Abstract
Elevated levels of coagulation factor VIII have been associated with increased risk for venous thromboembolism (VTE). The role of Von Willebrand factor (VWF) in VTE is unclear. In this study, we investigated the association of raised levels of VWF and factor VIII in patients with VTE. 100 patients of VTE and 100 age and gender matched controls were tested for levels of VWF antigen, coagulation factor VIII:C, Protein C, Protein S, antithrombin, antiphospholipid antibodies and for the presence of Factor V Leiden mutation. The mean level of VWF was significantly higher amongst patients of VTE (177.3 IU/dl) when compared to controls (129.7 IU/dl) (P < 0.001). Similarly, the mean level of FVIII:C was significantly higher amongst patients of VTE (199.1 IU/dl) when compared to controls (145.2 IU/dl) (P < 0.001). On logistic regression analysis, after adjusting for FVIII:C, Protein S, factor V Leiden and trauma, the association of raised VWF with VTE remained significant (P = 0.001). Similarly higher levels of FVIII:C persisted to have an independent association with VTE (P = 0.004). Both, higher levels of VWF and FVIII:C levels, are independently associated with VTE.
Keywords: VWF antigen, Venous thromboembolism, Factor VIII:C
Introduction
Venous thromboembolism (VTE) is thought to be caused by an imbalance between thrombogenic stimuli and antithrombotic mechanisms. The complex multifactorial pathogenesis of VTE includes genetic and acquired risk factors. Surgery, hospitalization, prolonged immobilization, trauma, pregnancy, cancer, obesity, antiphospholipid antibody syndrome, hyperhomocysteinemia and the use of female sex hormones are some of the known acquired risk factors. The genetic risk factors include the factor V Leiden (FVL), Prothrombin gene G20210A mutation, Antithrombin (AT), Protein C (PC), Protein S (PS) deficiency and dysfibrinogenemia [1]. These known genetic and acquired factors have been associated with VTE in about 50–60% cases. However, there remains a sizeable number of patients with VTE in whom these risk factors are not present [2, 3] and the etiology of VTE in these patients remains unknown.
Raised levels of plasma coagulation factors have been shown to be associated with VTE. Increased levels of factor VIII have been associated with VTE [2, 4–12]. Von Willebrand factor (VWF) is a plasma glycoprotein that acts as carrier for factor VIII. Elevated levels of VWF are associated with elevated coagulation factor VIII levels. An increased VWF level in plasma is found to be associated with VTE [4, 5, 11, 13]. The role of VWF levels in VTE is currently unclear as some studies show an independent association of VWF levels with VTE [4, 5, 11], while others report that the effect of VWF levels in VTE is dependent on factor VIII levels [7, 9].
ABO blood groups may contribute to the risk of VTE. Individuals with non-O blood group are more susceptible to VTE [5, 14–16]. One of the reasons for this association maybe that the non-O blood group individuals have higher factor VIII and VWF levels in plasma [14, 17–19]. To the best of our knowledge, there is currently no data on VWF antigen (VWF Ag) levels in Indian patients with VTE and this study was undertaken to ascertain the relationship between high VWF Ag and FVIII:C levels in the North Indian patients with VTE.
Patients and Methods
Study Design
Consecutive patients aged between 16 and 60 years, referred to the coagulation laboratory for work up the thrombophilia risk factors from June 2013 to December 2016 with deep vein thrombosis (DVT) and/or pulmonary thromboembolism (PE) confirmed objectively by imaging studies i.e. Doppler ultrasound or computerized tomographic angiography, were enrolled in the study. Age and sex matched healthy volunteers were included as controls in the study. All tests were performed at-least 4 weeks after cessation of anticoagulant therapy. Patients presenting in the acute phase of VTE, i.e., within 6 weeks of symptoms, pregnant women and patients with liver disease were excluded from the study. The minimum interval between onset of symptoms and sampling was 6 weeks. History regarding the risk factors including surgery, trauma, smoking, prolonged immobilization, pregnancy or post-partum state, oral contraceptive pills (OCPs) and hormone replacement therapy (HRT) was obtained at the time of sampling. Informed consent was obtained from the patients and controls. Approval for the study was obtained from the ethics committee of the institute (Reference number NK/1126/MD/11252).
