Abstract
Outcomes with contemporary cochlear implants (CI) depend partly upon the survival and condition of the cochlear spiral ganglion (SG) neurons. Previous studies indicate that CI stimulation can ameliorate SG neural degeneration after deafness, and brain-derived neurotrophic factor (BDNF) delivered by an osmotic pump can further improve neural survival. However, direct infusion of BDNF elicits undesirable side effects, and osmotic pumps are impractical for clinical application. In this study, we explored the potential for two adeno-associated viral vectors (AAV) to elicit targeted neurotrophic factor expression in the cochlea and promote improved SG and radial nerve fiber survival. Juvenile cats were deafened prior to hearing onset by systemic aminoglycoside injections. Auditory brainstem responses showed profound hearing loss by 16–18 days postnatal. At ~ 4 weeks of age, AAV2-GFP (green fluorescent protein), AAV5-GFP, AAV2-hBDNF, or AAV5-hGDNF (glial-derived neurotrophic factor) was injected through the round window unilaterally. For GFP immunofluorescence, animals were studied ~ 4 weeks post-injection to assess cell types transfected and their distributions. AAV2-GFP immunofluorescence demonstrated strong expression of the GFP reporter gene in residual inner (IHCs), outer hair cells (OHCs), inner pillar cells, and in some SG neurons throughout the cochlea. AAV5-GFP elicited robust transduction of IHCs and some SG neurons, but few OHCs and supporting cells. After AAV-neurotrophic factor injections, animals were studied ~ 3 months post-injection to evaluate neural survival. AAV5-hGDNF elicited a modest neurotrophic effect, with 6 % higher SG density, but had no trophic effect on radial nerve fiber survival, and undesirable ectopic fiber sprouting occurred. AAV2-hBDNF elicited a similar 6 % increase in SG survival, but also resulted in greatly improved radial nerve fiber survival, with no ectopic fiber sprouting. A further study assessed whether AAV2-hBDNF neurotrophic effects would persist over longer post-injection periods. Animals examined 6 months after virus injection showed substantial neurotrophic effects, with 14 % higher SG density and greatly improved radial nerve fiber survival. Our results suggest that AAV-neurotrophin gene therapy can elicit expression of physiological concentrations of neurotrophins in the cochlea, supporting improved SG neuronal and radial nerve fiber survival while avoiding undesirable side effects. These studies also demonstrate the potential for application of cochlear gene therapy in a large mammalian cochlea comparable to the human cochlea and in an animal model of congenital/early acquired deafness.
Keywords: adeno-associated viral vector, auditory deprivation, auditory nerve, BDNF, GDNF, gene therapy, cochlear implant
INTRODUCTION
Contemporary cochlear implants (CIs) are highly successful for remediation of severe to profound sensorineural hearing loss, but there is still great variability in outcomes among CI recipients. Poor results are likely due, at least in part, to poor nerve survival after deafness. Consequently, there has been considerable recent interest in exploring potential therapies to ameliorate neuronal loss. When the hair cells are damaged in sensorineural hearing loss, the radial nerve fibers (distal axons) of the SG neurons retract, and the SG cell somata within the cochlear modiolus degenerate. Moreover, the SG neurons can be damaged directly by various insults and undergo primary degeneration independent of hair cell loss (Liberman et al. 2015; Kujawa and Liberman 2015; Fernandez et al. 2015) including in humans (Mackary et al. 2011; Viana et al. 2015).
Previous studies from our laboratory have shown that in early-deafened cats, electrical stimulation with a multichannel CI over several months results in significantly improved SG neural survival (Leake et al. 1999, 2008, 2007, 2013) compared to the contralateral ear. However, CI stimulation only prevents part of the SG degeneration that occurs after deafness (Leake et al. 2008, 2013), so there also has been interest in neurotrophic treatments that could be used with a CI to further enhance auditory nerve survival (for review, see Staecker et al. 2010). Of particular interest is the nerve growth factor family of proteins, or neurotrophins (NTs), including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5. The survival-promoting effects of depolarization and NTs have been studied in cultured SG neurons, and findings have demonstrated multiple intracellular signaling mechanisms (Hansen et al. 2001; Hegarty et al. 1997; Zha et al. 2001). L-type voltage-gated Ca2+ channels mediate the neurotrophic effects of depolarization through an autocrine neurotrophin mechanism, by cAMP production, and through Ca2+/calmodulin-dependent protein kinase (CaMk)-mediated phosphorylation of the transcription factor CREB. The neurotrophins BDNF and NT-3 are expressed by immature SG neurons and promote their survival by an autocrine mechanism that is additive with depolarization effects.
In numerous animals studies, infusion of exogenous NTs into the cochlea has been shown to improve SG survival after various insults (see Ramekers et al. 2012). Highly significant neurotrophic effects in deafened guinea pigs have been demonstrated with BDNF (Agterberg et al. 2009; Glueckert et al. 2008; Miller et al. 2007; Shepherd et al. 2008; Wise et al. 2005; Ramekers et al. 2015), as well as with neurotrophic factors such as glial cell line-derived neurotrophic factor or GDNF (Kanzaki et al. 2002; Maruyama et al. 2008; Yagi et al. 2000; Ylikoski et al. 1998) and fibroblast growth factor or FGF (Glueckert et al. 2008).
Because neurotrophins regulate neuronal differentiation and survival during development (Fariñas et al. 2001; Fritzsch et al. 1999; Gao et al. 1995; Korsching 1993; Rubel and Fritzsch 2002; Tessarollo et al. 2004; Yang et al. 2011), exogenous NTs are particularly effective in developing animals after early-onset hearing loss. In cats deafened as neonates and implanted at 4 weeks of age, intracochlear infusion of BDNF elicited markedly improved SG survival (Leake et al. 2011). Moreover, the survival-promoting effects of NTs may be enhanced by concomitant electrical stimulation and protective effects are maintained after cessation of NT infusion (Kanzaki et al. 2002; Shepherd et al. 2008, 2005; Leake et al. 2013; Ramekers et al. 2015). Combined BDNF + ES over a 6-month period “rescued” more than 50 % of the SG neurons and radial nerve fibers, relative to the contralateral untreated cochlea (Leake et al. 2013), which could be quite beneficial for CI outcomes. However, substantial ectopic and disorganized sprouting of radial fibers into the scala tympani also occurred, which would likely be deleterious to implant function due to degradation of their precise frequency organization.
Further, although findings to date are encouraging, osmotic pumps are impractical for clinical application because of concerns about infection and the disorganized sprouting of radial nerve fibers. Gene delivery to the cochlea may provide a means for a one-time injection of virus to elicit sustained and localized expression of neurotrophic factors by cells within the target tissue. Adeno-associated virus (AAV) offers significant advantages for this potential application, in that it is non-replicating and already has been shown to be safe and effective in the cochlea (Lustig and Akil 2012; Grieger and Samulski 2012). AAV-mediated expression should elicit more physiological NT concentrations, preventing deleterious side effects seen with high levels of BDNF from osmotic pumps (angiogenesis, increased peri-implant fibrosis, and especially disorganized sprouting of radial nerve fibers).
Several prior studies have demonstrated that neurotrophin cochlear gene therapy with either adenovirus (Nakaizumi et al. 2004; Wise et al. 2010) or AAV can promote improved survival of the cochlear SG neurons after deafness (Khalin et al. 2015; Pfingst et al. 2017). The previous studies, however, have been conducted in rodents. The present research assessed the potential for using gene therapy in the much larger cat cochlea, making results more relevant to the human cochlea. Another novel aspect of the present work is that studies were conducted in developing, neonatally deafened animals, because long-term nerve survival and improved outcomes are particularly important for pediatric CI recipients. Further, we compared the efficacy of two viral vectors, AAV5-GDNF and AAV2-BDNF, in eliciting neurotrophic effects in the deafened cochlea. Our hypothesis was that cochlear gene therapy may provide an efficient, clinically practical option for ameliorating neuronal degeneration following early deafness. Specifically, can a single injection of either AAV5-hGDNF or AAV2-hBDNF transfect a sufficient number of cells within the cochlea to elicit significant neurotrophic effects over the long term? Our data suggest that unilateral injections of either vector resulted in significant improvement in SG neuronal survival compared to the contralateral side. AAV2-hBDNF also resulted in substantially improved radial nerve fiber survival, which is likely important for optimum CI function. In contrast, AAV5-hGDNF did not elicit improve radial fiber survival, but did elicit undesirable disorganized sprouting in all injected cochleas. Based upon the more encouraging results with AAV2-BDNF, a further study evaluated the persistence of neurotrophic effects over a prolonged post-injection period of 6 months. This long-term study demonstrated a substantial, highly significant improvement in SG neuronal survival after a single injection of virus and marked improvement in radial fiber survival with no evidence of undesirable ectopic sprouting.
METHODS
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco and conformed to NIH guidelines. All animals included in this study were bred in closed colonies maintained at UCSF and at UC Davis.
Deafening Procedure
Cats were deafened by injections of an ototoxic aminoglycoside antibiotic, neomycin sulfate (60 mg/kg, SQ), SID beginning 3–6 days after birth (depending on birth weight) and continuing until kittens were 16 days old. Auditory brainstem response (ABR) testing was then performed (Leake et al. 2011). Briefly, acoustic stimuli (100 μs clicks, 20/s) were delivered by a canister headphone (STAX, model SMR-1/MK-2) through a hollow ear bar inserted into the external ear canal. If a profound hearing loss occurred (absence of ABR at 100 dB peak SPL), neomycin injections were discontinued. If residual hearing was observed, neomycin injections were continued in 2- to 3-day increments, and ABR testing was repeated until hearing loss was profound. The individual histories are presented in Table 1 for animals in the AAV-NT groups. Neomycin administration varied from 10 to 13 days for individual subjects.
