Abstract
The Near East cattle are adapted to different agro-ecological zones including desert areas, mountains habitats, and humid regions along the Tigris and Euphrates rivers system. The region was one of the earliest and most significant areas of cattle husbandry. Currently, four main breeds of Iraqi cattle are recognized. Among these, the Jenoubi is found in the southern more humid part of Iraq, while the Rustaqi is found in the middle and drier region of the country. Despite their importance, Iraqi cattle have up to now been poorly characterized at the genome level. Here, we report at a genome-wide level the diversity and signature of positive selection in these two breeds. Thirty-five unrelated Jenoubi cattle, sampled in the Maysan and Basra regions, and 60 Rustaqi cattle, from around Baghdad and Babylon, were genotyped using the Illumina Bovine HD BeadChip (700K). Genetic population structure and diversity level were studied using principal component analysis (PCA), expected heterozygosity (He), observed heterozygosity (Ho), and admixture. Signatures of selection were studied using extended haplotype homozygosity (EHH) (iHS and Rsb) and inter-population Wright’s Fst. The results of PCA and admixture analysis, including European taurine, Asian indicine, African indicine, and taurine indicate that the two breeds are crossbreed zebu × taurine, with more zebu background in Jenoubi cattle compared with Rustaqi. The Rustaqi has the greatest mean heterozygosity (He = 0.37) among all breeds. iHS and Rsb signatures of selection analyses identify 68 candidate genes under positive selection in the two Iraqi breeds, while Fst analysis identifies 220 candidate genes including genes related to the innate and acquired immunity responses, different environmental selection pressures (e.g., tick resistance and heat stress), and genes of commercial interest (e.g., marbling score).
Keywords: Bos taurus, Bos indicus, genetic structure, diversity, positive selection, immune responses, adaptive genes
Introduction
Archeological and genetic studies support two centers of cattle domestication, the Fertile Crescent and the Northern part of the Indian subcontinent including the Indus Valley (Loftus et al., 1994; Bradley et al., 1998; Troy et al., 2001; Helmer et al., 2005; Bradley and Magee, 2006; Zeder, 2008; Chen et al., 2010; Magee et al., 2014). The earliest archeological evidence of humpless cattle was found in the Fertile Crescent, dated to around 10,000 bc. The first evidence of domestic humped cattle is from the Indus Valley region around 8,000 bc (Felius et al., 2015). From these two heartlands of domestication, two main cattle types, Bos taurus (humpless taurine) and Bos indicus (humped zebu), dispersed across the world, with taurine cattle reaching Africa, Europe, and East Asia and indicine cattle migrating to Africa, South Asia, and South-East Asia (Hanotte et al., 2002; Diamond and Bellwood, 2003; Freeman et al., 2006a; Gifford-Gonzalez and Hanotte, 2011; Magee et al., 2014). The domestication process of animals was essentially a form of symbiosis with humans enabling the dissemination of domesticated cattle throughout the world (Diamond and Bellwood, 2003).
Cattle husbandry was part of the ancient civilizations of Mesopotamia, modern-day Iraq, at an early time with the earliest available evidence of domestic cattle in this region dating to around 6000 bc. There are many archeological evidence of the antiquity and importance of cattle husbandry in central Mesopotamia, including cylinder seals (Read, 2015). These animals were of the humpless taurine type. Further south, closer to the Indus Valley center of cattle domestication, archeological evidence of domestic cattle in Mesopotamia is much fewer. It includes artistic depictions from the royal tombs of Ur (South of Iraq) including domestic animals (Supplementary Figure S1A, B), with most of the ancient agricultural settlements along the Tigris and Euphrates now buried under flooded plains (Read, 2015).
Despite the high cattle number worldwide, it is estimated that 17% of cattle breeds are facing extinction, following changing environmental and production conditions (Rischkowsky and Pilling, 2007; Taberlet et al., 2011; FAO, 2015; Felius et al., 2015). Endangered breeds of cattle are mostly found in developing countries (Joost et al., 2015). For instance, 32% of the recognized indigenous African breeds are at risk of extinction, and another 22% are already considered extinct (Mwai et al., 2015).
Several studies have explored the genome diversity and adaptation of cattle breeds using either high-density SNP chips (Bovine high density SNPs BeadChip (777,962 SNPs) (e.g., Xu et al., 2014; Bahbahani et al., 2017) or full genome sequence analysis (e.g., Choi et al., 2015; Kim et al., 2017). However, the cattle genomes of the Fertile Crescent (including Iraq) have not yet been characterized. The available studies are a microsatellite study (Ates¸ et al., 2014) and mitochondrial DNA analysis for a few breeds (Freeman et al., 2006a, Freeman et al., 2006b; Edwards et al., 2007; Ateş et al., 2014). These studies show evidence of zebu introgression within the Near East taurine, in particular within the Iraqi and Anatolian breeds.
Today, four cattle breeds are officially recognized in Iraq (Al-Murrani et al., 2003) (Supplementary Table S1). Karradi and Sharabi in the northern part of the country, Rustaqi in the central part, and further south, Jenoubi. Phenotypically, Rustaqi may be classified as taurine and Jenoubi as zebu. Living in different agro-ecologies, they will be expected to be adapted to different environmental challenges, including external–internal parasites or infectious diseases, heat, and humidity. Alongside, some commercial breeds have been introduced over the years, including Jersey, Hereford, Ayrshire, and Holstein-Friesian cattle (Al-Murrani et al., 2003) with crossbreeding between Sharabi and Friesian cattle documented (Dabdoub, 2005; Maaroof, 2011; Nasser et al., 2013; Nassar et al., 2014). Until now, none of these Iraqi native breeds have been documented at a genome-wide level, despite the uniqueness of the country in the region of taurine cattle domestication, and its historic importance as major center of civilization in the past. We report for the first time at the autosomal genome-wide level, the genome diversity and candidate signatures of positive selection (e.g., tick resistance genes) in two Iraqi cattle breeds (Jenoubi and Rustaqi), providing new insights on the past and present breeding dynamics and evolutionary forces that shaped the genome of the cattle population in the region.
Materials and Methods
Population Samples
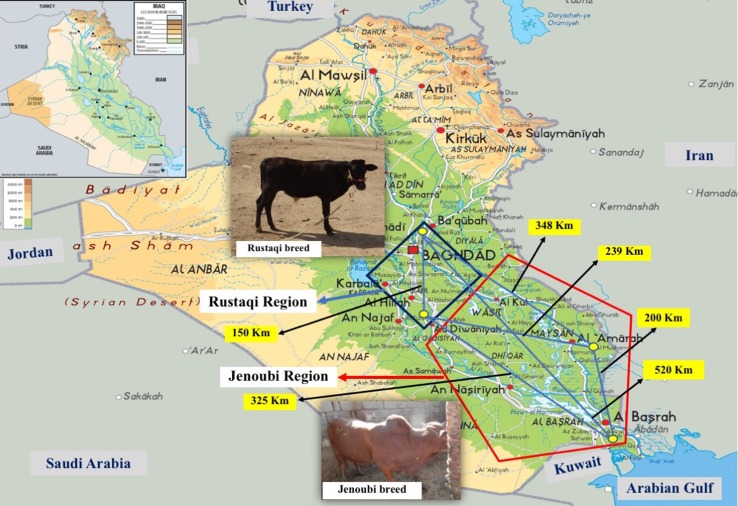
We collected blood samples spotted on FTA paper (Whatman Technology®) from two indigenous Iraqi breeds, Rustaqi and Jenoubi ( Figure 1 ). In particular, for the Rustaqi breed, 60 blood samples were collected: 20 samples from the Baghdad region, in central Iraq, and 40 samples from the Babylon area (80 km south of Baghdad). For the Jenoubi breed, 35 blood samples were collected from the southern regions of Iraq, including an area close to Basra (n = 9) (560 km South of Baghdad) and in Maysan (n = 26) (400 km South of Baghdad). The middle region of Iraq (Baghdad and Babylon) is characterized by a hot and arid climate, while the climate in the southern part of Iraq (Basra and Maysan regions) close to the marshes is hot and more humid (https://www.accuweather.com/en/iq/national/satellite). Samples were shipped to a private company (Deoxi Biotecnologia, http://www.deoxi.com.br/) for genotyping using the Illumina Bovine HD Genotyping BeadChip (700K) (http://www.illumina.com). The geographic location and global positioning system (GPS) coordinates of the Iraqi cattle samples can be found at Supplementary Table S2A, B. Aerial distances in kilometers between sampling sites were calculated using the geographic information system (GIS) ArcGIS® software Esri (www.esri.com).
Figure 1.
Geographic distribution and sampling location of Rustaqi and Jenoubi breeds. Blue square: Rustaqi breed distribution. Red pentagon: Jenoubi breed distribution. Yellow dots: sampling locations. Geographical distances between sampling locations are indicated in black digits. Physical map obtained from http://www.mapsland.com/asia/iraq.
SNP Genotyping
Ninety-five samples of Iraqi breeds (Rustaqi, n = 60; Jenoubi, n = 35) were genotyped with the Illumina Bovine HD Genotyping BeadChip (http://www.illumina.com) including 777,962 SNPs mapped to the UMD 3.1; please see section of Data Accessibility for more details (EVA: PRJEB32975; Datadryad.org:dryad.t35r32q). High-density SNP data for references cattle breeds, Holstein-Friesian (European taurine, n = 30), Jersey (European taurine, n = 32), Nellore (Asian zebu, n = 35), Gir (Asian zebu, n = 30), Sahiwal (Asian zebu, n = 13), EASZ (East African Short horn zebu, n = 30), Sheko (East African zebu × taurine cross, n = 18), Ankole (East African, n = 25), Adamawa Gudali (West African zebu, n = 25), Muturu (African taurine, n = 12), Red Bororo (West African zebu, n = 22), and N’Dama Guinea (African taurine, n = 24) were obtained from Bahbahani et al. (2017).
Data Analysis
Autosomal SNP datasets were prepared using R (https://www.r-project.org) and PLINK 1.9 (Purcell et al., 2007) software. SNPs with minor allele frequency (MAF) of less than 5% (–maf 0.05) and missing genotype data higher than 0.05% (–geno 0.05) were excluded from the analysis. It removed 63,451 variants and 34,387 markers, respectively, leaving 680,124 SNPs for analysis. Also, one sample from Rustaqi, EASZ, and Red Bororo breeds were removed due to their low call rate (<95%).
