Abstract
Patients with late‐stage Kellgren‐Lawrence knee osteoarthritis received a single intra‐articular injection of 1, 10, or 50 million bone marrow mesenchymal stromal cells (BM‐MSCs) in a phase I/IIa trial to assess safety and efficacy using a broad toolset of analytical methods. Besides safety, outcomes included patient‐reported outcome measures (PROMs): Knee Injury and Osteoarthritis Outcome Score (KOOS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC); contrast‐enhanced magnetic resonance imaging (MRI) for cartilage morphology (Whole Organ MRI Scores [WORMS]), collagen content (T2 scores), and synovitis; and inflammation and cartilage turnover biomarkers, all over 12 months. BM‐MSCs were characterized by a panel of anti‐inflammatory markers to predict clinical efficacy. There were no serious adverse events, although four patients had minor, transient adverse events. There were significant overall improvements in KOOS pain, symptoms, quality of life, and WOMAC stiffness relative to baseline; the 50 million dose achieved clinically relevant improvements across most PROMs. WORMS and T2 scores did not change relative to baseline. However, cartilage catabolic biomarkers and MRI synovitis were significantly lower at higher doses. Pro‐inflammatory monocytes/macrophages and interleukin 12 levels decreased in the synovial fluid after MSC injection. The panel of BM‐MSC anti‐inflammatory markers was strongly predictive of PROMs over 12 months. Autologous BM‐MSCs are safe and result in significant improvements in PROMs at 12 months. Our analytical tools provide important insights into BM‐MSC dosing and BM‐MSC reduction of synovial inflammation and cartilage degradation and provide a highly predictive donor selection criterion that will be critical in translating MSC therapy for osteoarthritis. stem cells translational medicine 2019;8:746&757
Keywords: Arthritis, Mesenchymal stem cells, Monocyte, Cellular therapy, Clinical trials, Selectable marker
Significance Statement.
This clinical trial advances the field of stromal cell research in osteoarthritis by providing insights into clinical mechanisms of action. This study used different tools including imaging, biomarkers, patient‐reported outcomes, and molecular biology to quantify changes to patients’ disease state, which provided important insights even in a small‐sized trial. These tools will be more effective in larger, powered trials and should be used to illustrate the multifactorial behavior of stromal cells in osteoarthritis.
Introduction
Osteoarthritis (OA) is a progressive and debilitating joint disease that is expected to affect up to 25% of the population by 2040 and represents an enormous socioeconomic health care burden 1. Patients are initially treated with a combination of physical therapy, activity modification, weight loss, nonsteroidal anti‐inflammatories, and/or intra‐articular injections with the goal of symptom modification 2. When OA progresses to the end stages and nonoperative care fails, total joint replacement can be an effective alternative.
Mesenchymal stromal cells (MSCs) are a leading investigational therapeutic product because of their multiple mechanisms of action 3. MSCs have been tested in a number of smaller‐phase trials and are effective in alleviating pain and patient symptoms 4, 5, 6, 7. In some cases there are reported improvements to cartilage morphology 8, 9, 10. Although clinical trials of BM‐MSCs have reported efficacy at higher doses ranging from 25 × 106 cells 6 and 40 × 106 cells 4, 5, efficacy has been reported with lower doses of adipose tissue‐derived MSC 7, 11 suggestive of tissue‐specific differences in dosing efficacy.
Preliminary efficacy results in early‐phase trials have created tremendous enthusiasm and a rush to commercialize MSC products in treating patients with knee osteoarthritis (KOA) ahead of a fundamental understanding of how MSCs work. As a result, there remains a gap in our collective knowledge of MSC mechanism of action in OA and whether these cells are limited to inflammation resolution versus chondroprotective and/or regenerative effects. Unanswered questions related to MSC dosing as well as selection criteria to identify potent donor MSCs also persist.
In order to address some of these shortcomings, we initiated the first Canadian clinical MSC trial using autologous BM‐MSCs to treat 12 patients with moderate to severe KOA (ClinicalTrials.gov Identifier: NCT02351011). Given that this was the first MSC trial in Canada, the primary aim was to assess clinical safety. We also used a broad toolkit of analytical methodologies including patient‐reported outcomes, imaging, biomarkers, and molecular fingerprinting to obtain a better understanding of MSC dosing, mechanisms of action, and donor selection.
