Abstract
The aim of work was to check and make comparison of efficacy for five approaches for petroleum crude contaminated agricultural soil remediation by making use of soil microcosms. Concerning the published literature in our information, this is the first report comparing five approaches i.e. abiotic losses, native microbial flora, nutrient amendments and pre-adapted native microbial culture and concurrent amendments of nutrients + pre-adapted native microbial culture for agricultural soil bioremediation using Pseudomonas aeruginosa NCIM 5514 by performing soil microcosm experiments. 96.00 ± 0.18% degradation of petroleum hydrocarbon fractions in 60 days of the experiment was observed when nutrients and P. aeruginosa NCIM 5514 were applied concomitantly. In nutrients- and P. aeruginosa NCIM 5514-added microcosm reduction in nitrogen, organic carbon, and phosphorus was noted. P. aeruginosa NCIM 5514, can be applied as a prospective bioremediation agent to remediate petroleum crude contaminated soil.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00814-0) contains supplementary material, which is available to authorized users.
Keywords: Weathering effects, Agriculture, Indigenous microorganisms, P. aeruginosa NCIM 5514, Nutrient amendment, Soil microcosm
Introduction
Terrestrial crude oil spills due to activities of the petroleum industry is a burning research topic for environmental scientists and engineers and hence is the most perspective field of research [1]. The demand for petroleum products has been increasing drastically in the past two decades. For this petroleum, crude has been produced, explored and refined globally [2, 3]. Therefore, the risk of soil contamination due to oil spills is increasing at an alarming rate [2, 4].
Petroleum crude exploration and processing activities in the oilfields of India are apparent, very old petroleum crude operation in India has been started in Southern Gujarat. Oil and Natural Gas Corporation Limited (ONGC), a very well-known oil and gas exploration company has installed many units related to petroleum activities in Ankleshwar. Therefore, this region is more susceptible to environmental pollution from petroleum oil spills. This exuberant of petroleum crude in Gujarat represent both blessing and harm because petroleum crude exploration as well as drilling locations and group gathering stations have been situated near human settlings including agricultural areas/belts. During petroleum industrial activities petroleum crude leakage leads to the contamination of adjacent agricultural areas/fields and water resources [5]. Accidental and purposeful petroleum crude spillage and environmental pollution is a top-tier threat for soil ecosystem and its biota, as the transfer of harmful components of petroleum crude takes place into the food chain, which leads biomagnification of petroleum hydrocarbons due to their recalcitrant and persistent nature [3, 6, 7]. Contamination of agricultural soil by harmful chemicals present in petroleum crude makes it of no use for its landowners as it causes negative consequences on fertility of soil, because of this soil losses its applicability to be used for agriculture. Therefore, remediation of agricultural soil contaminated with petroleum is a global issue for agricultural land restoration and its use [1, 8].
Physico-chemical and thermal methods used for remediation of contaminated soil with terrestrial oil spills due to their reported drawbacks have become less favorable [9]. Biological technologies particularly the application of microorganisms because of their sustainable nature have been favored by researchers globally for petroleum crude contaminated soil remediation works [10–12]. Degradation of hydrocarbons after oil spill, occurs by weathering processes or indigenous microorganisms [13]. Natural attenuation has been reported as a long-time process as compared to the engineered processes [14]. Bioremediation approaches including biostimulation and bioaugmentation have become thrust area of research for environmental biotechnologists because of its fewer energy requirements and comparable operating costs [6, 15]. Altogether it has the benefit of soil restoration ability [10, 16]. However, during the bioremediation process, toxic metabolites may be generated affects the microbial growth [17, 18].
Bioaugmentation approach follows the addition of exogenously enriched microbes (which may be native to the site or may be engineered for desired characteristics) to the contaminated soil for its bioremediation [11, 19]. However, it has been reported that in biostimulation approach favorable growth conditions for better growth of microorganisms in the contaminated soil have been provided [13, 15]. It has been reported that for faster, notable and efficient degradation of petroleum compounds synchronization of biostimulation and bioaugmentation is required [15, 17, 18, 20, 21].
Numerous laboratory studies have been performed for application of Pseudomonas aeruginosa on petroleum crude degradation worldwide. The study was focused to compare five remediation technologies for biological remediation of artificially contaminated agricultural soil using soil microcosm trials. Biodegradation study has been performed by applying gravimetric (for qualitative analysis) and gas-chromatography (for quantitative analysis) methods. The degradation of petroleum hydrocarbon compounds reported in this study is faster than the reported degradation (of petroleum hydrocarbons) by other researchers, shows distinctiveness of this study. The study was emphasized to check the feasibility of this bacterial culture to increase petroleum crude degradation using nutrient amendments.