Laboratory Parameters
Blood was collected from the antecubital vein using sterile precautions and was transferred into 3.2% citrate, EDTA and a plain vacuum tube. The citrated sample was centrifuged at 2000g for 20 min and the supernatant plasma was processed for screening coagulation tests {Prothrombin time, activated partial thromboplastin time (APTT) and fibrinogen} in STA Compact coagulation analyser (Diagnostica Stago, Asnieres, France) on the same day and the remaining plasma was aliquoted and stored at − 20 °C. Fibrinogen assay was performed using the Clauss method. One of the aliquots was spun an additional time and the supernatant plasma stored separately for lupus anticoagulant (LA) testing. Serum was separated and stored for anti Cardiolipin Antibody (ACA) and anti beta 2 Glycoprotein 1 (aβ2GP1) antibody at − 20 °C. Tests for PC, PS, AT, LA, VWF Ag, FVIII:C, ACA and aβ2GP1 were performed in batches within 1 month of storage for both patients and control samples.
Deficiencies of natural coagulation inhibitors like PC, PS and AT were tested in the STA Compact Coagulation analyzer (Diagnostica Stago, Asnieres, France) using the kits from the manufacturer: STA Staclot PC, STA Staclot PS and STA Stachrom ATIII. The clot based kits were used for estimation of functional activity of PC and PS while that for AT was chromogenic. The presence of LA was detected by two methods which included an “in house” Kaolin clotting test (KCT) and commercial dilute Russel Viper Venom Test (dRVVT) kits in accordance with the recommended guidelines. The assays for antibodies for ACA and aβ2GP1 were carried out using commercial ELISA kits (Orgentec, GMBh). FVL was tested by Polymerase chain reaction- restriction fragment length polymorphism using Mnl1 restriction endonuclease enzyme. VWF Ag levels were estimated using immunoturbidimetric method in the STA Compact Coagulation analyzer (Diagnostica Stago, Asnieres, France) using the latex immune agglutination test kit. Calibration curves were obtained from the instrument using VWF calibrator with traceability against a secondary reference standard of the International Reference Standard 97/586. Factor VIII:C activity was measured by one stage clot based assay on the same coagulation analyzer. PC, PS, AT, VWF Ag, FVIII:C, ACA and aβ2GP1 testing in the laboratory is assessed by participation in the WHO EQAS programmes for Coagulation and Immunology and Immunochemistry from the UK at regular intervals. The cut-off levels to define deficiencies of PC was 63%, PS was 67% in males and 55% in females and AT was 80%. Low values for PC, PS and AT were repeat tested after 4–6 weeks in patients who were not on any anticoagulants, and results were confirmed. The cut-off levels for ACA IgG and IgM were < 10 GPLU/ml and < 7 MPLU/ml respectively and for aβ2GP1 IgG and IgM were < 5 IU/ml. Positivity for antiphospholipid antibodies were repeated once after 12 weeks of first sampling and positivity reported only if repeat sample was also positive.
Statistical Analysis
The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 18.0 for Windows). Normality of data was checked by measures of Kolmogorov–Smirnov tests of normality. Mean for measures of dispersion for VWF Ag and factor VIII:C were calculated.
For the purpose of statistical analysis, PC, PS, AT were categorized into deficient and normal levels. LA, ACA, aβ2GP1 were categorized as positive and negative. FVL was categorized into normal and heterozygous. Blood group was categorized into O and non-O groups. In the absence of available cut off levels for coagulation factors in literature, the 90th percentile of control subjects was used. Using 90th percentile of control, VWF Ag and FVIII:C levels were categorized into elevated and not elevated. Analysis of categorical data was done using Chi square test. A P value < 0.05 was taken as significant. Predictors for thromboembolism were identified on univariate analysis and the significant ones were subjected to multivariate logistic regression analysis. Stratification of FVIII:C and VWF Ag levels was done and the odds ratio was calculated taking an arbitrary baseline value.