Materials and Methods
Study Design and Patients
This is a nonrandomized, open‐label, dose‐escalation phase I/II clinical trial conducted from April 2015 to December 2017 that investigated the role of autologous BM‐MSCs in the treatment of KOA. The protocol was approved by the Research and Ethics Board (REB 14–7909) and Health Canada. Patients were eligible if they (a) were between 40 and 65 years of age; (b) had radiographically confirmed symptomatic Kellgren‐Lawrence III–IV KOA; (c) failed a nonoperative treatment regimen for a minimum of 6 months; (d) had a clinically stable knee; and (e) had neutral alignment as measured by 4′ standing anteroposterior x‐rays (supplemental online Table 1). Twelve patients were enrolled in four cohorts; three patients in each of the first three cohorts received 1 × 106, 10 × 106, and 50 × 106 BM‐MSCs in 6.5 ± 1.5 mL of excipient. In cohort number 4, each patient (n = 3) received either 1 × 106, 10 × 106, or 50 × 106 BM‐MSCs. Clinical follow‐up was carried out at 48 hours, 1 week, 2 weeks, 6 weeks, 3 months, 6 months, 12 months, and 24 months after BM‐MSCs injection (supplemental online Table 2). Physical examination, adverse events, and patient‐reported outcome measures (PROMs) were recorded at each time point. Magnetic resonance imaging (MRI) was conducted at baseline, 6 months, and 12 months; synovial fluid was collected at baseline and 3 months; blood and urine was collected at baseline, 2 weeks, 6 weeks, 3 months, 6 months, and 12 months.
BM‐MSC Isolation and Preparation
Patients underwent aspiration of 50 mL of bone marrow (BM) from the posterior superior iliac spine under local anesthetic. A blood sample of approximately 25 mL was collected for generating autologous serum. The blood was collected for each patient using an additive‐free vacutainer (catalog number 366408; Becton Dickinson, Franklin Lakes, NJ) and was left for at least 1 hour at room temperature to clot. The autologous serum was collected after centrifugation and was then stored at −80°C until the day of MSC injection. The BM was processed for isolation of mononuclear cells (MNCs) by density gradient centrifugation using Good Manufacturing Practice‐grade Ficoll‐Paque Premium (GE Healthcare, Chicago, IL). Approximately 30 × 106 MNCs were plated in 175 cm2 flask(s) in defined medium, that is, DMEM low glucose (Life Technologies, Carlsbad, CA), 1% Glutamax (Life Technologies), and 10% screened fetal bovine serum (HyClone, GE Healthcare, Chicago, IL) and denoted as passage 0 (P0). After BM harvest (day 0), every 3–4 days the medium was changed, or cells were passaged at a ratio 1:3 up to P3 (day 30) or P4 (day 37; supplemental online Fig. 1).
BM‐MSC Characterization
BM‐MSCs Harvest and Injection
BM‐MSCs were harvested by cell dissociation with TrypLE Select. Cells were washed three times (two times in Plasmalyte A and one time in excipient) and suspended in an excipient of 6.5 ± 1.5 mL (2.5% of the patient's autologous serum in Plasmalyte A). Extra cells were cryopreserved for research purposes. The final product was retained in a sterile syringe aseptically in a class 100 biosafety cabinet at 15°C–25°C until the final release criteria (supplemental online Table 3) were met (2–4 weeks before harvest: negative for growth and mycoplasma; on the day of harvest: negative for endotoxin, cell viability over 70%, negative for stat gram stain, immunophenotyping meeting the International Society for Cellular Therapy criteria). We established the following storage conditions to be adequate: 15°C–25°C during the first 8 hours after first contact with Plasmalyte A and then 2°C–10°C for the next 24 hours. Patients received an ultrasound‐guided intra‐articular injection of BM‐MSCs.
BM‐MSC Immunophenotyping
BM‐MSCs were characterized just before infusion, by cell surface expression of CD73‐PE, CD90‐PE, CD19‐PE, CD34‐PE, and CD45‐PE (BioLegend, San Diego, CA); CD105‐PE, HLADR‐PE, and CD14‐PE (Becton Dickinson); propidium iodide (PI; Sigma); and isotype IgG1‐PE and isotype IgG2a‐PE (Becton Dickinson). For immunolabeling, cells were incubated for 45 minutes at 2°C–8°C. PI‐negative BM‐MSCs (viable) were characterized using FC500 flow cytometer (Beckman Coulter, Mississauga, ON, Canada) and analyzed using FlowJo (Treestar).