Table 1
| a. Neonatally Deafened Cats 3 Months after AAV5-hGNDF Injections | |||||||
| Subject | # days neomycin | Neomycin started | Age at injection (weeks) | AAV5-GDNF (weeks) | Age at study (weeks) | SG density (% normal) | |
| AAV5-GDNF | Contra. | ||||||
| K400 | 10 | P5 | 6 | 12 | 18 | 54 % | 44 % |
| K409 | 13 | P6 | 4 | 14 | 18 | 64 % | 56 % |
| K410 | 13 | P6 | 4 | 14 | 18 | 57 % | 49 % |
| K412 | 13 | P6 | 4 | 13 | 17 | 39 % | 40 % |
| K413 | 12 | P5 | 5 | 14 | 19 | 60 % | 50 % |
| Mean (n = 5) | 12 | P6 | 4.5 weeks | 13.5 weeks | 18 weeks | 55 % | 49 % |
| b. Neonatally Deafened Cats ~3 Months after AAV2-hBNDF Injections | |||||||
| Subject | # days neomycin | Neomycin started | Age at injection (weeks) | AAV2 BDNF (weeks) | Age at study (weeks) | SG density (% normal) | |
| AAV2-BDNF | Contra. | ||||||
| K399 | 10 | P5 | 3 | 14 | 17 | 76 % | 71 % |
| K401 | 10 | P5 | 3 | 14 | 17 | 74 % | 72 % |
| K403 | 10 | P5 | 3 | 14 | 17 | 48 % | 38 % |
| K407 | 13 | P5 | 4 | 9 | 13 | 50 % | 47 % |
| K419 | 11 | P5 | 4 | 14 | 18 | 68 % | 57 % |
| Mean (n = 5) | 11 | P5 | 3.5 weeks | 13 weeks | 16 weeks | 63 % | 57 % |
| c. Neonatally Deafened Cats ~6 Months after AAV2-hBDNF Injections | |||||||
| Subject | # days neomycin | Neomycin started | Age at injection (weeks) | AAV2 BDNF (weeks) | Age at study (weeks) | SG density (% normal) | |
| AAV2-BDNF | Contra. | ||||||
| K414 | 12 | P4 | 4 | 22 | 26 | 58 % | 40 % |
| K415 | 12 | P4 | 4 | 24 | 28 | 36 % | 39 % |
| K418 | 11 | P5 | 4 | 25 | 26 | 74 % | 37 % |
| K421 | 12 | P5 | 4 | 22 | 26 | 47 % | 39 % |
| Mean (n = 4) | 12 | P4.5 | 4 weeks | 23 weeks | 27 weeks | 53 % | 39 % |
Viral Vectors and Method of Injection
Recombinant AAV serotype 5 vectors containing cDNA encoding either green fluorescent protein (GFP) or human GDNF under control of the CBA promoter were produced and provided to the investigators by uniQure Biopharma b.v. (Amsterdam, The Netherlands). The AAV5-GFP (final titer of 1.4E14 GC/ml) and AAV5-GDNF (1.8E14 GC/ml) were generated according to dedicated procedures using the baculo-platform of uniQure.
Recombinant AAV serotype 2 encoding either GFP (2 × 10E12 GC/ml) or human BDNF (3 × 10E12 GC/ml) under control of the CGA promoter was generated by triple transfection of HEK293 cells with CsCL purification by the Vector Core at the University of North Carolina. The BDNF vectors were donated to the investigators by Dr. Krystof Bankiewicz, UCSF Department of Neurological Surgery.
Our group previously developed cochlear implant electrodes containing a miniature cannula for combined intracochlear drug delivery and electrical stimulation in chronically implanted cats (Rebscher et al. 2007). These electrodes provided the basis for fabricating cannulas used for intracochlear virus delivery. Each cannula consisted of a 20-mm length of fine teflon tubing (Sub-Lite-Wall™ PTFE extruded tubing, 0.010” OD, 0.005” ID; Zeus Industrial Products, Inc.), one end of which was sealed into a ~ 10-in. length of larger Silastic™ silicone elastomer tubing (0.037” OD, 0.020” ID; Dow Corning Inc.). During fabrication, approximately 5–6 mm of the fine PTFE tubing was threaded into the larger silicone tubing while RTV silicone adhesive (MED-1137; NuSil Technology) was applied. The external tip of the PTFE tubing was beveled at approximately 45 ° to facilitate insertion. The distal end of the Silastic tubing was attached to a 25-ga blunt tip needle, and the cannula and needle assembly was packaged for sterilization (SterradR sterilization system).
Deafened animals were injected unilaterally with a viral vector at ~ 4 weeks of age. Buprenorphine (0.01–0.03 mg/kg) was administered as a preanesthetic 30–60 min prior to surgery. A surgical plane of anesthesia was induced with isoflurane; animals were intubated, and anesthesia was maintained with isoflurane throughout the procedure. An aseptic surgical procedure was performed. A small incision was made in the skin behind the right pinna (after infiltration with bupivacaine). The neck muscles were reflected and the bulla opened to expose the round window of the cochlea. The cannula/needle assembly was mounted on a glass gas-tight 50-μl syringe, and a 10-μl aliquot of viral vector was drawn up into the cannula. Both the PTFE and silicone tubing were translucent, allowing observation of the movement of the fluid meniscus; the 10-μl aliquot filled about 4 in. of the silicone tubing. In these young animals, it was necessary to cauterize fine vessels on the round window membrane to prevent bleeding into the scala tympani. The fine tip of a high-temperature, ophthalmic cautery pen (MedlineR) was approximated close to the vessel(s), but not touching the surface, until blanching occurred. A 30-ga needle was then used to create a small opening in the round window membrane and small wedges of Weck-CelR cellulose spears (Beaver-Visitec International) were used to wick the perilymph, until an air bubble formed under the membrane, inside the scala tympani. (This was done to decrease fluid loss from the round window during the injection of the viral vector. There was substantial individual variability in the amount of perilymph produced after opening the round window and during injections, which could account for some variability in efficacy of transfection among subjects.) Just prior to use, the small PTFE tubing was gently formed into a curve (similar to the curve of the feline CI electrodes used previously) to facilitate deeper insertion to a depth of 6–8 mm. This positioned the tip at about the midpoint of the cochlear spiral. (Basilar membrane length is about 24 mm in cats, but a large hook region of 4–5 mm winds around the round window basal to the point of insertion; thus, an insertion of 7–8 mm placed the cannula tip at ~ 12 mm.) The viral vector was manually injected slowly (over a 15–25-min period). The cannula was left in place for 5 min, then gently withdrawn. The round window membrane was sealed with a small piece of fat/connective tissue glued to the inferior margin of the window (Tissuemend II™ Veterinary Products Laboratories, Phoenix, AZ). The incision was closed in layers. Meloxicam (0.1–0.3 mg/kg) was administered at the time of the procedure and 24 h later for pre-emptive analgesia. After recovery from anesthesia, animals were returned to the queen. They continued to nurse and gain weight normally over the ensuing survival period prior to study.
Immunocytochemistry for Demonstration of GFP
Initial experiments using AAV5-GFP and AAV2-GFP evaluated and compared the efficacy of transfection of the viral vectors. Injections were done when animals were about 4 weeks old, and the cochlear specimens were examined about 4 weeks later. Immunocytochemistry was performed for detection of the transgene using protocols reported previously (Akil et al. 2019), with procedures adapted for the larger size and greater amount/density of bone of the cat cochlea. Fixation of the cochlear specimens was performed by transcochlear perfusion of 4 % paraformaldehyde (0.1 M phosphate buffer, pH 7.4) via the round and oval windows, in vivo with the animal heavily anesthetized. The animals were then humanely euthanized by an overdose of sodium pentobarbital, and transcardiac perfusion performed. After immersion in the same fixative (2 h, 4 °C), cochleae were rinsed thoroughly in phosphate buffer. Specimens were dissected (the otic capsule bone above the scala vestibuli was thinned using diamond dental burrs, then carefully removed; the round window was enlarged and the eighth nerve was removed) to facilitate full and rapid decalcification (5 % EDTA in 0.1 M PBS, 2–3 days). Specimens were then soaked in 30 % sucrose until saturated, embedded in OCT Tissue Tek Compound (Miles Scientific), and cryo-sectioned at 10 mm in a plane parallel to the modiolus.
For immunofluorescence staining, sections from injected and control cochleae were incubated for 1 h in PB containing 0.25 % Triton X-100 and 5 % normal goat serum (blocking buffer), then incubated overnight at 4 °C with a rabbit anti-GFP antibody (Invitrogen Cat# A11122 or Fitzgerald Cat #20R-GR011 diluted to 1:250 in PB). The sections were rinsed twice for 10 min with PB, incubated for 2 h in goat anti-rabbit IgG conjugated to Cy2 (Jackson ImmunoResearch, 111- 165-003) diluted to 1:2000 in PB, and rinsed again in PB twice for 10 min. After mounting on glass slides (mounting solution containing DAPI), sections were observed under an Olympus model 1X71 microscope with confocal immunofluorescence to assess efficacy of transduction, types of cells transduced, and their distribution throughout the cochlea.
Histological Preparation of Cochleae for Evaluation of Neurotrophic Effects
Methods for cochlear histology and analyses of survival of SG neurons and radial nerve fibers have been described previously (Leake et al. 2011, 2013). Briefly, animals were deeply anesthetized (sodium pentobarbital, I.V.), the stapes was removed and round window opened, and cochleae were perfused directly with mixed aldehyde fixative (1.5 % glutaraldehyde and 2.5 % paraformaldehyde in 0.1 M phosphate buffer, pH 7.4). An overdose of sodium pentobarbital was administered, and transcardiac perfusion was performed with the same fixative. The temporal bones were placed in fixative overnight, then transferred to phosphate buffer. The cochlea was isolated, the otic capsule bone was thinned using diamond dental burrs and opened over the scala vestibuli in several locations, and the cochlear nerve removed to facilitate decalcification and embedding. Specimens were post-fixed in 1 % phosphate-buffered osmium tetroxide with 1.5 % potassium ferricyanide (to enhance contrast), decalcified in 0.2 M EDTA for 36–48 h, dehydrated, and embedded in Aradite 502 resin (Electron Microscopy Sciences, Fort Washington, PA). Thick surface preparations containing the organ of Corti and SG were mounted with epoxy resin on glass slides. The basilar membrane was measured, and small segments (< 1 mm) of the organ of Corti and adjacent SG were excised from each 10 % cochlear sector. These specimens were remounted for sectioning in the radial plane. For morphometric analyses, sections were cut at a thickness of 5 μm, stained with toluidine blue, and examined with a Zeiss Axioskop II. At selected locations, additional semi-thin (2 μm) sections were cut for photomicroscopy to illustrate specific histological findings. The organ of Corti was evaluated for the presence of hair cells, condition of supporting cells, and evidence of infection and/or inflammatory response.
Histological and Morphometric Analyses
Morphometric analyses assessed SG neuron survival, cell soma size, and radial nerve fiber density and size.
Unbiased Stereology to Assess Spiral Ganglion Cell Numerical Density
Survival of the SG neurons was assessed by morphometric analysis of the density of cell somata in Rosenthal’s canal at nine cochlear locations, using paired comparisons between the injected contralateral cochleae. Many previous studies (Agterberg et al. 2009, 2008; Glueckert et al. 2008; Shepherd et al. 2008; Leake et al. 2011, 2013) have reported that direct intracochlear infusion of BDNF results in larger SG cell somata. Therefore, we utilized a physical disector stereological method that accurately determines the number of objects (nucleoli of SG neurons) in a given volume of tissue, independent of their size (Mouton 2002). The term “numerical density” is used to distinguish these labor-intensive stereological measurements from more usual cell density data obtained by simple counts of the number of cells or nuclei per unit area of tissue. Four sets of six serial sections (5 μm thickness) were collected at each of nine locations representing 10 % sectors of the cochlea. In adjacent serial sections, counts were made to determine the number of “new” nucleoli appearing only in the second section. Images were acquired using an Axiocam MRc5 camera, and a 40X/0.95 NA lens. In the native files, a 330 by 250 μm field was equal to 2584 by 1936 pixels and the system acquired images at 150 dpi (resolution, 7 pixels/μm). Pairs of serial sections were imaged at a resolution of 3.2 pixels/μm, 300 dpi, and a screen magnification of ~ ×3000. In Adobe Photoshop™ TIFF images, the “wand” tool was used to select all cells with a visible nucleolus in the reference section, preserving cytoplasmic boundaries but excluding the nucleus, and other reference structures (e.g., vessels) were also selected. The selected structures were copied, creating a template that was inserted into a file with the image of the second serial section. The two images were then matched and aligned, and only the “new” nucleoli in the second section were counted. A minimum of four pairs of serial sections (~ 50 μm separation between pairs) were analyzed at each location to obtain a data set of at least 50 cells. In the same images, the “magnetic lasso” tool was used to outline Rosenthal’s canal. The canal outlines and images of cells were converted to monochrome, imported into NIH ImageJ (version 1.37v), and canal areas (used to calculate area fraction/numerical density) and cell areas were measured using the “particle analysis” function. Image selection and all analyses were performed with the observer blinded to experimental conditions.