Materials and Methods
Soil Sampling and Its Physico-Chemical and Bacteriological Characterization
Oil and Natural Gas Corporation Limited (ONGC) is the largest petroleum exploration company handled by Govt. of India and carrying crude oil exploration activities at different locations in India since 1960. Ankleshwar with 21.6264°N, 73.0152°E coordinates in the Bharuch district in south Gujarat, India is a hub for industrial units [2]. ONGC has developed pipeline network for transporting petroleum from oil well(s) to its group gathering stations [22]. Because of this petroleum oil drilling, storage, transportation, production, exploration, and processing are potential sources of agricultural soil contamination [1, 2].
Keeping this in mind pristine soil samples (equal quantity) from different five sites were collected from an agricultural field near ONGC site, Ankleshwar. Samples were collected in sterilized plastic bags, labeled and transported to the laboratory (Storage: 4 °C). The composition of soil in terms of organic carbon, nitrogen and pH was performed using standard procedures such as ASTM and IS, as explained earlier [22]. Total viable count and hydrocarbon utilizing bacterial count of soil sample were performed by dilution plate-counting technique. For total viable count and hydrocarbon utilizing bacterial count, nutrient agar plates and crude oil supplemented Bushnell–Haas (BH) plates were used, respectively. Percentage of hydrocarbon utilizing bacteria (HUB) in the total viable count of heterotrophic bacteria (TVC) was calculated by the following formula [22]:
Crude Oil Sample Collection Site and Characterization of Crude Oil
Cambay basin is located in the Western states of India and has been actively explored approximately 55 years for oil exploration and production by ONGC. Important fields in the Cambay Basin are Ankleshwar, Gandhar, Sobhasan, and Kalol [22]. The crude oil sample was collected from oil well of Kalol oilfield, ONGC. TLC–FID detector (HYDROSCAN MK-6s) was used to perform SARA analysis of the crude oil. Other physicochemical parameters such as viscosity, density and API gravity has been performed using the methods as described previously [9].
Source of Oleophilic P. aeruginosa NCIM 5514
Petroleum crude utilizing and biosurfactant producing P. aeruginosa NCIM 5514 was isolated by authors before 6 years. Enrichment culture technique has been utilized to obtain the said isolate from crude oil contaminated soil sample of Ankleshwar asset of ONGC. Culture preservation, activation and growth parameters have been explained earlier [22–25]. Authors have previously reported effective utilization of petroleum crude (qualitative) by P. aeruginosa NCIM 5514 employing a redox indicator 2,6 dichlorophenolindophenol. More details on biodegradation (of petroleum crude) using this organism in a laboratory experiment have been published earlier [25].
Soil Microcosms Study: Experimental Design
To evaluate the efficacy of remediation for artificially contaminated agriculture soil using the P. aeruginosa NCIM 5514, simulated conditions of crude oil contaminated field plots in form of the microcosms were created in 30 cm × 23 cm × 6 cm size (without lid) containers. Five soil samples collected from nearby agricultural land of Ankleshwar oilfield, ONGC were mixed to have composite sample, which was considered as representative soil sample and used for microcosm studies i.e. bioremediation work.
Preparation of soil sample for further experiment has been reported earlier [5]. In each microcosm 1 kg artificially crude oil contaminated soil was kept and specific treatment was given. Microcosm experiments were conducted at room temperature (Experiment time: 60 days). Abiotic control was prepared by adding of 2 g of HgCl2 to the microcosm 1 as a biocide [26]. Activity of indigenous bacterial flora was observed in microcosm 2. For bioaugmentation, the active culture of P. aeruginosa NCIM 5514 (2%, w/v) was used in respective microcosms (microcosms 4 and 5). This strain was inoculated in microcosm(s) to achieve 107 CFU/g HUB count. In microcosms 3 and 5, for biostimulation nutrient amendment was done to attain proportion of C:N:P as 100:10:1. NH4NO3 and Na2HPO4 were used as bio-stimulants.
Moisture of soil was monitored two times in a week and required amount of water was supplied to microcosms to keep 20% water content. Tilling was used every week to perform manual aeration. The purpose of tilling was to homogenize soil by breaking lumps, making fine particles and increase air circulation, which in turn increases contact between soil amendments, homogenous distribution of oil contaminants and microorganisms.
Petroleum Hydrocarbons Extraction and Gravimetric Analysis
The concentration of petroleum hydrocarbons in each soil microcosms was analyzed by method as previously described [2]. In brief soil sample(s) (10 g) from each microcosm (in triplicates) were taken periodically e.g. 0, 15, 30, 45 and 60 days. Hydrocarbons from soil(s) were extracted as described earlier using chloroform, hexane and methylene chloride [2, 5]. All extracts were mixed and moisture was removed using anhydrous Na2SO4. Evaporation of remaining solvents was performed by drying and residual petroleum carbon fractions recovered were determined by gravimetric quantitation. Percentage of crude oil reduction was estimated by the following equation [22, 25]:
Statistical analytical software SPSS 16.0 was used for statistical analysis of the results obtained. Analyses were performed in triplicates and results are represented as mean ± standard deviation [25].
