Abstract
The production of cheap and effective compound for medicinal application is a major challenge for scientific community. So, several biological materials have been explored for the possible application in material synthesis which are useful in biomedical uses. Here, biomolecules from green tea leaves were functionalized on the surface of silicon dioxide nanoparticles (GSiO2 NPs). Next, the decoration silver (Ag) NPs on the surface of the GSiO2 NPs was observed in very short time of incubation in aqueous AgNO3. Ultraviolet–visible spectroscopy confirmed the formation of Ag NPs and the high-resolution transmission and scanning electron microscopies confirmed the decoration of spherical Ag NPs of 10 to 15 nm size on the surface of GSiO2 NPs. The antimicrobial activity of the Ag–GSiO2 NPs was determined against Staphylococcus aureus and Escherichia coli. The Ag–GSiO2 NPs displayed sustainable antimicrobial activity compared to Ag ions. The results indicate the potential value of Ag–GSiO2 NPs in surgical material and food processing.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00812-2) contains supplementary material, which is available to authorized users.
Keywords: Biosynthesis, Silver nanoparticles, Green tea, Biomolecules, Antimicrobial
The various forms of the nanoparticles have been demonstrated for the different applications as the physical, chemical and biological properties of the nanoparticles varies with their shapes, size and surface properties [1–3]. Among the various nanomaterials, noble metals and noble metal-based nanomaterials have been not only synthesized and studied also extensively applied in various industrial and healthcare fields like medicinal, catalyst, energy, diagnostic, electronics, and other fields [4]. The platinum (Pt), palladium (Pd), silver (Ag) NPs, and gold (Au) NPs based nanomaterials have gained most interest due to their optical, physical and electrical properties over the other metal nanoparticles [5]. Among the various supporting materials, silicon dioxide (SiO2) NPs have the most favored inorganic material, which is inert, thermally stable, carry negative change in neutral pH, and less interference in magnetic and optical fields [6–9]. Synthesis of Ag NPs to decorate the surface of SiO2 NPs has been explored in the application of coating on antimicrobial materials. Several reported methods for formation of Ag–SiO2 NPs have involved the use of hazardous chemicals like sodium borohydride [10], expensive instruments like microwave devices [11] and sonicators [12], and time-consuming microbial methods [13]. Thus, to date, the approaches to synthesize Ag–SiO2 NPs have proven to be expensive and potentially hazardous. With the goal of devising a rapid, safe, and cost-effective method for Ag–GSiO2 NP synthesis, active reducing molecules were used for the functionalization of SiO2 NPs in this study. These molecules were then used for the reduction of Ag+. The biosynthesized silver–silica composite was tested against Staphylococcus aureus and Escherichia coli to demonstrate the its effective antimicrobial activity. The Ag@GSiO2 nanostructure formation was achieved as demonstrated in the schematics using biofunctionalized SiO2 NPs (Fig. S1). Briefly, the SiO2 NPs were synthesized and functionalized with amine groups as previously described [10]. The alkaline ethanolic extract was prepared from the commercial green tea leaves as previously reported [14]. The amine-functionalized SiO2 NPs were dispersed in the extract and incubated for 12 h. The alcoholic extract of green tea was enriched in polyphenols and displayed antioxidant activity [15]. During incubation, these active biomolecules of green tea covalently attached to the amine-functionalized SiO2 NPs to form functionalized SiO2 (GSiO2) NPs. Deligiannakis et al. demonstrated the efficient functionalization of aminopropyl–SiO2 NPs with gallic acid, which retained their antioxidant capacity [16]. The epicatechin and polyphenols present in green tea can reduce Ag+ for the synthesis of Ag NPs [17]. The GSiO2 NPs were dispersed in aqueous AgNO3. After a 120-min reaction, the color change from greyish-white to brown was observed indicating Ag NPs synthesis on GSiO2 NPs surface. The biomolecules present on the surface of the SiO2 NPs reduced Ag+ to Ag NPs and resulted in the formation of Ag–GSiO2 NPs.