For overall group comparisons to assess the effects of viral vectors on SG cell survival, the mean number of nucleoli in a [100 μm]3 volume of Rosenthal’s canal was calculated for each of nine cochlear sectors.
Measurements of Cross-Sectional Areas of Spiral Ganglion Cells
Measurements of SG cell somata areas were made at the same time and in the same images as the SG numerical densities analysis described above. The Photoshop images were imported as Tiff files into ImageJ. The “analyze particles” function was used to estimate cross-sectional areas of all SG cell somata containing a nucleolus, as previously selected for the disector analysis. The perimeter of the SG cell cytoplasmic boundary was measured, excluding the somatic myelin and satellite cells. Approximately 50 cells were measured for each cochlear sector. SG cell area and numerical density data for normal adult cats obtained previously using these same methods (Leake et al. 2011) were used to express data as percentages of normal.
Radial Nerve Fiber Counts and Cross-Sectional Areas
Radial nerve fiber survival and size were evaluated in a separate series of sections cut orthogonal to the radial plane. Three cochlear regions in the basal turn at about 3–6, 7–9, and 11–13 mm (matched between the left and right sides for each animal) were selected in which the blocks used for SG cell counts still had remaining tissue. Serial 5 μm sections were cut starting at the habenula perferata (edge of basilar membrane) and proceeding 135 μm inward toward the modiolus. Every third section was imaged (7.6 pixels per μm, 150 dpi), providing a total of nine samples per location. In Photoshop™, the “rectangular marquee” tool (fixed size) was used to count the number of cross-sectional profiles of fibers in a 100-μm length of the osseous spiral lamina. A reference structure was identified in all nine sections of the series to ensure that counts sampled the same region. Data were averaged for the 9 samples and multiplied by 10 to estimate of the number of fibers per millimeter. Fiber size was assessed in these same 9 samples, using the “wand” tool to select axonal profiles of the first 10 fibers at one side of each image that had a roughly cross-sectional (round) profile. The selected profiles were imported into ImageJ and their areas measured using the “wand” tool, which outlined the outer perimeter of the myelin by demarcating the boundary between the higher pixel-density of the myelin compared to the adjacent tissue. Values were averaged for nine sections at each cochlear location.
It is possible that these fiber counts may include both peripheral axons of SG neurons and terminal branches of myelinated medial olivocochlear (efferent) fibers. However, a prior study showing sprouting after direct delivery of NT to the cochlea used immunohistochemical techniques to demonstrate that the sprouted fibers were afferent peripheral processes of SG neurons, not efferents (Glueckert et al. 2008). Further, because the efferent fibers are much fewer in number, even if they are present it is unlikely to significantly affect the results.
Statistical Analyses
For most of the statistical analyses performed, the data were normally distributed, and showed equal variance among the samples and thus did not violate the assumptions of the tests; any exceptions are noted in the text. All statistical analyses were performed using Sigma Stat™ (version 2.03).
RESULTS
GFP Immunofluorescence
Initial experiments conducted with AAV5-GFP and AAV2-GFP evaluated and compared the transfection efficacy of the two viral serotypes. Virus injections were made when animals were 4–5 weeks of age, and cochleae were examined 4 weeks later. We hypothesized that findings would be similar to previous studies conducted in newborn mice after AAV5 injections (Akil et al. 2019). Specifically, we expected that any surviving inner and outer hair cells in these deafened cochleae, supporting cells (particularly inner phalangeal cells), and spiral ganglion neurons would express the transgene throughout the cochlea after injections made through the round window.
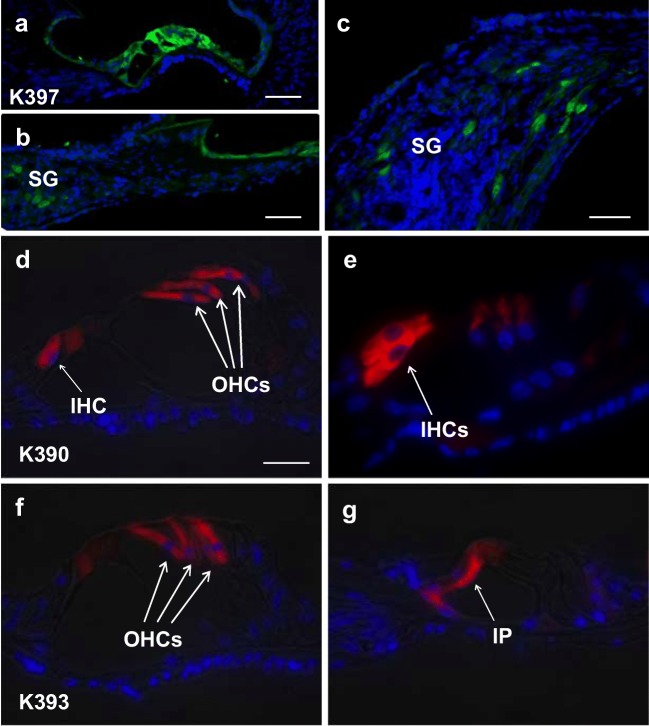
Figure 1a–c shows immunocytochemistry for detection of the GFP transgene in cochlear sections from a representative neonatally deafened cat 4 weeks after injection of AAV5-GFP. Transfected cells were distributed throughout the cochlea. GFP expression was seen in the majority of the supporting cells, but only a relatively modest number (roughly 5–10 %) of the SG neurons expressed GFP. AAV5-GFP also elicited strong transduction in most of the residual inner hair cells (IHCs) that survived only at the apex in these deafened animals, but few outer hair cells (OHCs) expressed GFP. Following AAV2-GFP injections (Fig. 1d–g), immunofluorescence demonstrated strong expression of GFP in the majority of both inner IHCs and OHCs, inner and outer pillar cells, and in about 5–10 % of SG cells, similar to the extent of SG transfection seen with AAV5.
Fig. 1.
Immunofluorescence (green) in cochlear sections from a neonatally deafened cat examined 4 weeks after intracochlear injection of AV5-GFP at 4 weeks of age. Transfection of supporting cells (a) and some of the spiral ganglion neurons (SG) was observed throughout the cochlea, as demonstrated in a low magnification image from the basal coil (b) and at higher magnification in a section from the apex (c). Cochlear sections from two other neonatally deafened cats, K390 (d, e) and K393 (f, g), studied ~ 5 weeks after cochlear injections of AAV2-GFP made in deafened animals at 4 weeks of age. Immunofluorescence (red) demonstrates strong viral transfection and robust GFP expression in residual inner (IHC) and outer hair cells (OHC) near the cochlear apex and in some inner pillar cells (IP)
In summary, AAV-GFP (both serotypes) elicited GFP expression in IHCs, outer pillar cells and some SG neurons. AAV2-GFP additionally transduced OHCs, and some inner pillar cells.
AAV-Neurotrophin Experimental Groups
The potential for neurotrophin expression elicited by AAV transfection in the cochlea to promote improved SG survival was assessed in three groups of neonatally deafened cats. The experimental histories for individual animals are presented in Table 1. Virus injections were again performed when animals were about 4 weeks old. Five animals were included in the AAV5-hGDNF and AAV2-hBDNF experimental groups examined about 3 months after virus injections. In addition, a longer-term study included four cats studied roughly 6 months after AAV2-hBDNF injections.
AAV5-hGDNF Elicited Improved SG Survival in Early-Deafened Animals 3 Months Post-Injection, but Potentially Deleterious Side Effects Also Occurred
Evaluation of the organ of Corti in histological sections confirmed that no intact hair cells were present throughout most of the cochlear spiral in any of these deafened animals, although a few surviving hair cells were often seen in the apical coil. Moreover, it is important to note that no evidence of infection or inflammation was observed in the cochlea after injection of the AAV5-hGDNF virus directly into the scala tympani.
The physical disector method was used to estimate the numerical density of SG neurons in Rosenthal’s canal, with data collected for nine cochlear sectors from base to apex. The data for animals examined 3 months after AAV5-hGDNF (Fig. 2) indicate that SG density in the injected ears was greater than contralateral in seven of nine cochlear sectors. The overall mean cell density after hGDNF treatment was about 55 % of normal, compared to 49 % in the contralateral cochleae, representing rescue of about 6 % of the SG population. Calculated as percentage increase normalized to contralateral, SG neuronal survival was improved by about 12 % in the injected ears. Table 1a presents the overall mean SG numerical density values for individual animals in the AAV5-hGDNF group. SG survival in the control non-injected ears varied from 40 to 56 % of normal, reflecting variability in the ototoxic drug effect. Four of the five animals showed higher SG survival in the injected ear, but one subject (K412) had equivalent survival on both sides. Consequently, individual variability was even greater in the hGDNF-treated ears with SG survival ranging from 39 to 64 % of normal. But if we eliminate the one animal in which the injection apparently failed, then individual variability is substantially less, ranging from 54 to 64 % of normal.
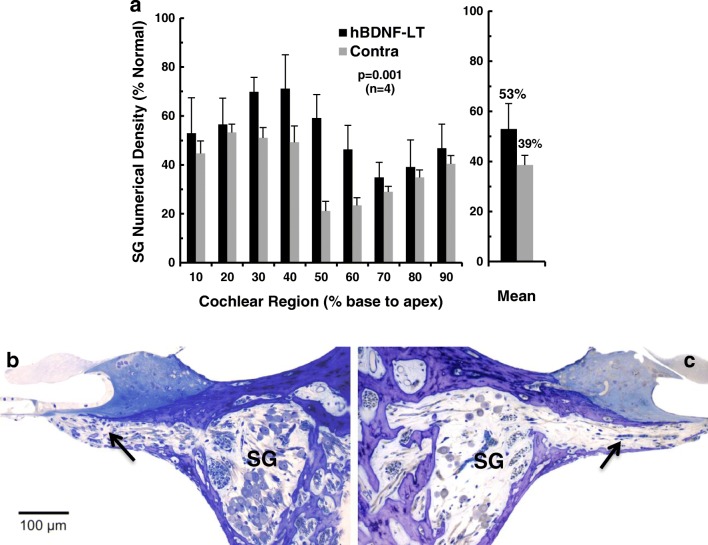
Fig. 2.