Estimation of the Level of Genetic Diversity
To assess the level of genetic diversity, the mean of expected heterozygosity (He) and observed heterozygosity (Ho) were computed using PLINK 1.9 (Purcell et al., 2007). The genetic diversity was estimated for each population of Iraqi indigenous cattle and several breeds of references. These breeds are Iranian breeds (Sarabi, Kurdi, Taleshi, Pars, Sistani, and Najdi), East Asian native cattle [from Vietnam, Myanmar, Bangladesh, Bhutan, Korea (Hanwoo), Japan (Polled), and Mongolia (MON)], and African breeds [N’Dama Guinea and Sheko (Ethiopian breed)]. Additionally, two important main breeds that represent the main lineage of cattle were used: Holstein-Friesian (Bos taurus) and Nellore (Bos indicus) (Uzzaman et al., 2014; Karimi et al., 2016; Yonesaka et al., 2016). Furthermore, He was assessed under the assumption of Hardy–Weinberg equilibrium, and Ho was averaged over loci (Nei, 1978).
Principal Component Analysis (PCA)
The PLINK 1.9 software was used for principal component analysis (PCA) (Purcell et al., 2007). The autosomal data of the two indigenous Iraqi breeds (Rustaqi and Jenoubi) and several reference breeds [European taurine (Holstein-Friesian, Jersey), Asian zebu (Nellore, Sahiwal, and Gir), African zebu and their crossbreed (East African Shorthorn zebu (EASZ), Sheko, Ankole, Adamawa Gudali, and Red Bororo], and African taurine (Muturu and N’Dama Guinea) were used. The plotting of the PCA results was done using the Genesis software version (0.2.6b) (https://github.com/shaze/genesis).
Admixture
In order to estimate the ancestry and the genetic structure of the Iraqi cattle population, we used Admixture version 1.3.0 (Alexander et al., 2015) using the following breeds: Rustaqi, Jenoubi, Holstein-Friesian, Jersey, Nellore, Sahiwal, Gir, Brahman, EASZ, Sheko, Ankole, Adamawa Gudali, Red Bororo, and N’Dama Guinea. The analysis was conducted at genome-wide autosomal level, first with Iraqi breeds and four reference breeds (Holstein-Friesian, N’Dama Guinea, Sheko, and Nellore) and then including the entire set of breeds. We performed K = 2 to K = 10 as ancestral modes in order to identify the optimal number of ancestral populations by detecting the lowest value of cross-validation error. We plot our admixture results using the Genesis software (version 0.2.6b) (http://www.bioinf.wits.ac.za/software/genesis/).
Positive Candidate Signature of Selection
To construct haplotype files for signature selection analysis, haplotype data of Iraqi breeds (Rustaqi and Jenoubi) and other cattle references were reconstructed by phasing the genotyped SNPs using the SHAPEIT software (v2.8 37) (O’Connell et al., 2014).
Rehh (iHS and Rsb) Analysis
Identification of signature selection was based on the extended haplotype homozygosity (EHH) tools, using the Rehh package in R. Two analyses were performed (i) based on within-population statistics using Integrated Haplotype Score (iHS) (Voight et al. 2006); and (ii) relative integrated EHH of a site between populations (Rsb) (Sabeti et al., 2002). The iHS test was applied to Rustaqi and Jenoubi. Rsb test was conducted between i) Jenoubi and Rustaqi and ii) between each Iraqi cattle (Jenoubi and Rustaqi) with three reference breeds (Holstein-Friesian, N’Dama Guinea, and Nellore). The standardized Rsb and iHS values were normally distributed, so a Z-test was applied to identify statistically significant SNPs under selection. One-sided upper-tail P-values were derived as 1 − Φ (Rsb) from the Gaussian cumulative density function Φ. For Iraqi breeds, we set a threshold of −log10 P-value = 4 and −4 for the iHS test and a threshold of −log10 P-value = 5 and −5 for the Rsb test for the candidate regions. All annotated genes within the region were considered as candidate changes. Then, we examined commonly detected iHS and Rsb genes for the Iraqi cattle (Jenoubi and Rustaqi) as well as the Rsb results of Iraqi cattle and the three reference breeds. A Venn diagram online tool (http://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to check the overlap of candidate genes (Oliveros, 2015).
Fst Analysis
Inter-population Wright’s Fst analyses were conducted between the two Iraqi cattle breeds. Fst summarizes the genetic differentiation among populations, through estimation of the allele frequency between populations relative to the total variance of these populations (Wright, 1951; Holsinger and Weir, 2009). The calculation was performed on sliding windows of 60 SNPs, overlapping by 30 SNPs. The above 0.2 of the distribution of Fst values was arbitrarily chosen as the significant threshold.
Gene Function and Gene Pathway Identification Within Candidate-Selected Regions
Gene identification was based on the database of Ensembl Genes 91—Bos taurus genes (UMD 3.1) using the BioMart tool (http://www.ensembl.org/biomart). PANTHER 11.0 (http://www.pantherdb.org/) and the Enrichr (http://amp.pharm.mssm.edu/Enrichr/) tools were used to explore protein families, molecular functions, biological processes, cellular components, and pathways (Chen et al., 2013; Kuleshov et al., 2016; Mi et al., 2017). Moreover, the list of bovine quantitative trait locus (QTL) regions was downloaded from http://www.animalgenome.org/cgi-bin/QTLdb/index (Hu et al., 2016). Up-to-date information for some specific genes annotation was sourced from Gene Cards (http://www.genecards.org/) and most recent literature, integrating Google Scholar (https://scholar.google.com). To determine over-represented ontology terms for candidate genes following Fst analysis, we used DAVID version 6.7 (https://david.ncifcrf.gov/), which detects enriched functional terms (Huang da et al., 2009a; Huang da et al., 2009b) with an enrichment score of 1.3, equivalent to the Fisher exact test P = 0.05, as the significant threshold.
Results
Genomic Diversity
The highest values of mean He and Ho were found in Rustaqi and Sheko breeds, while the lowest values were obtained in N’Dama and Nellore ( Table 1 and Supplementary Figure S2). In particular, mean He and Ho were 0.37 and 0.36 for Rustaqi, respectively, while they were 0.32 (He and Ho) in Jenoubi. Our findings indicate that Iraqi breeds possess significant diversity compared to Asian (e.g., Korean Hanwoo), African (e.g., N’Dama), and European breeds (e.g., Holstein-Friesian) (Supplementary Tables S3, S4, and S5).
Table 1.
Number of animals, mean of expected heterozygosity (He), and observed heterozygosity (Ho).
| Breed | n | He (Mean/SD) | Ho (Mean/SD) |
|---|---|---|---|
| Jenoubi | 35 | 0.32/ ± 0.15 | 0.32/ ± 0.16 |
| Rustaqi | 59 | 0.37/ ± 0.12 | 0.36/ ± 0.13 |
| Holstein-Friesian | 30 | 0.31/ ± 0.17 | 0.31/ ± 0.19 |
| Nellore | 35 | 0.22/ ± 0.18 | 0.23/ ± 0.19 |
| N’Dama Guinea | 24 | 0.23/ ± 0.19 | 0.23/ ± 0.20 |
| Sheko | 18 | 0.36/ ± 0.12 | 0.37/ ± 0.15 |
Genetic Structure
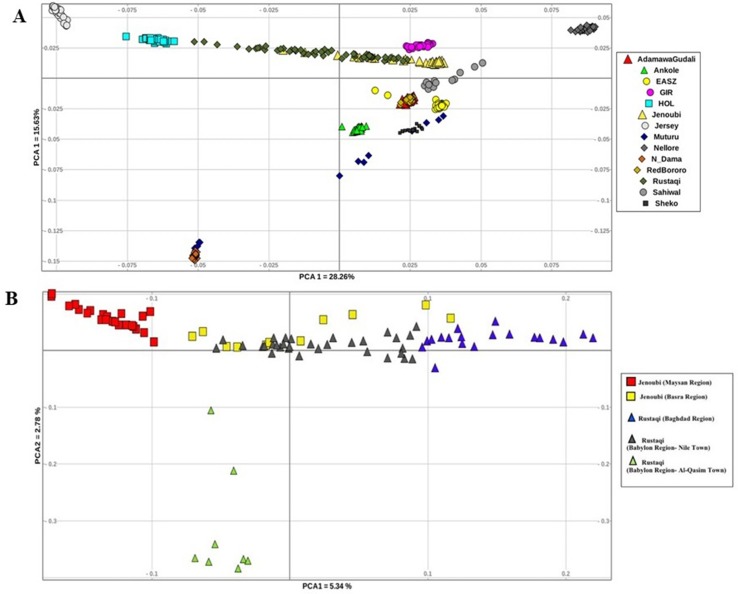
The genetic structure across and within breeds was first assessed using PCA. We conducted two analyses, first between Iraqi breeds and the reference breeds, and then within Iraqi breeds only. For all the breed analysed ( Figure 2A ), PC1 accounts for 28.26% of the total variation. It separates the Asian reference zebu population (Nellore) from the taurine breed (Holstein-Friesian); between the two, we do find the Rustaqi animal to be closer to taurine Holstein-Friesian, and the Jenoubi animals to be closer to the indicine Nellore. PC2, which accounts for 15.63% of the total variation, separates the African zebu breeds (Ankole, Sheko, Adamawa Gudali, and EASZ) and the African taurine (N’Dama and Muturu) from the other cattle populations (non-African breeds). The second PCA implemented for the Iraqi breeds only (Rustaqi and Jenoubi) reveals substructuring within each breed ( Figure 2B ). The first component, which accounts for 5.34% of the total variation, separates the Jenoubi animals sampled in the Maysan region from Rustaqi sampled around Baghdad, while Jenoubi from the Basra region and Rustaqi animals sampled around Babylon region are positioned between these two populations. The second component, which explains 2.78% of the total variation, separates Rustaqi animals from Al-Qasim town (Babylon region) from the other animals.
Figure 2.