Results
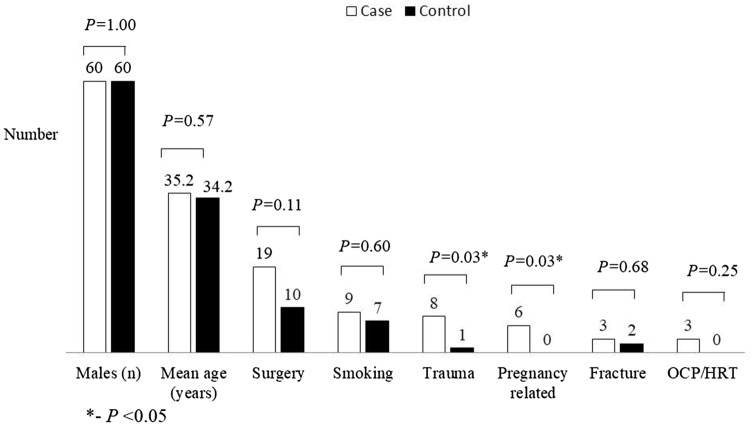
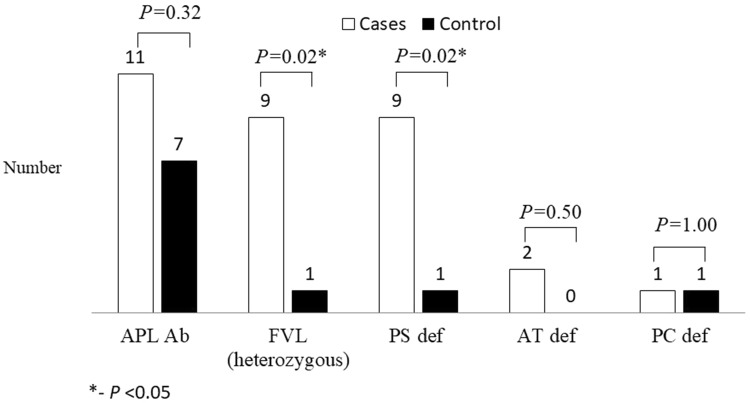
A total of consecutive 152 patients with VTE were screened for entry into the study, of which 100 met the inclusion criteria. Of these data pertaining to PC, PS, AT, FVL and FVIII:C for 25 cases has been reported previously [2]. One hundred age and sex matched healthy volunteers were sampled. The comparison of the demographic data and risk factors between cases and controls are depicted in Figs. 1 and 2. The mean age of cases of VTE was 35.2 (range 16–60) years and 60% (n = 60) were males. The study cohort included 93 (93%) patients with isolated DVT, 1 (1%) patient with isolated PE and 6 (6%) patients with combined DVT and PE. Fifty five (55%) patients were found to have any of the underlying predisposing risk factors listed were labelled “provoked” while the remaining 45 (45%) of the patients where no factor was positive were labelled “unprovoked”. Surgery and post-surgery immobilisation were the most common predisposing factor seen in 19 (19%) patients. Twenty one (21%) patients had inherited thrombophilic risk factors amongst which PS deficiency (9, 9%) and FVL (6, 6%) were the commonest. History of recurrent fetal loss was seen in 9 (22.5% of female patients). APL antibodies were seen in 11 (11%) patients and 7 (7%) controls.
Fig. 1.
Circumstantial risk factors in controls and patients of venous thromboembolism. OCP/HRT oral contraceptive pills/hormone replacement therapy
Fig. 2.
