Abstract
We previously demonstrated that intracardiac delivery of autologous peripheral blood‐derived CD34+ stem cells (SCs), mobilized by granulocyte‐colony stimulating factor (G‐CSF) and collected by leukapheresis after myocardial infarction, structurally and functionally repaired the damaged myocardial area. When used for cardiac indication, CD34+ cells are now considered as Advanced Therapy Medicinal Products (ATMPs). We have industrialized their production by developing an automated device for ex vivo CD34+‐SC expansion, starting from a whole blood (WB) sample. Blood samples were collected from healthy donors after G‐CSF mobilization. Manufacturing procedures included: (a) isolation of total nuclear cells, (b) CD34+ immunoselection, (c) expansion and cell culture recovery in the device, and (d) expanded CD34+ cell immunoselection and formulation. The assessment of CD34+ cell counts, viability, and immunophenotype and sterility tests were performed as quality tests. We established graft acceptance criteria and performed validation processes in three cell therapy centers. 59.4 × 106 ± 36.8 × 106 viable CD34+ cells were reproducibly generated as the final product from 220 ml WB containing 17.1 × 106 ± 8.1 × 106 viable CD34+ cells. CD34+ identity, genetic stability, and telomere length were consistent with those of basal CD34+ cells. Gram staining and mycoplasma and endotoxin analyses were negative in all cases. We confirmed the therapeutic efficacy of both CD34+‐cell categories in experimental acute myocardial infarct (AMI) in immunodeficient rats during preclinical studies. This reproducible, automated, and standardized expansion process produces high numbers of CD34+ cells corresponding to the approved ATMP and paves the way for a phase I/IIb study in AMI, which is currently recruiting patients. stem cells translational medicine 2019;8:822&832
Keywords: CD34+, Cellular therapy, Hematopoietic stem cells, Peripheral blood stem cells, Cardiac, Cell culture
Significance Statement.
The authors showed in a previous study that intracardiac injection of peripheral blood CD34+ stem cells allows a functional and structural cardiac regeneration of the ischemic lesion after myocardial infarction. This study presents a simplified and standardized production process yielding high amounts of CD34+ cells, avoiding leukapheresis processing. This process will improve the ability of the patient to withstand the therapeutic procedure and allow its extension worldwide. The production of such CD34+ cells, recognized as advanced therapy medicinal products by the regulatory authorities, opened the way for a clinical phase I/IIb, which is currently recruiting patients with severe acute myocardial infarction.
Introduction
The work of Orlic et al., documenting the efficacy of murine lineage‐negative c‐kit‐positive (Linˉ c‐kit+) bone marrow (BM) cell transplantation for repairing experimental myocardial infarction 1, triggered its clinical application for patients with ischemic cardiac diseases. Various sources of stem cells (SCs) have been assessed in randomized trials, which have varied widely in design. They confirmed the feasibility and safety of cell‐reinfusion procedures, but the improvement in heart function was globally less than that expected 2, 3, 4, 5.
These divergent outcomes can be explained by differences in the type(s), source(s), doses of reinjected stem/progenitor cells, cell injection route and timing, choice of efficiency criteria, previous or concomitant reperfusion of the infarcted zone, and type of cardiac disease (acute myocardial infarct [AMI], congestive heart failure, refractory angina), which separately or together, could have influenced the results.
A pilot study conducted by our group 6 demonstrated very long‐term functional and structural regeneration of the cardiac lesion in patients with AMI after direct intracardiac reinjection of autologous peripheral blood (PB)‐CD34+ SCs, previously mobilized by granulocyte‐colony stimulating factor (G‐CSF). However, the procedure was difficult and demanding for the patient and could have only been performed in a very few specialized centers, which would limit access to a small number of AMI patients. Furthermore, regulatory authorities now consider CD34+ cells used for cardiac repair as Advanced Therapy Medicinal Products (ATMPs). A robust process that would allow reproducible good manufacturing practice (GMP) production of cell grafts was thus necessary to improve patient access. We simplified and standardized the cell‐graft production process by developing an automated device (StemXpand) that allows GMP SC expansion starting from whole blood (WB) samples withdrawn after G‐CSF mobilization, resulting in CD34+ SC numbers at least equivalent to those collected during one leukapheresis procedure (LKP).
Our aim was to establish acceptance criteria of CD34+ cell grafts obtained after a 9‐day expansion process through the StemXpand integrated device under GMP conditions, as required by regulatory authorities before clinical delivery. Thus, we assessed the characteristics (CD34+‐cell numbers, purity/impurity profile, and viability) and safety (sterility, pyrogen, and mycoplasma content) of the expanded cells. Functionality of expanded cells (eCD34+) has been assessed during an in vivo preclinical study in rats.
Materials and Methods
Healthy Donors and Cell‐Production Centers
Thirty‐three healthy male volunteers (average age: 30 years, range 20–53) were enrolled in this study after approval by the French regulatory agency Agence Nationale de Sécurité du Médicament et des produits de santé and the regional ethics committee. All volunteers provided signed informed consent. Each volunteer first underwent daily subcutaneous (s.c.) administration of 10 μg/kg per day G‐CSF (Lenograstim) for 4 days. A WB sample of 440 ml ± 10 ml was withdrawn at the Clinical Investigation Center (CIC‐Paris Est) of the Pitié‐Salpêtrière Hospital (Paris, France) on the morning of the fifth day by simple venous puncture and collected in a blood bag and immediately shipped at ambient temperature to the “Unité d'Ingéniérie Tissulaire et Cellulaire (UITC)” in Créteil (France). Its content was divided into equal volumes of 220 ml into two labeled bags, of which one was transported at 4°C–8°C to the CellProthera facilities in Mulhouse (France), whereas the other was either maintained at 4°C–8°C in the Creteil UITC facilities or sent to CellProthera, depending on the requirements of the study. The manufacturing process was started on the sixth day, after overnight storage of the WB sample at 4°C–8°C, using the StemXpand automated integrated system and StemPack disposable kits developed by CellProthera. Additionally, the Nantes Cell Therapy Center (CTC; Atlantic Bio GMP) participated in the validation of the GMP process.