Petroleum Hydrocarbon Composition by Gas Chromatography
GC-FID technique was used for determination of carbon compounds present in extracted crude oil (Method: ASTM D7169) [25, 27]. 0.2 μl petroleum crude was injected for one cycle of GC which was having total run time 53.3 min. Other conditions for GC have been explained earlier [5]. Calibration of the GC instrument was checked by running ASTM D2887. Quantitative calibration mix (for C6–C44 fractions) and polywax 655 (for higher paraffins) were used as standards. Biodegradation efficiency was calculated by following formula [27].
Hydrocarbon Utilizing Bacterial Enumeration
Enumeration of HUB in soil samples which were taken at desired time intervals was performed using dilution plate—counting technique. For this soil (10 g) was withdrawn from each soil microcosm on 0, 15, 30, 45 and 60 days, and was mixed with 90 mL of normal saline. Vortex mixer was used to shake it. Thereafter aliquots of 0.1 mL were plated on oil agar plate (Incubation: 37 ± 2 °C for 24–48 h). HUB density was determined in terms of CFU/g for soil samples [22]. Statistical analytical software SPSS 16.0 was used to perform statistical analysis and results were noted as described earlier [24].
Results and Discussion
Properties of Agricultural Soil and Crude Petroleum
Characteristics of soil including nutrient profiling have been summarized in table S1. It showed 0.0015% HUB in TVC. The crude oil used for presented works was collected from petroleum crude well of Kalol, Gujarat, India. Kalol is a city having 25.42 km2 area and also known as an industrial city. Petroleum crude showed 16% of moisture content and 6.1429 of Saturates/Aromatics. Density at 15.5 °C was noted 867.5 kg/m3. However, specific gravity and API gravity at 15.5 °C were 868.3 kg/m3 and 31.46° API, respectively. The viscosity of petroleum crude was 1885.02 cp. It also showed the presence of resins (6%) and asphaltenes (4%).
Count of Hydrocarbon Utilizing Bacteria (HUB) in Soil Microcosms
Soil microcosm 1 because of treatment with HgCl2 did not show any microbial count throughout the experiment. Microcosm 2 in which artificially contaminated agricultural soil was kept, showed 1.47 ± 0.05 × 104 CFU/g of indigenous HUB population at 60 days. In soil microcosm 2, HUB count was observed indicates growth of indigenous HUB of agricultural soil. Soil microcosm 3 showed higher HUB population than that for soil microcosm 2 because of nutrient addition, this indicates the influence of stimulation by nutrients (Table 1). Microcosm 4 showed 0.08 ± 0.01 × 108 CFU/g HUB population at end of the experiment. Microcosm 5 at the initiation of the experiment showed HUB population 4.82 ± 0.11 × 107 CFU/g. HUB count reached up to 4.0 ± 0.80 × 109 CFU/g at 30 days experiment. After this decrease in HUB population was noted which was 1.3 ± 0.06 × 108 and 1.07 ± 0.15 × 108 CFU/g at 45 and 60 days, respectively (Table 1). Increasing HUB count in soil samples from microcosms 4 and 5 reveals the ability of added P. aeruginosa culture to be alive or exist in the system and utilize petroleum crude for its growth.
Table 1.
Hydrocarbon utilizing bacterial count in soil microcosms
| Soil microcosm no. | Hydrocarbon utilizing bacterial count (CFU/g)a | ||||
|---|---|---|---|---|---|
| Time (days) | |||||
| 0 | 15 | 30 | 45 | 60 | |
| Soil microcosm 2 | 0.53 ± 0.11 × 102 | 1.29 ± 0.02 × 103 | 0.68 ± 0.17 × 104 | 2.20 ± 0.01 × 104 | 1.47 ± 0.05 × 104 |