BM‐MSC Tri‐Lineage Differentiation Assay
BM‐MSCs were tested for trilineage differentiation potential using StemPro differentiation kits (Life Technologies) for osteogeneic, adipogeneic, and chondrogeneic differentiation as per manufacturer's instructions. Staining was performed using Oil Red O (Sigma) stain for adipogenesis, Alizarin Red S (Sigma) for osteogenesis, and Alcian Blue 8GX (Sigma) for chondrogenesis.
Gene Expression of IFN‐γ‐Licensed MSCs
Extra BM‐MSCs from 12 patients (thawed from frozen samples at early passages, P3 or P4), were stimulated with 30 ng/mL interferon‐gamma (IFN‐γ; Peprotech) at 37°C, 5% CO2 for 18–20 hours. After stimulation, total RNA was isolated using TRIzol Reagent (Life Technologies) and converted into cDNA with High‐Capacity cDNA RT Kit (Life Technologies). Real‐time reverse transcription polymerase chain reaction was performed using FastStart Universal SYBR Green Master mix (Roche, Indianapolis, IN). Samples were analyzed in triplicate for β2‐microglobulin (B2M), cyclooxygenase 2, programmed death‐ligand 1, interleukin 10, hepatocyte growth factor (HGF), transforming growth factor beta, and indoleamine 2,3‐dioxygenase expression (supplemental online Table 4). These markers were selected based on review of the literature for factors through which BM‐MSCs exert their actions 12, 13, 14. Thermal cycling was performed with 7900HT System (Life Technologies): 95°C for 2 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 20 seconds. Relative expression levels were calculated using the 2^−ΔΔCt method 15, with B2M as housekeeping gene.
TSG‐6 Protein Expression of TNF‐α‐Licensed MSCs
We also analyzed the secretion of tumor necrosis factor alpha (TNF‐α)‐stimulated gene (TSG‐6) by TNF‐α‐stimulated BM‐MSCs (thawed from frozen patient samples), as TSG‐6 is a potential immunomodulatory agent secreted by BM‐MSCs 16. Twelve patients’ BM‐MSCs were stimulated with 6 ng/mL TNF‐α (Peprotech) at 37°C, 5% CO2 for 18–20 hours. After stimulation, cell supernatant was collected and analyzed using TSG‐6 ELISA kit (RayBio Human TSG‐6 ELISA kit, ELH‐TSG‐6) as per manufacturer's instructions.
Outcome Measures
Primary Outcome
Safety was measured by the documentation of local and systemic adverse events. A combination of vital signs, physical examination, and laboratory tests were used, and adverse events were categorized using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 scale 17.
Secondary Outcomes
Secondary outcomes included clinical, radiological, and biomarker assessments.
Clinical
An assessment of changes in knee‐joint‐specific function and disease‐specific quality of life as determined by the Knee Injury and Osteoarthritis Outcome Score (KOOS) 18 and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) 19, respectively, at 2 weeks, 6 weeks, 3 months, 6 months, 12 months, and 24 months was performed. The analysis in this paper was done up to 12 months to allow correlation with the blood and urine biomarkers.
Radiological
A radiologist evaluated hyaline cartilage on all MRI images according to the Whole Organ MRI Score (WORMS). Briefly, cartilage was scored from 0 to 6 in 14 subdivisions (6 within the medial compartment, 6 within the lateral compartment, and 2 within the patella). The baseline, 6‐month, and 12‐month images were scored concurrently with the reviewer blinded to the time point and the dose of MSC administered.
Synovitis was scored based on post‐gadolinium T1‐weighted fat‐suppressed images as per the methodology of Guemazi et al. 20. Synovitis was scored semiquantitatively from grade 0 to 2 based on the maximal synovial thickness at predefined locations (grade 0: <2 mm, grade 1: 2–4 mm, grade 2: >4 mm). The sites evaluated included medial parapatellar, lateral parapatellar, suprapatellar, infrapatellar, intercondylar, medial perimeniscal, lateral perimeniscal, adjacent to the posterior cruciate ligament and the anterior cruciate ligament, and, if applicable, adjacent to loose‐bodies and within a Baker's cyst. The sum of these individual synovitis scores yielded a total synovitis score. The baseline, 6‐month, and 12‐month images were scored concurrently with the reviewer blinded to the time point and dose of BM‐MSCs.
T2 maps were reconstructed for each time point. Each femoral condyle was divided into five subcompartments and each tibial plateau was subdivided into three subcompartments according to the methodology of Bae et al. 21. In addition, two subcompartments were also evaluated in the patella, yielding a total of 18 subcompartments. For each subcompartment, an area was chosen whereby no significant chondral defects were identifiable on the corresponding morphological images on three consecutive images in order to avoid variations due to partial volume. Regions of interest were drawn on each of the three slices; the T2 values recorded and the mean of the three slices were calculated for each time point. All time points were assessed concurrently but the reviewer was blinded to the time point.