ProtheraCytes Preparation
Starting from the initial WB sample (Supporting Information Fig. S1), red blood cell (RBC) sedimentation was performed for total nuclear cell (TNC) isolation, adapting the gelatin method previously described for cord blood TNC preparation 7. Briefly, 440 ml of WB/phosphate‐buffered saline 1:1 solution (PBS; Macopharma, Mouvaux, France) was mixed with 440 ml of 4% gelatin (Gelofusine, BBraun, Melsungen, Germany) in two 600‐ml transfer bags, which were hung for 20 minutes to facilitate RBC sedimentation. RBCs remaining in the pellet were again mixed with 4% gelatin for a second 20‐minute sedimentation period. The two supernatants were pooled and centrifuged at 400g for 10 minutes at room temperature to pellet the TNC, from which basal (b)‐CD34+ SCs were purified using the CliniMACS system (Magnetic‐Activated Cell Sorting, Miltenyi Biotec, Bergisch Gladbach, Germany). The purified b‐CD34+ SC suspension bag was immediately connected to the machine kit to undergo a 9‐day culture period in our proprietary StemFeed medium into the StemXpand incubator, in which the expansion steps are automatically programmed and controlled: first, predetermined volumes of StemFeed culture medium, cytokine mix (composed of interleukin [IL]6, IL3, Stem Cell Factor, ThromboPoietin, and Fms‐Like Tyrosin kinase 3 Ligand at various concentrations), and the previously immunoselected CD34+ SCs were successively distributed into the dedicated culture bag placed on the agitator contained in the device incubator room. The bag was then gently agitated for 30 seconds to disperse the cell mixture, which was then incubated at 37°C in a 5% CO2‐controlled atmosphere for a 9‐day cell expansion period, without any further intervention. At the end of incubation, the cell suspension was dispersed by gentle agitation, followed by adjustment of the agitator tray to an 80° inclination to facilitate distribution of the cell suspension into two collection bags in equal volumes. Samples were collected at day 0 and day 7 to analyze sterility after dispersing the cell suspension and 50° inclination of the agitator tray.
At the end of the 9‐day period, the culture product was collected, centrifuged, and immunoselected using the CliniMACS system for the purification of eCD34+ SC, which constituted the final product (ProtheraCytes) once resuspended in 15 ml PBS/2% human serum albumin (HSA) and conditioned in three syringes of 5 ml each.
Flow Cytometry Evaluation
Total CD34+ Cell Enumeration
CD34+ cell counts were performed at each stage of the manufacturing process, that is, on WB, before the first round of immunoselection, before expansion, before the second round of immunoselection, and at the end of the process as in‐process controls. They were performed using the Stem Cell Enumeration kit and the Stem Cell Control kit (both from BD Biosciences, San Jose, CA) and analyzed with a Fluorescence Activated Cell Sorting (FACS) Canto II analyzer (BD Biosciences) and FACS DIVA software following the manufacturer's instructions and ISHAGE guideline 8. Additionally, we also looked for CD133+ coexpression to determine the proportion of immature CD34+ cells. Cells (2 × 105) were labeled with a combination of Peridinin Chlorophyll Protein (PerCP)‐conjugated CD45, Fluorescein IsoThioCyanate (FITC)‐conjugated CD34 (BD Biosciences, clone 8G12), and AlloPhycoCyanin (APC)‐conjugated CD133 (Miltenyi Biotec, clone AC133).
PerCP/APC/FITC‐conjugated mouse immunoglobulin G1 (IgG1) antibodies were used as isotype controls to ensure specificity. A minimum of 50,000 gated events were analyzed in each sample.
Determination of the Percentage of Cell Impurities
TNCs (2 × 105) were labeled with a combination of PerCP‐conjugated CD45 (BD Biosciences, clone 2D1) and APC‐conjugated CD34 (BD Biosciences, clone 8G12) plus: FITC‐conjugated CD14 (BD Biosciences, clone MSE2) for monocyte detection; PhycoErythrin (PE)‐conjugated CD56 (BD Biosciences, clone MY31), FITC‐conjugated CD2 and CD3 (BD Biosciences, clone MOPC21 and UCHT1), FITC‐conjugated CD19 and CD20 (BD Biosciences, clone HIB19 and 2H7) for lymphocyte detection; and FITC‐conjugated CD15 (BD Biosciences, clone MMA) for granulocyte detection. The percentage of cell impurities was calculated as the percentage of CD45+ events after the exclusion of CD34+ events using a FACS Canto II analyzer (BD Biosciences) and FACS DIVA software (Supporting Information Fig. S2).
Microarray Study
Cell Sample Collection and RNA Preparation
Total RNA from bCD34+ and eCD34+ cells collected from four donors was isolated in RLT plus buffer followed by purification over a Qiagen RNeasy column (Qiagen, Hilden, Germany).
Microarray Hybridization and Data Acquisition
This step was subcontracted to Helixio (Saint‐Beauzire, France). cDNA was reverse transcribed from each of the eight RNA samples using the Low Input Quick Amp WT Labeling Kit, One color (Agilent Technologies, Santa Clara, CA) kit. cRNA was then transcribed and fluorescently labeled with Cyanin3 (Cy3) and purified using the RNeasy kit (Qiagen). The Cy3‐labeled and amplified cRNA (600 ng) was hybridized to an Agilent SurePrint G3 Human Gene Expression 8x60K v2 Microarray (AMADID 039494, Agilent Technologies) and processed according to the manufacturer's instructions. The array was scanned using an Agilent G2505C DNA microarray scanner. The image files were extracted using Agilent Feature Extraction software version 11.5.1.1.
Microarray Data Analysis
Data were filtered and normalized using Agilent Genespring GX software v12.0 and a t test was performed on the filtered probes. Hierarchical clustering was performed on probes for which the p value was <.001 after t test analysis. The distance was determined, Pearson centered, and the average linkage determined.
Cell Diameter
Cell diameter analyses were performed on bCD34+ and eCD34+ SC from six healthy donors. A cell suspension (100 μl) was spread on a glass slide and covered with a coverslip. Cell size was evaluated using a Zeiss Axiostar Plus microscope (Zeiss, Oberkochen, Germany). At least three representative images of the cell population were analyzed using Axiocam Icc1 software to calculate cell diameter.
Evaluation of CD34+ Cell Stemness
CD34 Epitope Quantification
CD34 epitopes were quantified with a FACS Canto II analyzer using QifiKit (Dako/Agilent Technology, Glostrup, Denmark) following the manufacturer's instructions. Briefly, 2 × 105 CD34+ SCs were labeled with a saturating concentration of nonconjugated CD34 antibody (BD Biosciences, clone 8G12). Cell specimens (bCD34+ or eCD34+ SC) and set‐up and calibration beads were labeled in parallel with a fluorescein‐conjugated antimouse secondary antibody, used at saturating concentrations. Fluorescence intensity was directly correlated with the number of epitopes present on the cell surface or the beads. The antibody binding capacity was calculated by calibration curve interpolation.