| Soil microcosm 3 | 0.54 ± 0.11 × 102 | 1.81 ± 0.01 × 103 | 1.35 ± 0.19 × 104 | 3.09 ± 0.23 × 104 | 1.84 ± 0.11 × 104 |
| Soil microcosm 4 | 4.95 ± 0.13 × 107 | 5.36 ± 0.31 × 107 | 6.76 ± 0.08 × 107 | 0.93 ± 0.39 × 108 | 0.08 ± 0.01 × 108 |
| Soil microcosm 5 | 4.82 ± 0.11 × 107 | 8.32 ± 0.11 × 108 | 4.0 ± 0.80 × 109 | 1.30 ± 0.06 × 108 | 1.07 ± 0.15 × 108 |
CFU/g colony forming unit per gram
aValues are mean of three replicates noted during the experiment, error bars indicate standard deviation
Influence of Nutrients Addition and Bioaugmentation
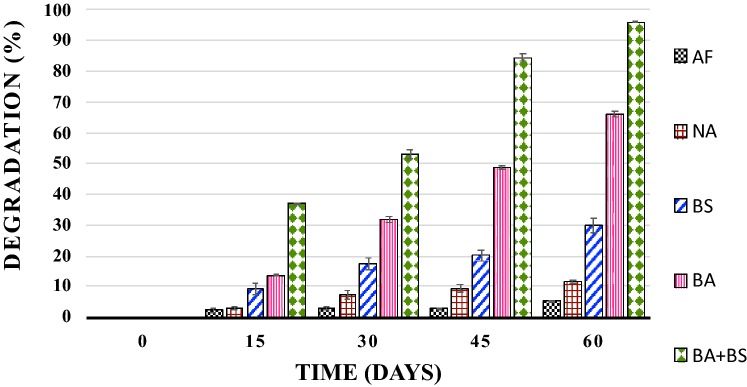
Degradation (Qualitative) of hydrocarbons in crude petroleum oil samples from agricultural soil was performed using gravimetric analysis as depicted in Fig. 1. Petroleum crude degradation (quantitative) was performed by means of fingerprinting petroleum hydrocarbons employing gas chromatography technique. Influence of natural attenuation on degradation of hydrocarbons in crude oil samples extracted from microcosms 2 has been summarized in table S2. Role of nutrients addition, bioaugmentation and nutrients addition + bioaugmentation have been shown in tables S3, 2 and 3. As shown in Fig. 1 in case of soil microcosm 1, 5.18% degradation (gravimetric analysis) of petroleum hydrocarbons was noted at end of experiment. In microcosm 5 at 60 days, 96.00% and 95.22% degradation of crude oil by gravimetric and gas chromatography analysis, respectively was noted (Fig. 1 and Table 3) As shown in Table 3, at 60 days most of the normal alkane peaks were decreased in soil microcosm 5 which was given a concurrent treatment for addition of P. aeruginosa NCIM 5514 culture and nutrients. Normal alkanes fractions of petroleum crude of soil microcosms for natural attenuation (microcosm 2) and Bioaugmentation + Biostimulation (microcosm 5) have been summarized in tables S2 and 3, respectively. At 60 days in weathering control soil microcosm C8 has been removed totally, showing the role of abiotic factors in the degradation of petroleum hydrocarbons (Data not shown). In soil microcosm 2 i.e. in case of natural attenuation, at 60 days of incubation C8–C10 have been removed totally which indicates the role of native hydrocarbon utilizers present in soil and abiotic factors (Table S2). For soil microcosm 5 in which P. aeruginosa NCIM 5514 and nutrients were added, C8–C14 and C16–C17 compounds were totally utilized which indicates an influence of external culture and nutrient addition (Table 3). At 60 days in soil microcosm 5, C8–C14 and C16–C17 were not detected but very less amount of C15 was detected, if taken as a whole then the trend by gravimetric analysis indicates an increase in the percentage of petroleum crude degradation as shown in Fig. 1 and Table 3. C15 compound’s presence may be due to conversion/transformation of compounds having high molecular weight to low molecular weight compounds. Highest and nearly complete biotic removal (99.92%) was observed for C20 and C12 (41.96%) was noted to be least biodegraded. C13–C15, C17–C24, C30–C32 and C34–C35 were degraded more than 95%. C16, C26, C28, C29, C33 and C36 + petroleum hydrocarbons were biodegraded from 85 to 95% (Table 3).
Fig. 1.
Petroleum crude (% degradation) in soil microcosms for different bioremediation approaches; Values are mean of three replicates noted during 60 days of the experiment, error bars indicate standard deviation
Table 3.