Plots of principal component analysis. (A) PCA 1 versus PCA 2 analysis of Iraqi cattle and reference breeds. (B) PCA 1 versus PCA 2 analysis of Iraqi cattle (Rustaqi and Jenoubi).
Admixture
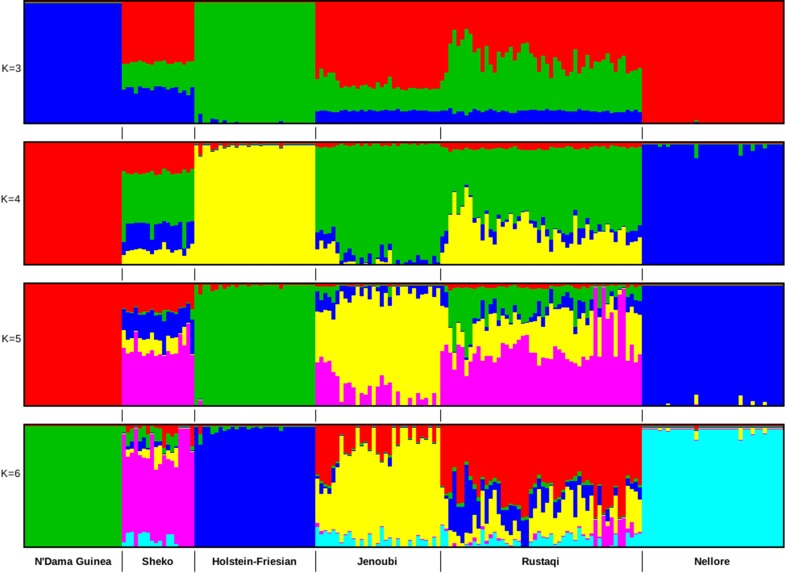
Admixture analysis was based on 680,124 SNPs after QC. We performed two admixture analyses: first among Iraqi cattle and four main cattle reference breeds (European taurine Holstein-Friesian, Asian zebu Nellore, African crossbreed Sheko, and African taurine N’Dama Guinea) and then including also Jersey, Sahiwal, Gir, East African Shorthorn zebu (EASZ), Ankole, Adamawa Gudali, and Red Bororo. In the first analysis, the selected breeds are representative of the main lineages of cattle (European taurine, African taurine, zebu, and Asian zebu). The optimal number of clusters was here defined as K = 4 (it has the lowest cross-validation value). As shown in Figure 3 , for ancestry K = 3, we do observe in Rustaqi and Jenoubi an European taurine and an African taurine shared ancestry as well as an Asian indicine one, for the latter higher in Jenoubi than in Rustaqi. At K = 4, shared ancestry with Holstein-Friesian is observed in Rustaqi but much less so in Jenoubi. Shared ancestries with the Nellore and African cattle are present in both Iraqi breeds, but these are low. At K = 5 and K = 6, both Rustaqi and Jenoubi show admixed background, however, less so for the former than the latter. For the second analysis, the optimal number of clusters was defined as K = 7 (Supplementary Figure S3). The results obtained here support the previous admixture results with more zebu ancestry in Jenoubi and more taurine ancestry in Rustaqi.
Figure 3.
Clustering assignments of five cattle populations based on ADMIXTURE 1.3.0 (Alexander et al., 2015) for inferred K values ranging from 2 to 6. Each individual is represented by a single vertical line divided into K colored segments, where K is the number of ancestral populations.
Genetic Signature of Positive Selection in Iraqi Breeds
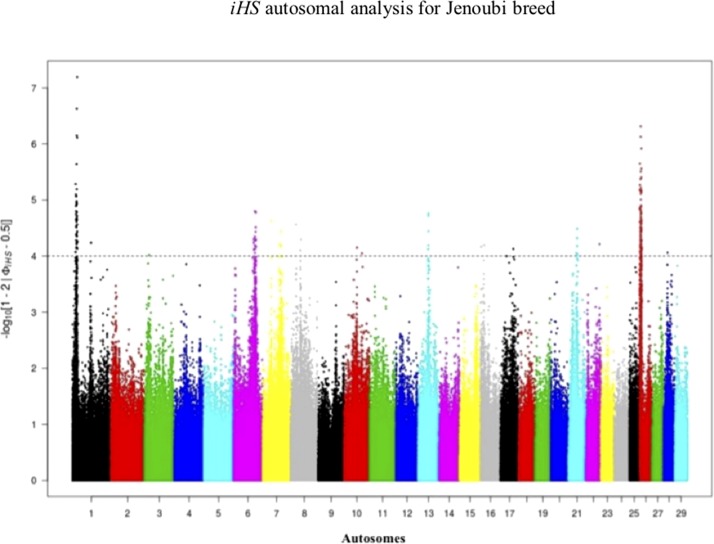
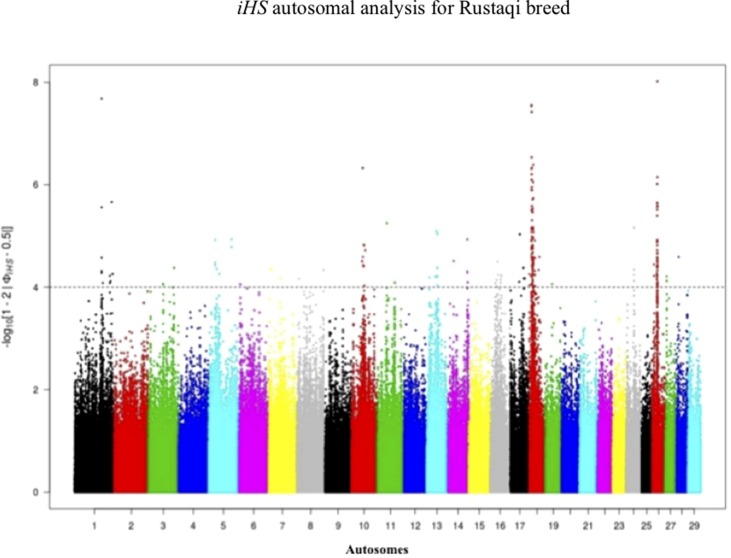
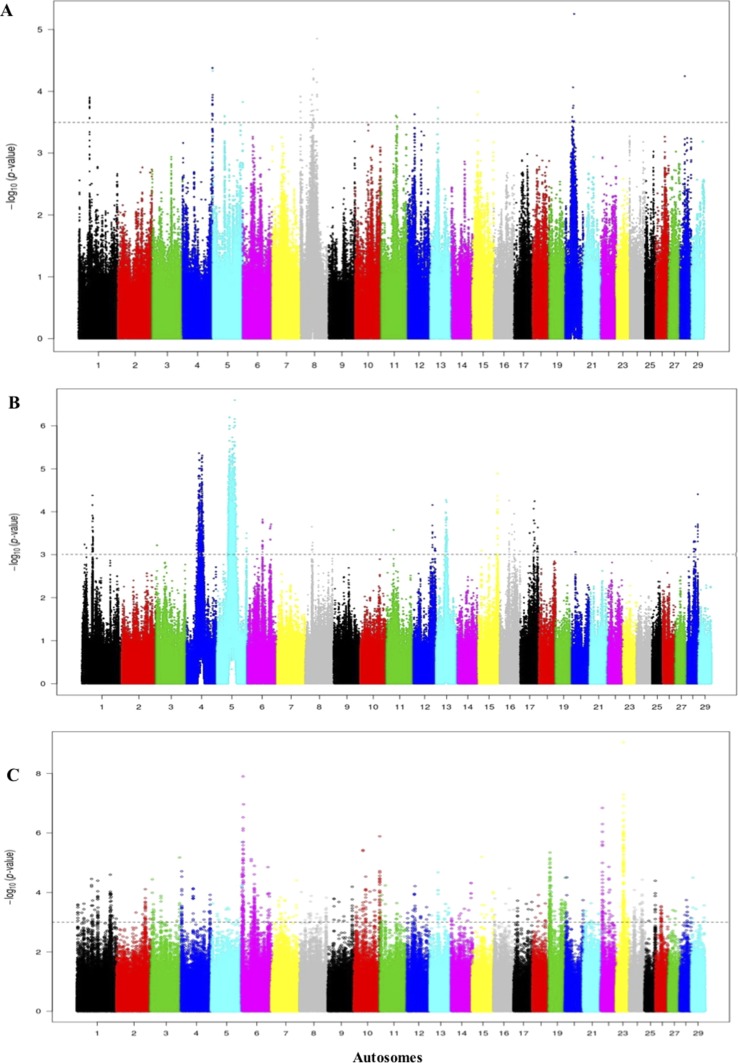
Footprints of selection for Iraqi breeds (Jenoubi and Rustaqi) were analyzed using iHS and Rsb statistics, based on extended haplotype homozygosity (EHH). Results are presented in Figures 4 and 5 . In Jenoubi, we observe the strongest evidence of selection on BTA1 with an iHS score of −5.40 and on BTA26 with iHS score of −5.0. Several genes are present within these regions, on BTA1 NCAM2, TMPRSS15, and CHODL and on BTA26 PRKG1. In Rustaqi, we observe the strongest evidence of selection on BTA1 with iHS score of −5.60 and on BTA18 with iHS score of −5.03. Genes present within these significant regions include on BTA1 PPM1L and IGSF5 and on BTA18 PLCG2, CDH13, NOVEL, OSGIN1, TLDC1, CRISPLD2, IRF8, JPH3, KLHDC4, SLC7A5, CA5A, BANP, GALNS, CBFA2T3, ABCC12, ZNF423, and LPCAT2. The Jenoubi breed has 13 candidate-selected regions derived and six ancestral, compared with 11 and 16, respectively, for Rustaqi (Supplementary Figures S4 and S5).
Figure 4.
Manhattan plot of genome-wide iHS autosomal analysis for Jenoubi. Significance threshold (dashed line) set at −log10 > 4.
Figure 5.
Manhattan plot of genome-wide iHS autosomal analysis for Rustaqi. Significance threshold (dashed line) set at −log10 > 4.