Telomere Length
Relative telomere length (RTL) was determined using the telomere peptide nucleic acid (PNA) kit/FITC for flow cytometry (FCM; Dako). bCD34+ or eCD34+ SCs (2 × 105) were mixed with an equal number of control cells (1301 cell line: human T‐cell leukemia, Sigma–Aldrich, St. Louis, MO), denatured for 10 minutes at 82°C, either in a hybridization solution without probe or a hybridization solution containing a fluorescein‐conjugated PNA probe specific for telomere repeats. Hybridization was performed overnight, in the dark, at room temperature and followed by two 10‐minute washes at 40°C. The DNA staining solution included in the kit was used for the identification of G0/1 cells. The intensity of CD34+ cell fluorescence relative to that of control cells and unstained controls was measured using a BD FACS Canto II analyzer.
RTL values were calculated as the ratio between the telomere signal of each CD34+ SC sample and that of control cells with a correction for the DNA index of G0/1 cells to standardize the number of telomere ends per cell and thus telomere length per chromosome.
The DNA index was calculated according to Vindeløv et al. 9 using propidium iodide staining (BD Bioscience) and two internal controls: chicken erythrocyte nuclei (BD Bioscience, DNA QC particles) and trout erythrocytes nuclei (iCyt Mission Technology, Inc., Champaign, IL), which have a DNA content of 35% and of 80%, respectively, of that of human diploid cells.
Karyotype
Constitutional Karyotypes on WB
Metaphases were obtained after 72 hours of culture of WB (1 ml) in Roswell Park Memorial Institute medium (Sigma, St. Louis, MO) with phytohemagglutinin. Reverse banding using Heat and Giemsa (RHG) banding was performed on chromosome preparations. For each control, 20 metaphases were analyzed and the karyotype expressed following International System for Human Cytogenomic Nomenclature 2013 guidelines.
Postexpansion Karyotypes (D9): Direct Technique
CD34+ cells (5–10 × 106) were seeded in Marrow Max medium (Invitrogen, Paisley, U.K.) supplemented with 4 μg/ml colchicine (final concentration, 0.04 μg/ml; Sigma) for 90 minutes at 37°C. After centrifugation (10 minutes, 500g), cells were incubated in a prewarmed (37°C) hypotonic solution (5.6 g/l KCl [Sigma]) for 30–40 minutes and fixed in a mixture of methanol and glacial acetic acid (Carlo Erba Reagents, Val de Reuil, France). The fixed suspension was added dropwise onto glass slides in a dedicated chamber (Thermotron, Holland, MI) at 22°C and 45% hygrometry to perform RHG banding.
Microscopy
Slides were examined with a light microscope Axiophot (ZEISS, Oberkochen, Germany) equipped with Ikaros software (Metasystems, Altlußheim, Germany).
In vivo Preclinical Study
The study included 32 athymic male rats (Charles River, Crl: NIH‐Foxn1rnu), which were at least 9 weeks old and weighed between 247 and 339 g. The rats were divided into four experimental groups: sham‐operated, placebo, bCD34+ SC, and eCD34+. Experimental AMI was performed by permanent left anterior descending coronary artery ligation (CAL) for all, except the sham‐operated group. Direct intramyocardial delivery of 5 × 105 bCD34+ or eCD34+ cells, resuspended in 100 μl PBS/2% HSA or PBS/2% HSA alone (placebo) was performed 1 week after CAL. Four injections of 25 μl each were performed in the left ventricle (LV) bordering the ischemic lesion, irrespective of the group.
A microtip pressure‐volume conductance calibrated catheter (SPR‐838NR, Millar Instruments, Houston, TX) was repeatedly introduced into the LV via the carotid artery and left ascending aorta under closed‐chest conditions. After stabilization, volume and pressure signals were continuously recorded using a PV conductance system (MPVS‐Ultra, Millar Instruments) connected to a PowerLab 4/35 acquisition system (ADinstruments, Dunedin, New Zealand) to establish the pressure/volume (P/V) relationship. All P/V loop data were analyzed using LabChart 7 software version 7.3.7 (ADinstruments).
Animals were euthanized 2 months postinjection by exsanguination under isoflurane gas anesthesia and quickly necropsied. Hearts were excised, fixed in 4% vol/vol formalin/phosphate buffer for 24 hours, and preserved in 70% ethanol until histological analysis. Four transverse sections, from apex to base, were adjusted to fit in individual cassettes for histology and embedded in paraffin, from which 12 slides of equal thickness (4 μm) were prepared and further used for Masson trichrome staining.
Statistical Analysis
In Vitro Study
Statistical analyses were performed using GraphPad Prism version 7.03 for Windows (GraphPad Software, La Jolla, CA) and R (version 3.0.2, http://cran.r-project.org/) software. All values are expressed as the mean ± SD. After checking whether the data had a normal distribution (Shapiro–Wilk test), Student's t test or nonparametric tests (Kruskal–Wallis or Mann–Whitney) were used to compare the bCD34+ and eCD34+ SC groups. p < .05 was considered to be significant.
Preclinical Study
Student's test was used to compare the cardiac functional parameters between the groups. Results are presented as the mean ± SEM. p < .05 was considered to be significant.
Results
CD34+‐Cell Mobilization
Only mild and commonly reported adverse event was observed following G‐CSF administration that was easily managed with paracetamol: bone pain (93%), headache/asthenia (42%), and elevated lactate dehydrogenase (17%). In addition, mild allergic erythema was observed after the first injection in one volunteer.
G‐CSF administration mobilized 67.5 ± 34.8 viable CD34+ SC per microliter of WB (Fig. 1B), representing 17.6 × 106 ± 11.5 × 106 viable CD34+ cells in 220 ml WB. CD34+ SC mobilization tended to be influenced by the age of the donors, although it was not statistically significant (Fig. 1A; Pearson correlation; p = .0517, r 2 = 0.12, n = 33), but not tobacco consumption (smokers: 62.98 ± 37.82 CD34+ per microliter, nonsmokers: 65.45 ± 32.9 per microliter; F = 0.15, p > .05).
Figure 1.