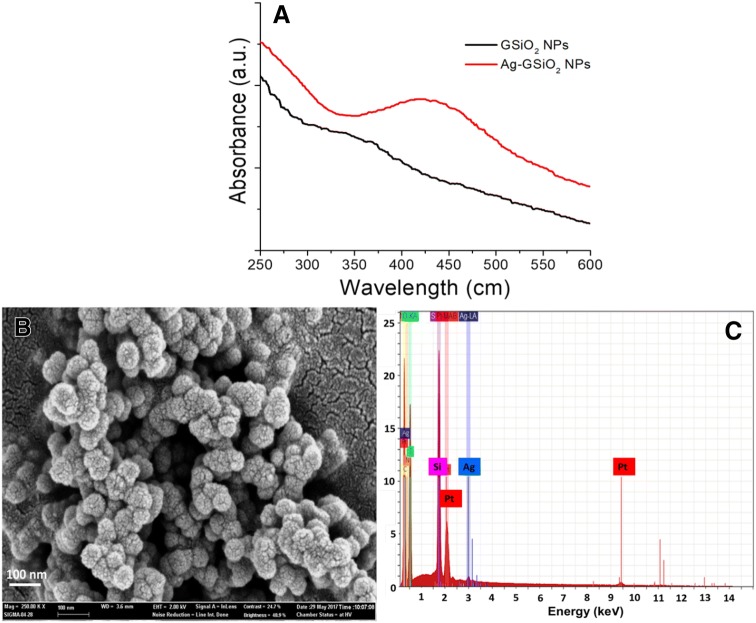
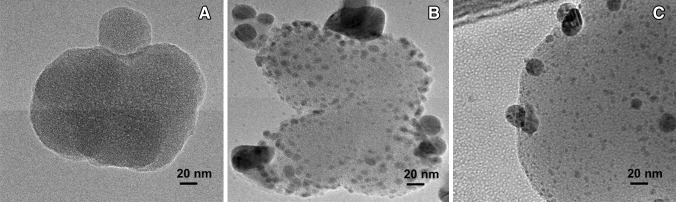
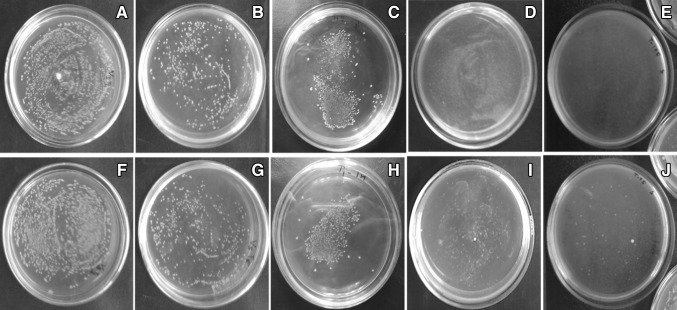
The synthesis of Ag NPs from aqueous AgNO3 using GSiO2 NPs was confirmed by the absorbance in the visible light region with UV–visible spectroscopy. In the UV–visible spectra for GSiO2 and Ag–GSiO2 NPs, GSiO2 NPs did not show any absorbance in 300–700 nm range (Fig. 1a). In contrast, at 425 nm the Ag–GSiO2 NPs was having absorbance of Ag NPs, which confirmed formation Ag NPs on the GSiO2 NPs surface [18]. The XRD analysis of the Ag–GSiO2 NPs demonstrated in Fig. S2 revealing its crystalline nature and phase conformation of the Ag NPs. The face centric cubic (FCC) crystal structure was obtained from the diffractogram with (111), (002), (022), and (113) planes of metallic silver corresponding to peaks at 2θ angles of 37.8°, 43.9°, 63.9°, and 76.8°, respectively (JCPDS No. 96-901-3048) [19]. The Ag–GSiO2 NPs morphology was analyzed by the field emission scanning electron microscopy (FE-SEM). The rough surface of the spherical GSiO2 NPs confirmed that the surface of GSiO2 NPs was covered by Ag NPs (Fig. 1b). The elemental compositional analysis was determined using Energy-dispersive X-ray spectroscopy (EDS) to confirm the presence of elemental Ag at approximately 3 keV (Fig. 1c) [20]. The detailed morphology and size of the NPs were confirmed by high resolution transmission electron microscopy (Hr-TEM) (Fig. 2a). The GSiO2 NPs were spherical in shape and agglomerated due to presence of the biomolecules. After reduction reaction, the Ag NPs became dispersed on the surface of the GSiO2 NPs (Fig. 2b). The spherical Ag NPs varied in size ranging from 5 to 40 nm (Fig. 2c). The antimicrobial activity of the Ag–GSiO2 NPs is due to the Ag NPs present on the surface. The antimicrobial activity of Ag–GSiO2 NPs was confirmed using S. aureus and E. coli. Different concentrations of Ag–GSiO2 (0–30 mg L−1) were added in 100 mL of LB broth containing 106–107 CFU mL−1 of S. aureus or E. coli, and these mixtures were incubated for 24 h at 37 °C. Serial dilutions were prepared, and 0.1 mL aliquots were dispensed in LB broth and incubated for 24 h. The colonies were enumerated and the numbers of viable bacteria calculated. Figure 3 displays representative colonies of S. aureus (Fig. 3a) and E. coli (Fig. 3f) developed without treatment of Ag–GSiO2 NPs as control plates. As the concentration of the Ag–GSiO2 NPs increased up to 30 mg L−1, growth of both bacteria was adversely affected (Fig. 3b–d, g–i). At 30 mg mL−1, Ag–GSiO2 NPs completely inhibited the growth of S. aureus (Fig. 3e) with slight growth of E. coli evident (Fig. 3j). The LB broth medium was used to determine the minimum inhibitory concentration (MIC). The MIC of Ag–GSiO2 NPs was 0.3 mg mL−1 for S. aureus and 0.35 mg mL−1 for E. coli. The results clearly demonstrated the antimicrobial efficiency of Ag–GSiO2 NPs inhibiting gram-negative and gram-positive bacterial growth.