Briefly, following the method in Vega et al. 4, Poor Cartilage Index (PCI) was estimated using a cutoff of ≤50 for the T2 values to indicate good cartilage and calculating the proportion of the 18 sites below the cutoff. PCI values with no T2 estimates were assumed to be poor cartilage and were set to zero for the dichotomization. Changes in the T2 values for section and time point were averaged across the three slices, and differences were taken between 12 months and baseline. The differences were then averaged across sites for the medial, lateral, and patellar regions separately and in all regions.
Biomarkers
Biomarkers of interest were measured using enzyme‐linked immunosorbent assays (ELISAs), as per manufacturer's recommendations, in (a) plasma (hyaluronan [HA]; Cisbio US; cartilage oligomeric matrix protein [COMP]; US biologicals), (b) serum (C1‐2C collagen type I and II cleavage [C1‐2C]; IBEX Pharmaceuticals Inc), and (c) urine (C‐telopeptide of type II collagen [CTX‐II]; Immunodiagnostic Systems Holdings PLC; collagen type II cleavage [C2C] sandwich ELISA, IBEX) at baseline and at 2, 6, 12, 24, and 48 weeks after injection (supplemental online Table 5). Blood was collected at different times over the 12‐month follow‐up period (supplemental online Table 6), and urine was collected at second morning void to best control for diurnal variations; however, two of the samples obtained at baseline (003 and 006) were third‐morning voids. Urine was corrected for creatinine levels.
Soluble factor levels in the synovial fluid (SF) of seven patients (patient number 1, 3, 5, 9, 11, 12, and 13) that had SF draws at both baseline and 3 months after MSC injection were measured. Matrix metalloproteinases (3, 9, 13; collagenases), tissue inhibitor of metalloproteinases (inhibitor of collagenases), C‐X‐C chemokine motifs (CX3CL1, CXCL1, and CC2, chemokines that recruit monocytes/macrophages), vascular endothelial growth factor (VEGF; angiogenic factor), interleukins (IL12p40, IL6, IL8, inflammatory cytokines), HGF (growth factor), soluble receptors (sCD163 and sCD14, soluble monocyte/macrophage receptors), prostaglandin, and adiponectin, adipsin, leptin, and resistin (lipid mediators) were tested using immunoassays (supplemental online Table 5) as per manufacturer's instructions.
Biomarkers: Synovial Fluid Cell and Soluble Factor Characterization
SFs were acquired at the baseline and 3 months after BM‐MSC injection, stored at 4°C, and processed within 24 hours of acquisition. SF samples obtained from seven patients were analyzable (patients 1, 3, 5, 9, 11, 12, and 13); the other samples from five patients were not analyzable because of insufficient volume. SFs were used for characterizing inflammatory cytokines and chemokines; SF cell characterization was also performed except on patients 5 and 11, who had insufficient cell numbers or presented obvious blood contamination, respectively.
To obtain SF cells, SFs were centrifuged at 12,000g for 15 minutes, followed by removal of SF supernatant, which was stored at −80°C for subsequent soluble factor analysis. The cell pellet was resuspended in 100 U/mL hyaluronidase in Roswell Park Memorial Institute medium (∼1 SF:1 hyaluronidase solution) to reduce SF viscosity. Hyaluronidase treatment was performed after the first centrifugation, as the pellet remained in 100–200 μL of SF and needed enzymatic digestion to reduce cells loss.
Samples were diluted in flow cytometry buffer, filtered through a 70 μm cell strainer, and washed to obtain SF cells. Erythrocytes present in any of the samples were excluded based on size or CD45+ labelling. For cell immunolabeling, SF cells were incubated for 20–30 minutes on ice with the isotype, monocyte/macrophage (MΦ, a mixed population of both monocytes and macrophages) antibodies (all antibodies used were from Biolegend, unless otherwise specified). The isotype panel had matched isotypes to the antibodies used in the MΦ panel. The MΦ panel contained CD163‐FITC (clone GHI/61), CD16‐PE (clone B73.1) or BV510, HLA‐DR‐PerCPCy5.5 (clone L243), and CD14‐PECy7 (clone 61D3; eBioscience) for all samples. MΦs were CD14+ and/or CD16+ while expressing human leucocyte agent D related (HLA‐DR) at different levels. MΦs subpopulations were identified based on Abeles et al. 22 and A. Gomez‐Aristizabal, R. Gandhi, N.N. Mahomed et al. (manuscript submitted for publication), using FlowJo v10.