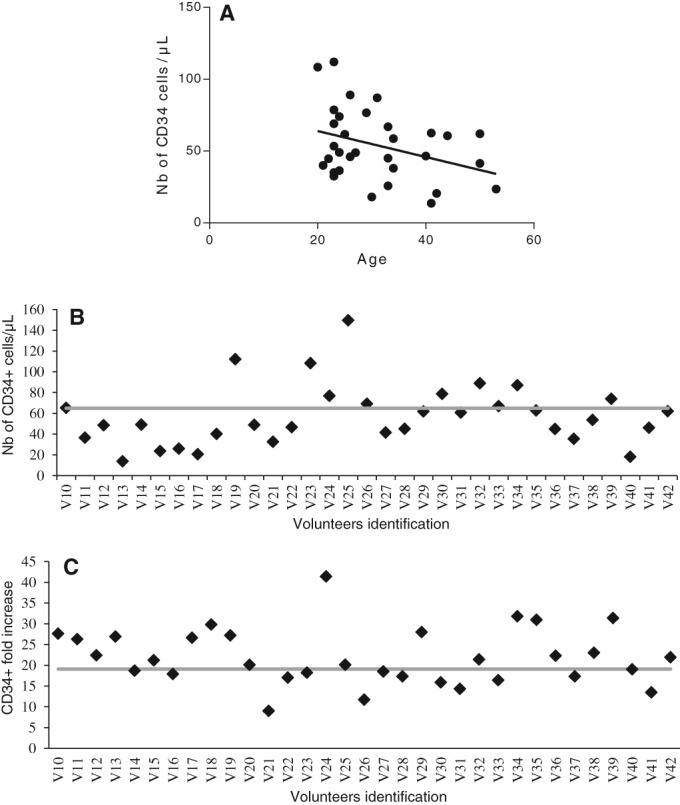
CD34+ cells mobilization and expansion. (A): Correlation between CD34+ cell mobilization and donor age. (B): Number of CD34+ cells per microliter counted in the blood of healthy donors after granulocyte‐colony stimulating factor mobilization (average: 67.5 ± 34.8). (C): Fold increase in the number of CD34+ cells obtained with the StemXpand device (average: 19.1 ± 7.5).
In‐process Controls and Definition of Graft Acceptance Criteria
Thirty‐three manufacturing runs were performed to complete process development. In average, CD34+ SC recoveries after initial RBC sedimentation and the first immunoselection were 84.2% ± 16.6% and 72.7% ± 17.4% (with a purity of 73.4% ± 14.1%), respectively. Although eCD34+ cells only represented 18.0% ± 6.5% of the total CD45+ population, due to asymmetrical division, the cell expansion process increased viable CD34+ SC numbers up to 158.0 × 106 ± 87.7 × 106, corresponding to a 19.1‐fold ± 7.5‐fold increase (Fig. 1C). The second immunoselection step achieved a recovery of 39.3% ± 12.5% viable CD34+ SC with a purity of 79.7% ± 10.4%. The viable CD34+ SC obtained at the end of the process reached 57.9 × 106 ± 41 × 106.
Although the age of the donor tended to influence CD34+ cell mobilization, it did not affect the rate of CD34+ cell expansion or the final number of recovered eCD34+ cells (Fig. 2A, 2B; p = .9217 and p = .1505, respectively).
Figure 2.
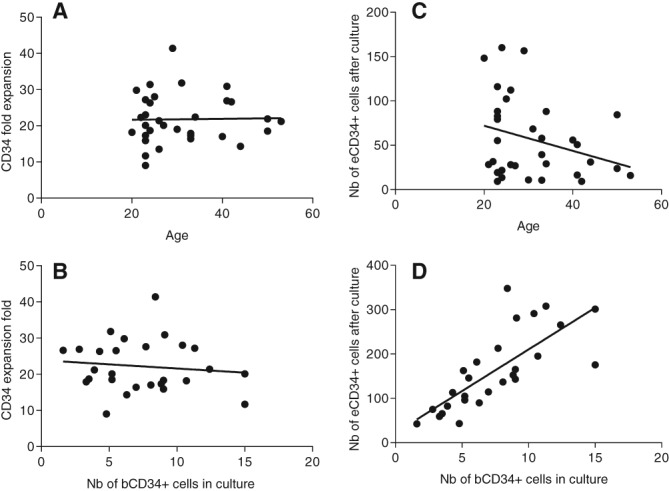
Influence of age and number of bCD24+ cells in culture on fold expansion of CD34+ cells. (A): Correlation between the fold expansion of CD34+ stem cell (SC) and the donor age. (B): Correlation between the fold expansion of CD34+ SC and the number of bCD34+ SC seeded. (C): Correlation between the number of eCD34+ SC obtained after culture and the donor age. (D): Correlation between the number of eCD34+ SC obtained after culture and the number of bCD34+ SC seeded.
Furthermore, the number of initially seeded bCD34+ SC did not affect the expansion rate (p = .5723), although it affected the final number of eCD34+ cells obtained (p < .0001); indeed, the more bCD34+ SC initially seeded, the more eCD34+ cells finally obtained (Fig. 2C, 2D). After expansion, 74.0 × 106 ± 12.3 × 106 CD34+ cells coexpressed CD133, corresponding to a sixfold increase in this more immature population.
To complete the study, cellular impurities were assessed as percentage of CD45+ CD34− cells. Common markers of blood cells were tested: monocytes (CD14), granulocytes (CD15), and lymphocytes (T: CD2, CD3; B: CD19, CD20; NK: CD56). Only monocytes (10.9% ± 8.4%) and granulocytes (2.5% ± 2.1%) were found as impurities.
Graft acceptance criteria were determined from the analysis of those 33 ProtheraCytes batches, as follows: viability ≥95%, purity ≥85%, monocytes ≤10%, granulocytes ≤5%, lymphocytes ≤3%, sterile, and pyrogen and mycoplasma free (Table 1).
Table 1.
Graft acceptance criteria and results obtained during validation of the process
| Tests | Acceptance criteria | Sites | ||
|---|---|---|---|---|
| Créteil | Mulhouse | Nantes | ||
| Color | Colorless to pale yellow | Conform | Conform | Conform |
| Appearance | Clear to opalescent | Conform | Conform | Conform |
| CD34+ purity | ≥85% | 91.4 ± 4.1 | 86.2 ± 4.4 | 94.3 ± 2.1 |
| CD34+ viability | ≥95% | 99.3 ± 0.4 | 98.8 ± 0.3 | 95.6 ± 1.6 |
| CD34+ count | ≥1 × 107 viable cells | 2.7 × 107 ± 1.4 × 107 | 2.7 × 107 ± 1.4 × 107 | 6.2 × 107 ± 1.3 × 107 |
| Monocytes | ≤10% | 2.35 ± 2.31 | 8.4 ± 3.1 | 1.6 ± 1.2 |
| Granulocytes | ≤5% | NR | 2.9 ± 1.3 | 2.5 ± 0.4 |
| Lymphocytes | ≤3% | NR | <1 | <1 |
| Sterilitya | Sterile | Sterile | Sterile | Sterile |
| Pyrogens | ≤12.5 EU/ml | Conform | Conform | Conform |
| Mycoplasma | No detection | Conform | Conform | Conform |
Sterility was verified at days 0, 7, and 9 (Ph.Eur.2.6.27).
Abbreviation: NR, not realized.