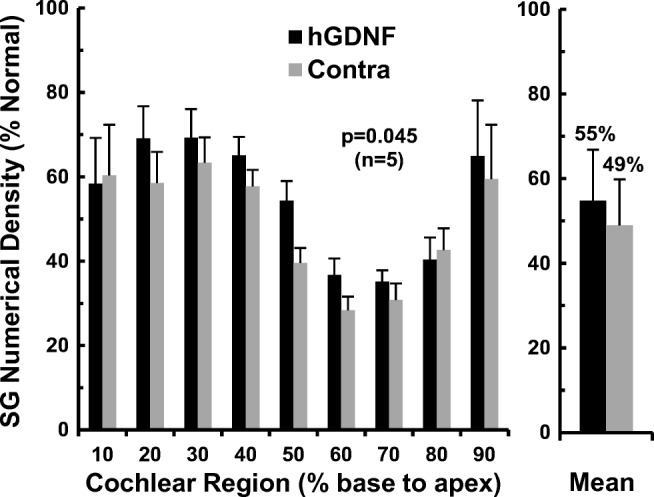
Numerical density of SG neurons 3 months after unilateral injections of AAV5-hGDNF. Data are shown for 10 % cochlear sectors from base to apex, with values expressed as percentage of normal for the group of five cats. The injected cochleae show higher densities for most cochlear intervals compared to paired data for the contralateral ears, although significant neural degeneration occurred on both sides. Averaged over all cochlear sectors, SG cell survival is modestly, but significantly, improved at about 55 % of normal after AAV5-hGDNF, and about 49 % on the opposite side (p = 0.045). (Error bars indicate standard errors of the means)
To test whether this modest improvement in SG neuronal survival elicited by injection of AAV5-hGDNF was statistically significant, the overall mean SG numerical densities in the injected and contralateral cochleae were compared using a paired Student’s t test. This indicated that the difference was statistically significant (t(5) = 2.89, p = 0.045)).
Because BDNF has been reported to elicit substantial increases in SG cell size (Leake et al. 2011, 2013), we evaluated cross-sectional areas of SG cell somata in the same histological sections used to determine numerical density. After AAV5-hGDNF injections, the mean SG cell area was 208 μm2 (78 % of normal), as compared to 211 μm2 (79 % of normal) in the contralateral, non-injected ears (data not shown). Thus, although the SG neurons in these early-deafened animals were substantially smaller than normal, cell size was virtually identical between sides.
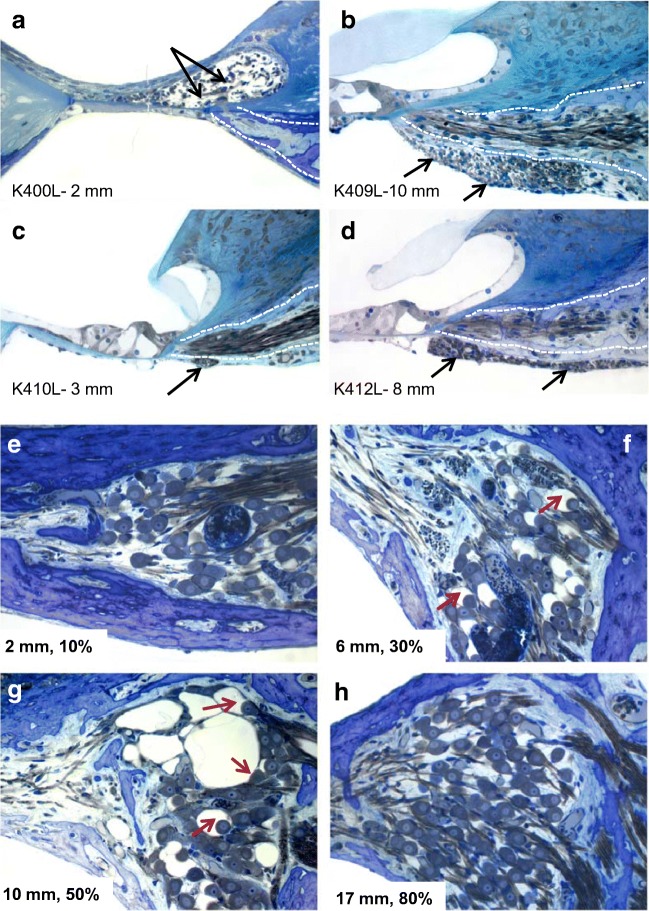
In the normal cochlea, the myelinated radial nerve fibers pass from their parent SG neurons in the modiolus through the osseous spiral lamina; at the habenula perforata, they become unmyelinated and course up into the organ of Corti where they innervate the hair cells. In these deafened animals, ectopic (i.e., found in locations where they do not normally occur) myelinated radial nerve fibers were observed in the AAV5-hGDNF-injected cochleae of all five cats examined, whereas no ectopic fibers were observed in the contralateral ears. As illustrated in Fig. 3(a–d), these fibers either sprouted into the region of the degenerated organ of Corti (K400) or grew downward through the lower bony plate of the osseous spiral lamina into the scala tympani, forming fascicles under the basilar membrane and/or osseous spiral lamina (K409, K410, K412, K413). In all of these cochleae, with the exception of K410, the sprouting observed was substantial, with up to roughly 200–300 fibers coursing over several millimeters, and many fibers had round profiles, indicating a longitudinal orientation. Such extensive sprouting could clearly degrade the normal strict radial organization of these fibers, which is the basis of the precise frequency organization of the cochlea, and could be detrimental to cochlear implant function. This potentially deleterious side effect of hGDNF may be due to the very high viral titer and transfection efficacy of the AAV5-hGDNF viral vector (see Discussion).
Fig. 3.
In the normal cochlea, the radial nerve fibers pass from their parent SG neuron in the modiolus through the osseous spiral lamina (dotted white lines) to innervate the hair cells in the organ of Corti. In deafened cats examined 3 months after injection of AAV5-hGDNF, ectopic radial nerve fibers (black arrows) were observed in all injected cochleae, as shown in exemplary histological sections from four animals. Ectopic fibers sprouted either into the region of the degenerated organ of Corti as illustrated in subject K400 (a) or down into the scala tympani in fascicles coursing under the osseous spiral lamina as shown in three other animals, K409, K410, K412 (b–d). Such sprouting clearly would degrade the normal strict radial organization of these fibers, which is the basis of the precise frequency organization of the cochlea, and could be detrimental to cochlear implant function. e–h SG neurons in a cochlea examined 3 months after AAV5-hGDNF injection (K400L) are shown in histological sections from four cochlear regions. In the basal (2 mm) and apical regions (17 mm), some SG cell loss is seen due to ototoxic deafening, but the remaining cells appear normal. In the middle cochlear sectors (6 and 10 mm) nearest the virus injection site, satellite cells exhibit vacuoles and appear distended and hypertrophied (arrows). We hypothesize that this pathology is the result of hGDNF overexpression elicited with the very high titer of this virus
Another interesting finding after AAV5-hGDNF injections was that varying degrees of pathology were observed in the SG of all five injected ears. This pathology was limited to a 3–5-mm region in the middle of the cochlea, near where the tip of the cannula delivered the virus. Figure 3e–h illustrates this finding in one subject (K400), in histological sections of SG neurons from several different regions of the injected cochlea. In the basal (Fig. 3e) and apical cochlear regions (Fig. 3h), some SG cell loss was seen, but remaining cells appeared relatively normal. However, in the middle cochlear sectors (6 and 10 mm from the base, Fig. 3f, g), many SG cell bodies appear very shrunken and pyknotic and the satellite cells that surround them exhibit severe vacuoles and appear distended and hypertrophied. Again, we hypothesize that this pathology is the result of hGDNF overexpression elicited by the extremely high titer of the uniQure viral vector.
AAV2-hBDNF Promotes Improved Neuronal Survival in the Deafened Cochlea with No Deleterious Side Effects
In the second experimental group studied ~ 3 months after AAV2-hBDNF injections, histology of the organ of Corti again confirmed the absence of intact hair cells throughout the cochlear spiral, with the exception of a few surviving hair cells in the extreme apex. Further, there was no evidence of infection or inflammation in the cochlea after AAV2-hBDNF injections (as was also true for AAV5-hGDNF).
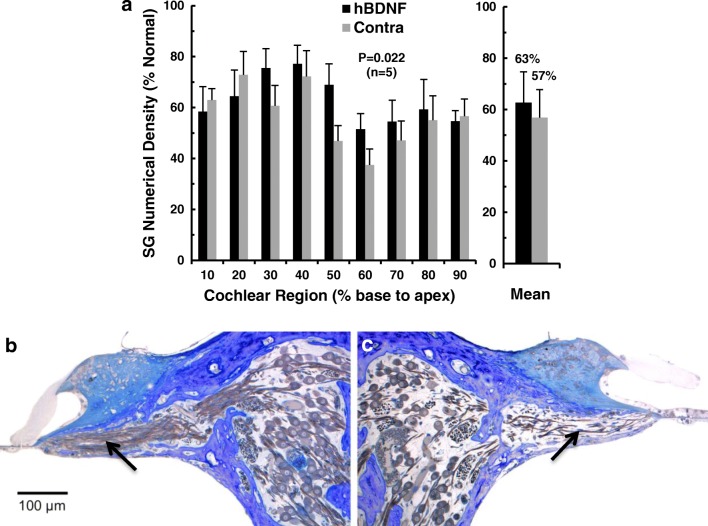
Figure 4a illustrates the physical dissector data estimating SG survival for the five animals in this AAV2-hBDNF group. Numerical density is shown for nine cochlear sectors from base to apex, along with overall means for injected and control cochleae. Survival of SG neurons is higher in the AAV2-hBDNF-treated ears than on the contralateral side throughout most of the cochlea, but not in the two most basal sectors. (The lack of an effect here is likely due to damage caused by cauterizing vessels on the round window, done to prevent bleeding during virus injections in these young animals. This 3-month AAV2-hBDNF group was the first experimental series the investigators completed. After observing these initial results, in later experiments the round window was opened with a 30-ga needle and perilymph removed to create a non-conducting air bubble behind the round window prior to cauterizing the vessels, thereby preventing this damage.) Interestingly, the greatest improvement in SG survival occurred in the middle cochlear sectors (40–50 and 50–60 % from the base), near where the tip of the cannula delivered the viral vector and thus where the highest concentration of virus would be expected. Averaged over the entire cochlea, mean SG survival for the five subjects was 63 % of normal for the injected cochleae and 57 % for the contralateral side, representing rescue of about 6 % of the SG population. Expressed as percentage increase normalized to survival on the contralateral side, SG neuronal survival was improved by about 11 % after AAV2-BDNF injections.
Fig. 4.
a Numerical densities of SG neurons in five cats examined ~ 3 months after unilateral injections of AAV2-hBDNF, with data expressed as percentage of normal. Averaged over all cochlear sectors, SG cell survival is significantly, but modestly, improved at about 63 % of normal after AAV2-hBDNF treatment as compared to about 57 % on the opposite side (p = 0.022). (Error bars indicate standard errors of the means.) b Light microscopic images of histological sections of the cochleae from one neonatally deafened animal (K403) illustrating neurotrophic effects 14 weeks after unilateral injection of AAV2-hBDF. The 40–50 % sector of the injected left cochlea (b) and the paired region from the contralateral control ear (c) illustrate the higher density of SG cells in Rosenthal’s canal (roughly 50 % of normal in the injected cochlea here and about 25 % for the control in this sector). Maintenance of a greater number of radial nerve fibers within the osseous spiral lamina (arrows) is also apparent after AAV2-hBDNF treatment. Note the lack of evidence of inflammation within the scala tympani. Scale bar = 100 μm
Table 1b presents the overall mean SG densities for individual animals in the AAV2-hBDNF group. SG survival in the five hBDNF-treated ears ranged from 48 to 76 % of normal, whereas survival in the control non-injected ears varied from 38 to 71 % of normal, reflecting substantial individual variability in the ototoxic drug effect. Three of the five animals showed higher SG survival on the injected side, but two animals (K401 and K407) showed roughly equivalent survival on the two sides. The modest improvement in SG survival elicited by injection of AAV2-hBDNF was statistically significant (t (5) = 3.644, p = 0.022; Student’s t test, paired).