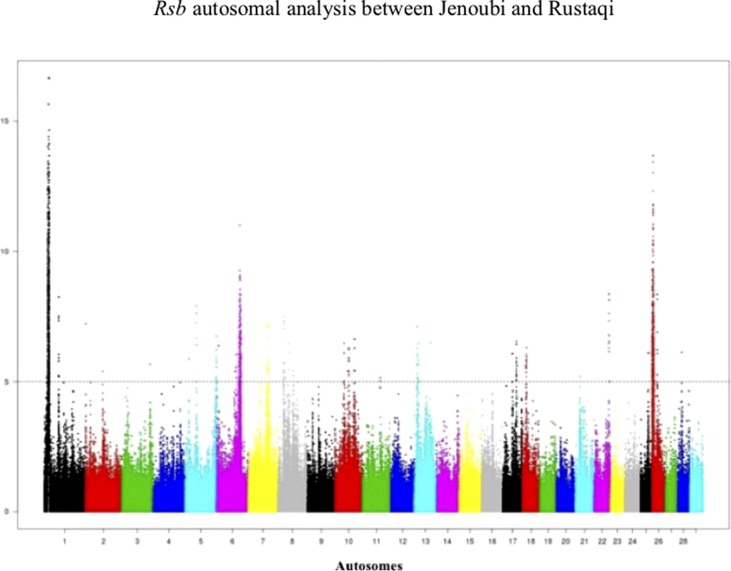
Rsb results between Rustaqi and Jenoubi show a total of 209 SNPs in Jenoubi and 236 in Rustaqi above the significant threshold. The Rsb plots show strong signals of positive selection on BTA1, BTA6, BTA7, BTA8, BTA10, BTA17, BTA22, and BTA26 in Jenoubi, and on BTA1, BTA5, BTA13, BTA18, and BTA26 in Rustaqi ( Figure 6 ).
Figure 6.
Manhattan plot of the genome-wide Rsb autosomal analysis between Jenoubi and Rustaqi. Significance threshold (dashed line) set at −log10 > 5.
Candidate Genes at Genomic Regions
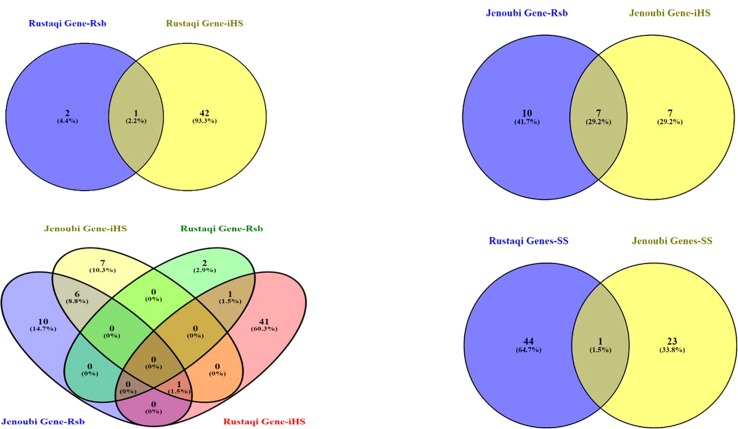
Candidate regions under positive selection in Jenoubi include 24 annotated genes (14 genes following iHS analysis, 17 genes following Rsb analysis, and 7 genes present in genome region commonly identified in both tests), while candidate regions under positive selection in Rustaqi include 45 annotated genes (43 genes following iHS analysis, 3 genes following Rsb analysis, and 1 gene present in genome region commonly identified in both tests); see Supplementary Table S6.
So a total of 68 annotated genes are present within the two Iraqi breeds at the candidate regions defined by the iHS and Rsb analyses (see Figure 7 for a Venn diagram showing the number of unique and shared genes found within candidate signature selection regions). The BTA18 regions have the largest number of genes (18 genes), followed by BTA6 with 10 genes, and finally BTA26 with 8 genes.
Figure 7.
Venn diagram showing the unique and shared genes found within candidate signature selection regions in Jenoubi and Rustaqi. (SS: signature selection genes of iHS&Rsb)
Overall, the results of genes functions/annotation analysis reveal 19 genes related to the immune system response; 5 genes for Jenoubi, 15 genes for Rustaqi, and 1 overlapping gene between Jenoubi and Rustaqi breeds. This gene is PRKG1 associated with tick resistance in cattle (Mapholi et al., 2016; Vajana et al., 2018). These immune response genes were involved in both the acquired and innate immune responses, for example, IRF8 (Rustaqi; BTA18) and ABCC2 (Rustaqi; BTA26) linked to the acquired immune response to protozoan and bacterial infections (Giagu, 2016) and gastrointestinal nematodes (Li et al., 2011), respectively; PARM1, an innate immune response gene (Jenoubi breed; BTA6), associated with anti-apoptotic activity especially during fertility stage (Cochran et al., 2013); and ATG7 (innate response gene, Jenoubi; BTA22), linked to the autophagy process (Aboelenain et al., 2015).
The remaining genes are related to other environmental adaptation or production characteristics. For instance, SLC24A3 (Rustaqi breed; BTA13) is related to fertility traits (Moran et al., 2017), and NCAM2 (Jenoubi, BTA1) is linked to fat, protein, and milk yield (Venturini et al., 2014). Supplementary Table S7 summarizes the log (P-value) of the most significant SNPs within the different significant regions in both breeds. In Jenoubi, the most significant SNPs (n = 58 SNPs, maximum SNP log P-value = 13.01; BTA26) are within the PCDH15 gene region involved in the maintenance of the integrity of the intestinal membrane. Then, NCAM2 (n = 20 SNPs, maximum SNP log P-value = 12.95, BTA1) and TMPRSS15 (n = 58 SNPs, maximum SNP log P-value = 12.93, BTA1) are linked to fat, protein, and milk yield.
In Rustaqi, the most significant SNPs (n = 25 SNPs, maximum SNP log P-value = 8.01; BTA18) are within the DNMBP gene region. This gene has been shown to contribute the milk-fat composition (Buitenhuis et al., 2014). Last but not least, the highest number of significant SNP value found in both Rustaqi and Jenoubi is at PRKG1 (n = 25 SNPs, maximum SNP log P-value = 7.93; BTA26).
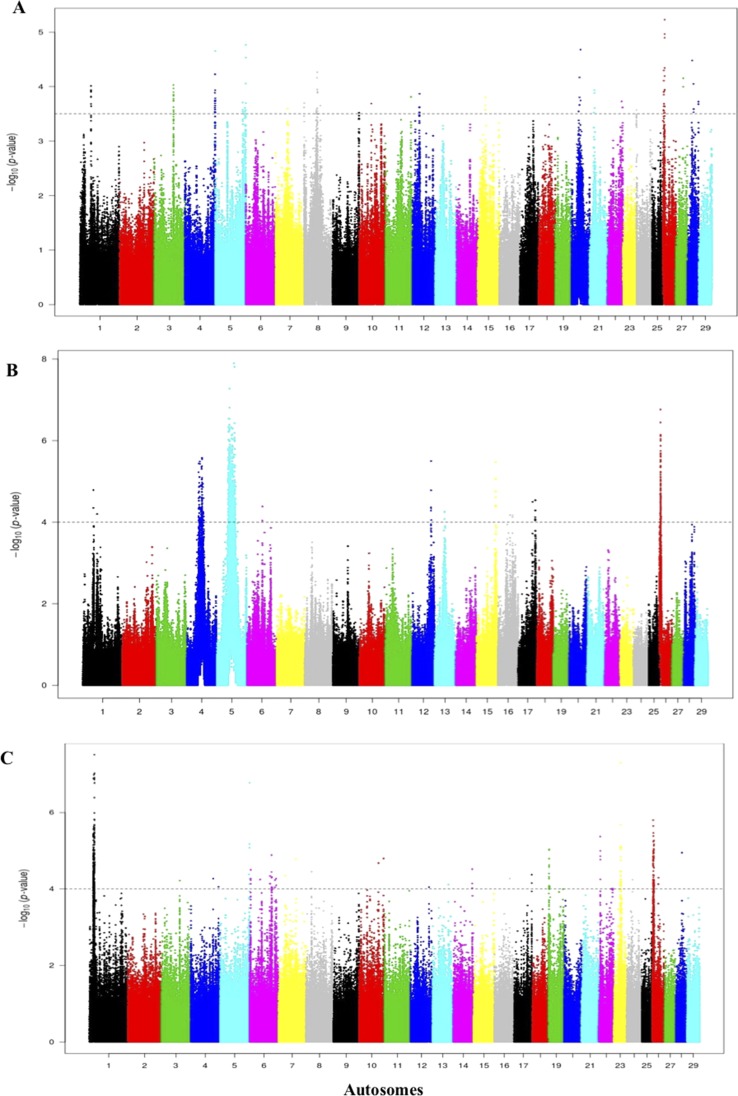
For the comparisons of the Jenoubi with Holstein-Friesian, Nellore, and N’Dama, we used a threshold for the Rsb of >3.5, 4, and 4, respectively ( Figures 8A–C ). We identified 161, 100, and 272 candidate regions for the Jenoubi Rsb comparison with Holstein-Friesian, Nellore, and N’Dama, respectively. The most significant SNP values are 5.3 on BTA26 (Jenoubi versus Holstein-Friesian), 7.8 on BTA5 (Jenoubi versus Nellore), and 7.6 on BTA1 (Jenoubi versus N’Dama).
Figure 8.
Manhattan plot of the genome-wide Rsb autosomal analysis between Jenoubi versus (A) Holstein-Friesian, (B) Nellore, and (C) N’Dama, respectively. Significance threshold (dashed line) set at −log10 > 3.5, 4, and 4, respectively.
A total of 38 candidate annotated genes are present in significant Jenoubi versus Holstein-Friesian Rsb regions. Among these, four candidate genes overlap with previous Iraqi cattle analysis (iHS and Rsb). These candidate genes are ATG7 (autophagy control), PRKG1 (tick resistance), PCDH15 (maintenance of intestine membrane), and TMEM132B (control of brain physiology). The remaining 34 genes were considered to be new genes not identified in our previous analysis of Iraqi breeds. Thirteen genes are included in the Jenoubi–N’Dama Guinea comparison, including eight genes overlapping with the Iraqi breeds analysis (iHS and Rsb) (e.g., NCAM2, TMPRSS15, PCDH15, PRKG1, and FOCAD). Only one gene PCDH15 is present for Rsb Jenoubi versus Nellore analysis. (Supplementary Table S8).