GMP Process Validation
The manufacturing process was validated by performing four runs in the Créteil UITC, three in the CellProthera laboratory, and four in the Nantes CTC. Data analyses from in‐process controls confirmed that the GMP manufacturing process was robust and reproducible: there were no differences in the performance criteria of any steps between the three manufacturing sites (data not shown). Moreover, these validation runs allowed the production of grafts that met our acceptance criteria (Table 1).
Comparison of Basal and Expanded CD34+ Cell Characteristics
We analyzed and compared the morphological, stemness, molecular, and karyotypic characteristics of the bCD34+ and eCD34+ SC.
Cell size
The average diameter of the eCD34+ SC was greater than that of the bCD34+ SC (11.18 μm ± 0.49 μm versus 8.78 μm ± 0.11 μm; p = .01; Fig. 3A).
Figure 3.
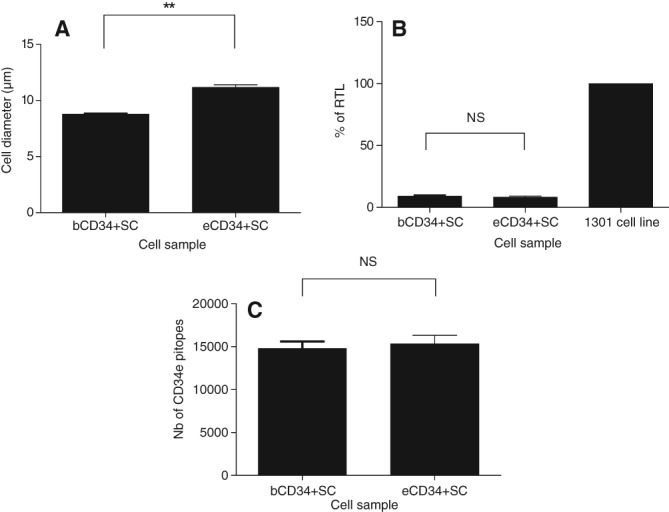
CD34+ stem cell (SC) characterization. Comparison of bCD34+ and eCD34+ SC characteristics: (A) Cell diameter (n = 6). (B): % of Relative Telomere Length (n = 6) compared with that of a positive control (1301 cell line). (C): Number of cell‐surface CD34 epitopes (n = 8). Statistical analysis: **, p < .01. Abbreviation: NS, not significant.
CD34+ Cell Stemness
CD34 antigen site numbers were similar before and after cell expansion (14,804 ± 2,294 epitopes versus 15,363 ± 2,730 epitopes; p > .05; Fig. 3C). bCD34+ and eCD34+ SC telomere lengths were also similar (9.17% ± 1.32% versus 8.35% ± 2.07% RTL; p > .05; Fig. 3B), confirming that eCD34+ SC maintained their proliferative capacity.
Microarray Analysis
Of the 23,448 probes filtered, 3,808 were differentially expressed in bCD34+ and eCD34+ cells (p < .001; Fig. 4). Among cardiac progenitor markers, the mesp mesoderm marker was most highly expressed in eCD34+ cells (p < .01), and kit expression shared the same tendency, but it was not significant (p > .05). Among cardiomyocyte differentiation markers (mef2c, nkx2.5, sirpa), only nkx2.5 was expressed at lower levels in eCD34+ cells (p < .05).
Figure 4.
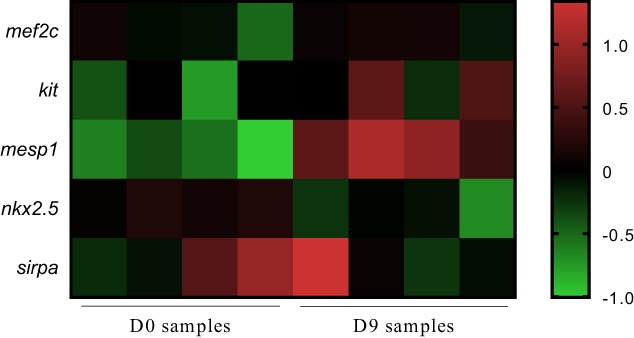
Average linkage cluster of mRNA expressed in bCD34+ stem cell (SC) and eCD34+ SC. Genes involved in cardiomyocyte differentiation are presented. The color bar is a logarithmic indicator of the fold difference in the expression of each gene from the average, that is, 1 indicates a >1‐fold increase and −1 indicates a <0.5‐fold decrease.
Karyotype
All bCD34+ SC showed a normal male constitutional karyotype. After expansion, six samples (19%) had no analyzable metaphases at D9 (Supporting Information Fig. S3). Among the rest, 16 metaphases were analyzed in 9 samples (28%) and 2–16 in 17 samples (53%), of which 7 had between 10 and 16 analyzable metaphases. There were no detectable chromosomal aberrations or structural rearrangements in any sample, except one, which showed monosomy 19 in three metaphases.
Preclinical Study
LV function was significantly impaired after CAL in the animals of all but the sham group, as shown by reduced left ventricular ejection fraction (LVEF) and increased end diastolic left ventricular pressure (eDLVP; Figs. 5, 6). eDLVP tended to be higher in the eCD34+ than placebo and bCD34+ groups (12 ± 2.4 versus 9.1 ± 1.6 and 9 ± 1.2 mmHg, respectively). However, the inverse was true at 2 months (15.6 ± 3.3 versus 17.3 ± 6.2 [placebo] and 18 ± 3 mmHg [bCD34+ cells]; Fig. 5B). Two months after injection, LVEF was consistently higher in animals that received bCD34+ or eCD34+ SC than those that received the placebo (Fig. 5A). The ischemic area surface was significantly reduced and associated with a significant increase in ventricle wall thickness for both CD34+ cell groups relative to the placebo. However, these morphological improvements were even more significant in the eCD34+ group (Fig. 6).
Figure 5.
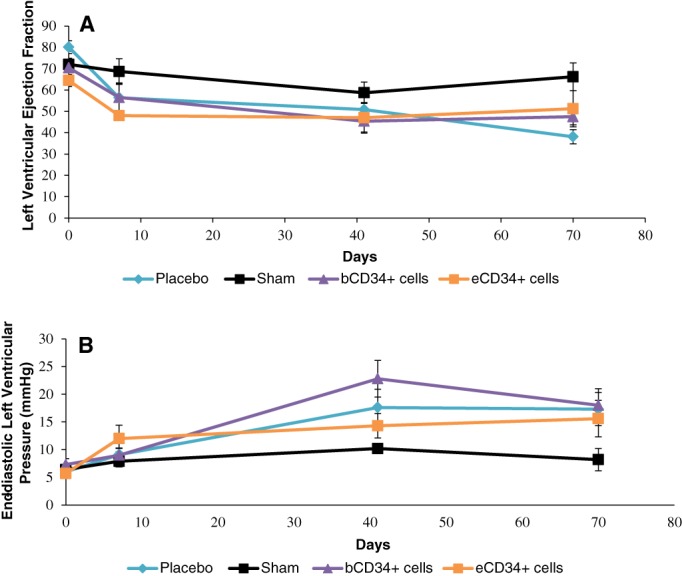
Ejection fraction (A) and end diastolic left ventricular pressure (B) kinetics after sham (n = 7), injection of placebo (n = 5), bCD34+ stem cell (SC; n = 10), or eCD34+ SC (n = 9) in a rat acute myocardial infarct model.
