Abstract
Immobilization of enzymes through metal-based system is demonstrated as a promising approach to enhance its properties. In this study, the influence of metals ions, including copper, cobalt and zinc (Zn) on the immobilization of β-glucosidase (BGL) through the synthesis of protein-inorganic hybrid was evaluated at 4 °C. Among these metal ions-based hybrids, Zn showed the highest encapsulation yield and relative activity of 87.5 and 207%, respectively. Immobilized BGL exhibited higher pH and temperature stability compared to free form. Thermal stability of hybrid improved up to 26-fold at 60 °C. After 10 cycles of reuse, immobilized enzyme retained 93.8% of residual activity. These results suggested that metal ions played a significant role in the enzyme immobilization as a protein-inorganic hybrid. Overall, this strategy can be potentially applied to enhance the properties of enzymes though effective encapsulation for the broad biotechnological applications.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00796-z) contains supplementary material, which is available to authorized users.
Keywords: β-Glucosidase, Immobilization, Protein-inorganic hybrid, Reusability, Stability
The biocatalytic transformation reactions success is largely influenced by the properties of the enzyme. Primarily, the use of costly free form of enzyme has resulted in low process efficiency due to their lower stability and limitation for subsequent reuses. In order to solve these problems, immobilization of enzymes has been demonstrated using different methods, including adsorption, covalent, cross-linking or encapsulation [1–8]. The enzyme properties such as residual activity and stability are significantly influenced by the immobilization strategies [2, 7]. From past few years, the protein and metal ions-based enzyme immobilization through protein-inorganic hybrid as a nanoflowers has been shown as a potential approach [9–13]. The synthesis of protein-inorganic hybrids occurs through the three steps mechanism of nucleation, aggregation and anisotropic growth. Overall, the synthesis conditions, types of metal and enzyme played a key role in the efficient immobilization [9, 12, 13]. In addition, the structural properties such as size and morphology of synthesized hybrid vary a lot with the modifications in conditions for synthesis and enzymes [9, 13]. The nano-flower based-system has shown major advantages such as retention of higher efficiency and improved stability of the enzyme over their immobilization through typical approaches [1, 10, 14]. It is due to the effective confinement of enzymes and high surface area of the synthesized hybrid. In contrast, the long incubation periods required for the synthesis and low structural stability during repeated uses of the synthesized nano-flowers are the critical disadvantages [9, 13].
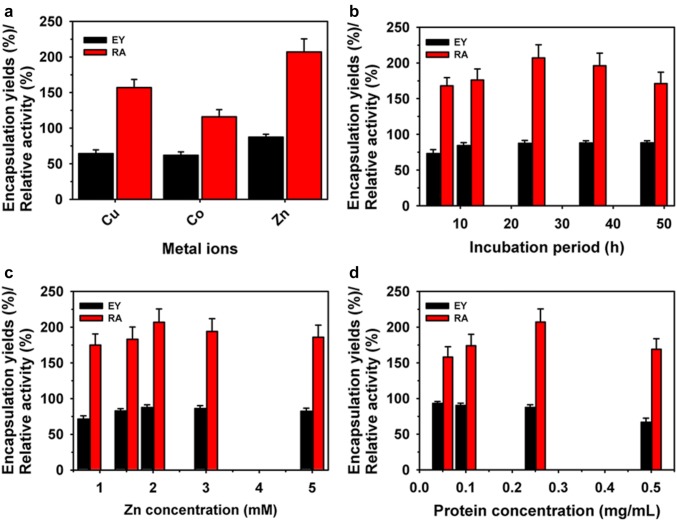