The threshold for the Rustaqi Rsb analysis was >3.5, 3, and 3.5 for the Holstein-Friesian, Nellore, and N’Dama, respectively. Fifty-seven, 18, and 98 candidate regions were identified from the Rustaqi breed versus Holstein-Friesian, Nellore, and N’Dama analyses, respectively ( Figures 9A–C ). Additionally, the strongest SNP values were 5.3 on BTA20 (Rustaqi versus Holstein-Friesian), 6.8 on BTA5 (Rustaqi versus Nellore), and 7.2 on BTA6 (Rustaqi versus N’Dama). While 12 genes are present in the comparison Rustaqi versus Holstein-Friesian, none overlapped with previous genes identified in the Iraqi analysis (iHS and Rsb). Rsb analysis of Rustaqi against Nellore uncovered five genes, again with no shared genes with our previous Iraqi analysis (iHS and Rsb). Rsb Rustaqi versus N’Dama Guinea found 26 genes, with one gene (CD96) previously identified in Iraqi breeds (Supplementary Table S9).
Figure 9.
Manhattan plot of the genome-wide Rsb autosomal analysis between Rustaqi versus (A) Holstein-Friesian, (B) Nellore, and (C) N’Dama, respectively Significance threshold (dashed line) set at −log10 > 3.5, 3, and 3.5, respectively.
Gene Ontology Analysis
The PANTHER analysis of the biological processes for Jenoubi (iHS genes) reveals the following significant categories: biological regulation, molecular function, cellular components, protein class, and pathways (Supplementary Figure S6; Supplementary Table S10A–D). On the other hand, the Enrichr tool reveals the following biological processes: positive regulation of apoptotic process, sensory perception of light stimulus, and regulation of GTPase activity. Molecular function analysis shows three enriched levels of gene clusters [apoptotic process (GO: 0043065), transmembrane–ephrin receptor activity (GO:0005005), and calcium channel regular activity (GO:0005246)] (Supplementary Figure S7A and B; Supplementary Table S11A and B).
The PANTHER analysis for Rustaqi (iHS genes) identifies the same categories (biological regulation, molecular function, cellular components, protein class, and pathway) (Supplementary Figure S8). However, the Enrichr tool analysis reveals two enriched clusters: regulation of positive chemotaxis (GO:0050926) and glomerular epithelial cell development (GO:0072310). On the other hand, the results of molecular function analysis indicate one enriched cluster [O-acetyltransferase activity (GO: 0016413)] (Supplementary Figure S9A and B).
For the Rsb analysis, the PANTHER for Jenoubi indicates eight clusters in biological categories: biological regulation (GO:0065007) (5 genes), biological adhesion (GO:0022610) (3 genes), cellular process (GO:0009987) (12 genes), localization (GO:0051179) (6 genes), metabolic process (GO:000 8152) (5 genes), cellular component organization or biogenesis (GO:0071840) (2 genes), multicellular organismal process (GO:0032501) (1 gene), and response to stimulus (GO:0050896) (1 gene). On the other hand, molecular function shows binding (GO:0005488) (6 genes), catalytic activity (GO:00 03824) (3 genes), and transporter activity (GO:0005215) (3 genes). The Enrichr tool reveals the following biological processes: vitamin D metabolic process and growth factor activity. These ontologies and others (cellular components, protein class, and pathway) are further shown at Supplementary Figures S10 and 11.
The PANTHER analysis for Rustaqi (Rsb analysis) indicates the following two biological process supported with two genes, cellular process (GO:0009987) and metabolic process (GO:0008152), and the biological process binding (GO:0005488) with one gene. The Enrichr analysis identifies three enriched terms: small GTPase-mediated signal transduction (GO:0007264), cell–matrix adhesion (GO:0007160), and positive regulation of hydrolase activity (GO:0051345). On the other hand, the molecular function analysis recognizes one enriched term, Rho guanyl-nucleotide exchange factor activity (GO:0005089).
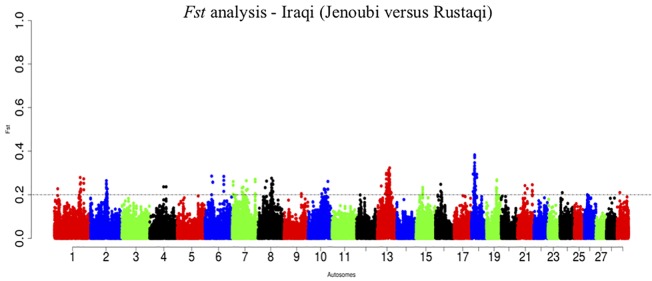
Fst Candidate Gene Regions
The overall genome differentiation of Fst values between Iraqi breeds is Fst = 0.28 ( Figure 10 ). The Fst analysis reveals regions with candidate genes differentiated between Jenoubi and Rustaqi (Supplementary Table S12). DAVID bioinformatics analysis for Fst results shows 16 annotation clusters, but only one of them (metal thiolate and mineral absorption cluster) representing 51 genes has an enrichment score of 4.89, largely above the threshold of 1.3 (P = 0.05), with the next cluster, enrichment score 1.29 (metal binding cluster), just below the significant threshold level considered.
Figure 10.
Manhattan plots of the pairwise genome-wide autosomal Fst analyses between Rustaqi and Jenoubi for autosomes. The significant thresholds (dashed line) are set at above 0.2 of the Fst windows distribution.
Discussion
In this study, we report at a genome-wide level for the first time the genetic structure, diversity, and candidate signatures of positive selection in two Iraqi cattle breeds, Jenoubi and Rustaqi. At the crossroad of the zebu and taurine centers of domestication, Iraqi cattle may be expected to show high diversity of both taurine and zebu origin. This is confirmed in our study with the presence of both indicine and taurine ancestry in the two breeds, although in different proportions for each of them. Jenoubi cattle are classified as zebu following their humped cattle phenotypes. Our principle component and admixture analyses support such classification, but they also reveal a small proportion of taurine ancestry in their genetic backgrounds. Two factors may have contributed in the shaping of the genetic make-up of Jenoubi. The taurine background within this breed may be corresponding to ancient admixture events from the putative Near East cattle taurine domestication centers, and/or it is the consequence of recent exotic taurine introgression. At K = 3, the Jenoubi taurine background is shared with N’Dama and European taurine. However, at K = 4, the optimal K, the ancestry with European taurine largely disappears. The native habitat of the Jenoubi breed (South-Eastern of Iraq) is away from areas where exotic breeds (e.g., Holstein-Friesian) have been introduced in Iraq in the recent past (Al-Bayatti et al., 2016), supporting ancient taurine introgression events rather than a more recent one from European exotic taurine.
In contrast, with a significant proportion of European ancestry, as revealed in our admixture analysis, recent gene flow from exotic cattle origin likely occurred in Rustaqi. The geographic origin of this breed is central Iraq. It is close to the capital Baghdad, and crossbreeding with exotic taurine might have been driven by the pressures to increase milk production in response to the consumer demands from the city. Admixture analysis indicates also some low zebu background in Rustaqi. Likewise, with the taurine introgression in Jenoubi breed, it may be of ancient origin and the consequence of past trading networks not only between central Iraq and southern Iraq but also further north and south, linking the ancient civilization of the Fertile Crescent and the Indus Valley (Magee et al., 2014).
Interestingly, genetic studies in Anatolian cattle (Anatolian Black, South Anatolian Red, Anatolian Southern Yellow, and Turkish Grey) have also revealed taurine × zebu admixture (Ateş et al., 2014). Also, Karimi et al. (2016) have mentioned that indigenous Iranian cattle from the western part of the country, near the Iraqi border, have more taurine genetic background than southwest Iranian cattle on the south-east border with Iraq, which are more indicine in their genetic background. Our study together with previous ones illustrates the pattern and gradient of zebu and taurine genetic admixture in the region. In terms of genome diversity, the Rustaqi He is higher compared with all the Iranian cattle population examined by Karimi et al. (2016). Similarly, comparison of the Jenoubi and Rustaqi with African taurine (N’Dama Guinea), Asian zebu (Nellore), and European Holstein-Friesian shows that Iraqi breeds possess higher genome diversity. It is expected for a crossbred population compared with the non-admixed taurine and zebu population. We also do observe higher genome diversity in Jenoubi and Rustaqi compared with the Sheko, an African zebu × taurine admixed breed (Bahbahani et al., 2017). It may be explained by the closer proximity of Iraqi cattle to the centers of cattle domestication, and therefore the center diversities of domestic cattle compared with the African breeds.
Our signature of selection results in both Iraqi breeds, are suggesting that environmental challenges including diseases pressures have shaped the genomes of Rustaqi and Jenoubi breeds, but not in an identical way, with important differences between the two breeds according to our findings from the iHS and Rsb analyses.
The iHS results in Rustaqi indicate more candidate-selected regions with genes involved in innate and acquired immunities, compared with the results obtained in Jenoubi. Interestingly, among the 14 immune response-related genes unique to Rustaqi, we do find that OSGIN1 and CBFA2T3 previously showed to be part to the cattle immune response to mammary gland inflammation (Gilbert et al., 2012; Wang et al., 2012; Osińska et al., 2014). Furthermore, IRF8 plays an important role against bacterial (e.g., Salmonella) and protozoan infections (Gautier et al., 2009; Porto-Neto et al., 2013; Giagu, 2016). The results suggest that the importance of the Rustaqi breed in milk production may have shaped, at least partly, the candidate signatures of selection observed here.
Nevertheless, the iHS results in Jenoubi breed have revealed five important immunity genes. TNFAIP8 and FOCAD candidate genes are known to play a role in immune homeostasis and tumor suppression (Hadisaputri et al., 2012; Iwata, 2016). Another crucial gene is ATG7, an autophagy gene that contributes to the regulation of the cell death process through elimination of unwanted or dead cells (Aboelenain et al., 2015).
Among the genes identified in both breeds, PRKG1, which has been reported previously in two other studies (Mapholi et al., 2016; Vajana et al., 2018), is associated with the tick resistance–tolerance phenotype, a major issue in the pastoral areas of the middle and southern regions of Iraq (Al-Ramahi and Kshash, 2011; Mohammad, 2015). Our study is adding further support to the importance of this gene in relation to disease resistance traits. Similarly, ABCC2 identified in Rustaqi breed has been related to resistance–tolerance to gastrointestinal nematode parasites infection.