Statistical Analysis
To identify changes between baseline and 12‐month follow‐up for PROMs and synovitis scores, the Wilcoxon signed‐rank test was performed. For multiple comparisons between baseline and 3‐month post‐BM‐MSC treatment data for synovial fluid, Friedman test was used, followed by post hoc analysis using Dunn's tests. Because of the exploratory nature of the latter analysis (n < 12), we did not adjust for multiple comparisons.
We also performed an exploratory analysis to identify possible factors that affect BM‐MSC therapy outcomes using general estimating equations (GEE) and linear modeling to detect differences between doses; we did not adjust for multiple comparisons as it was an exploratory analysis. Transformations of the data were done, if required, to conform with the assumption that residuals resulting from the models follow a normal distribution: logarithmic transformation, Gpower, and hyperbolic transformations were used 23. GEE with an autoregressive‐1 correlation structure was used for analyses over time. To decrease the dimensionality of BM‐MSC gene expression and to use these data as predictor of clinical outcomes, we used principal component analysis.
The changes in the observed T2 values were also analyzed by estimating the differences between baseline and 12 months, evaluated using a paired t test. The effect of different dosages on changes in the T2 values was also analyzed as the difference between the 1, 10, and 50 BM‐MSCs dosages and was evaluated by a standard t test.
The effect of the PCI on each of the KOOS‐derived outcomes was assessed in a univariate model, including data at all available time points (baseline, 6 months, and 12 months) using GEE with an AR1 correlation structure for subjects to estimate significance. Correlation structure was selected based on the correlation of the observed data at the different time points.
Results
Fifty patient charts were reviewed for eligibility; 14 patients consented, and 12 were enrolled in the trial (supplemental online Table 7). There were seven males and five females with ages ranging from 45 to 65 years, with a mean age of 56 years. The body mass index (BMI) ranged from 19.0 to 29.9 kg/m2, with a mean of 25.8. Of 12 patients, 11 were Kellgren‐Lawrence grade III. Ten patients had varus or valgus malalignment <5°, and two patients had varus or valgus malalignment <7.5°. All patients had an effusion present on baseline MRI. Supplemental online Tables 8 and 9 provide patient baseline characteristics and adverse events. Four patients had minor local transient adverse events (pain and/or swelling at site of injection), which subsided without intervention (supplemental online Table 8). There were no serious and/or systemic adverse events.
Clinical Outcomes
We observed significant improvement in KOOS pain (p = .025), symptoms (p = .033), and quality of life (QOL; p = .037) and WOMAC stiffness (p = .021) 12 months after BM‐MSC treatment relative to baseline (Fig. 1A, 1B). All four patients in the 50 million BM‐MSC dose group achieved the minimal clinically important difference (MCID; defined as an increase of >10 points 24) in all scores except KOOS sports (2/4 patients had improvements) and QOL (3/4 patients had improvements) at 12‐month follow up (Fig. 1C). At least half (2/4) of patients in the 1 million dose and at least one in the 10 million dose showed MCID in all scores (Fig. 1C). However, the 50 million dose cohort had the greatest number of patients achieving the MCID for PROMs (Fig. 1C).
Figure 1.