Fig. 1.
Characterization of Ag–GSiO2 nanoparticles a UV–visible spectra of GSiO2 nanoparticles and Ag–GSiO2 nanoparticles showing the characteristic absorbance at 425 nm, b X-ray diffraction pattern of the Ag–GSiO2 nanoparticles, c field emission scanning electron microscopy to study the morphology of the Ag–GSiO2 nanoparticles, d energy-dispersive X-ray spectra of the Ag–GSiO2 nanoparticles
Fig. 2.
Morphology and shape of the nanoparticles analyzed with high resolution transmission electron microscopy a GSiO2 nanoparticles, b, c Ag–GSiO2 nanoparticles
Fig. 3.
Antimicrobial activity of Ag–GSiO2 nanoparticles against Staphylococcus aureus (a–e) and Escherichia coli (f–j) exposed to concentrations of control (a, d), 15 mg L−1 (b, g), 20 mg L−1 (c, h), 25 mg L−1 (d, i) and 30 mg L−1 (e, j)
In summary, we developed a novel green tea leaf-based method which is rapid and easy for the synthesis silver–silica composite material. The active biomolecules present in the green tea extract functionalized SiO2 NPs retained their capacity to reduce Ag+, leading to formation of Ag NPs that decorated GSiO2 NPs. Hr-TEM confirmed the synthesis of Ag NPs on GSiO2 NPs surface. The Ag–GSiO2 NPs displayed pronounced antimicrobial activity against S. aureus and E. coli after a short exposure time of 120 min. The green chemistry method allowed the rapid formation of nanohybrid structures, potentially useful in biomedical applications. However, further studies, including toxicological studies, are necessary to confirm the practical application of these Ag–GSiO2 NPs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by Basic Science Research Program (2013M3A6A8073184, NRF-2018H1D3A2001746) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning. This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2018.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
In-Won Kim, Phone: 82-2-450-3508, Email: inwon@konkuk.ac.kr.
Jung-Kul Lee, Phone: 82-2-450-3505, Email: jkrhee@konkuk.ac.kr.