β-glucosidase (BGL) is an industrially important enzyme for the broad applications, including production of biofuels from agricultural wastes, food processing, wine or beverage industry and synthesis of glucosides [15, 16]. Still, immobilization of BGL as protein-inorganic hybrid has not been reported. Therefore, immobilization of BGL using different metal ions copper (Cu), cobalt (Co) and zinc (Zn) as protein-inorganic hybrid has been investigated in this study. The synthesis of BGL hybrids was performed using metal ions (Cu, Co or Zn) and enzyme in the phosphate buffer saline (PBS) incubated for 24 h at 4 °C, as reported previously [12]. The synthesized hybrids exhibited encapsulation yield (EY) and relative activity (RA) in the range of 61.9–87.5 and 116–207%, respectively (Fig. 1a). Here, Zn metal ions-based hybrid exhibited both higher EY and RA as compared with Cu and Co. Here, variations in these properties might be associated with differential influence of metals ions on BGL. Remarkably, synthesized BGL-Zn3(PO4)2 hybrid showed high RA of 207% compared to those of free enzyme. Further, the optimization of the synthesis conditions, including incubation period (4–48 h), metal (0.8–5.0 mM) and protein (0.05-0.50 mg/mL) concentrations were evaluated to improve the immobilization of enzyme using Zn as a metal ion in the PBS (pH, 7.4) (Fig. 1b–d). The optimum incubation period, metal ions and protein concentrations were observed to be 24 h, 2.0 mM and 0.25 mg/mL for effective immobilization of BGL to achieve EY and RA of 89.2 and 207%, respectively. Overall, protein-inorganic hybrid of BGL showed significantly higher efficiency of 207% compared to those due to immobilization of BGL using solid supports [17–19]. In addition, the synthesis of BGL required shorter incubation period in comparison to 3 days of incubation used for immobilization of enzymes thorough protein-inorganic hybrid using different metal ions and enzymes [9, 11, 20]. In contrast, BGL immobilized on solid supports including silica and magnetic nanoparticles exhibited significantly lower RA in the range of 19.6-41.4% [18, 19].
Fig. 1.
Immobilization of BGL as protein-inorganic hybrid using different metal ions at a concentration of 2 mM (a). Effect of incubation period (b), protein (c) and metal ions (d) concentrations on the immobilization of BGL using Zn metal ions
The synthesized BGL-Zn3(PO4)2 hybrid using 0.25 mg/mL of protein and 2.0 mM of Zn metal ions were characterized to evaluate the properties of hybrids (Fig. 2). The flower like morphology of BGL-Zn3(PO4)2 hybrids was confirmed through field emission scanning electron microscopy analysis. The average size of hybrids was observed to be ~ 6 µm. The presence of C, P and Zn in the energy dispersive spectrometry analysis confirmed the synthesis of hybrid (Table S1). Further, the green fluorescence of fluorescein isothiocyanate labelled BGL in the confocal laser scanning analysis confirmed the immobilization of enzyme as reported previously [12].
Fig. 2.
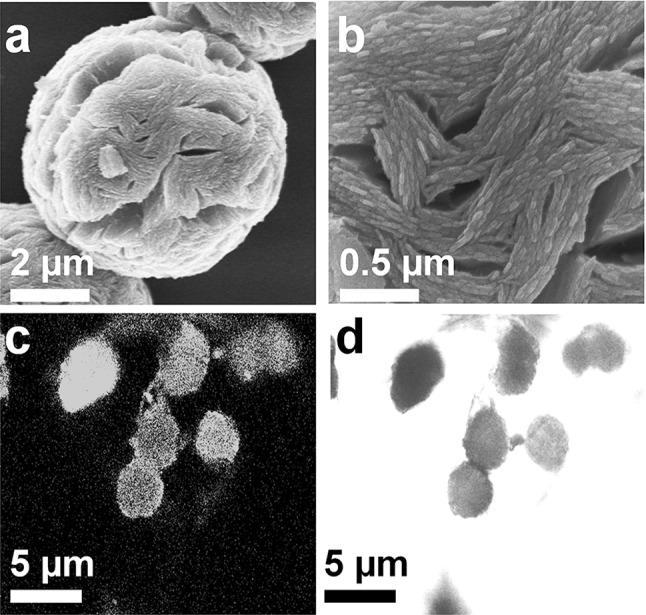
Characterization of synthesized BGL-Zn3(PO4)2 hybrid. Field emission scanning electron microscopy image (a), in high resolution (b), confocal microscopy image of synthesized hybrid using FITC-labelled BGL in green channel (c) and bright channel (d)
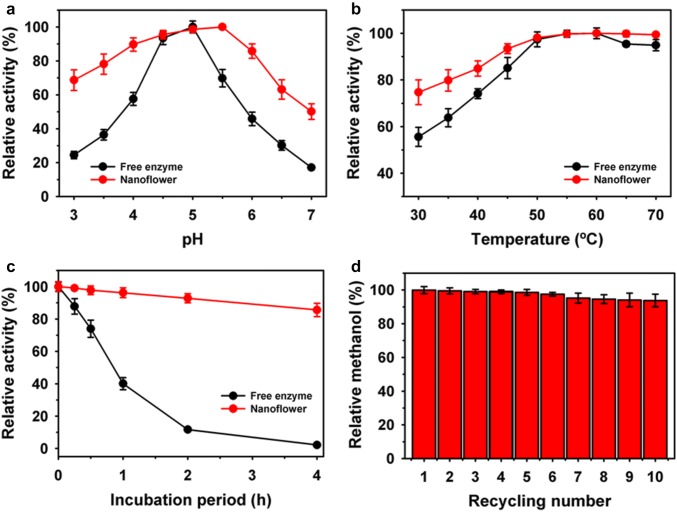