Thrombophilic risk factors in controls and patients of venous thromboembolism. APL Ab anti phospholipid antibody, FVL factor V Leiden, PS def Protein S deficiency, AT def antithrombin deficiency and PC def Protein C deficiency
VWF Antigen and Factor VIII:C Levels in VTE
The mean levels of VWF Ag in patients with VTE was 177.3 IU/dl (range 73–703 IU/dl) compared to 129.7 IU/dl (range 34–214 IU/dl) in controls. The mean level of factor VIII:C in patients with VTE was 199.1 IU/dl (range 37.5–437 IU/dl) and was 145.2 IU/dl (range 44–284 IU/dl) in controls. In univariate analysis, this difference in the mean levels between the cases and control groups for both VWF Ag (P < 0.001) and factor VIII:C (P < 0.001) were statistically significant. For both VWF Ag and factor VIII:C, 90th percentile was calculated by using data of controls. These 90th percentile for both VWF Ag and factor VIII:C were used to define the cut off levels. 90th percentile for VWF Ag and factor VIII:C were 176.3 IU/dl and 206 IU/dl respectively. 39 patients of VTE and 10 controls had VWF Ag levels above 90th percentile (P < 0.001), while 41 patients with VTE and 10 controls had levels of factor VIII:C above 90th percentile (P < 0.001). Both VWF Ag and factor VIII:C were significantly elevated in cases as compared to controls. In cases who had VWF Ag and/or FVIII:C levels above the cut-off, consent for repeat sampling was requested. For such cases where repeat sampling was done, the lower value among the two values was taken as final value. Among the 13 patients who had given consent for repeat sampling for VWF Ag, 10 (76.9%) had persistent rise in VWF Ag levels while in the remaining 3 (23.1%) the levels were below 90th percentile on repeat testing. Similarly, 19 patients gave consent for a repeat factor VIII:C testing. Thirteen (68.4%) patients had persistently elevated levels of factor VIII:C while in the remaining 6 (31.6%) the levels were below 90th percentile on repeat testing. To see the trend of increasing VWF Ag and factor VIII with VTE, sub-categorization into five strata was done and the stratum with levels of less than 100% was considered baseline. For both VWF Ag and factor VIII:C a dose response relationship with risks of VTE was established as shown in Table 1. VWF Ag levels are known to rise during acute phase states, hence samples were taken at-least 6 weeks after an acute episode. To ensure elevation of VWF Ag was not due acute phase reaction, correlation of VWF Ag and fibrinogen was checked and was not found to be significant (P = 0.17).
Table 1.
Association of VWF Ag and FVIII:C levels with venous thromboembolism before and after logistic regression analysis
| VWF Ag levels (IU/dl) | Patients N |
Controls n |
OR (95% CI) | Adjusted OR (95% CI) | FVIII:C levels (IU/dl) | Patients n |
Controls n |
OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| ≤ 100 | 5 | 21 | 1a | 1a | ≤ 100 | 7 | 17 | 1a | 1a |
| 101–150 | 30 | 51 | 2.52 (0.86–7.39) | 3.07 (0.84–11.26) | 101–150 | 17 | 45 | 0.92 (0.32–2.60) | 0.83 (0.24–2.84) |
| 151–200 | 45 | 24 | 7.88 (2.63–23.51) | 12.17 (3.28–45.19) | 151–200 | 31 | 26 | 2.90 (1.04–8.05) | 2.56 (0.75–8.71) |
| 201–250 | 9 | 4 | 9.45 (2.05–43.61) | 13.43 (2.49–72.36) | 201–250 | 24 | 11 | 5.30 (1.71–16.46) | 4.65 (1.27–17.10) |
| ≥ 251 | 10 | Nil | Not calculated | Not calculated | ≥ 251 | 21 | 1 | 51.00 (5.70–456.01) | 31.29 (3.09–316.44) |
OR odds ratio, CI confidence interval
aBaseline value
Multivariate logistic regression analysis was performed on parameters that were significant in univariate analysis including trauma, PS deficiency, FVL, VWF Ag (for FVIII:C) and FVIII:C (for VWF Ag). Levels of both VWF Ag and FVIII:C were found to have an independent, dose response relationship with presence of VTE. The odds ratio in different strata of VWF Ag and FVIII:C remained unchanged after multivariate analysis as shown in Table 1. FVIII:C and/or VWF Ag elevation were seen in 22% (n-22) patients who did not have any other risk factors. Among the 22 patients 5% cases (n = 5) had isolated elevation of factor VIII:C and VWF Ag each while 12% cases (n = 12) had combined elevation of VWF Ag and factor VIII:C levels.
Blood Groups and Levels of VWF Ag and FVIII
The mean levels of VWF Ag among blood group non-O (156.9 IU/dl) was higher compared to that in blood group O (141.3 IU/dl), but the difference was not statistically significant (P = 0.15). The mean levels of FVIII:C among blood group non-O (178.6 IU/dl) was significantly higher when compared to blood group O (148.5 IU/dl) (P = 0.01).
Discussion
VTE is a multifactorial disease caused by interaction between various environmental and genetic risk factors. Inspite of having many known risk factors for the disease, 40–50% of cases have no identifiable risk factor [2, 3]. Our patients seem to be comparable with the universally known data since 45% cases were “unprovoked” and without any risk factors.