Figure 6.
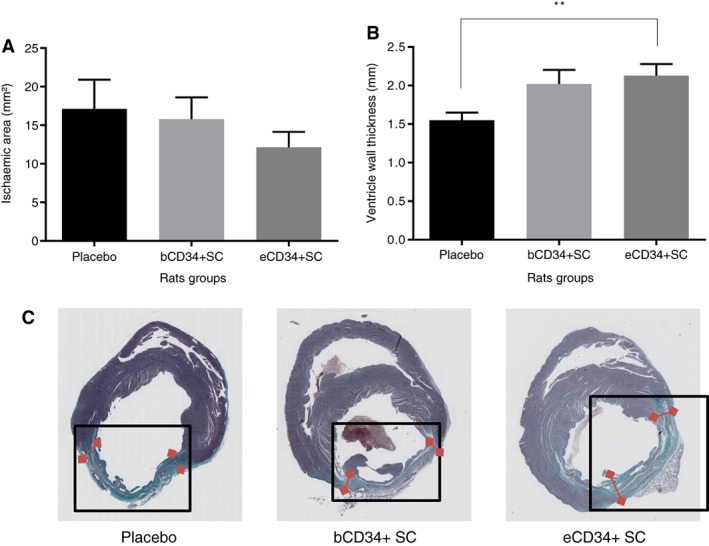
Comparison of (A) ischemic surface and (B) ventricle wall thickness after placebo (n = 14), bCD34+ stem cell (SC; n = 32), and eCD34+ SC (n = 31) injections. **, p < .01. (C): Illustration of ischemic area size (in green, framed) and ventricle wall thickness (red arrows) among the different groups.
Discussion
CD34 antigen has long been considered to be a specific marker of HSCs and hematopoietic progenitor cells 10, but is now well‐established also as a marker of various nonhematopoietic cell types 6, 11, 12. At the end of the 90s, Asahara et al. reported the intracardiac homing of CD34+ cells after experimental AMI in nude mice 13. Human CD34+ cells delivered to athymic nude rats undergoing experimental AMI differentiated more abundantly into cardiomyocytes and endothelial cells in the infarcted myocardium and augmented ischemic neo‐vascularization on day 28 post‐transplant than unselected MNCs (mononuclear cells). They exhibit superior efficacy than unselected MNCs in inhibiting LV remodeling and preserving myocardial integrity and function after AMI 14, 15, 16. A large proportion of CD34+ cells isolated from human PB after G‐CSF mobilization stain positively for c‐troponin T when transplanted directly into the scar of rats with experimental AMI, indicating that they may also differentiate into cardiomyocytes 17.
In humans, an increase in the release of endogenous CD34+ cardiac and endothelial progenitor cell subpopulations by the BM into the blood has been reported within the first hours following AMI and persists for up to several days 18. A return to normal circulating levels occurs within 1 week, presumably related to the homing and consumption of these cells within the ischemic lesion 19, confirming experimental observations of Asahara et al. 13. Although the release of such cells appears to be a physiological response to AMI, its probable effect on limiting ischemic scar formation is not actually measurable and it is clearly unable to compensate for the loss of infarcted cardiomyocytes.
Such experimental outcomes encouraged clinical trials using CD34+ cells. In 2002, our group conducted the first pilot study to use G‐CSF‐mobilized and immunoselected PB‐CD34+ cells in AMI patients with a poor prognosis 6. They received trans‐epicardial CD34+ cell injections directly into the ischemic scar at the end of a coronary artery bypass grafting for compassionate reasons. All but one patient, showed a marked and sustained improvement of LVEF from baseline values 4 years after the procedure (mean: +21%, range: +4% to +42%) associated with cardiac tissue regeneration. Additionally, we detected the presence of small subpopulations by FCM that coexpressed c‐troponin T (a cardiac marker) or CD133+/VEGFR‐2 (Vascular Endothelial Growth Factor Receptor) (an endothelial marker) in mobilized CD34+ cells from AMI patients, whereas they were almost undetectable in those of controls.
Among randomized studies using CD34+ cells in AMI, CD34+ cells were purified and enriched by immunomagnetic selection either from BM aspirates or PB‐CD34+ cells previously mobilized by G‐CSF and collected by LKP 2, 20. The therapeutic results were dose‐dependent 21. Intracoronary (IC) injection of a threshold dose of >10 million CD34+ cells was associated with a significantly greater improvement in cardiac function parameters than when lower doses were used 22. In the PreSERVE AMI study, 8–40 × 106 (mean: 14.9 ± 8) autologous BM‐CD34+/CXCR4+ cells or placebo were delivered IC to patients with LV dysfunction post‐STEMI (ST segment elevation myocardial infarction) 23. There was no significant improvement in LVEF, except for patients who received more than 20 × 106 CD34+/CXCR4+ cells. In our pilot study, a significant (p < .05) average improvement of LVEF of +18% from baseline values was observed 2 years after the injection of an average of 52 × 106 CD34+ (range 29–107 × 106) cells 8.
The number of PBSC (peripheral blood stem cells) can be increased for cell therapy by a number of means, including ex vivo expansion. Various hematopoietic stem cell‐expansion techniques have been proposed, but mostly with the goal to engage cell differentiation along appropriate hematopoietic lineages 24, 25. A few groups have worked on clinical scale PB CD34+ SC expansion to ensure that these cells retain their long‐term self‐renewal capacity. Only one has done it for the performance of cell cardiomyoplasty, but did not obtain more than a fivefold to eightfold increase in the number of CD34+ SC 26.
The automated process we have developed, multiplied the number of CD34+ SC obtained by 19.1‐fold ± 7.5‐fold, reaching a production of 158 × 106 ± 87.7 × 106 CD34+ SC. Such a level of expansion of CD34+ SC, while retaining their stemness potency, is unique. However, the number of cells obtained after expansion varied, likely related to individual differences in terms of cell mobilization and the use of a constant volume of StemFeed medium, regardless of the number of cells seeded. CD34+ SC expansion did not correlate with donor age, but our study involved mostly young volunteers, which may have negatively affected the correlation results.