References
- 1.Otari SV, Patel SKS, Kim S-Y, Haw JR, Kalia VC, Kim IW, Lee JK. Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol. 2019;59:105–108. doi: 10.1007/s12088-018-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SKS, Choi SH, Kang YC, Lee JK. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 3.Patel SKS, Otari SV, Li J, Kim DR, Kim SC, Cho B-K, Kalia VC, Kang YC, Lee JK. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Sau TK, Rogach AL, Jäckel F, Klar TA, Feldmann J. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv Mater. 2010;22:1805–1825. doi: 10.1002/adma.200902557. [DOI] [PubMed] [Google Scholar]
- 5.Patel SK, Kalia VC, Choi JH, Haw JR, Kim IW, Lee JK. Immobilization of Laccase on SiO2 nanocarriers improves the its stability and reusability. J Microbiol Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Singh R, Sivakumar D, Guo Z, Li J, Chen F, He Y, Guan X, Kang YC, Lee JK. An artificial synthetic pathway for acetoin, 2,3-butanediol, and 2-butanol production from ethanol using cell free multi-enzyme catalysis. Green Chem. 2018;20:230–242. doi: 10.1039/C7GC02898A. [DOI] [Google Scholar]
- 7.Kumar A, Park GD, Patel SKS, Kondaveeti S, Otari SV, Anwar MZ, Kalia VC, Singh Y, Kim SC, Cho B-K, Sohn J-H, Kim DR, Kang YC, Lee JK. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. doi: 10.1016/j.cej.2018.11.052. [DOI] [Google Scholar]
- 8.Kim TS, Patel SKS, Selvaraj C, Jung W-S, Pan C-H, Kang YC, Lee JK. A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzounis L, Contreras-Caceres R, Schellkopf L, Jehnichen D, Fischer D, Cai C, Uhlmann P, Stamma M. Controlled growth of Ag nanoparticles decorated onto the surface of SiO2 spheres: a nohybrid system with combined SERS and catalytic properties. RSC Adv. 2014;4:17846–17855. doi: 10.1039/C4RA00121D. [DOI] [Google Scholar]
- 10.Karimipour M, Shabani E, Mollaei M, Molaei M. Microwave synthesis of Ag@SiO2 core–shell using oleylamine. J Nanopart Res. 2015;17:2–9. doi: 10.1007/s11051-014-2832-1. [DOI] [Google Scholar]
- 11.Abbas M, Torati SR, Kim CG. A novel approach for the synthesis of ultrathin silica-coated iron oxide nanocubes decorated with silver nanodots (Fe3O4/SiO2/Ag) and their superior catalytic reduction of 4-nitroaniline. Nanoscale. 2015;7:12192–12204. doi: 10.1039/C5NR02680F. [DOI] [PubMed] [Google Scholar]
- 12.Das SK, Khan MMR, Guha AK, Naskar N. Bio-inspired fabrication of silver nanoparticles on nanostructured silica: characterization and application as a highly efficient hydrogenation catalyst. Green Chem. 2013;15:2548–2557. doi: 10.1039/c3gc40310f. [DOI] [Google Scholar]
- 13.Song J, Kim H, Jang Y, Jang J. Enhanced antibacterial activity of silver/polyrhodanine-composite decorated silica nanoparticles. ACS Appl Mater Interfaces. 2013;5:11563–11568. doi: 10.1021/am402310u. [DOI] [PubMed] [Google Scholar]
- 14.Otari SV, Shinde VV, Gao H, Patel SKS, Kalia VC, Kim IW, Lee JK. Biomolecule-entrapped SiO2 nanoparticles for ultrafast green synthesis of silver nanoparticle-decorated hybrid nanostructures as effective catalyst. Ceram Int. 2019;45:5876–5882. doi: 10.1016/j.ceramint.2018.12.054. [DOI] [Google Scholar]
- 15.Rodrigues MJ, Nevesa N, Martins A, Rauter AP, Neng NR, Nogueira JMF, Varel J, Barreir L, Custódio L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: a comparison with green tea (Camellia sinensis) Food Chem. 2016;200:322–329. doi: 10.1016/j.foodchem.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 16.Deligiannakis Y, Sotiriou GA, Pratsinis SE. antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl Mater Interfaces. 2012;4:6609–6617. doi: 10.1021/am301751s. [DOI] [PubMed] [Google Scholar]
- 17.Moulton MC, Braydich-Stolle LK, Nadagouda MN, Kunzelman S, Hussain SM, Varma RS. Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols. Nanoscale. 2010;2:763–770. doi: 10.1039/c0nr00046a. [DOI] [PubMed] [Google Scholar]
- 18.Otari SV, Patil RM, Nadaf NH, Ghosh SJ, Pawar SH. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater Lett. 2012;72:92–94. doi: 10.1016/j.matlet.2011.12.109. [DOI] [Google Scholar]
- 19.Otari SV, Patil RM, Waghmare SR, Ghosh SJ, Pawar SH. A novel microbial synthesis of catalytically active Ag-alginate biohydrogel and its antimicrobial activity. Dalton Trans. 2013;42:9966–9975. doi: 10.1039/c3dt51093j. [DOI] [PubMed] [Google Scholar]
- 20.Otari SV, Patil RM, Ghosh SJ, Pawar SH. Green phytosynthesis of silver nanoparticles using aqueous extract of Manilkara zapota (L.) seeds and its inhibitory action against Candida species. Mater Lett. 2014;116:367–369. doi: 10.1016/j.matlet.2013.11.066. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.