In order to characterize the immobilized BGL, the properties of BGL-Zn3(PO4)2 hybrid nanoflower were compared with the free form of enzyme (Fig. 3). A shift in the higher optimum pH activity of BGL nanoflower was observed to 5.5 as compared to free enzyme of 5.0. Whereas, a similar optimum temperature of 60 °C was recorded for both free and immobilized BGL. Remarkably, BGL nanoflower exhibited higher residual activities over broad ranges of pH and temperature. In contrast, BGL immobilized through zeolitic imidazolate framework-8 (ZIF-8) showed lower residual activity at higher pH and temperature to those of free enzyme [18]. The thermal stability was measured at 60 °C for an incubation period of 4 h. The nanoflowers hybrid retained significantly higher residual activity of 85.6% as compared to 2.2% residual activity of free enzyme. Here, half-life value of nanoflower was found to improve 26-fold. These results suggested better suitability of immobilized enzyme over free form for application at higher temperatures. Primarily, the significant reduction in residual activity of immobilized enzymes during reusability is a major limiting factor despite of efficient immobilization. However, the reusability of BGL-Zn3(PO4)2 hybrid nanoflower was assessed up to 10 cycles of reuses under standard assay conditions. BGL nanoflower retained residual activity of 98.6 and 93.8% after five and 10 cycles of reuse, respectively. Previous studies have shown that, BGL immobilized on the silica and magnetic particles retained residual activity in the range of 38.0–67.0% [18, 19]. In contrast, a significant reduction up to 80% residual activity was reported for BGL immobilized through ZIF-8 [18].
Fig. 3.
Activity profile of immobilized BGL-Zn3(PO4)2 hybrid at different pH (a), and temperature (b). Thermal stability at 60 °C (c), and reusability (d)
In summary, the immobilization of BGL was reported through the protein-inorganic hybrids using different metal ions, including Cu, Co and Zn. The metals ions significantly influenced the EY and RA of immobilized BGL. Interestingly, Zn showed very efficient immobilization of BGL to achieve high EY and RA. BGL nanoflowers exhibited flower like morphology and showed improved pH and temperature profiles, and stability compared to those of free enzyme. In addition, BGL nanoflowers exhibited significantly higher reusability than those previously reported on the various solid supports and ZIF-8 hybrids. Therefore, synthesis of efficient protein-inorganic hybrid using different metals ions may be employed to improve the properties of industrially important enzymes for biotechnological applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This paper was supported by Konkuk University Researcher Fund in 2018. This research was supported by Basic Science Research Program (2019R1C1C1009766) and by the Intelligent Synthetic Biology Center of Global Frontier Project (2013M3A6A8073184) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning. This research was supported by the KU Research Professor program of Konkuk University.
Compliance with Ethical Standards
Conflict of interest
There is no conflict of interest among the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Myung-Seok Choi, Email: mchoi@konkuk.ac.kr.
Jung-Kul Lee, Email: jkrhee@konkuk.ac.kr.