We also identified several regions including genes that may be linked to environmental agro-climatic adaptation in both breeds following iHS analysis. Rustaqi animals are raised in a relatively dry and hot environment, and accordingly, adaptation to heat stress may be expected. Here, we do find UCN3 involved in the genetic control of heat tolerance and oxidative stress, including in Holstein-Friesian cattle (Zheng et al., 2014). Also, in Jenoubi, we identified within candidate-selected regions two genes related to nutrition, SLC4A4, which plays a crucial role in the rumen development (Connor et al., 2013), and EPHA5, contributing to the improvement of the feed conversion rate from rumination (Santana et al., 2016). This suggests that the breed, largely free grazing, may be particularly adapted to the local availability of feeds.
Although overlap regions are few between Rustaqi and Jenoubi breeds, the same gene pathways may have been under selection pressures in both breeds. For example, NCAM2, TMPRSS15, and SLC4A4 within candidate regions in Jenoubi breed have functions related to milk quality and production, with the latter also found under a candidate signature of selection region in Holstein cattle (Li et al., 2010; Venturini et al., 2014). The same regions are not significant in Rustaqi, but here, other genes linked to milk quality and production are found in other significant regions, such as LPCAT2 and DNMBP, two genes linked to protein and fat content in milk (Ogorevc et al., 2009; Buitenhuis et al., 2014; Venturini et al., 2014). Interestingly, we note also the presence in Jenoubi of PCDH15, a gene involved in meat quality within a candidate-selected region (Ryu and Lee, 2014).
The outputs of the Enrichr analysis for Jenoubi (iHS analysis) indicate that the most enriched cluster among the biological process category is the gene ontology term apoptotic process. Programmed cell death (apoptosis) is part of the immune adaptive response of an organism. In particular, positive regulation of the apoptotic process has been shown to play a role in the immune response of blood cells to trypanosome infection in cattle (Hill et al., 2005), as well as meat quality through elimination of the dead cells, and in maintaining the rumination process of cattle through conserving rumen cells activity (Herrera-Mendez et al., 2006; Connor et al., 2013; Shabtay, 2015). In the Canchim Brazilian beef cattle, the apoptotic process was also among the most enriched clusters (Urbinati et al., 2016). Furthermore, Taye et al. (2017) have mentioned that apoptosis as a response to external stress may be involved in thermotolerance in cattle. Another enriched cluster is sensory perception to light stimulus, which reflects adaptation to vision, one of the cognitive functions of an animal. Such adaptation may be of relevance in particular for outdoor grazing animals (Kim et al., 2017). The GTPase activity cluster found in Jenoubi breed, which plays a significant role in inflammatory reaction following nematode infection (Huang et al., 2007; Kim et al., 2015), and in relation to milk and fertility traits (Kasarapu et al., 2017), has also been found in Yiling yellow Chinese cattle (Ling et al., 2017).
Enrichr results for Rustaqi (iHS analysis) indicated biological process related to gene upregulation (clusters regulation of positive chemotaxis and glomerular epithelial cell development) (Pokharel et al., 2018), as well as genes playing a crucial role in defense mechanism against bacterial infection, and growth function processes (Flori et al., 2009; Gautier et al., 2009; Porto-Neto et al., 2013; Giagu, 2016).
PANTHER analysis of Jenoubi Rsb results reveals several clusters linked to biological process. For instance, cluster genes of metabolic process are associated with milk production, metabolism of water-soluble vitamins, and regulation of actin cytoskeleton (Raven et al., 2016). Another important cluster is biological regulation including genes involved in rumen and muscle development (Feng et al., 2007; Li et al., 2010). Enrichr analysis identified one enriched category in the biological process cluster (vitamin D metabolic process) and one enriched category in molecular function cluster (growth factor activity). Both clusters may be linked to the health of the animals. For example, vitamin D contributes to the protection of the body from autoimmune diseases with deficiency in vitamin D linked to pathologies, such as osteoporosis and skin or coating diseases (Adorini and Penna, 2008; Bikle, 2014).
Regarding Rsb results of Rustaqi breed, the more important ontology term from the Enrichr analysis is the cell–matrix adhesion cluster that regulates tissue construction and cell activity (Lodish et al., 2000). For example, this cluster includes MYRFL-201, which protects the myelinated central nervous system, and DNMBP, responsible for milk quality traits such as fat composition (Buitenhuis et al., 2014; Koenning, 2015).
Interestingly, among the regions differentiated between the two breeds (Fst analysis), DAVID tool identifies the significance cluster metal-thiolate function important for metabolism detoxification activities (e.g., after zinc and copper ingestions) (Richards, 1989). It supports that the two breeds are exposed to different feeds with different toxicity, and they may have responded to such selection pressures accordingly.
In conclusion, we have reported here for the first time at a genome-wide level the genetic structure, diversity, and candidate signatures of positive selection in two Iraqi cattle breeds. Our results support the phenotypic classification of Jenoubi cattle as zebu, and Rustaqi cattle as taurine but with introgression from the other cattle subspecies in each of them. In addition, the results show a significant level of genetic diversity in indigenous Iraqi cattle in line with their history. Genome-wide analysis unravels the genes that play an important role in immunity and other environmental adaptive traits, including in relation to parasitic, bacterial disease challenges, and heat tolerance. This study illustrates the uniqueness of these two indigenous breeds, while the information obtained is expected to help the control of diseases, conservation, management, and utilization of the indigenous Iraqi cattle genetic resources.
Ethics Statement
The animals used in this study are owned by farmers. Prior to sampling, the objectives of the study were explained to them in their local languages so that they could make an informed decision regarding giving consent to sample their animals. Government veterinary, animal welfare, and health regulations were observed during sampling of the populations analyzed here. The procedures involving animal sample collection also followed the recommendation of directive 2010/63/EU. Collection of blood samples was permitted by the Iraqi Ministry of Agriculture.
Author Contributions
AA and OH conceived and designed the experiment. AA and OH performed the experiment. AA, AE, and SA-B collected samples. AA analyzed the data. AA and OH wrote the manuscript. All authors have agreed on the contents of the manuscript.
Funding
We would like to extend our sincere gratitude to the Iraqi Ministry of Higher Education and Scientific Research (MOHESR, Grant 1362), Iraqi cultural attaché, for sponsoring this work
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the Animal Genetics Resources Department, Directorate of Animal Resources – Baghdad, Directorate of Veterinary Medicine/Baghdad, the Ministry of Iraqi Agriculture for supporting the fieldworks of this research as well as the CGIAR CRP Research Program on Livestock.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00609/full#supplementary-material
References
- Aboelenain M., Kawahara M., Balboula Z., Montasser M., Zaabel M., Okuda K., et al. (2015). Status of autophagy, lysosome activity and apoptosis during corpus luteum regression in cattle. J. Reprod. Dev. 61 (3), 229. 10.1262/jrd.2014-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L., Penna G. (2008). Control of autoimmune diseases by the vitamin D endocrine system. Nat. Rev. Rheumatol. 4 (8), 404. 10.1038/ncprheum0855 [DOI] [PubMed] [Google Scholar]
- Al-Bayatti S., Essa A., Nasef N., Salman S., Abdulkader N., Al-Tameemi R., Mahdi L. (2016). Phenotypic characterisation of Rustaqi cattle breed within its natural environment. Proceeding of Regional Conference for Animal Genetic Resources Conservation, Oman, pp. 22.