SG cell size was evaluated in the same histological sections used for SG density estimates. After AAV2-hBDNF injections, the mean cross-sectional area of SG cells measured 238 μm2 (89 % of normal) in the injected cochleae and 232 μm2 (87 % of normal) for contralateral ears (data not shown). Thus, as for AAV5-hGDNF, SG cells in these early deafened cats were substantially smaller than normal, but there was no difference between the treated and contralateral ears. (Because there is a systematic difference in cell size with larger SG cells at the cochlear base and progressively smaller cells toward the apex [Leake et al. 2013], we used a two-way ANOVA matching the cell size data from the nine cochlear intervals to compare the injected vs control: F(1,72) = 1.803, p = 0.184).
The effects of AAV2-hBDNF injections are illustrated in histological sections (Fig. 4b, c) from the middle of the cochlea (40–50 % from the base). Note that this representative ear shows no evidence of infection or inflammatory response to the viral injection. The higher density of SG cells within Rosenthal’s canal is evident in the treated ear (Fig. 4b; ~ 50 % of normal SG density) as compared to the same region in the contralateral ear (Fig. 4c; 26 % of normal). Careful inspection of the osseous spiral lamina in these images also suggests a higher density of radial nerve fibers in the injected cochlea as compared to the opposite side (arrows), but no ectopic radial nerve fibers were observed in this, or any of the AAV2-hBDNF-injected cochleae.
Radial Nerve Fiber Number, Size, and Distribution 3 Months After Injection of AAV5-hGDNF or AAV2-hBDNF
The survival of peripheral processes of the SG neurons—the radial nerve fibers within the osseous spiral lamina—is likely to be beneficial to optimum function of a cochlear implant. These fibers were assessed quantitatively in both AAV5-hGDNF and AAV2-hBDNF series described above. Tangential cross-sections of the osseous spiral lamina (orthogonal to the radial plane) were cut from a subset of the blocks used for SG numerical density assessments as described previously (Leake et al. 2011, 2013). Specifically, samples were evaluated at three different cochlear locations, roughly 5, 8, and 12 mm from the cochlear base, corresponding to 20–30, 30–40, and 50–60 % sectors, respectively. The number of cross-sectional profiles of radial nerve fibers in a 1-mm sector was calculated and normal data from the same locations were used to express the data as percentage of normal.
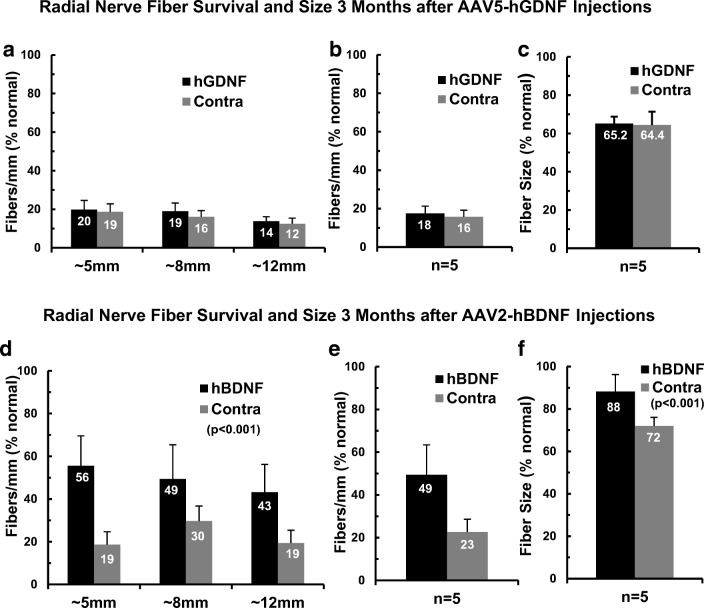
In the subjects examined after injection of AAV5-hGDNF, the survival of radial nerve fibers was very slightly higher on the injected side at all three locations (Fig. 5a). The overall mean values (averaged for the three cochlear sectors) were 436 fibers/mm or 18 % of normal in the injected ears as compared 380 fibers/mm or 16 % of normal on the control side (Fig. 5b). But this small difference was not statistically significant (two-way ANOVA on fiber density at three cochlear sectors: F(1,24) = 1.24; p = 0.276). The cross-sectional areas of the radial nerve fibers were determined in the same images, and fiber size was virtually identical between the two sides, with mean values of 65.2 and 64.4 % of normal for the injected and deafened control sides, respectively (Fig. 5c).
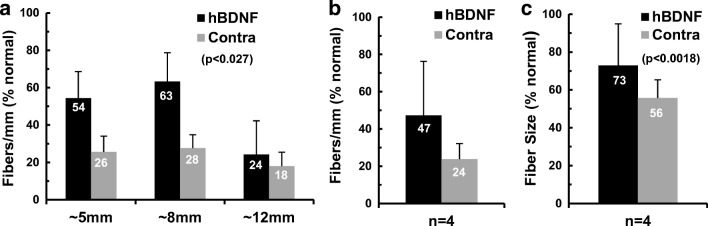
Fig. 5.
Histological sections were cut tangential to the usual radial plane to evaluate survival of the peripheral axons of the SG neurons (radial nerve fibers). a Counts of radial nerve fiber profiles at three different cochlear locations within the basal turn demonstrated severe degeneration and roughly equivalent numbers of fibers in the cochleae examined 3 months after AAV5-hGDNF injections as compared to contralateral. b Averaged over the three sectors, there was no significant left-right difference in fiber survival with means of 18 % of normal vs 16 %, respectively. c The cross-sectional areas of the surviving radial nerve fibers were much smaller than normal, averaging only about two thirds of normal values, but again there was no difference between the two sides. d In contrast to findings with AAV5-hGDNF, animals examined ~ 3 months after transfection with AAV2-hBDNF showed substantially greater numbers of fibers maintained at all locations in the injected cochleae, as compared to contralateral ears. e Radial nerve fibers averaged 49 % of normal for the AAV2-BDNF-treated ears vs 23 % contralateral, and this difference was highly significant (p < 0.001). f Measurements of the cross-sectional areas of the radial nerve fibers also demonstrated a highly significant difference, with fiber size averaging 88 % of normal after treatment, vs 72 % of normal for controls (p < 0.001)
In contrast to the data for AAV5-hGDNF, the radial nerve fibers counts in the AAV2-hBDNF group showed consistently higher survival in the injected cochleae in all three cochlear sectors compared to contralateral (Fig. 5d). The highest density was observed in the most basal cochlear sector, where fiber counts averaged 56 % of normal (roughly 1190 fibers/mm) after AAV2-hBDNF injections as compared to only 19 % of the normal (~ 390 fibers/mm) on the opposite side. Substantial differences were also observed in the other two cochlear sectors examined. Overall mean fiber counts in the AAV2-hBDNF-treated ears averaged more than double the values seen contralaterally (49 % of normal vs 23 %; Fig. 5e), and this difference was highly significant (two-way ANOVA: F(1,24) = 17.27, p < 0.001).
In addition to the improved survival of radial nerve fibers, measurements of their cross-sectional areas (outer perimeter of myelin) revealed that fibers were also substantially larger in the AAV2-hBDNF-injected cochleae than in the contralateral ears (Fig. 5f; two-way ANOVA: F(1,24) = 14.69, p < 0.001). The fibers averaged 88 % of normal after AAV2-hBDNF treatment, compared to 72 % of normal on the contralateral side. Because the fiber counts and area measurements were made at the light microscopic level, no attempt was made to assess potential pathological alterations in the myelin or the axoplasm of these fibers. Therefore, some fibers that were undergoing initial stages of degeneration may have been included in both fiber counts and area data.
AAV2-hBDNF Promotes Substantial Improvement in Long-Term Survival of SG Neurons and Radial Nerve Fibers
Given the promising results in the AAV2-hBDNF experimental group studied 3 months after virus injections, we elected to conduct a final study using the identical protocols, but extending the post-injection survival period to 6 months. The objectives were to increase the number of subjects evaluated after AAV2-hBDNF injections (given the small numbers of cats necessarily included in each series) and to determine whether the effects observed initially would persist over a longer duration.
As in both of the previous experimental groups, evaluation of the organ of Corti in histological sections in this long-term survival group confirmed the absence of intact hair cells throughout most of the cochlea, and very few surviving hair cells were seen even at the apex in these animals 6 months post-deafening. And again there was no evidence of infection or inflammation within the cochlea.
Figure 6a illustrates the SG numerical density data for the four animals examined 6 months after AAV2-hBDNF injections. Survival of the SG neurons was greater in the AAV2-hBDNF-treated ears than on the contralateral side in all cochlear sectors. Moreover, the greatest improvement in SG survival occurred in the middle cochlear sectors, 30–40, 40–50, and 50–60 % from the base, closest to where the tip of the cannula injected the viral vector, and thus where the highest viral concentration would be expected. The overall mean SG survival for the four subjects was about 53 % of normal for the injected cochleae and 39 % contralaterally, representing the rescue of about 14 % of the normal SG population. Expressed as percentage increase normalized to contralateral survival, this represents an improvement in neuronal survival of more than 35 % in the injected ears, relative to control.
Fig. 6.
a Morphometric data illustrating the mean numerical densities of SG neurons in 10 % sectors from base to apex of the cochlea for the four neonatally deafened cats examined ~ 6 months after AAV2-hBDNF injections. The injected ears show higher densities throughout the cochlea than the paired data for the contralateral ears. Averaged over all cochlear sectors, SG cell survival shows a marked and highly significant improvement from 39 % of normal for the control side to 53 % of normal after AAV2-hBDNF transfection (p = 0.022). (Error bars indicate standard errors.) b, c Light microscopic images of histological sections from the cochleae of one neonatally deafened animal (K414), illustrating neurotrophic effects 26 weeks after unilateral injection of AAV2-hBDNF. The 40–50 % sector of the injected left cochlea (b) and the paired region from the contralateral control cochlea (c) illustrate the higher density of SG cells in Rosenthal’s canal (48 % of normal, in the injected cochlea and 12 % for the control in this sector). Careful inspection of the images also suggests survival of more radial nerve fibers within the osseous spiral lamina (arrows) after AAV2-hBDNF treatment. Scale bar = 100 μm
Table 1c shows the mean overall SG numerical density data for the four individual animals in the 6-month AAV2-hBDNF group. SG survival in the control non-injected ears after this longer survival interval was quite uniform and varied from 37 to 40 % of normal. Clearly, neuronal degeneration in the control ears had progressed significantly with the extended survival period of 6 months, compared to the two groups studied at 3 months (57 % of normal for AAV5-hGDNF control ears and 49 % for AAV2-hBDNF controls). Three of the four animals showed substantially higher SG survival in the AAV2-hBDNF-treated cochlea, but the virus injection was apparently ineffective in one subject (K415), in which survival was equivalent on the two sides. Consequently, mean overall SG survival in the hBDNF-injected ears of the four subjects showed much greater variation, ranging from 36 to 74 % of normal. To test whether the substantial improvement in SG neuronal survival elicited by AAV2-hBDNF was statistically significant, the overall mean SG density for each injected cochleae was compared to contralateral using a paired Student’s t test. Results indicated that the difference elicited by injection of AAV2-hBDNF was not statistically significant, but the power of this statistical test was quite low due to the great variability in the data for the injected ears (particularly because of the injection failure in one subject), and the small n of this group. Therefore, we next performed a two-way ANOVA comparing the AAV2-hBDNF treated and contralateral SG numerical density data for the nine cochlear sectors from base to apex. This statistical analysis showed a highly significant main effect of AAV2-hBDNF treatment (two-way ANOVA: F(1,54) = 14.588, p < 0.001) with no significant interaction between treatment and cochlear interval.
