Schoenmakers N, van Trotsenburg AS, Oostdijk W, Voshol P, et al. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nat Genet 2012;44: 1375–81. doi: 10.1038/ng.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tajima T, Nakamura A, Ishizu K. A novel mutation of IGSF1 in a Japanese patient of congenital central hypothyroidism without macroorchidism. Endocr J 2013;60: 245–9. doi: 10.1507/endocrj.EJ13-0009 [DOI] [PubMed] [Google Scholar]
- 29.Nakamura A, Bak B, Silander TL, Lam J, Hotsubo T, Yorifuji T, et al. Three novel IGSF1 mutations in four Japanese patients with X-linked congenital central hypothyroidism. J Clin Endocrinol Metab 2013;98: E1682–91. doi: 10.1210/jc.2013-1224 [DOI] [PubMed] [Google Scholar]
- 30.Asakura Y, Abe K, Muroya K, Hanakawa J, Oto Y, Narumi S, et al. Combined growth hormone and thyroid-stimulating hormone deficiency in a Japanese patient with a novel frameshift mutation in IGSF1. Horm Res Paediatr 2015;84: 349–54. doi: 10.1159/000438672 [DOI] [PubMed] [Google Scholar]
- 31.Hughes JN, Aubert M, Heatlie J, Gardner A, Gecz J, Morgan T, et al. Identification of an IGSF1-specific deletion in a five-generation pedigree with X-linked Central Hypothyroidism without macroorchidism. Clin Endocrinol (Oxf) 2016;85: 609–15. doi: 10.1111/cen.13094 [DOI] [PubMed] [Google Scholar]
- 32.Nishigaki S, Hamazaki T, Fujita K, Morikawa S, Tajima T, Shintaku H. A Japanese family with central hypothyroidism caused by a novel IGSF1 mutation. Thyroid 2016;26: 1701–5. doi: 10.1089/thy.2016.0005 [DOI] [PubMed] [Google Scholar]
- 33.Tenenbaum-Rakover Y, Turgeon MO, London S, Hermanns P, Pohlenz J, Bernard DJ, et al. Familial central hypothyroidism caused by a novel IGSF1 gene mutation. Thyroid 2016;26: 1693–700. doi: 10.1089/thy.2015.0672 [DOI] [PubMed] [Google Scholar]
- 34.Van Hulle S, Craen M, Callewaert B, Joustra S, Oostdijk W, Losekoot M, et al. Delayed Adrenarche may be an Additional Feature of Immunoglobulin Super Family Member 1 Deficiency Syndrome. J Clin Res Pediatr Endocrinol 2016;8: 86–91. doi: 10.4274/jcrpe.2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García M, Barrio R, García-Lavandeira M, Garcia-Rendueles AR, Escudero A, Díaz-Rodríguez E, et al. The syndrome of central hypothyroidism and macroorchidism: IGSF1 controls TRHR and FSHB expression by differential modulation of pituitary TGFβ and Activin pathways. Sci Rep 2017;7: 42937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinen CA, Zwaveling-Soonawala N, Fliers E, Turgeon MO, Bernard DJ, van Trotsenburg ASP. A Novel IGSF1 mutation in a boy with short stature and hypercholesterolemia: A case report. J Endocr Soc 2017;1: 731–6. doi: 10.1210/js.2017-00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roche EF, McGowan A, Koulouri O, Turgeon MO, Nicholas AK, Heffernan E, et al. A novel IGSF1 mutation in a large Irish kindred highlights the need for familial screening in the IGSF1 deficiency syndrome. Clin Endocrinol (Oxf) 2018;89: 813–23. doi: 10.1111/cen.13827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi T, Hothubo T, Morikawa S, Nakamura A, Mori T, Tajima T. A Japanese patient with congenital central hypothyroidism caused by a novel IGSF1 mutation. J Pediatr Endocrinol Metab 2018;31: 355–9. doi: 10.1515/jpem-2017-0144 [DOI] [PubMed] [Google Scholar]
- 39.Oguma M, Kobayashi M, Yamazaki M, Yokoyama K, Morikawa S, Yamaguchi T, et al. Two siblings with congenital central hypothyroidism caused by a novel mutation in the IGSF1 gene. Clin Pediatr Endocrinol 2018;27: 95–100. doi: 10.1297/cpe.27.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joustra SD, Heinen CA, Schoenmakers N, Bonomi M, Ballieux BE, Turgeon MO, et al. IGSF1 Clinical Care Group. IGSF1 deficiency: lessons from an extensive case series and recommendations for clinical management. J Clin Endocrinol Metab 2016;101: 1627–36. doi: 10.1210/jc.2015-3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joustra SD, Andela CD, Oostdijk W, van Trotsenburg AS, Fliers E, Wit JM, et al. Mild deficits in attentional control in patients with the IGSF1 deficiency syndrome. Clin Endocrinol (Oxf) 2016;84: 896–903. doi: 10.1111/cen.12947 [DOI] [PubMed] [Google Scholar]
- 42.Turgeon MO, Silander TL, Doycheva D, Liao XH, Rigden M, Ongaro L, et al. TRH Action Is Impaired in Pituitaries of Male IGSF1-Deficient Mice. Endocrinology 2017;158: 815–30. doi: 10.1210/en.2016-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinen CA, Losekoot M, Sun Y, Watson PJ, Fairall L, Joustra SD, et al. Mutations in TBL1X are associated with central hypothyroidism. J Clin Endocrinol Metab 2016;101: 4564–73. doi: 10.1210/jc.2016-2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev 2000;14: 1048–57. [PMC free article] [PubMed] [Google Scholar]
- 45.Bassi MT, Ramesar RS, Caciotti B, Winship IM, De Grandi A, Riboni M, et al. X-linked late-onset sensorineural deafness caused by a deletion involving OA1 and a novel gene containing WD-40 repeats. Am J Hum Genet 1999;64: 1604–16. doi: 10.1086/302408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García M, Barreda-Bonis AC, Jiménez P, Rabanal I, Ortiz A, Vallespín E, et al. Central hypothyroidism and novel clinical phenotypes in hemizygous truncation of TBL1X. J Endocr Soc 2018;3: 119–28. doi: 10.1210/js.2018-00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satoh T, Monden T, Ishizuka T, Mitsuhashi T, Yamada M, Mori M. DNA binding and interaction with the nuclear receptor corepressor of thyroid hormone receptor are required for ligand-independent stimulation of the mouse preprothyrotropin-releasing hormone gene. Mol Cell Endocrinol 1999;154: 137–49. doi: 10.1016/S0303-7207(99)00032-5 [DOI] [PubMed] [Google Scholar]
- 48.Tagami T, Madison LD, Nagaya T, Jameson JL. Nuclear receptor corepressors activate rather than suppress basal transcription of genes that are negatively regulated by thyroid hormone. Mol Cell Biol 1997;17: 2642–8. doi: 10.1128/MCB.17.5.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takamizawa T, Satoh T, Miyamoto T, Nakajima Y, Ishizuka T, Tomaru T, et al. Transducin β-like 1, X-linked and nuclear receptor co-repressor cooperatively augment the ligand-independent stimulation of TRH and TSHβ gene promoters by thyroid hormone receptors. Endocr J 2018;65: 805–13. doi: 10.1507/endocrj.EJ17-0384 [DOI] [PubMed] [Google Scholar]