- Al-Murrani W., Majid S., Alkas J. (2003). Animal genetic resources in Iraq. Country scientific report. Baghdad: Iraq Agricultural Ministry Publication, 1–55. [Google Scholar]
- Al-Ramahi H., Kshash Q. (2011). Epidemiological study of some factors affecting prevalence of clinical theileriosis in cows in southern Baghdad. Euphrates J. Agric. Sci. 3 (3), 22–28. [Google Scholar]
- Alexander D., Novembre J., Lange K. (2015). Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664. 10.1101/gr.094052.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateş A., Türkay G., Akiş I., Esen Gürsel F., Yardibi H., Öztabak K. (2014). Genotype and allele frequencies of polymorphisms in ABCG2, PPARGC1A and OLR1 genes in indigenous cattle breeds in Turkey. Acta Vet. 64, 73–80. 10.2478/acve-2014-0008 [DOI] [Google Scholar]
- Bahbahani H., Tijjani A., Mukasa C., Wragg D., Almathen F., Nash O., et al. (2017). Signatures of selection for environmental adaptation and zebu × taurine hybrid fitness in East African Shorthorn Zebu. Front. Genet. 8, 68. 10.3389/fgene.2017.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D. (2014). Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 21 (3), 319–329. 10.1016/j.chembiol.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D., Magee D. (2006). Genetics and origins of domestic cattle. Documenting domestication: new genetic and archaeological paradigms 22, 317–328. [Google Scholar]
- Bradley D., Loftus R., Cunningham C., MacHugh D. (1998). Genetics and domestic cattle origin. Evolutionary Anthropology Issues News Rev. 6 (3), 79–86. [DOI] [Google Scholar]
- Buitenhuis B., Janss L., Poulsen A., Larsen B., Larsen K., Sørensen P. (2014). Genome-wide association and biological pathway analysis for milk-fat composition in Danish Holstein and Danish Jersey cattle. BMC Genomics 15 (1), 1112. 10.1186/1471-2164-15-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lin B., Baig M., Mitra B., Lopes R., Santos A., et al. (2010). Zebu cattle are an exclusive legacy of the South Asia Neolithic. Mol. Biol. Evol. 27, 1–6. 10.1093/molbev/msp213 [DOI] [PubMed] [Google Scholar]
- Chen Y., Tan M., Kou Y., Duan Q., Wang Z., Meirelles V. (2013). Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 14, 128. 10.1186/1471-2105-14-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W., Choi H., Lee H., Lee S., Kim H. C., Yu D., et al. (2015). Whole-genome resequencing analysis of Hanwoo and Yanbian cattle to identify genome-wide SNPs and signatures of selection. Mol. Cells 38 (5), 466. 10.14348/molcells.2015.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran D., Cole B., Null J., Hansen J. (2013). Discovery of single nucleotide polymorphisms in candidate genes associated with fertility and production traits in Holstein cattle. BMC Genet. 14 (1), 49. 10.1186/1471-2156-14-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. E., Baldwin R. L., VI, Li C.-j., Li R. W., Chung H. (2013). Gene expression in bovine rumen epithelium during weaning identifies molecular regulators of rumen development and growth. Funct. Integr. Genomics 13 (1), 133–142. 10.1007/s10142-012-0308-x [DOI] [PubMed] [Google Scholar]
- Dabdoub S. (2005). Mortality and birth weight in Friesian, Sharabi and crossbred calves. Iraqi J. Vet. Sci. 19 (2), 91–98. 10.33899/ijvs.2005.46744 [DOI] [Google Scholar]
- Diamond J., Bellwood P. (2003). Farmers and their languages: the first expansions. Science 300, 597–603. 10.1126/science.1078208 [DOI] [PubMed] [Google Scholar]
- Edwards C., Baird J., Machugh D. (2007). Taurine and Zebu admixture in near eastern cattle: a comparison of mitochondrial, autosomal and Y-chromosomal data. Anim. Genet. 38, 520–4. 10.1111/j.1365-2052.2007.01638.x [DOI] [PubMed] [Google Scholar]
- Felius M., Theunissen B., Lenstra J. (2015). Conservation of cattle genetic resources: the role of breeds. J. Agric. Sci. 153 (01), 152–162. 10.1017/S0021859614000124 [DOI] [Google Scholar]
- Feng Z., Tang L., Li K., Liu B., Yu M., Zhao H. (2007). Molecular characterization of the BTG2 and BTG3 genes in fetal muscle development of pigs. Gene 403 (1), 170–177. 10.1016/j.gene.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Flori L., Fritz S., Jaffrézic F., Boussaha M., Gut I., Heath S., et al. (2009). The genome response to artificial selection: a case study in dairy cattle. PLoS One 4 (8), e6595. 10.1371/journal.pone.0006595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations [FAO] (2015). The second report on the state of the world’s animal genetic resources for food and agriculture. Rome: Food and Agriculture Organization of the United Nations, 1–20. Available online at: http://www.fao.org/3/a-i4787e.pdf. [Google Scholar]
- Freeman A., Bradley D., Nagda S., Gibson J., Hanotte O. (2006. a). Combination of multiple microsatellite data sets to investigate genetic diversity and admixture of domestic cattle. Anim. Genet. 37, 1–9. 10.1111/j.1365-2052.2005.01363.x [DOI] [PubMed] [Google Scholar]
- Freeman R., Hoggart J., Hanotte O., Bradley G. (2006. b). Assessing the relative ages of admixture in the bovine hybrid zones of Africa and the Near East using x chromosome haplotype mosaicism. Genetics 173 (3), 1503–1510. 10.1534/genetics.105.053280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier M., Flori L., Riebler A., Jaffrézic F., Laloé D., Gut I., et al. (2009). A whole genome Bayesian scan for adaptive genetic divergence in West African cattle. BMC Genomics 10 (1), 550. 10.1186/1471-2164-10-550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagu A. (2016). Immunopathological aspects in human and bovine tuberculosis: immunopathological changes in IRF-8–/– mice during Mycobacterium tuberculosis infection—histopathological and biomolecular evaluations in cattle tuberculin skin test positive slaughtered according to the regional eradication program. Doctoral dissertation. Italy: Università Degli Studi Di Sassari; Available online at: https://core.ac.uk/download/pdf/33724226.pdf. [Google Scholar]
- Gifford-Gonzalez D., Hanotte O. (2011). Domesticating animals in Africa: implications of genetic and archaeological findings. J. World Prehistory 24 (1), 1–23. 10.1007/s10963-010-9042-2 [DOI] [Google Scholar]
- Gilbert I., Robert C., Vigneault C., Blondin P., Sirard A. (2012). Impact of the LH surge on granulosa cell transcript levels as markers of oocyte developmental competence in cattle. Reproduction 143 (6), 735–747. 10.1530/REP-11-0460 [DOI] [PubMed] [Google Scholar]
- Hadisaputri E., Miyazaki T., Suzuki S., Yokobori T., Kobayashi T., Tanaka N., et al. (2012). TNFAIP8 overexpression: clinical relevance to esophageal squamous cell carcinoma. Ann. Surg. Oncol. 19 (3), 589–596. 10.1245/s10434-011-2097-1 [DOI] [PubMed] [Google Scholar]
- Hanotte O., Bradley D., Ochieng J., Verjee Y., Hill E., Rege J. (2002). African pastoralism: genetic imprints of origins and migrations. Science 296 (5566), 336–339. 10.1126/science.1069878 [DOI] [PubMed] [Google Scholar]
- Helmer D., Gourichon L., Monchot H., Peters J., Segui S. (2005). “Identifying early domestic cattle from pre-pottery Neolithic sites on the Middle Euphrates using sexual dimorphism,” in The first steps of animal domestication: new archeological approaches (Oxford: Oxbow Books; ), 86–95. [Google Scholar]
- Herrera-Mendez C. H., Becila S., Boudjellal A., Ouali A. (2006). Meat ageing: reconsideration of the current concept. Trends Food Sci. Technol. 17 (8), 394–405. 10.1016/j.tifs.2006.01.011 [DOI] [Google Scholar]
- Hill W., O’Gorman M., Agaba M., Gibson P., Hanotte O., Kemp J., et al. (2005). Understanding bovine trypanosomiasis and trypanotolerance: the promise of functional genomics. Vet. Immunol. Immunopathol. 105 (3–4), 247–258. 10.1016/j.vetimm.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Holsinger E., Weir S. (2009). Genetics in geographically structured populations: defining, estimating and interpreting. Nat. Rev. Genet. 10 (9), 639–650. 10.1038/nrg2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Park A., Reecy M. (2016). Developmental progress and current status of the animal QTLdb. Nucleic Acids Res. 44 (D1), D827–D833. 10.1093/nar/gkv1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W., Sherman B. T., Lempicki R. A. (2009. a). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. 10.1186/s12711-016-0254-5 [DOI] [PubMed] [Google Scholar]
- Huang da W., Sherman T., Lempicki A. (2009. b). Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Sherman T., Tan Q., Kir J., Liu D., Bryant D., et al. (2007). DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 35 (suppl.2), 169–175. 10.1093/nar/gkm415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H. (2016). Age-associated events in bovine oocytes and possible countermeasures. Reprod. Med. Biol. 15 (3), 155–164. 10.1007/s12522-015-0233-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost S., Colli L., Bonin A., Biebach I., Allendorf F., Hoffmann I., et al. (2015). Promoting collaboration between livestock and wildlife conservation genetics communities. Conserv. Genet. Resour. 3, 785–788. 10.1007/s12686-011-9457-9 [DOI] [Google Scholar]
- Karimi K., Strucken M., Moghaddar N., Ferdosi H., Esmailizadeh A., Gondro C. (2016). Local and global patterns of admixture and population structure in Iranian native cattle. BMC Genet. 17 (1), 108. 10.1186/s12863-016-0416-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasarapu P., Porto-Neto R., Fortes S., Lehnert A., Mudadu A., Coutinho L., et al. (2017). The Bos taurus–Bos indicus balance in fertility and milk related genes. Plos One 12 (8), 1–21. 10.1371/journal.pone.0181930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Cho S., Bhak J., O’Brian J., Yeo H. (2017). Perspectives provided by leopard and other cat genomes: how diet determined the evolutionary history of carnivores, omnivores, and herbivores. BMB Rep. 50 (1), 3–4. 10.5483/BMBRep.2017.50.1.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Sonstegard T., Da Silva B., Gasbarre C., VanTassell P. (2015). Genome-wide scan of gastrointestinal nematode resistance in closed angus population selected for minimized influence of MHC. PLoS One 10 (3), 1–18. 10.1371/journal.pone.0119380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenning M. (2015). Transcriptional control of myelin maintenance in the adult CNS. PhD thesis. Centre for Neuroscience, Australia: Melbourne University; Available online at: https://minerva-access.unimelb.edu.au/handle/11343/56415. [Google Scholar]
- Kuleshov V., Jones R., Rouillard D., Fernandez F., Duan Q., Wang Z., et al. (2016). Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44 (W1), W90–W97. 10.1093/nar/gkw377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang Z., Moore S., Schenkel S., Stothard P. (2010). Genome-wide scan for positional and functional candidate genes affecting milk production traits in Canadian Holstein Cattle. Proc 9th WCGALP, Leipzig, Germany, 26. [Google Scholar]
- Li W., Rinaldi M., Capuco V. (2011). Characterization of the abomasal transcriptome for mechanisms of resistance to gastrointestinal nematodes in cattle. Vet. Res. 42 (1), 114. 10.1186/1297-9716-42-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling X., Wen-gang Z., Jun-ya L., De-jiang Z., Xiao-cheng X., Yan-zi T., et al. (2017). Genetic background analysis and breed evaluation of Yiling yellow cattle. J. Integr. Agric. 16 (10), 2246–2256. 10.1016/S2095-3119(17)61679-4 [DOI] [Google Scholar]
- Lodish H., Berk A., Zipursky S. L., Matsudaira P., Baltimore D., Darnell J. (2000). “Cell–cell adhesion and communication. Section 22.2,” in Cell–matrix adhesion, 4th. Ed (New York: W. H. Freeman; ). Available from: https://www.Ncbi.nlm.nih.gov/books/NBK21539/. [Google Scholar]
- Loftus R., Machugh D., Bradley D., Sharp P., Cunningham P. (1994). Evidence for two independent domestications of cattle. PNAS 91 (7), 2757–2761. 10.1073/pnas.91.7.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaroof N. (2011). Performance of Sharabi cattle. Emir. J. Food Agric. 4 (1), 110–119. 10.9755/ejfa.v4i1.5116 [DOI] [Google Scholar]
- Magee A., MacHugh E., Edwards J. (2014). Interrogation of modern and ancient genomes reveals the complex domestic history of cattle. Anim. Front. 4 (3), 7–22. 10.2527/af.2014-0017 [DOI] [Google Scholar]
- Mapholi O., Maiwashe A., Matika O., Riggio V., Bishop C., MacNeil D., et al. (2016). Genome-wide association study of tick resistance in South African Nguni cattle. Ticks Tick-borne Dis. 7 (3), 487–497. 10.1016/j.ttbdis.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Mi H., Huang X., Muruganujan A., Tang H., Mills C., Kang D., et al. (2017). PANTHER version 11: expanded annotation data from Gene Ontology and reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 45 (D1), D183–D189. 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad M. (2015). Distribution of ixodid ticks among domestic and wild animals in central Iraq. Bull. Iraq Nat. Hist. Mus. 13 (2), 23–30. [Google Scholar]
- Moran B., Butler T., Moore G., MacHugh E., Creevey J. (2017). Differential gene expression in the endometrium reveals cytoskeletal and immunological genes in lactating dairy cows genetically divergent for fertility traits. Reprod. Fertil. Dev. 29 (2), 274–282. 10.1071/RD15128 [DOI] [PubMed] [Google Scholar]
- Mwai O., Hanotte O., Kwon Y., Cho S. (2015). African indigenous cattle: unique genetic resources in a rapidly changing world. Australas. J. Anim. Sci. 28 (7), 911. 10.5713/ajas.15.0002R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar A., Shamsaldain Q., Aboo N. (2014). Effect of using rations different in nitrogen sources on growth and blood parameters for growing local Sharabi calf under environment condition of Nineveh. Diyala Agric. Sci. J. 6 (2), 18–26. [Google Scholar]
- Nasser A., Al-dain Q., Abou N., Mahmood A. (2013). Using fenugreek seeds powder as a feed additive in rations of Sharabi local cows and its effect on some hematological and biochemical parameters. Iraqi J. Vet. Sci. 27 (1), 13–19. 10.33899/ijvs.2013.82854 [DOI] [Google Scholar]
- Nei M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89 (3), 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell J., Gurdasani D., Delaneau O., Pirastu N., Ulivi S., Cocca M., et al. (2014). A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 10 (4), e1004234. 10.1371/journal.pgen.1004234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogorevc J., Kunej T., Razpet A., Dovc P. (2009). Database of cattle candidate genes and genetic markers for milk production and mastitis. Anim. Genet. 40 (6), 832–851. 10.1111/j.1365-2052.2009.01921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros J. C. (2015). Venny. An interactive tool for comparing lists with Venn’s diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html.