Petroleum crude carbon fractions in soil microcosm 5: effectiveness of nutrients + pre-adapted P. aeruginosa NCIM 5514 addition
| Petroleum crude composition | % Variation of petroleum crude composition | % Removal | Removal (%) by P. aeruginosa NCIM 5514 | |
|---|---|---|---|---|
| 0 day | 60 days | |||
| C8 | 3802.70 | 0.00 | 100.00 | 0.00 |
| C9 | 7237.61 | 0.00 | 100.00 | 0.00 |
| C10 | 2003.70 | 0.00 | 100.00 | 0.00 |
| C11 | 14,921.60 | 0.00 | 100.00 | 49.84 |
| C12 | 23,780.03 | 0.00 | 100.00 | 41.96 |
| C13 | 20,938.79 | 0.00 | 100.00 | 95.57 |
| C14 | 20,981.00 | 0.00 | 100.00 | 95.21 |
| C15 | 14,077.98 | 559.32 | 96.03 | 99.38 |
| C16 | 16,859.91 | 0.00 | 100.00 | 94.27 |
| C17 | 18,624.03 | 0.00 | 100.00 | 97.99 |
| C18 | 11,184.52 | 2312.00 | 79.33 | 97.75 |
| C19 | 12,065.61 | 1184.19 | 90.19 | 99.35 |
| C20 | 13,664.26 | 567.00 | 95.85 | 99.92 |
| C21 | 12,851.33 | 3250.03 | 74.71 | 97.04 |
| C22 | 16,256.66 | 542.06 | 96.67 | 98.27 |
| C23 | 19,167.19 | 41.71 | 99.78 | 94.62 |
| C24 | 13,908.54 | 52.59 | 99.62 | 99.86 |
| C26 | 12,546.89 | 22.58 | 99.82 | 90.21 |
| C27 | 14,221.29 | 11.30 | 99.92 | 76.76 |
| C28 | 9006.22 | 1331.42 | 85.22 | 91.37 |
| C29 | 8806.69 | 1062.45 | 87.94 | 89.35 |
| C30 | 7143.20 | 42.40 | 99.41 | 97.74 |
| C31 | 5534.87 | 324.39 | 94.14 | 96.08 |
| C32 | 3890.80 | 212.18 | 94.55 | 96.38 |
| C33 | 3253.79 | 288.02 | 91.15 | 90.81 |
| C34 | 3008.78 | 64.30 | 97.86 | 98.30 |
| C35 | 2000.21 | 701.48 | 64.93 | 97.47 |
| C36+ | 6748.64 | 74.65 | 98.89 | 85.43 |
| Total | 318,486.84 | 12,644.07 | ||
| Degradation (%) | 0.00 | 95.22 | ||
Effects of Pre-adapted P. aeruginosa NCIM 5514 Culture- and Nutrients-Addition on Selected Nutrients in Microcosm
Sandy loam soil (colour: brown) was used in the study. Soil properties and its nutrients profile have been enlisted in table S1. Soil characteristics and selected nutrients at bioremediation site (soil from microcosm 5) have been depicted in Table 4. At the bioremediation site, a significant change in pH during the experiment was not observed. On the 60th day of experiment decrease in nutrients such as organic carbon, nitrogen and phosphorus have been observed. The nitrogen, organic carbon, and phosphorus were recorded 0.25 ± 0.05%, 0.96 ± 0.03%, and 264.33 ± 11.72 mg/kg on last day of experiment (Table 4).
Table 4.
Influence of pre-adapted P. aeruginosa NCIM 5514 culture—and nutrients—addition on characteristics of soil withdrawn from microcosm 5
| Characteristics | Valuesa | |
|---|---|---|
| 0 day | 60 days | |
| pH | 7.25 ± 0.09 | 7.60 ± 0.25 |
| O.C. (%) | 3.48 ± 0.03 | 0.96 ± 0.03 |
| Nitrogen (%) | 0.34 ± 0.08 | 0.25 ± 0.05 |
| Phosphorus (mg/kg) | 346.33 ± 4.04 | 264.33 ± 11.72 |
O.C. organic carbon
aValues have been reported as the mean of three replicates ± standard deviation
Significance of environmental factors, available nutrients and biological factors in petroleum compounds’ biodegradation has been reported globally [5, 7, 13]. The work reported in this manuscript also encourages the importance of environmental, biological and nutritional factors for rapid biodegradation of hydrocarbon compounds present in petroleum oil (used for the said work). It has been reported that microbial activities are faster (may be due to efficient nutrient delivery) in the presence of sufficient water indicates the significance of soil moisture content [28]. For soil microcosm study presented here, 20% moisture level was maintained by addition of sterile water in all microcosms during the whole experiment. 25–33 °C temperature (temp.) was noted for all microcosms during the experiment. Maintaining temp. is not possible to achieve it on open land, therefore, biodegradation efforts using P. aeruginosa NCIM 5514 shall be performed when temp. is suitable for bioremediation works to be carried.
Most conducive C:N:P ratio to have notable crude petroleum biodegradation is 100:10:1 [29]. Pristine agricultural soil used to perform the study did not show reported optimum C:N:P ratio. Hence, nutrients were added and C:N:P was maintained at 100:10:1 for providing required optimum nutrients concentration to native microbes (microcosm 3) and native microbes + P. aeruginosa NCIM 5514 (microcosm 5) for their growth. This in-turn leads decomposition of hydrocarbons present in petroleum crude, which was evidenced as the highest and speedy degradation of hydrocarbons in soil microcosm 5 (Tables 2, 3, S2 and S3). The decrease in petroleum hydrocarbons and increase in HUB population has been reported by researchers earlier [2, 30]. This supports results obtained in the study presented here. Biodegradation can be consequential when hydrocarbon degrading microbial population in the soil sample is greater than 105 CFU/g [28]. Hence, if HUB count is less than 105 CFU/g of soil, then to increase hydrocarbon utilizing bacterial count treatment is required by means of either biostimulation or bioaugmentation or both [13, 15]. The agricultural soil used for the said work showed very less HUB count (0.66 ± 0.15 × 102) suggesting treatment to the soil is needed to increase HUB count. Therefore, artificially contaminated agricultural soil was bioaugmented with P. aeruginosa NCIM 5514 and biostimulation was performed using nutrient supplements such as NH4NO3 (nitrogen source) and Na2HPO4 (Phosphorous source).