Measurements of the cross-sectional areas of SG cell somata 6 months after AAV2-hBDNF injections determined that mean cell area was 269 μm2 or 100 % of normal in the AAV2-BDNF-injected cochleae, as compared to 235 μm2 or 88 % of normal for the contralateral control ears (data not shown). Because there is a systematic difference in cell size in the normal cochlea, with larger SG cells at the cochlear base and progressively smaller cells toward the apex (Leake et al. 2013), we ran a two-way ANOVA on cell size data from the nine cochlear intervals to compare the injected vs control sides: F(1,54) = 5.619, p = 0.021). However, although the p value suggested that the treatment effect was significant, the data failed the normality test. Review of the data for individual cases revealed the reason for this failure. In three of the four subjects, the cell areas were quite similar in the left and right ears, but in one subject (K418) there was a very substantial difference in cell size, with a mean of 380 μm2 in the injected ear compared to 253 μm2 in the contralateral cochlea. Thus, no conclusion can be drawn regarding the possible long-term effect of AAV2-hBDNF on SG soma size. It is interesting to note, however, that this single animal with markedly larger cells in the injected cochlea was also the subject with the highest SG density in the injected ear and the greatest difference in SG survival between the injected and control sides (74 vs 37 % of normal).
Figure 6b, c presents histological sections illustrating the effects of AAV2-hBDNF in light microscopic images from the middle of the cochlea near the injection site (subject K414; cochlear sector 40–50 %). The higher density of SG cell somata within Rosenthal’s canal is apparent in the injected ear (Fig. 6b; ~ 48 % of normal SG density) as compared to the same region in the contralateral ear (Fig. 6c; 12 % of normal). Inspection of the osseous spiral lamina in these images also suggests a higher density of radial nerve fibers in the injected cochlea as compared to the opposite side (arrows). As mentioned previously and shown here, no ectopic sprouting of the radial nerve fibers was observed in any of the AAV2-hBDNF-injected cochleae.
The survival of the radial nerve fibers was again quantified by counting the profiles of myelinated axons in sections cut orthogonal to the radial plane at three cochlear locations. In this 6-month AAV2-hBDNF injection group, fiber survival was consistently higher in the injected cochleae at all three cochlear locations as compared to contralateral (Fig. 7a). The greatest effect was seen in the middle cochlear sector, where fiber counts averaged 63 % of normal (1613 fibers/mm) with hBDNF treatment as compared to only 28 % (~ 700 fibers/mm) in the same region of the opposite ear. With the consistent difference at all three cochlear regions, overall fiber counts in the AAV2-hBDNF-treated ears averaged double that in the contralateral deafened controls (47 % of normal vs 24 %; Fig. 7b), and this difference was statistically significant (two-way ANOVA: F(1,18) = 5.82, p = 0.027).
Fig. 7.
Radial nerve fiber counts in animals examined ~ 6 months after transfection with AAV2-hBDNF. a Substantially greater numbers of fibers survived in the transfected ears, as compared to the contralateral deafened cochleae at all three cochlear locations examined. b Mean fiber density averaged 47 % of normal for the AAV2-BDNF-treated ears vs 24 % contralateral, a highly significant improvement in survival (p < 0.001), and quite similar to the values recorded 3 months after AAV2-BDNF treatment (Fig. 5d–f). c Measurements of the cross-sectional areas of the radial nerve fibers also demonstrated a highly significant difference, with fiber size averaging 73 % of normal after treatment, vs 56 % of normal in control ears (p < 0.001)
In addition to the improved survival of radial nerve fibers, measurements of their cross-sectional areas revealed that fibers were also significantly larger in the AAV2-hBDNF-injected cochleae than on the contralateral side (Fig. 7c; two-way ANOVA: F(1,18) = 6.72, p = 0.018). On average, fibers averaged 73 % of normal after AAV2-hBDNF treatment, compared to 56 % of normal contralaterally.
DISCUSSION
GFP Immunofluorescence Reveals Several Cell Types Are Transfected with AAV
Our results of the GFP immunofluorescence studies conducted 2–4 weeks after injections of either AAV2-GFP or AAV5-GFP demonstrated that both viral serotypes elicited expression of the reporter gene in surviving hair cells (at the apex) of deafened cochleae, in some supporting cells, and in a relatively small number (5–10 %) of the SG neurons. These results are quite similar to the pattern of expression shown in newborn mice in a previous study using the same AAV5-GFP viral vector (Akil et al. 2019). However, it should be noted that transfection of SG neurons was more robust in the mouse cochlea, with the majority of neurons expressing GFP. It seems likely that the smaller size of the mouse cochlea and different injection procedure used may account for this difference. Specifically, in newborn mice the virus is injected by inserting a glass micropipette through the intact bulla and round window, thus reducing the likelihood of backflow or leakage of virus once injected into the scala tympani. Further, the very small volume of the scala tympani in newborn mice may ensure a higher concentration and more uniform distribution of virus.
AAV-Mediated Neurotrophin Gene Therapy with Both AAV5-hGDNF and AAV2-hBDNF Promotes Improved SG at 3 Months Post-Injection
The significant neurotrophic effects elicited by both AAV5-hGDNF and AAV2-hBDNF on SG neuronal survival 3 months after virus injections are highly encouraging. Although the overall effect was a relatively modest increase of 11–12 % relative to contralateral, it should be noted that both groups were compromised by animals in which transfection apparently failed and SG survival was equivalent between sides. If we omit the data from these individuals (K412, K401; see Table 1a, SG density for the remaining four animals after AAV5-hGDNF treatment averaged 59 % of normal vs 50 % contralateral, or an increase in SG survival of about 18 % re: control. And for the four animals with effective AAV2-hBDNF injections, mean SG density was 61 % of normal after treatment vs 53 % contralateral, an overall increase of about 15 % relative to the deafened control condition.
Several factors could lead to injection failure or transfection inefficacy. Variability in the animals’ blood pressure and excessive production of perilymph could cause dilution and backflow/leakage of the virus at the RW during injection, or inadequate sealing of the round window after the injection could result in perilymph leakage during recovery from surgery. Further, we cannot completely rule out leakage of the injection system (microsyringe or cannula) and/or inactivation of the virus during preparation or injection. In addition to the neurotrophic effects, it is also encouraging that careful histological assessment showed no evidence of inflammatory response or infection with either viral vector.
It is unclear what caused the localized SG pathology observed following injections of AAV5-hGDNF, with distention, hypertrophy, and vacuolation of the satellite cells surrounding the SG neurons. However, the satellite cells are glial cells, so their hypertrophy is consistent with expected effects of GDNF. Pathology was limited to the middle cochlear sectors, near where the tip of our cannula delivered the virus, and did not occur in more basal or apical regions. Thus, we believe this finding may be associated with the very high titer (1.8E14 GC/ml) of the undiluted virus employed. A prior study in newborn mice demonstrated extremely high levels of hGDNF overexpression after cochlear injections of undiluted virus (Akil et al. 2019), and severe deleterious side effects occurred. These side effects were eliminated when the virus was diluted at a 1/20 dilution prior to injection, and qPCR showed more appropriate levels of hGDNF expression (48,000-fold amplification over native mouse GDNF).
Radial Nerve Fiber Number, Size, and Distribution 3 Months After Injection of AAV5-hGDNF or AAV2-hBDNF
Although the AAV5-hGDNF and AAV2-hBDNF both elicited quantitatively similar neurotrophic effects on the SG cells, it is important to note that the two viral vectors marked showed markedly different effects on the radial nerve fibers. Specifically, AAV5-hGDNF elicited significant ectopic sprouting of radial nerve fibers, either down into the scala tympani or into the region of the degenerated organ of Corti, whereas no such sprouting was observed after AAV2-hBDNF injections. These contrasting findings are particularly interesting and important because such disorganized, ectopic fibers would be deleterious to the optimum function of a cochlear implant, which depends on the strict cochleotopic organization of these fibers for frequency selective (spectral resolution) of electrical “channels” of the implant. A previous study with direct cochlear delivery of hBDNF in chronically implanted, deafened animals demonstrated a significant deterioration in the selectivity of activation by individual CI channels that was associated with such sprouting (see Leake et al. 2013 for review).
Interestingly, the sprouted fibers in the AAV5-GDNF-injected cochleae were associated with Schwann cells that often appeared hypertrophied and/or more numerous than usual (Fig. 4a–c). Large and numerous Schwann cell nuclei invested fibers both within the osseous spiral lamina and in the regions where they had sprouted. Schwann cells are glial cells, and their hypertrophy is consistent with observed effects on the SG satellite cells and with the expected effects of hGDNF expression. In prior studies evaluating the effects of direct cochlear infusion of hBDNF, transmission electron microscopy of such sprouted fibers and the associated Schwann cells demonstrated the presence of both myelinated and unmyelinated profiles that aggregated into fascicles surrounded by hypertrophied Schwann cells (Leake et al. 2011), similar to the effects observed here with sprouting elicited by AAV5-hGDNF. These findings of fiber sprouting and glial cell activation are consistent with the well-known functions of GDNF in neuronal axon guidance and maintenance and function of glial cells (see Ibanez and Andressoo 2017 for review).
Another potentially important difference between the two AAV vectors is that AAV5-hGDNF failed to elicit any improvement in the overall survival of radial nerve fibers, whereas transfection with AAV2-hBDNF resulted in substantially improved survival of these fibers. In fact, AAV2-hBDNF-treated cochlea had fiber densities that were more than double the values observed on the contralateral side (~ 50 % of normal vs 23 %). AAV2-hBDNF injections also resulted in significantly larger (closer to normal), mean fiber diameters.