In the literature, there are few studies that show independent association of VWF with VTE [5, 11], irrespective of factor VIII levels. However few others show association of VWF with VTE in univariate analysis only while this association disappears when other known variables are considered [7, 9]. Few other studies have shown independent association of VWF with VTE, however, have not investigated FVIII levels [4, 20]. Further, VWF Ag levels may be raised in as an acute phase reactant. In order to ensure that the rise in VWF was not merely due to an acute phase reaction, a minimum interval of 6 weeks after acute event of VTE or any other acute illness was ensured before sampling. Further, fibrinogen was measured in the cases and controls. Since there was no statistically significant correlation with fibrinogen in the current study, it is unlikely that the raised levels of the coagulation factors and VWF were the result of an acute phase response. Similar means have been used to exclude the effect of acute phase reaction effect on the levels of VWF and FVIII in other studies [5, 9]. In the present study, we have found an independent and dose dependent relationship between both VWF Ag and FVIII in patients with VTE in the Indian subcontinent.
The pathogenesis of elevated VWF leading to increased odds of VTE is not clearly understood. Each VWF monomer has two binding domains (D’ and D3 domains) that bind non-covalently to factor VIII and protect it from proteolysis by the activated PC system [21]. This helps in prolonging the half-life of factor VIII which is then efficiently delivered at the site of vascular injury to participate in coagulation. In addition, VWF also co-localizes with factor VIII and platelets in venous thrombosis [21]. Plasma levels of VWF have shown variations with various genetic polymorphisms in cohorts and controls of cardiovascular disease. Few of these polymorphisms in promotor as well as coding region have shown an increase in VWF levels [22]. No direct correlation has been seen between VWF polymorphism and VTE [12]. The biological mechanism of how raised VWF can have an independent association with VTE is not clear. However, due to this correlation and potential causative mechanism, VWF is being explored as a target for anti-thrombotic therapy [22].
We noted a persistent effect of raised FVIII:C levels on VTE even after adjusting for VWF, PS, FVL and trauma in multivariate analysis. An independent association of FVIII with VTE has been found in many studies [2, 5, 6, 8–10]. FVIII belongs to intrinsic pathway of coagulation, which on activation by thrombin, dissociates from VWF and forms a complex with activated factor IX. This activates factor X which converts prothrombin to thrombin which furthers converts fibrinogen to fibrin. Thus increased levels of FVIII could possibly increase the rate of fibrin and thrombin formation [23, 24]. This mechanism is substantiated by marked elevation of thrombin generation seen in patients with elevated FVIII and VTE as well as a positive correlation between elevated FVIII levels and markers of thrombin generation (including prothrombin fragments 1 + 2 and thrombin-antithrombin complex) [9]. Another possible mechanism is that elevated FVIII interferes with natural anticoagulation mechanism, i.e., it induces a degree of “acquired” activated PC resistance [25]. Amplification of FVIII gene copy number is associated with both elevated FVIII levels and VTE [26]. Studies on correlations of polymorphisms in FVIII gene with FVIII levels or VTE. have failed to demonstrate any such association [12, 27].
The mean levels of both VWF and factor VIII were higher in the general population of North India as compared to other populations studied (Table 2) [5, 7, 11]. Higher levels of FVIII:C in both controls and VTE patients has also been reported from south India [6]. The higher levels of VWF Ag and FVIII:C in both cases as well as healthy volunteers may be suggestive of a true biological variation of VWF and FVIII in the North Indian population. A higher baseline FVIII:C and VWF Ag may increase the odds for VTE across the population. Caution therefore needs to be exercised in extrapolating the cut off levels for FVIII:C used in the western literature to the Indian population.
Table 2.