Table 2.
Petroleum crude carbon fractions in soil microcosm 4: effectiveness of pre-adapted P. aeruginosa NCIM 5514
| Petroleum crude composition | % Variation of petroleum crude composition | % Removal | |
|---|---|---|---|
| 0 day | 60 days | ||
| C8 | 3802.70 | 0.00 | 100.00 |
| C9 | 7237.61 | 0.00 | 100.00 |
| C10 | 2003.70 | 0.00 | 100.00 |
| C11 | 14,921.60 | 0.00 | 100.00 |
| C12 | 23,780.03 | 0.00 | 100.00 |
| C13 | 20,938.79 | 0.00 | 100.00 |
| C14 | 20,981.00 | 0.00 | 100.00 |
| C15 | 14,077.98 | 568.24 | 95.96 |
| C16 | 16,859.91 | 4303.38 | 74.48 |
| C17 | 18,624.03 | 16,815.59 | 9.71 |
| C18 | 11,184.52 | 9154.32 | 18.15 |
| C19 | 12,065.61 | 7118.22 | 41.00 |
| C20 | 13,664.26 | 1543.71 | 88.70 |
| C21 | 12,851.33 | 6030.39 | 53.08 |
| C22 | 16,256.66 | 7241.60 | 55.45 |
| C23 | 19,167.19 | 18,802.57 | 1.90 |
| C24 | 13,908.54 | 9882.61 | 28.95 |
| C26 | 12,546.89 | 3092.20 | 75.35 |
| C27 | 14,221.29 | 8901.48 | 37.41 |
| C28 | 9006.22 | 2994.49 | 66.75 |
| C29 | 8806.69 | 5662.51 | 35.70 |
| C30 | 7143.20 | 2931.38 | 58.96 |
| C31 | 5534.87 | 2024.47 | 63.42 |
| C32 | 3890.80 | 267.12 | 93.13 |
| C33 | 3253.79 | 385.20 | 88.16 |
| C34 | 3008.78 | 721.18 | 76.03 |
| C35 | 2000.21 | 660.57 | 66.97 |
| C36+ | 6748.64 | 1244.28 | 81.56 |
| Total | 318,486.84 | 110,345.51 | |
| Degradation (%) | 0.00 | 65.35 | |
The growth of hydrocarbon-degrading microorganisms in the soil after it’s bioaugmentation in microcosms prepared for bioremediation experiments is very important for degradation of petroleum crude [5, 14, 19]. Results of the present study demonstrate that biodegradation in 2nd and 3rd microcosms is because of native microorganisms and native microorganisms + nutrient(s) supplementation, respectively. However, for 4th and 5th soil microcosms biodegradation was because of exogenously introduced P. aeruginosa NCIM 5514 and P. aeruginosa NCIM 5514 + nutrient(s) supplementation, respectively. For microcosms 2–4, HUB count increased with time till 45 days, but at the end of the experiment, the count of the HUB was decreased. For 5th microcosm, HUB count increased with time till 30 days thereafter it’s decrease was noted till the end of the experiment. However, at the corresponding time interval, higher HUB count was noted in microcosm 5 than that for microcosm 4. Similar results were obtained for microcosms 2 and 3 i.e. higher HUB count at a particular time was noted for microcosm 3 than that for microcosm 2 indicating the effect of nutrient amendments. This can be due to the utilization of nutrients provided and hydrocarbons present in petroleum crude, which leads to the increased microbial count and the simultaneous decrease in petroleum hydrocarbons (Table 4).
Soil microcosm experiments to study the efficacy of various amendments in the soil including nutrients (NH4NO3 and KH2PO4) addition, soil grinding + nutrients (NH4NO3 and KH2PO4) addition and simultaneous bioaugmentation and biostimulation on biodegradation of crude oil carbon fractions have been studied [13]. They have reported the highest degradation of petroleum crude carbon fractions and increased hydrocarbon degraders count when concurrent treatment of nutrient amendments and bioaugmentation was given. The similar type of results i.e. highest results for biodegradation of petroleum crude has been reported in which researchers have concluded that apart from bioaugmentation and biostimulation by nutrients addition of biosurfactants gives better results [15]. In a study presented here, P. aeruginosa culture used for bioaugmentation produced a good amount of rhamnolipid as reported earlier [23, 24]. Hence biosurfactant addition was not performed for this study. Increased rate of degradation of petroleum hydrocarbons and enhanced HUB growth was recorded in microcosm 5 when collate to microcosm 1, showing the efficacy of biostimulation and bioaugmentation. Globally various reports are available with similar results [15, 17, 30]. The presented study looks similar with previously reported studies indicating better biodegradation in a situation where biostimulation and bioaugmentation have been applied in a synchronous way [13, 14, 20].