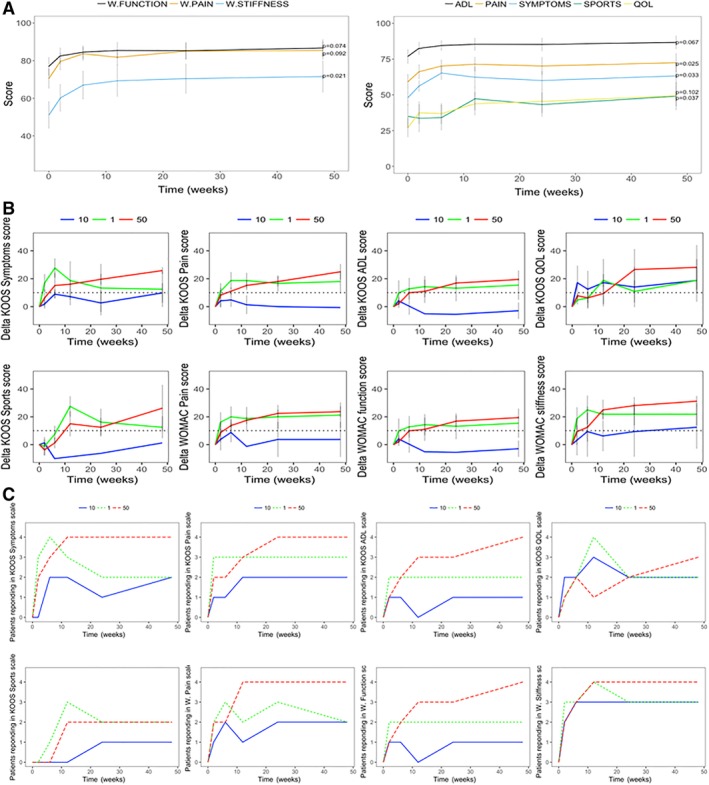
Patient‐reported outcomes at baseline and after BM‐MSC injection. (A): Progression in WOMAC (left) and KOOS (right) subscales after BM‐MSG injection. WOMAC scores have been normalized such that higher WOMAC scores are indicative of better outcomes. p values shown for comparisons between baseline and 1‐year follow‐up using Wilcoxon signed‐rank test. (B): Delta in patient‐reported outcome measures (PROMs) relative to baseline. Dotted line indicates minimal clinical important difference threshold of 10. Green indicates 1 million, blue 10 million, and red 50 million BM‐MSC dose group. Error bars indicate SEM. (C): Number of patients from each group (four in each group) having a minimal clinically significant improvement (>10 in delta relative to baseline for each PROM) in each PROMs. Abbreviations: ADL, activity of daily living; KOOS, Knee Injury and Osteoarthritis Outcome Score; QOL, quality of life; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MRI Outcomes
Morphological MRI cartilage scores (WORMS) at 6‐ and 12‐month follow‐ups showed no significant changes at any time point, relative to baseline (Fig. 2A). There were no changes in T2 scores from baseline to 12 months in any regions of the cartilage. The patella region had a marginally insignificant increase in T2 scores (p = .055 for a two‐sided test, nonadjusted). There were no differences in T2 changes (baseline to 12 months) between the 1, 10, or 50 million BM‐MSC cohorts.
Figure 2.
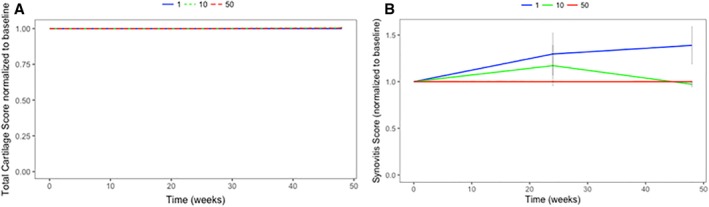
Cartilage and synovitis composite scores over 12 months. (A): Cartilage morphology does not change after mesenchymal stromal cell (MSC) treatment. Fold change relative to baseline is shown. n = 4 for each MSC dose. (B): Synovitis score (shown as fold change relative to baseline) differs slightly between doses. n = 4 for 50 and n = 3 for 10 and 1 million MSC dose. No significant differences were found between baseline and 1‐year follow‐up.
For the derived PCI, none of the KOOS‐derived outcomes were correlated once adjusted for multiple comparisons. Sport Score and QOL score was nominally correlated at an unadjusted p value of .031 and .046, respectively, using all sample time points.
Synovitis scores also did not significantly change between baseline and 12‐month follow‐up (Fig. 2B). However, statistical analysis of the effects of dose adjusted for both time and the baseline levels of synovitis showed that the 50 million BM‐MSC dose (effect estimate [B] = −1.828, p = .002) maintained synovitis at lower levels than the 1 million BM‐MSC dose. Similar to our observations on PROMs, the 50 million BM‐MSC dose demonstrated a better outcome.
Biomarker Assessment
Plasma Levels of Cartilage Catabolic Factors
Levels of plasma COMP (fragments of COMP, a noncollagenous component of articular cartilage is indicative of cartilage degradation) and plasma HA (a sensitive marker of synovitis 25) did not change significantly during the 12‐month follow‐up (Fig. 3A).
Figure 3.