The second CD34+ SC immunoselection recovery, consistently lower than that usually obtained (~30% instead of ~60%), can be explained by low CD34 antigen expression at the surface of numerous eCD34+ cells which, likely already committed, were not sufficiently bound by the immunomagnetic beads and discarded during the immunoselection phase. However, the final number of 57.9 × 106 ± 41 × 106 CD34+ SC achieved by the full process was in accordance with our expected goal, which was to reinject an average of ~50 × 106 CD34+ cells, as in our pilot study, and well above the threshold of 10 × 106 viable CD34+ SC to be injected, adapted from the literature 21. Moreover, this process allows the reproducible production of CD34+ cell grafts that meet stringent criteria:
Similar numbers of CD34+ epitopes on the cell surface and similar telomere lengths in bCD34+ and eCD34+ demonstrate that stemness was not impaired by the expansion process. The larger diameter of eCD34+ cells was probably due to the fact that all eCD34+, but not bCD34+ SC, were cycling 27.
The presence of endothelial and cardiac progenitor populations has been demonstrated in CD34+ cells 6, 14, 28. Although CD133+ cells were largely expanded with our process, we failed to detect endothelial VEGFR‐2 and cardiac (c‐troponin T) markers in bCD34+ or eCD34+ cells from the first healthy volunteers enrolled in the study and did not further pursue this line of investigation. Indeed, we did not expect to detect them, as the cells were obtained from healthy donors who were not exposed to AMI: CD34+ SC commitment along the endothelial and cardiac pathways is strongly dependent on the changes that occur in myocardial stiffness after AMI 29. Thus, such commitment does not occur under steady‐state conditions (for example in healthy donors): the results of our pilot study, showing a fourfold higher number of c‐troponin T+ cells in the LKP of cardiac patients than in those of controls, support this hypothesis.
Nkx2.5 (a marker of cardiac progenitor cells) was expressed at lower levels in eCD34+ SC. However, the higher expression of mesoderm layer markers (mesp1 and kit), from which cardiac progenitor cells are derived, and the stability of cardiac differentiation marker expression (mef2c and sirpa) by the eCD34+ cells, both suggest that they retained their cardiac differentiation capacity.
We systematically performed karyotypes on bCD34+ and eCD34+ cells from each healthy donor to verify that the cells did not acquire any clonal chromosomal abnormalities after expansion. The direct technique was applied to eCD34+ cells to avoid a possible bias related to a supplementary culture step. This choice, and the fact that cytogenetic studies were performed when cell proliferation reached a plateau, likely explains the relatively low number of metaphases obtained after expansion, as well as the failure rate (19%). It could also explain the monosomy 19 observed in three metaphases in one sample. Indeed, our experience on trophoblasts and BM suggest that the direct technique can generate incomplete metaphases and rare artifacts, such as nonclonal, random losses of chromosomes (personal data).
Thus, eCD34+ SC obtained with the StemXpand device have similar genetic properties, proliferative capacity, and CD34 antigen expression as bCD34+ SC, leading to expect that they would have similar cardiac repair capacity in vivo. Indeed, the preclinical study showed a significant improvement of various cardiac function parameters in the two groups that received CD34+ cells relative to the placebo group, this improvement tending to be more pronounced in the eCD34+ group. Thus, eCD34+SC appears to retain a capacity for myocardial regeneration. These data confirm the positive effects of human expanded and nonexpanded CD34+ SC after experimental AMI in nude rats 14, 15, 30, as well as those obtained from BM 26 and cord blood 31.
Conclusion
We have developed a robust and reproducible process that allows the automated production of enough eCD34+ cells to constitute a suitable ATMP (ProtheraCytes) that meet stringent quality criteria, including sterility and pyrogenicity. bCD34+ and eCD34+ SC have similar genetic characteristics, proliferative capacity, stemness capacity, and CD34 antigen expression. The preclinical study has shown the capacity of CD34+ cells to restore function after experimental AMI in athymic rats. We have launched the randomized “EXCELLENT” phase I/IIb clinical trial, which is currently recruiting (NCT02669810/Eudract number 2014‐001476‐63), to demonstrate the safety and efficiency of ProtheraCytes in AMI patients.
Author Contributions
C.S.: conception and design of the study, collection and assembly of the data, data analysis and interpretation, writing and final approval of the manuscript; S.V., C.T., A.A.: conception and design of the study, data analysis and interpretation; A.M.: provision of study material or patients, collection and assembly of the data, data analysis and interpretation; C.V., L.D.: conception and design of the study; A.C., J.‐E.S., L.K., J.‐S.H.: provision of study material or patients; L.H., B.B., H.R.: conception and design of the study, collection and assembly of the data; C.H., E.J., A.‐G.C.‐L.: data analysis and interpretation; S.D.: collection and assembly of the data; S.J., E.M., S.R.: conception and design, data analysis and interpretation; P.H.: conception and design of the study, writing and final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
C.S., A.C. declared employment/leadership position and patent holder with CellProthera. S.V. declared employment/leadership position with CellProthera. P.H. declared employment/leadership position, ownership interest, and patent holder with CellProthera. L.D. declared employment/leadership position with EryPharm, patent licensing to CellProthera and EryPharm, consultant/advisory role with CellProthera, and ownership interest with CellProthera and EryPharm. The other authors indicated no potential conflicts of interest.
Supporting information
Figure S1 Scheme of the Protheracytes manufacturing process.
Figure S2 Gating strategy for determination of cellular impurities in Protheracytes preparation
After selective gating of CD34+ cells (1st line), percentage of CD14+, CD15+, CD2+, CD3+, CD19+, CD20+ and CD56+ has been gated on CD34− cell population
Figure S3 Karyotype stability. (A) constitutional karyotype realized on bCD34+ SC. (B) Corresponding post‐expansion karyotype.
Acknowledgments
This study was conducted with support of Chugai Pharma France, supplier of G‐CSF product for the trial. We thank the whole CellProthera team for their support, Mickaël Lecuyer and Laetitia Moeckes for their technical work, the entire IRHT team for their scientific and technical support. This study was performed and funded by CellProthera SAS. E.M. is currently affiliated with the Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany; L.D. is currently affiliated with the EryPharm, Paris, France.
Data Availability Statement:Data available on request from the authors.
Data Availability Statement
Data available on request from the authors.