Conclusions
The reported study here presents the feasibility of pre-adapted P. aeruginosa NCIM 5514 with simultaneous nutrients amendments for its application in agricultural soil bioremediation. The results of soil microcosm (BA + BS) study revealed nearly complete degradation of hydrocarbon fractions at the end of the experiment. To summarise order of the approaches used here for bioremediation from increasing to decreasing pattern: Bioaugmentation + Biostimulation > Bioaugmentation > Biostimulation > Natural attenuation > Weathering control. The degradation of hydrocarbons reported in this study is better both qualitatively and quantitatively as compared to that of reported by other researchers. From the results of microcosm study, it is concluded that P. aeruginosa NCIM 5514 can be employed as a potential bio-degrader to remediate soil contaminated with petroleum hydrocarbons.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the scientists and faculties of Institute of Reservoir Studies (IRS), ONGC, Ahmedabad; M.G. Science Institute, Ahmedabad; Indian Institute of Advanced Research (IIAR), Gandhinagar and Kadi Sarva Vishwavidyalaya, Gandhinagar for their support and co-operation.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whelan MJ, Coulon F, Hince G, Rayner J, McWatters R, Spedding T, Snape I. Fate and transport of petroleum hydrocarbons in engineered biopiles in polar regions. Chemosphere. 2015;131:232–240. doi: 10.1016/j.chemosphere.2014.10.088. [DOI] [PubMed] [Google Scholar]
- 2.Mishra S, Jyot J, Kuhad RC, Lal B. In situ bioremediation potential of an oily sludge-degrading bacterial consortium. Curr Microbiol. 2001;43:328–335. doi: 10.1007/s002840010311. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan A, Fazlurrahman OJG, Jain RK. Bacterial metabolism of polycyclic aromatic hydrocarbons: strategies for bioremediation. Indian J Microbiol. 2008;48:95–113. doi: 10.1007/s12088-008-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Liang J, Wang J, Gao S. Combining stable carbon isotope analysis and petroleum-fingerprinting to evaluate petroleum contamination in the Yanchang oilfield located on loess plateau in China. Environ Sci Pollut Res. 2018;25:2830–2841. doi: 10.1007/s11356-017-0500-6. [DOI] [PubMed] [Google Scholar]
- 5.Varjani S, Upasani VN. Influence of abiotic factors, natural attenuation, bioaugmentation and nutrient supplementation on bioremediation of petroleum crude contaminated agricultural soil. J Environ Manag. 2019;245:358–366. doi: 10.1016/j.jenvman.2019.05.070. [DOI] [PubMed] [Google Scholar]
- 6.Kaczorek E, Cieslak K, Bielicka-Daszkiewicz K, Olszanowski A. The influence of rhamnolipids on aliphatic fractions of diesel oil biodegradation by microorganism combinations. Indian J Microbiol. 2013;53:84–91. doi: 10.1007/s12088-012-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonfa MR, Grossman MJ, Mellado E, Durrant LR. Biodegradation of aromatic hydrocarbons by Haloarchaea and their use for the reduction of the chemical oxygen demand of hypersaline petroleum produced water. Chemosphere. 2011;84:1671–1676. doi: 10.1016/j.chemosphere.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Desforges JW, Sonne C, Levin M, Siebert U, Guise SD, Dietz R. Immunotoxic effects of environmental pollutants in marine mammals. Environ Int. 2016;86:126–139. doi: 10.1016/j.envint.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Mittal A, Singh P. Isolation of hydrocarbon degrading bacteria from soils contaminated with crude oil spills. Indian J Exp Biol. 2009;47:760–765. [PubMed] [Google Scholar]
- 10.Patowary K, Patowary R, Kalita MC, Deka S. Development of an efficient bacterial consortium for the potential remediation of hydrocarbons from contaminated sites. Front Microbiol. 2016;7:1–14. doi: 10.3389/fmicb.2016.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari S, Regar R, Bajaj A, Ch R, Satyanarayana GNV, Mudiam MKR, Manickam N. Simultaneous biodegradation of polyaromatic hydrocarbons by a Stenotrophomonas sp: characterization of nid genes and effect of surfactants on degradation. Indian J Microbiol. 2016;57:60–67. doi: 10.1007/s12088-016-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varjani SJ, Gnansounou E, Pandey A. Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere. 2017;188:280–291. doi: 10.1016/j.chemosphere.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Brassington KJ, Prpich G, Paton GI, Semple KT, Pollard SJT, Coulon F. Insights into the biodegradation of weathered hydrocarbons in contaminated soils by bioaugmentation and nutrient stimulation. Chemosphere. 