AAV2-hBDNF Promotes Substantial Improvement in Survival of SG Neurons and Radial Nerve Fibers 6 Months Post-Injection
When the post-injection survival period was extended to 6 months in a final experimental series with AAV2-hBDNF, data showed a substantial and highly significant (p < 0.001) improvement in neuronal survival, with SG cell numerical density averaging 53 % of normal after treatment vs 39 % contralateral. This is very encouraging and suggests that hBDNF expression is maintained over the longer term. Once again, among the four animals in this group, there was one subject (K415) in which the treatment was apparently ineffective and SG survival was equivalent in the two ears. If we eliminate this animal, the mean SG density in the injected ears was 60 % of normal 6 months after AAV2-hBDNF injections, almost identical to survival in the 3-month group, suggesting that the neural population initially rescued by treatment is fully maintained over the longer survival period. In contrast, SG degeneration in the contralateral non-treated ears progressed significantly. Thus, in the three animals in which injections were effective, AAV2-hBDNF rescued more than 20 % of the normal SG neuronal population (50 % increase in neuronal survival re: the contralateral side). These results are consistent with several prior studies showing that neurotrophin gene therapy with either adenovirus (Nakaizumi et al. 2004; Wise et al. 2010) or AAV can promote improved survival of the cochlear SG neurons after deafness (see Khalin et al. 2015 for review). Our results showing a few subjects in which injections were ineffective are similar to the findings of Pfingst et al. (2017), who reported that AAV-Ntf3 gene therapy in deafened guinea pigs elicited improved SG survival in 78 % of the injected cochleae as compared to deafened control ears. However, our data in the remaining animals appear to show more robust neurotrophic effects with BDNF than reported with NT-3, as also reported by Budenz et al. (2015). Our study extends the prior findings in adult rodents to an animal model of congenital profound hearing loss, showing highly significant results over the longer term and in a larger mammalian cochlea that is much closer in size and morphology to the human cochlea, making results more directly relevant to humans.
In addition, our data on the radial nerve fibers suggest a two-fold improvement in fiber survival with AAV2-hBDNF treatment. In fact, the fiber counts in the treated ears were quite similar in the 3- and 6-month survival groups (49 % of normal and 47 %, respectively) suggesting that neuronal survival is stabilized and maintained by ongoing hBDNF expression. Our findings are consistent with previous studies showing improved fiber survival after direct cochlear infusion of BDNF and NT-3 (Wise et al. 2005; Leake et al. 2011, 2013) or BDNF and FGF (Glueckert et al. 2008). Importantly, the latter study also used immunohistochemical techniques to demonstrate that these fibers were afferent peripheral processes of SG neurons, not efferents. Other studies have shown that adenoviral-mediated expression of BDNF (Shibata et al. 2010; Wise et al. 2010; Atkinson et al. 2014) or AAV-mediated expression of BDNF (Budenz et al. 2015) also can induce radial nerve fiber regrowth in the guinea pig cochlea. Improved survival of the radial nerve fibers is important because their maintenance will likely be helpful to optimizing CI function.
Clinical Implications
Current cochlear implant technology is generally highly effective, allowing average CI recipients with the latest devices to score around 80 % correct on speech recognition for high-context sentences. The majority of implant recipients are able to use a telephone (Zeng et al. 2008), and the most fortunate even enjoy listening to music (Drennan and Rubinstein 2008; Won et al. 2011; Jiam et al. 2019). But results on more difficult tests, such as recognition of single words, demonstrate extreme variability among CI users (Firszt et al. 2004, Holden et al. 2013), and especially among children using implants (Svirsky et al. 2000; Ortmann et al. 2017; Zhao et al. 2019). This variability in performance has re-focused attention on the importance of neural survival for CI outcomes and on neurotrophic agents that might be applied along with a CI to enhance neural survival (e.g., for review see Staecker et al. 2010; Akil et al. 2019; Khalin et al. 2015; Leake et al. 2013). Cochlear implant electrodes modified for intracochlear drug delivery in humans have already been designed (Hochmair et al. 2006).
The maintenance of auditory nerve survival is particularly important for congenitally deaf infants and very young deaf children who receive an implant, because they must use electrical hearing to acquire speech and language and must rely on a CI for their entire lifetime (Nicholas and Geers 2007). With pediatric implants, there is clear evidence that restoring auditory input as early as possible is more effective in allowing the developing auditory system to adapt to the CI input. It is quite encouraging that many children with implants ultimately can be mainstreamed into public schools (Geers 2004; Nicholas and Geers 2007; Svirsky et al. 2000; Tobey et al. 2013). But the same studies also show that too many other pediatric CI users remain far behind in language development. Therefore, it is extremely important to better understand the specific mechanisms underlying SG degeneration following early onset deafness and the potential role(s) of neurotrophins (for review see Ramekers et al. 2012; Khalin et al. 2015).
Our earlier studies in early deafened cats demonstrated that direct intracochlear delivery of exogenous hBDNF combined with electrical stimulation from a CI elicited substantially improved cochlear SG survival in this animal model of congenital deafness (Leake et al. 2013). Many other previous prior studies have shown SG protection from hBDNF in adult animals, and results also suggest that neurotrophic agents can be used successfully with a CI, offering the promise of clinical therapies that may promote improved outcomes in both adults and children. However, we suggest that osmotic pumps are impractical for clinical application due to concerns about infection with an implanted device in place, rapid degradation and short-term function of neurotrophins, and the finding that high concentrations of neurotrophins cause disorganized sprouting of radial nerve fibers (Staecker et al. 2010; Leake et al. 2013). Some recent studies have explored other strategies for direct cochlear delivery of neurotrophins (Hendricks et al. 2008; Richardson et al. 2008). These include cell-based therapies (Warnecke et al. 2007; Pettingill et al. 2008; Wise et al. 2011), hydrogels (Endo et al. 2005), as well as gene therapy using adenovirus-mediated expression of neurotrophic factors (Nakaizumi et al. 2004; Rejali et al. 2007; Chikar et al. 2008; Wise et al. 2010). Although these methods may provide alternatives in the future, they are still under development and concerns about potential side effects and risks have not yet been adequately addressed (for review, see Khalin et al. 2015; Ramekers et al. 2012).
Direct gene delivery to the cochlea offers the significant advantage that a one-time injection may elicit sustained expression of neurotrophic factors by cells within the target tissue. Stable and long-term expression is particularly important, given the slow time course of SG degeneration in the human cochlea and the long duration of expected implant use (especially in pediatric CI recipients). Recombinant AAV was selected for our studies because it is non-replicating, can efficiently transfer transgenes to the inner ear, and is not ototoxic (Ballana et al. 2008; Konishi et al. 2008; Lustig and Akil 2012; Gyorgy et al. 2017; Pfingst et al. 2017; Suzuki et al. 2017; Tao et al. 2018). AAV is not incorporated into the host genome; rather, the virus remains episomal and results in stable, long-term expression of the transgene (Xia et al. 2012). Moreover, AAV has already been utilized in clinical applications for neurological and neuromuscular diseases without adverse effects, including injections into the eye to treat Leber’s congenital amaurosis retinopathy (Bennett et al. 2012; Simonelli et al. 2010; Pierce and Bennett 2015) and choroideremia (Patricio et al. 2018). Finally, the cochlea is a favorable target organ for gene transfer because it is relatively isolated from surrounding tissues, limiting viral spread and exposure to the immune system; and inoculation of the cochlea with vectors is technically straightforward.
In conclusion, our findings suggest that AAV-mediated neurotrophin therapy can improve neural survival for optimized function of a CI. Recent advances in CI technologies, such as current focusing, virtual channel stimulation, and other complex stimulation strategies, rely on highly spatially restricted stimulation and will very likely benefit from improved neuronal survival. Moreover, the ectopic and disorganized sprouting of the radial nerve fibers induced by direct cochlear delivery of exogenous BDNF would be deleterious to these objectives and present a serious concern. These sprouted fibers survive over the long term and clearly could blur or distort the precise cochlear frequency map. Thus, it is particularly encouraging in our study that AAV2-mediated gene therapy elicited improved survival of SG neurons and radial nerve fibers and also maintained more normal neuroanatomy of the radial nerve fibers.
Finally, recent studies have shown that primary loss of SG neurons occurs in human ears as a function of increasing age, even when apparently normal inner and outer hair cells are present (Mackary et al. 2011; Zilberstein et al. 2012; Viana et al. 2015). The authors conclude that loss of SG neurons may underlie the debilitating decline in hearing-in-noise ability with aging or noise trauma. Thus, the potential intervention with AAV-mediated neurotrophin gene therapy may ultimately be an important first step toward using gene therapy to halt sensorineural hearing loss (or even restore hearing) by reconnecting radial nerve fibers to residual hair cells in ears with hearing loss due to loss of these connections, or even to regenerated hair cells.
Acknowledgements
UniQure Biopharma B.V. provided the AAV5-GFP and AAV5-hGDNF vectors for these studies. AAV2-GFP and AAV2-hBDNF were donated by Krystof Bankiewicz, MD, PhD (Dept. of Neurological Surgery, University of California San Francisco). The authors are grateful to Dr. Bankiewicz, Lawrence Lustig, MD, and Bas Blits, PhD for helpful discussions during the design and performance of this research. The authors also thank Andrew Tucker, Andrew Tauscher, and Christian Fahlman, Ph.D. for their significant and expert contributions to the project in sectioning and light microscopic imaging of cochlear specimens.