References
- 1. Orlic D, Kajstura J, Chimenti S et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–705. [DOI] [PubMed] [Google Scholar]
- 2. Martin‐Rendon E, Brunskill SJ, Hyde CJ et al. Autologous bone marrow stem cells to treat acute myocardial infarction: A systematic review. Eur Heart J 2008;29:1807–1818. [DOI] [PubMed] [Google Scholar]
- 3. Jeevanantham V, Butler M, Saad A et al. Adult bone marrow cell therapy improves survival and induces long‐term improvement in cardiac parameters: A systematic review and meta‐analysis. Circulation 2012;126:551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian T, Chen B, Xiao Y et al. Intramyocardial autologous bone marrow cell transplantation for ischemic heart disease: A systematic review and meta‐analysis of randomized controlled trials. Atherosclerosis 2014;233:485–492. [DOI] [PubMed] [Google Scholar]
- 5. Gyöngyösi M, Wojakowski W, Lemarchand P et al. Meta‐analysis of cell‐based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res 2015;116:1346–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasquet S, Sovalat H, Hénon P et al. Long‐term benefit of intracardiac delivery of autologous granulocyte‐colony‐stimulating factor‐mobilized blood CD34+ cells containing cardiac progenitors on regional heart structure and function after myocardial infarct. Cytotherapy 2009;11:1002–1015. [DOI] [PubMed] [Google Scholar]
- 7. Schwinger W, Benesch M, Lackner H et al. Comparison of different methods for separation and ex vivo expansion of cord blood progenitor cells. Ann Hematol 1999;78:364–370. [DOI] [PubMed] [Google Scholar]
- 8. Gratama JW, Kraan J, Keeney M et al. Validation of the single‐platform ISHAGE method for CD34(+) hematopoietic stem and progenitor cell enumeration in an international multicenter study. Cytotherapy 2003;5:55–65. [DOI] [PubMed] [Google Scholar]
- 9. Vindeløv LL, Christensen IJ, Nissen NI. Standardization of high‐resolution flow cytometric DNA analysis by the simultaneous use of chicken and trout red blood cells as internal reference standards. Cytometry 1983;3:328–331. [DOI] [PubMed] [Google Scholar]
- 10. Civin CI, Strauss LC, Brovall C et al. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG‐1a cells. J Immunol 1984;133:157–165. [PubMed] [Google Scholar]
- 11. Gordon MY, Levicar N, Pai M et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony‐stimulating factor. Stem Cells 2006;24:1822–1830. [DOI] [PubMed] [Google Scholar]
- 12. Sidney LE, Branch MJ, Dunphy SE et al. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014;32:1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 14. Kawamoto A, Iwasaki H, Kusano K et al. CD34‐positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation 2006;114:2163–2169. [DOI] [PubMed] [Google Scholar]
- 15. Yeh ETH, Zhang S, Wu HD et al. Transdifferentiation of human peripheral blood CD34+‐enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation 2003;108:2070–2073. [DOI] [PubMed] [Google Scholar]
- 16. Kawamoto A, Tkebuchava T, Yamaguchi J‐I et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003;107:461–468. [DOI] [PubMed] [Google Scholar]
- 17. Kim Y‐H. Intramyocardial transplantation of circulating CD34+ cells: Source of stem cells for myocardial regeneration. J Korean Med Sci 2003;18:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wojakowski W, Tendera M, Zebzda A et al. Mobilization of CD34(+), CD117(+), CXCR4(+), c‐met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT‐proBNP levels in patients with acute myocardial infarction. Eur Heart J 2006;27:283–289. [DOI] [PubMed] [Google Scholar]
- 19. Theiss HD, David R, Engelmann MG et al. Circulation of CD34+ progenitor cell populations in patients with idiopathic dilated and ischaemic cardiomyopathy (DCM and ICM). Eur Heart J 2007;28:1258–1264. [DOI] [PubMed] [Google Scholar]
- 20. Brunskill SJ, Hyde CJ, Doree CJ et al. Route of delivery and baseline left ventricular ejection fraction, key factors of bone‐marrow‐derived cell therapy for ischaemic heart disease. Eur J Heart Fail 2009;11:887–896. [DOI] [PubMed] [Google Scholar]
- 21. Poole JC, Quyyumi AA. Progenitor cell therapy to treat acute myocardial infarction: The promise of high‐dose autologous CD34(+) bone marrow mononuclear cells. Stem Cells Int 2013;2013:658480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quyyumi AA, Vasquez A, Kereiakes DJ et al. PreSERVE‐AMI: A randomized, double‐blind, placebo‐controlled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ Res 2017;120:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quyyumi AA, Waller EK, Murrow J et al. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J 2011;161:98–105. [DOI] [PubMed] [Google Scholar]
- 24. Lorenzon D, Mazzucato M, Abbruzzese L et al. Preclinical ex vivo expansion of peripheral blood CD34+ selected cells from cancer patients mobilized with combination chemotherapy and granulocyte colony‐stimulating factor. Vox Sang 2008;94:342–350. [DOI] [PubMed] [Google Scholar]
- 25. Ivanovic Z, Duchez P, Dazey B et al. A clinical‐scale expansion of mobilized CD 34+ hematopoietic stem and progenitor cells by use of a new serum‐free medium. Transfusion 2006;46:126–131. [DOI] [PubMed] [Google Scholar]
- 26. Gunetti M, Noghero A, Molla F et al. Ex vivo‐expanded bone marrow CD34(+) for acute myocardial infarction treatment: in vitro and in vivo studies. Cytotherapy 2011;13:1140–1152. [DOI] [PubMed] [Google Scholar]
- 27. Jorgensen P, Tyers M. How cells coordinate growth and division. Curr Biol 2004;14:R1014–R1027. [DOI] [PubMed] [Google Scholar]
- 28. Kawamoto A, Gwon HC, Iwaguro H et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001;103:634–637. [DOI] [PubMed] [Google Scholar]
- 29. Zhang S, Ma X, Yao K et al. Combination of CD34‐positive cell subsets with infarcted myocardium‐like matrix stiffness: A potential solution to cell‐based cardiac repair. J Cell Mol Med 2014;18:1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwasaki H, Kawamoto A, Ishikawa M et al. Dose‐dependent contribution of CD34‐positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation 2006;113:1311–1325. [DOI] [PubMed] [Google Scholar]
- 31. Schlechta B, Wiedemann D, Kittinger C et al. Ex‐vivo expanded umbilical cord blood stem cells retain capacity for myocardial regeneration. Circ J 2010;74:188–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Scheme of the Protheracytes manufacturing process.
Figure S2 Gating strategy for determination of cellular impurities in Protheracytes preparation
After selective gating of CD34+ cells (1st line), percentage of CD14+, CD15+, CD2+, CD3+, CD19+, CD20+ and CD56+ has been gated on CD34− cell population
Figure S3 Karyotype stability. (A) constitutional karyotype realized on bCD34+ SC. (B) Corresponding post‐expansion karyotype.
Data Availability Statement
Data available on request from the authors.