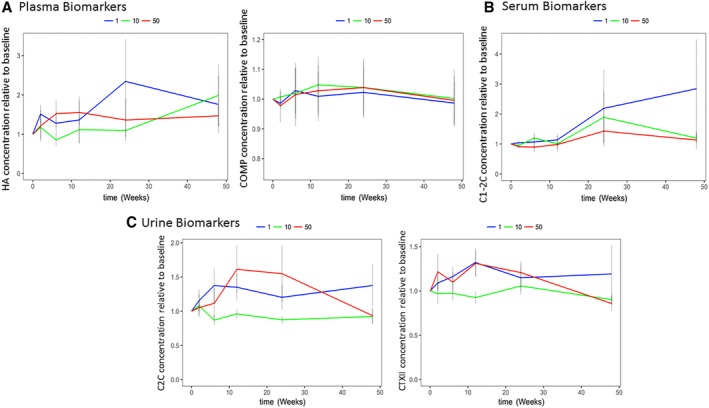
Changes in plasma, serum, and urine biomarkers after mesenchymal stromal cell (MSC) injection. (A): Plasma HA and COMP. (B): Serum C1‐2C. (C): Urine CTXII and C2C. (A–C): Data are shown as fold change relative to baseline (before MSC injection) at 2, 6, 12, 24, and 48 weeks after MSC treatment. Error bars represent SEM. Blue line, 1 million MSC dose; green line, 10 million MSC dose; red line, 50 million MSC dose. Statistics related to this figure are shown in Table 1. No significant differences were seen using pairwise comparisons of baseline versus 12 months; thus, no statistics are shown on the graphs. Abbreviations: C1‐2C, collagen type I and II cleavage; C2C, cleavage product specific to type II collagen; COMP, cartilage oligomeric matrix protein; CTXII, C‐terminal crosslinked telopeptide type II collagen; HA, hyaluronan.
Serum Levels of Cartilage Catabolic Factors
Serum levels of C1‐2C (a neoepitope generated by cleave of collage type I and II indicative of collagen fragments 26) showed a tendency to increase 12 months after BM‐MSC treatment (p = .125) in the 1 million dose; the other doses showed no changes (p > .5; Fig. 3B). GEE analysis of dose adjusted for patient variability, time, sex, and BMI showed that the 50 million dose leads to a significantly lower level of C1‐2C than both the 1 and 10 million doses over 12 months after BM‐MSC injection (B = −0.176, p = .002 and B = −0.156, p = .007, respectively; Table 1).
Table 1.
Effect of bone marrow mesenchymal stromal cells dose on clinical outcomes and MRI synovitis and established systemic biomarkers
| Outcome | Effect estimate, p value a | Effect estimate, p value a | Effect estimate, p value a |
|---|---|---|---|
| 1 vs. 10 million | 1 vs. 50 million | 10 vs. 50 million | |
| MRI synovitis | −0.700, p = .304 | −1.828, p = .002 | −1.128, p = .080 |
| Plasma HA | 0.127, p = .180 | 0.015, p = .870 | −0.112, p = .210 |
| Plasma COMP | −0.011, p = .747 | −0.001, p = .980 | 0.010, p = .784 |
| Serum C1‐2Cb | −0.020, p = .625 | −0.176, p = .002 | −0.156, p = .007 |
| Urine CTXII | −0.148, p = .008 | −0.026, p = .694 | 0.122, p = .018 |
| Urine C2Cb | −0.112, p ≤ .001 | −0.071, p = .035 | 0.040, p = .231 |
General estimating equation (GEE) with outcomes being changes in biomarkers (relative to baseline, delta) and predictors adjusting for dose and time.
Bolded values indicate statistical significance.
Nominal p values shown.
GEE adjusted for dose, time, and sex, as sex was found to be a significant predictor (p < .01). Hyperbolic power (α = 1) and Gpower transformations were used as indicated in supplemental online Table 9, as per Tsai et al. 50.
Abbreviations: C1‐2C, collagen type I and II cleavage; C2C, cleavage product specific to type II collagen; COMP, cartilage oligomeric matrix protein; CTXII, C‐terminal crosslinked telopeptide type II collagen; HA, hyaluronan; MRI, magnetic resonance imaging.
Urine Levels of Cartilage Catabolic Factors
Levels of urine C2C (cleavage product specific to type II collagen and indicative of pathology‐related cartilage collagen peptide 27) showed no overall changes at 12 months after BM‐MSC treatment (Fig. 3C). However, both the 10 and 50 million BM‐MSC doses led to overall lower urine C2C levels than the 1 million BM‐MSC dose (Table 1).
Levels of urine CTXII (a measure of C‐terminal crosslinked telopeptide type II collagen secreted into urine and indicative of disease progression) showed a tendency to decrease at the 50 million BM‐MSC dose at 12 months relative to baseline (p = .125; Fig. 3C); however, over all time points, the 10 million BM‐MSC dose appeared to lower urine CTXII levels compared with the 1 BM‐MSC dose, whereas the 50 million dose did not (Table 1).