References
- 1.Anwar MZ, Kim DJ, Kumar A, Patel SKS, Otari S, Mardina P, Jeong JH, Sohn JH, Kim JH, Park JT, Lee JK. SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep. 2017;7:15333. doi: 10.1038/s41598-017-15550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim T-S, Patel SKS, Selvaraj C, Jung W-S, Pan C-H, Kang YC, Lee J-K. A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Park GD, Patel SKS, Kondaveeti S, Otari S, Anwar MZ, Kalia VC, Singh Y, Kim SC, Cho B-K, Sohn J-H, Kim DR, Kang YC, Lee J-K. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. doi: 10.1016/j.cej.2018.11.052. [DOI] [Google Scholar]
- 4.Patel SKS, Kalia VC, Choi JH, Haw JR, Kim IW, Lee JK. Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J Microbiol Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 5.Patel SKS, Choi SH, Kang YC, Lee J-K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 6.Patel SKS, Choi SH, Kang YC, Lee J-K. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Inter. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 7.Patel SKS, Anwar MZ, Kumar A, Otari SV, Pagolu RT, Kim S-Y, Kim I-W, Lee J-K. Fe2O3 yolk-shel particle-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem Eng J. 2018;132:1–8. doi: 10.1016/j.bej.2017.12.013. [DOI] [Google Scholar]
- 8.Zhang B, Li P, Zhang H, Wang H, Li X, Tian L, Ali N, Ali Z, Zhang Q. Preparation of lipase/Zn3(PO4)2 hybrid nanoflower and its catalytic performance as an immobilized enzyme. Chem Eng J. 2016;291:287–297. doi: 10.1016/j.cej.2016.01.104. [DOI] [Google Scholar]
- 9.Ge J, Lei J, Zare RN. Protein-inorganic hybrid nanoflowers. Nat Nanotechnol. 2012;7:428–432. doi: 10.1038/nnano.2012.80. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Kim I-W, Patel SKS, Lee J-K. Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2. Indian J Microbiol. 2018;58:100–104. doi: 10.1007/s12088-017-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A, Patel SKS, Mardan B, Pagolu R, Lestari R, Jeong S-H, Kim T, Haw JR, Lim S-Y, Kim I-W, Lee J-K. Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol. 2018;28:638–644. doi: 10.4014/jmb.1710.10037. [DOI] [PubMed] [Google Scholar]
- 12.Patel SKS, Otari SV, Kang YC, Lee JK. Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/C6RA24404A. [DOI] [Google Scholar]
- 13.Patel SKS, Otari SV, Li J, Kim DR, Kim SC, Cho B-K, Kalia VC, Kang YC, Lee J-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Otari SV, Patel SKS, Kim S-Y, Haw JR, Kalia VC, Kim I-W, Lee J-K. Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol. 2019;59:105–108. doi: 10.1007/s12088-018-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salgado JCS, Meleiro LP, Carli S, Ward RJ. Glucose tolerant and glucose stimulated β-glucosidases—a review. Bioresour Technol. 2018;267:704–713. doi: 10.1016/j.biortech.2018.07.137. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran P, Jagtap SS, Patel SKS, Li J, Kang YC, Lee J-K. Role of the non-conserved amino acid asparagine 285 in the glycone-binding pocket of Neosartorya fischeri β-glucosidase. RSC Adv. 2016;6:48137–48144. doi: 10.1039/C5RA28017F. [DOI] [Google Scholar]
- 17.Coutinho TC, Rojas MJ, Tardioli PW, Paris EC, Farinas CS. Nanoimmobilization of β-glucosidase onto hydroxyapatite. Int J Biol Macromol. 2018;119:1042–1051. doi: 10.1016/j.ijbiomac.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Chen X, Zhang X, Pu S, Zhang X, Yang C, Li D. Comparative studies on ZIF-8 and SiO2 nanoparticles as carrier for immobilized β-glucosidase. Mol Catal. 2018;459:1–7. doi: 10.1016/j.mcat.2018.08.004. [DOI] [Google Scholar]
- 19.Park HJ, Driscoll AJ, Johnson PA. The development and evaluation of β-glucosidase immobilized magnetic nanoparticles as recoverable biocatalysts. Biochem Eng J. 2018;133:66–73. doi: 10.1016/j.bej.2018.01.017. [DOI] [Google Scholar]
- 20.Lopez-Gallego F, Yate L. Selective biomineralization of Co3(PO4)2-sponges triggered by His-tagged proteins: efficient heterogeneous biocatalysts for redox processes. Chem Commun. 2015;51:8753–8756. doi: 10.1039/C5CC00318K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.