- Osińska E., Wicik Z., Godlewski M., Pawłowski K., Majewska A., Mucha J., et al. (2014). Comparison of stem/progenitor cell number and transcriptomic profile in the mammary tissue of dairy and beef breed heifers. J. Appl. Genet. 55 (3), 383–395. 10.1007/s13353-014-0213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K., Mizukami Y., Watanabe K., Sakaguti S., Kadokawa H. (2017). Deep sequencing of the transcriptome in the anterior pituitary of heifers before and after ovulation. J. Vet. Med. Sci. 79 (6), 1003–1012. 10.1292/jvms.16-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pausch H., Wurmser C., Edel C., Emmerling R., Go¨tz U., Ruedi F. (2014). Exploiting whole genome sequence data for the identification of causal trait variants in cattle. Proc. 10th World Congress. Genetics Applied Livestock Production. Vancouver, Canada: WCGALP. 10.13140/2.1.1397.6966 [DOI] [Google Scholar]
- Pokharel K., Weldenegodguad M., Popov R., Honkatukia M., Huuki H., Lindeberg H., Kantanen J. (2018). Whole blood transcriptome analysis reveals footprints of cattle adaptation to sub-arctic conditions. Animal Genetics, 50 (3), 217-227. 10.1101/379925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Neto R., Sonstegard S., Liu E., Bickhart M., Da Silva V., Machado A., et al. (2013). Genomic divergence of zebu and taurine cattle identified through high-density SNP genotyping. BMC Genomics 14 (1), 876. 10.1186/1471-2164-14-876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M., Bender D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Human Genet. 81 (3), 559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven A., Cocks G., Kemper E., Chamberlain J., Vander Jagt J., Goddard E., et al. (2016). Targeted imputation of sequence variants and gene expression profiling identifies twelve candidate genes associated with lactation volume, composition and calving interval in dairy cattle. Mamm. Genome 27 (1–2), 81–97. 10.1007/s00335-015-9613-8 [DOI] [PubMed] [Google Scholar]
- Read J. (2015). Mesopotamia, British Museum. Eighth edition London, UK: British Museum Press. [Google Scholar]
- Richards P. (1989). Recent developments in trace element metabolism and function: role of metallothionein in copper and zinc metabolism. J. Nutr. 119 (7), 1062–1070. 10.1093/jn/119.7.1062 [DOI] [PubMed] [Google Scholar]
- Rischkowsky B., Pilling D. (2007). “The state of the world’s animal genetic resources for food and agriculture,” in Food and Agriculture Org Report (Rome: FAO publisher; ). Available online at: https://books.google.co.uk/books?hl=ar&lr=&id=Skpj197tU0oC&oi=fnd&pg=PR21&dq=The+state+of+the+world%27s+animal+genetic+resources+for+food+and+agriculture&ots=P54WgryASI&sig=l1GU-Ok6OcW1CX1cHNGpSSfluc4&redir_esc=yv=onepage&q=The%20state%20of%20the%20world’s%20animal%20genetic%20resources%20for%20food%20and%20agriculture&f=false. [Google Scholar]
- Ryu J., Lee C. (2014). Identification of contemporary selection signatures using composite log likelihood and their associations with marbling score in Korean cattle. Anim. Genet. 45 (6), 765–770. 10.1111/age.12209 [DOI] [PubMed] [Google Scholar]
- Sabeti C., Reich E., Higgins M., Levine Z., Richter J., Schaffner F., et al. (2002). Detecting recent positive selection in the human genome from haplotype structure. Nature 419 (6909), 832–837. 10.1038/nature01140 [DOI] [PubMed] [Google Scholar]
- Santana H., Junior O., Cesar M., Freua C., da Costa Gomes R., Silva L., et al. (2016). Copy number variations and genome-wide associations reveal putative genes and metabolic pathways involved with the feed conversion ratio in beef cattle. J. Appl. Genet. 57 (4), 495–504. 10.1007/s13353-016-0344-7 [DOI] [PubMed] [Google Scholar]
- Shabtay A. (2015). Adaptive traits of indigenous cattle breeds: the Mediterranean Baladi as a case study. Meat Sci. 109, 27–39. 10.1016/j.meatsci.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Taberlet P., Coissac E., Pansu J., Pompanon F. (2011). Conservation genetics of cattle, sheep and goats. Comptes Rendus Biol. 334 (3), 247–254. 10.1016/j.crvi.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Taye M., Lee W., Caetano-Anolles K., Dessie T., Hanotte O., Mwai O. A., et al. (2017). Whole genome detection of signature of positive selection in African cattle reveals selection for thermotolerance. Anim. Sci. J. 88 (12), 1889–1901. 10.1111/asj.12851 [DOI] [PubMed] [Google Scholar]
- Troy C., MacHugh D., Bailey J., Magee D., Loftus R., Cunningham P., et al. (2001). Genetic evidence for Near-Eastern origins of European cattle. Nature 410, 1088–91. 10.1038/35074088 [DOI] [PubMed] [Google Scholar]
- Urbinati I., Stafuzza B., Oliveira T., Chud S., Higa H., Regitano A., et al. (2016). Selection signatures in Canchim beef cattle. J. Anim. Sci. Biotechnol. 7, 29. 10.1186/s40104-016-0089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzaman M. R., Edea Z., Bhuiyan M. S. A., Walker J., Bhuiyan A. K. F. H., Kim K. S. (2014). Genome-wide single nucleotide polymorphism analyses reveal genetic diversity and structure of wild and domestic cattle in Bangladesh. Asian Australas. J. Anim. Sci. 27 (10), 1381. 10.5713/ajas.2014.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajana E., Barbato M., Colli L., Milanesi M., Rochat E., Fabrizi E., et al. (2018). Combining landscape genomics and ecological modelling to investigate local adaptation of indigenous Ugandan cattle to East Coast fever. Front. Genet. 9, 385. 10.3389/fgene.2018.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini C., Cardoso F., Baldi F., Freitas C., Aspilcueta-Borquis R., Santos A., et al. (2014). Association between single-nucleotide polymorphisms and milk production traits in buffalo. Genet. Mol. Res. 13 (4), 10256–68. 10.4238/2014.December.4.20 [DOI] [PubMed] [Google Scholar]
- Voight F., Kudaravalli S., Wen X., Pritchard K. (2006). A map of recent positive selection in the human genome. PLoS Biol. 4 (3), e72. 10.1371/journal.pbio.0040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Moisá S., Khan J., Wang J., Bu D., Loor J. (2012). MicroRNA expression patterns in the bovine mammary gland are affected by stage of lactation. J. Dairy Sci. 95 (11), 6529–6535. 10.3168/jds.2012-5748 [DOI] [PubMed] [Google Scholar]
- Wright S. (1951). The genetical structure of populations. Ann. Eugen. 15, 323–354. 10.1111/j.1469-1809.1949.tb02451.x [DOI] [PubMed] [Google Scholar]
- Xu L., Bickhart M., Cole J. B., Schroeder G., Song J., Tassell C., et al. (2014). Genomic signatures reveal new evidences for selection of important traits in domestic cattle. Mol. Biol. Evol. 32 (3), 711–725. 10.1093/molbev/msu333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonesaka R., Sasazaki S., Yasue H., Niwata S., Inayoshi Y., Mukai F., et al. (2016). Genetic structure and relationships of 16 Asian and European cattle populations using DigiTag2 assay. Anim. Sci. J. 87 (2), 190–196. 10.1111/asj.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeder M. (2008). Domestication and early agriculture in the Mediterranean Basin: origins, diffusion, and impact. PNAS 105 (33), 11597–11604. 10.1073/pnas.0801317105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Chen L., Zheng M., Li X., Wang L. (2014). Identification and bioinformatics analysis of microRNAs associated with stress and immune response in serum of heat-stressed and normal Holstein cows. Cell Stress Chaperones 19 (6), 973–981. 10.1007/s12192-014-0521-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.