Comparison of VWF and FVIII:C levels in present study and studies in literature in patients of venous thrombosis
| Mean VWF Ag levels (IU/dl) | Mean FVIII levels (IU/dl) | |||
|---|---|---|---|---|
| VTE patients | Controls | VTE patients | Controls | |
| Present study (n = 100) | 177.3 ± 75.3 | 129.7 ± 35.9 | 199.1 ± 75.2 | 145.2 ± 44.4 |
| LITE (ARIC cohort, n = 94) [11] | 156 ± 63 | 117 ± 47 | 151 ± 56 | 129 ± 39 |
| LITE (CHS cohort, n = 65) [11] | 165 ± 62 | 149 ± 56 | ||
| Koster et al. (n = 301) [7] | 133 | 119.6 | 127 | 111 |
| Flinterman et al. (n = 107) [5] | 138 ± 68 | 113 ± 49 | 131 ± 44 | 109 ± 37 |
The major limitation of the present study is the relatively small sample size. The test employed for VWF measurement was antigenic and not truly a measure of functional activity. In the deficiency state i.e. Von Willebrand disease, there may be significant differences between VWF Ag and Ristocetin cofactor activity levels. The same is not known in normal individuals or patients with VTE. Though the likelihood of acute phase reactivity leading to elevated VWF levels is remote as was demonstrated by the lack of correlation between VWF and fibrinogen levels, we were able to confirm persistently elevated VWF and VIIIC levels in only a fraction of our cases. The relationship of VWF Ag levels to blood group would have added a dimension to this analysis but the data was considered inappropriate for use due to inadvertent group matching done in cases vs. controls in this study.
Conclusions
Both VWF and factor VIII levels have an independent, dose dependent relationship with occurrence of VTE. This suggests that genetic or acquired causes of elevated VWF levels add to the thrombotic risk. Factor VIII and VWF antigen elevations were seen in 22% cases who did not have any other known risk factors. There is still an unmet need in the identification of other risk factors of VTE as no cause could be identified in 23% cases.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agarwala S, Bhagwat AS, Modhe J. Deep vein thrombosis in Indian patients undergoing major lower limb surgery. Indian J Surg. 2003;65:159–162. [Google Scholar]
- 2.Chougule A, Rajpal S, Ahluwalia J, et al. Coagulation factor VIII, IX and XI levels in north Indian patients with venous thromboembolism: first study from India. Blood Coagul Fibrinolysis. 2016;27:58–63. doi: 10.1097/MBC.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 3.Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167–1173. doi: 10.1016/S0140-6736(98)10266-0. [DOI] [PubMed] [Google Scholar]
- 4.Bucek RA, Reiter M, Quehenberger P, et al. Thrombus precursor protein, endogenous thrombin potential, von-Willebrand factor and activated factor VII in suspected deep vein thrombosis: is there a place for new parameters? Br J Haematol. 2003;120:123–128. doi: 10.1046/j.1365-2141.2003.03984.x. [DOI] [PubMed] [Google Scholar]
- 5.Flinterman LE, van Hylckama Vlieg A, Rosendaal FR, Doggen CJM. Venous thrombosis of the upper extremity: effect of blood group and coagulation factor levels on risk. Br J Haematol. 2010;149:118–123. doi: 10.1111/j.1365-2141.2009.08074.x. [DOI] [PubMed] [Google Scholar]
- 6.Hazra D, Sen I, Stephen E, et al. Evaluation of factor VIII as a risk factor in Indian patients with DVT. Surg Res Pract. 2015;2015:307879. doi: 10.1155/2015/307879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koster T, Blann AD, Briët E, et al. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–155. doi: 10.1016/S0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 8.Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost. 2000;83:5–9. doi: 10.1055/s-0037-1613747. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell J, Tuddenham EG, Manning R, et al. High prevalence of elevated factor VIII levels in patients referred for thrombophilia screening: role of increased synthesis and relationship to the acute phase reaction. Thromb Haemost. 1997;77:825–828. doi: 10.1055/s-0038-1656061. [DOI] [PubMed] [Google Scholar]
- 10.Ota S, Yamada N, Ogihara Y, et al. High plasma level of factor VIII: an important risk factor for venous thromboembolism. Circ J. 2011;75:1472–1475. doi: 10.1253/circj.CJ-10-1051. [DOI] [PubMed] [Google Scholar]