Funding Information
This work was supported by the National Institutes of Health, the National Institute on Deafness and Other Communication Disorders, Grant #5R01DC01306, The Epstein Endowment Fund, The Georgia Sullivan Endowment Fund, Hearing Research Inc., and a collaborative research agreement with uniQure Biopharma B.V. (Amsterdam, The Netherlands).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agterberg MJH, Versnel H, van Dijk LM, de Groot JC, Klis SF. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol. 2009;10:355–367. doi: 10.1007/s10162-009-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW, Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244:25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Akil O, Blits B, Lustig L, Leake P. Virally-mediated over-expression of GDNF elicits age- and dose-dependent neuronal toxicity and hearing loss. Hum Gene Ther. 2019;30(1):88–105. doi: 10.1089/hum.2018.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PJ, Wise AK, Flynn BO, Nayaqam BA, Richardson RT (2014) Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One 18, 9(7). 10.1371/journal.pone.0102077 [DOI] [PMC free article] [PubMed]
- Ballana E, Wang J, Venail F, Estivill X, Puel J-L, Arbones ML et al (2008) Efficient and specific transduction of cochlear supporting cells by adenoassociated virus serotype 5. Neurosci Lett 442(2):134–139. 10.1016/j.neulet.2008.06.060 [DOI] [PubMed]
- Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DD, McCaque S, Pierce EA, Chen Y, Bennicelli JL, Zhu X, Ying GS, Sun J, Wright JF, Auricchio A, Simonelli F, Shindler KS, Minqozzi F, high KA, Maquire AM. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4(120):120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budenz CL, Wong HT, Swiderski DL, Shibata SB, Pfingst BE, Raphael Y. Differential effects of AAV.BDNF and AAV.Ntf3 in the deafened adult guinea pig ear. Sci Rep. 2015;5:8619. doi: 10.1038/srep08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, Di Polo A, Raphael Y, Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear Res. 2008;245:24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan WR, Rubinstein JT. Music perception in cochlear implant users and its relationship with psychophysical capabilities. J Rehabil Res Dev. 2008;45:779–789. doi: 10.1682/JRRD.2007.08.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Nakagawa T, Kita T, Iguchi F, Kim TS, Tamura T, Iwai K, Tabata Y, Ito J. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 2005;115:2016–2020. doi: 10.1097/01.mlg.0000183020.32435.59. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Tessarollo L, et al. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35(19):7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firszt JB, Holden LK, Skinner MW, Tobey EA, Petersen A, Gaggl W, Runge-Samuelson CL, Wackym PA. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear Hear. 2004;25:375–387. doi: 10.1097/01.AUD.0000134552.22205.EE. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Gao WQ, Zheng JL, Karihaloo M. Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at later stages of cerebellar granule cell differentiation. J Neurosci. 1995;15:2656–2667. doi: 10.1523/JNEUROSCI.15-04-02656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AE. Speech, language, and reading skills after early cochlear implantation. Arch Otolaryngol Head Neck Surg. 2004;130:634–638. doi: 10.1001/archotol.130.5.634. [DOI] [PubMed] [Google Scholar]
- Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507:1602–1621. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- Grieger JC, Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. 2012;507:229–254. doi: 10.1016/B978-0-12-386509-0.00012-0. [DOI] [PubMed] [Google Scholar]
- Gyorgy B, Sage C, Indzhykulian AA, Scheffer DI, Brisson AR, Tan S, Wu X, Volak A, Mu D, Tamvakologos PI, Li Y, Fitzpatrik Z, Ericsson M, Breakefield XO, Corey DP, Maguire CA (2017) Rescue of hearing by gene delivery to inner-ear hair cells using exosomeo-associated AAV. Mol Ther 25(2):379–391. 10.1016/j.ymthe.2016 [DOI] [PMC free article] [PubMed]
- Hansen MR, Zha XM, Bok J, Green SH. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J Neurosci. 2001;21:2256–2267. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JL, Chikar JA, Crumling MA, Raphael Y, Martin DC. Localized cell and drug delivery for auditory prostheses. Hear Res. 2008;242:117–131. doi: 10.1016/j.heares.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmair I, Nopp P, Jolly C, Schmidt M, Schosser H, Garnham C, Anderson I. MED-EL cochlear implants: state of the art and a glimpse into the future. Trends Amplif. 2006;10:201–219. doi: 10.1177/1084713806296720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heydebrand G, Skinner MW (2013) Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 10.1097/AUD.0b013e3182741aa7 [DOI] [PMC free article] [PubMed]
- Ibanez CF, Andressoo JO. Biology of GDNF and its receptors—relevance for disorders of the central nervous system. Neurobiol Dis. 2017;97:80–89. doi: 10.1016/j.nbd.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Jiam NT, Deroche ML, Jiradejvong P, Limb CJ (2019) A randomized controlled crossover study of the impact of online music training on pitch and timbre perception in cochlear implant users. JARO. 10.1007/s10162-018-00704-0 [DOI] [PMC free article] [PubMed]
- Kanzaki S, Stöver T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Khalin I, Alyautdin R, Kocherga G, Abu Bakar M (2015) Targeted delivery of brain-derived neurotrophic factor for the treatment of blindness and deafness. Int J Nanomedicine 10:3245–3267. 10.2147/IJN.S77480.eCollection2015 [DOI] [PMC free article] [PubMed]
- Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T (2008) Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J Gene Med 10(6):610–618. 10.1002/jgm.1189 [DOI] [PubMed]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330(Pt B):191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Hetherington AM, Stakhovskaya O. Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol. 2011;519:1526–1545. doi: 10.1002/cne.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–562. doi: 10.1002/(SICI)1096-9861(19991004)412:4<543::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Vollmer M, Rebscher SJ. Neurotrophic effects of GM1 ganglioside and electrical stimulation on cochlear spiral ganglion neurons in cats deafened as neonates. J Comp Neurol. 2007;501:837–853. doi: 10.1002/cne.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Stakhovskaya O, Hradek GT, Hetherington AM. Factors influencing neurotrophic effects of electrical stimulation in the deafened developing auditory system. Hear Res. 2008;242:86–99. doi: 10.1016/j.heares.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Stakhovskaya O, Hetherington A, Rebscher SJ, Bonham B. Effects of brain-derived neurotrophic factor (BDNF) and electrical stimulation on survival and function of cochlear spiral ganglion neurons in deafened, developing cats. J Assoc Res Otolaryngol. 2013;14(2):187–211. doi: 10.1007/s10162-013-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Suzuki J, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol. 2015;16(2):205–219. doi: 10.1007/s10162-015-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig LR, Akil O (2012) Cochlear gene therapy. Curr Opin Neurol 25(1):57–60. 10.1097/WCO.0b013e32834f038c [DOI] [PMC free article] [PubMed]
- Mackary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12(6):711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama J, Miller JM, Ulfendahl M. Glial cell line-derived neurotrophic factor and antioxidants preserve the electrical responsiveness of the spiral ganglion neurons after experimentally induced deafness. Neurobiol Dis. 2008;29:14–21. doi: 10.1016/j.nbd.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007;85:1959–1969. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology: an introduction for bioscientists. Baltimore: Johns Hopkins University Press; 2002. [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9:135–143. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. J Speech Lang Hear Res. 2007;50:1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann M, Zwitserlood P, Knief A, Baare J, Crinkheetker S, Am Zehnhoff-Dinnesen A, Dobel C (2017) When hearing is tricky: speech processing strategies in prelingually deafened children and adolescents with cochlear implants having good and poor speech performance. PLoS One 12(1). 10.1371/journal.pone.0168655 [DOI] [PMC free article] [PubMed]
- Patricio MI, Barnard AR, Xue K, MacLaren RE (2018) Choroideremia: molecular mechanisms and development of AAV gene therapy. Expert Opin Biol Ther 18(7):807–820. 10.1080/14712598.2018.1484448 [DOI] [PubMed]
- Pettingill LN, Minter RL, Shepherd RK. Schwann cells genetically modified to express neurotrophins promote spiral ganglion neuron survival in vitro. Neuroscience. 2008;152:821–828. doi: 10.1016/j.neuroscience.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Swiderski DL, Hughes AP, Strahl SB, Sinan M, Raphael Y. Neurotrophin gene therapy in deafened ears with cochlear implants: long-term effects on nerve survival and functional measures. JARO. 2017;18:731–750. doi: 10.1007/s10162-017-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Bennett J (2015) The status of RPE65 gene therapy trials: Safety and efficacy. Cold Spring Har Perspect Med 5(9):a017285. 10.1101/cshperspect.a017285 [DOI] [PMC free article] [PubMed]
- Ramekers D, Versnel H, Grolman W, Klis SF (2012) Neurotrophins and their role in the cochlea. Hear Res. 10.1016/j.heares.2012.03.002 [DOI] [PubMed]
- Ramekers D, Versnel H, Strahl SB, Klis SFL, Grolman W. Temporary neurotrophin treatment prevents deafness-induced auditory nerve degeneration and preserves function. J Neurosci. 2015;35(36):12331–12345. doi: 10.1523/JNEUROSCI.0096-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebscher SJ, Hetherington AM, Snyder RL, Leake PA, Bonham BH. Design and fabrication of multichannel cochlear implants for animal research. J Neurosci Methods. 2007;166:1–12. doi: 10.1016/j.jneumeth.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RT, Wise AK, Andrew JK, O’Leary SJ. Novel drug delivery systems for inner ear protection and regeneration after hearing loss. Expert Opin Drug Deliv. 2008;5:1059–1076. doi: 10.1517/17425247.5.10.1059. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242:100–109. doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Cortez SR, Beyer LA, Wiler JA, Di Polo A, Pfingst BE, Raphael Y. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp Neurol. 2010;223:464–472. doi: 10.1016/j.expneurol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18(3):643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Jolly C, Garnham C. Cochlear implantation: an opportunity for drug development. Drug Discov Today. 2010;15:314–321. doi: 10.1016/j.drudis.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Hashimoto K, Xiao R, Vandenberghe LH, Liberman MC. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci Rep. 2017;7:45524. doi: 10.1038/srep45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirsky MA, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language development in profoundly deaf children with cochlear implants. Psychol Sci. 2000;11:153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Huang M, Shu Y, Ruprecht A, Wang H, Tang Y, Vandenberghe LH, Wang Q, Gao G, Kong WJ, Chen ZY (2018) Delivery of adeno-associated virus vectors in adult mammalian inner-ear cell subtypes without auditory dysfunction. Hum Gene Ther 29(4):492–506. 10.1089/hum.2017.120 [DOI] [PMC free article] [PubMed]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey EA, Thal D, Niparko JK, Eisenberg LS, Quittner AL, Wang N-Y, CDaCI Team Influence of implantation age on school-age language performance in pediatric cochlear implant users. Int J Audiol. 2013;52(4):219–229. doi: 10.3109/14992027.2012.759666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LM, O'Malley JT, Burgess BJ, Jones DD, Oliveira CA, Santos F, Merchant SN, Liberman LD, Liberman MC (2015) Cochlear neuropathy in human presbycusis: confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res 327:78–88. 10.1016/j.heares.2015.04.014 [DOI] [PMC free article] [PubMed]
- Warnecke A, Wissel K, Hoffmann A, Hofmann N, Berkingali N, Gro G, Lenarz T, Stover T. The biological effects of cell-delivered brain-derived neurotrophic factor on cultured spiral ganglion cells. Neuroreport. 2007;18:1683–1686. doi: 10.1097/WNR.0b013e3282f0b5d7. [DOI] [PubMed] [Google Scholar]
- Wise AK, Fallon JB, Neil AJ, Pettingill LN, Geaney MS, Skinner SJ, Shepherd RK. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics. 2011;8:774–787. doi: 10.1007/s13311-011-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O’Leary SJ, Shepherd RK, Richardson RT. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–1122. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of Guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Won JH, Jones GL, Drennan WR, Jameyson EM, Rubinstein JT (2011) Evidence of across-channel processing for spectral-ripple discrimination in cochlear implant listeners. J Acoust Soc AM 130(4):2088–2097. 10.1121/1.3624820 [DOI] [PMC free article] [PubMed]
- Xia L, Yin S, Wang J (2012) Inner ear gene transfection in neonatal mice using adeno-associated viral vector: a comparison of two approaches. Plos One 7(8):e43218. 10.1371/journal.pone.0043218 [DOI] [PMC free article] [PubMed]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B (2011) The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear Res 278:21–33. 10.1016/j.heares.2011.03.002 [DOI] [PMC free article] [PubMed]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998;124:17–26. doi: 10.1016/S0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Zeng F-G, Rebscher S, Harrison W, Sun X, Fen H. Cochlear implants: system design, integration, and evaluation. IEEE Rev Biomed Eng. 2008;1:115–142. doi: 10.1109/RBME.2008.2008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Bishop JF, Hansen MR, Victoria L, Abbas PJ, Mouradian MM, Green SH. BDNF synthesis in spiral ganglion neurons is constitutive and CREB-dependent. Hear Res. 2001;156:53–68. doi: 10.1016/S0378-5955(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li Y, Zheng Z, Li J, Nie X, Jin X, Zheng J, Zhang J, Chen M, Hao J, Yang Y, Liu W, Liu H, Ni X (2019) Health-Related quality of life in Mandarin-speaking children with cochlear implants. Ear Hear 40(3):605–614. 10.1097/AUD.0000000000000633 [DOI] [PubMed]
- Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci. 2012;32(2):405–410. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]