2016;161:300–307. doi: 10.1016/j.chemosphere.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margesin R, Schinner F. Bioremediation (natural attenuation and biostimulation) of diesel-oil-contaminated soil in an alpine glacier skiing area. Appl Environ Microbiol. 2001;67:3127–3133. doi: 10.1128/AEM.67.7.3127-3133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolopoulou M, Pasadakis N, Kalogerakis N. Evaluation of autochthonous bioaugmentation and biostimulation during microcosm-simulated oil spills. Mar Pollut Bull. 2013;72:165–173. doi: 10.1016/j.marpolbul.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Varjani SJ, Upasani VN. Crude oil degradation by Pseudomonas aeruginosa NCIM 5514: influence of process parameters. Indian J Exp Biol. 2017;55:493–497. [Google Scholar]
- 17.Di Gregorio S, Siracusa G, Becarelli S, Mariotti L, Gentini A, Lorenzi R. Isolation and characterization of a hydrocarbonoclastic bacterial enrichment from total petroleum hydrocarbon contaminated sediments: potential candidates for bioaugmentation in bio-based processes. Environ Sci Pollut Res. 2016;23:10587–10594. doi: 10.1007/s11356-015-5944-y. [DOI] [PubMed] [Google Scholar]
- 18.Varjani SJ. Microbial degradation of petroleum hydrocarbons. Bioresour Technol. 2017;223:277–286. doi: 10.1016/j.biortech.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Varjani SJ, Upasani VN. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int Biodeterior Biodegrad. 2017;120:71–83. doi: 10.1016/j.ibiod.2017.02.006. [DOI] [Google Scholar]
- 20.Szulc A, Ambrożewicz D, Sydow M, Ławniczak Ł, Piotrowska-Cyplik A, Marecik R, Chrzanowski Ł. The influence of bioaugmentation and biosurfactant addition on bioremediation efficiency of diesel-oil contaminated soil: feasibility during field studies. J Environ Manag. 2014;132:121–128. doi: 10.1016/j.jenvman.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Ramadass K, Megharaj M, Venkateswarlu K, Naidu R. Bioavailability of weathered hydrocarbons in engine oil-contaminated soil: impact of bioaugmentation mediated by Pseudomonas spp. on bioremediation. Sci Total Environ. 2018;636:968–974. doi: 10.1016/j.scitotenv.2018.04.379. [DOI] [PubMed] [Google Scholar]
- 22.Varjani SJ (2014) Hydrocarbon degrading and biosurfactants (bio-emulsifiers) producing bacteria from petroleum oil wells. Ph.D. thesis, Kadi Sarva Vishwavidyalaya, Gandhinagar, India
- 23.Varjani SJ, Upasani VN. Core flood study for enhanced oil recovery through ex situ bioaugmentation with thermo- and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour Technol. 2016;220:175–182. doi: 10.1016/j.biortech.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 24.Varjani SJ, Upasani VN. Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: production, characterization and surface active properties of biosurfactant. Bioresour Technol. 2016;221:510–516. doi: 10.1016/j.biortech.2016.09.080. [DOI] [PubMed] [Google Scholar]
- 25.Varjani SJ, Upasani VN. Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Bioresour Technol. 2016;222:195–201. doi: 10.1016/j.biortech.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Trzesicka-Mlynarz D, Ward OP. Degradation of fluoranthene in a soil matrix by indigenous and introduced bacteria. Biotechnol Lett. 1996;18:181–186. doi: 10.1007/BF00128676. [DOI] [Google Scholar]
- 27.Varjani SJ, Rana DP, Jain AK, Bateja S, Upasani VN. Synergistic ex situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int Biodeterior Biodegrad. 2015;103:11–124. doi: 10.1016/j.ibiod.2015.03.030. [DOI] [Google Scholar]
- 28.Forsyth JV, Tsao YM, Bleam RD (1995) Bioremediation: when bioaugmentation needed? In: Hinchee RE, Fredrickson J, Alleman BC (eds) Bioaugmentation for site remediation. Battelle Press, Columbus, pp 1–14. https://www.osti.gov/biblio/474234-bioremediation-when-augmentation-needed. Last Accessed 09 June 2019
- 29.Unites States Environmental Protection Agency (U.S. EPA) (2017) U.S. Environmental Protection Agency National contingency plan product schedule, January 2017. https://www.epa.gov/sites/production/files/2013-08/documents/schedule.pdf. Last Accessed 29 Apr 2019
- 30.Okparanma RN, Azuazu I, Ayotamuno JM. Assessment of the effectiveness of onsite ex situ remediation by enhanced natural attenuation in the Niger Delta region. J Environ Manag. 2017;204:291–299. doi: 10.1016/j.jenvman.2017.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.