- 11.Tsai AW, Cushman M, Rosamond WD, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) Am J Med. 2002;113:636–642. doi: 10.1016/S0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 12.Kamphuisen PW, Eikenboom JC, Rosendaal FR, et al. High factor VIII antigen levels increase the risk of venous thrombosis but are not associated with polymorphisms in the von Willebrand factor and factor VIII gene. Br J Haematol. 2001;115:156–158. doi: 10.1046/j.1365-2141.2001.03089.x. [DOI] [PubMed] [Google Scholar]
- 13.Karakaya B, Tombak A, Serin MS, Tiftik N. Change in plasma a disintegrin and metalloprotease with thrombospondin type-1 repeats-13 and von Willebrand factor levels in venous thromboembolic patients. Hematology. 2016;21:295–299. doi: 10.1080/10245332.2015.1125079. [DOI] [PubMed] [Google Scholar]
- 14.Morelli VM, De Visser MCH, Vos HL, et al. ABO blood group genotypes and the risk of venous thrombosis: effect of factor V Leiden. J Thromb Haemost. 2005;3:183–185. doi: 10.1111/j.1538-7836.2004.01071.x. [DOI] [PubMed] [Google Scholar]
- 15.Schleef M, Strobel E, Dick A, et al. Relationship between ABO and Secretor genotype with plasma levels of factor VIII and von Willebrand factor in thrombosis patients and control individuals. Br J Haematol. 2005;128:100–107. doi: 10.1111/j.1365-2141.2004.05249.x. [DOI] [PubMed] [Google Scholar]
- 16.Muellner SK, Haut ER, Streiff MB, et al. ABO blood group as a potential risk factor for venous thromboembolism in acutely injured patients. Thromb Haemost. 2010;105:5–13. doi: 10.1160/TH10-08-0504. [DOI] [PubMed] [Google Scholar]
- 17.Gallinaro L, Cattini MG, Sztukowska M, et al. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111:3540–3545. doi: 10.1182/blood-2007-11-122945. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler Thromb Vasc Biol. 2002;22:335–341. doi: 10.1161/hq0202.103997. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell J, Laffan MA. The relationship between ABO histo-blood group, factor VIII and von Willebrand factor. Transfus Med. 2001;11:343–351. doi: 10.1046/j.1365-3148.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson T, Mellbring G, Hedner U. Relationship between factor XII, von Willebrand factor and postoperative deep vein thrombosis. Acta Chir Scand. 1986;152:347–349. [PubMed] [Google Scholar]
- 21.van Schie MC, van Loon JE, de Maat MPM, Leebeek FWG. Genetic determinants of von Willebrand factor levels and activity in relation to the risk of cardiovascular disease: a review. J Thromb Haemost. 2011;9:899–908. doi: 10.1111/j.1538-7836.2011.04243.x. [DOI] [PubMed] [Google Scholar]
- 22.Calabrò P, Gragnano F, Golia E, Grove EL. von Willebrand factor and venous thromboembolism: pathogenic link and therapeutic implications. Semin Thromb Hemost. 2018;44:249–260. doi: 10.1055/s-0037-1605564. [DOI] [PubMed] [Google Scholar]
- 23.Machlus KR, Colby EA, Wu JR, et al. Effects of tissue factor, thrombomodulin and elevated clotting factor levels on thrombin generation in the calibrated automated thrombogram. Thromb Haemost. 2009;102:936–944. doi: 10.1160/TH09-03-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryland JK, Lawrie AS, Mackie IJ, Machin SJ. Persistent high factor VIII activity leading to increased thrombin generation—a prospective cohort study. Thromb Res. 2012;129:447–452. doi: 10.1016/j.thromres.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 25.De Mitrio V, Marino R, Scaraggi FA, et al. Influence of factor VIII/von Willebrand complex on the activated protein C-resistance phenotype and on the risk for venous thromboembolism in heterozygous carriers of the factor V Leiden mutation. Blood Coagul Fibrinolysis. 1999;10:409–416. [PubMed] [Google Scholar]
- 26.Shen W, Gu Y, Zhu R, et al. Copy number variations of the F8 gene are associated with venous thromboembolism. Blood Cells Mol Dis. 2013;50:259–262. doi: 10.1016/j.bcmd.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Mansvelt EP, Laffan M, McVey JH, Tuddenham EG. Analysis of the F8 gene in individuals with high plasma factor VIII:C levels and associated venous thrombosis. Thromb Haemost. 1998;80:561–565. [PubMed] [Google Scholar]