Taken together, the cartilage catabolic biomarkers appear to have a lower increase at higher (10 and 50 million BM‐MSC) doses than at the 1 million BM‐MSC dose.
Synovial Fluid Biomarkers
A selected list of cartilage catabolic, anabolic, angiogenic, chemokine, inflammatory cytokines, monocyte/macrophage‐recruiting chemokines, and shed receptors were assessed in the synovial fluid at baseline and 3 months after BM‐MSC injection. This list was selected based on recommendations of the Osteoarthritis Research Society 28. A 3‐month time point was chosen to be reflective of early changes to inflammatory markers. This time point also coincides with the timing when most patients report maximal improvements to their pain, symptoms, and function. Most soluble factors remain unchanged from baseline levels, but there were significant increases in levels of VEGF (an angiogenic factor), concomitant with decreases in IL12p40 (inflammatory cytokine) and adiponectin (a lipid mediator; Fig. 4).
Figure 4.
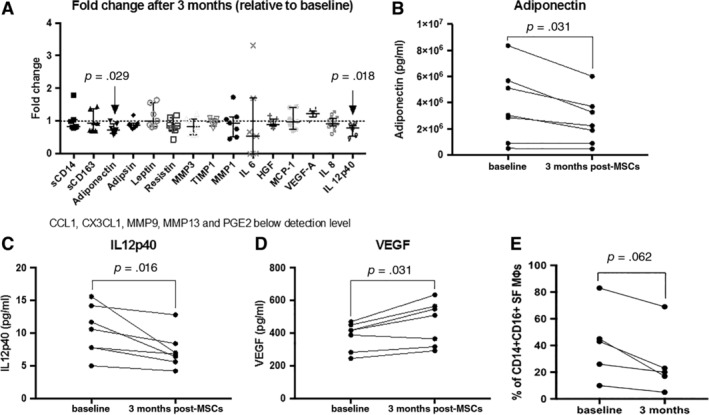
Synovial fluid changes 3 months after MSC treatment. (A): Only a few of the factors characterized in SF changed after MSC treatment. Fold change in SF soluble factors concentration after MSC treatment is displayed; dotted line indicates levels at baseline. Friedman test with Dunn's test as post hoc was used for identifying significant changes (n = 7). (B, C): Levels of adiponectin (B) and IL12p40 (C) in SF decrease after MSC treatment (n = 7). (D): Levels of VEGF increase after MSC treatment (n = 7). (E): Pro‐inflammatory CD14 + CD16+ monocyte/macrophages tend to decrease after MSC treatment (n = 5). (B–E): p values shown for Wilcoxon signed‐rank test. Abbreviations: CCL1, chemokine (C‐C motif) ligand 1; HGF, hepatocyte growth factor; IL, interleukins; MCP‐1, monocyte chemoattractant protein 1; MMP, matrix metalloproteinases; MSC, mesenchymal stromal cell; PGE2, prostaglandin; TIMP, tissue inhibitor of metalloproteinases; VEGF, vascular endothelial growth factor.
Importantly, we analyzed levels of different monocyte/macrophage subsets in the synovial fluid and noted a decrease tendency (p = .062) in levels of a pro‐inflammatory subset, identified as CD14 + CD16+ cells 29. Statistical significance was not achieved in this limited sample set (n = 5) as not all patients presented with synovial fluid at both baseline and 3 months after BM‐MSC infusion.
Characterization of BM‐MSCs
All BM‐MSCs were characterized prior to injection for cell surface markers as minimally recommended 30; all BM‐MSCs expressed CD105, CD73, and CD90, lacked CD45, CD34, CD14 or CD19, and HLA‐DR 30 (supplemental online Table 3), and were capable of tri‐lineage differentiation (supplemental online Fig. 2).
We characterized gene expression levels of selected anti‐inflammatory, antifibrotic, and anabolic factors 14, 31) in the 12‐patient‐derived BM‐MSCs licensed with either IFN‐γ or TNF‐α (Fig. 5A). BM‐MSC selected gene expression profile, represented by the principal component 1 (PC‐1), and TSG‐6 protein levels are significant predictors of patient‐reported outcomes (Table 2). Furthermore, selecting a 50 million BM‐MSC dose and a high PC‐1 is a better predictor of patient‐reported outcomes (Table 2).
Figure 5.
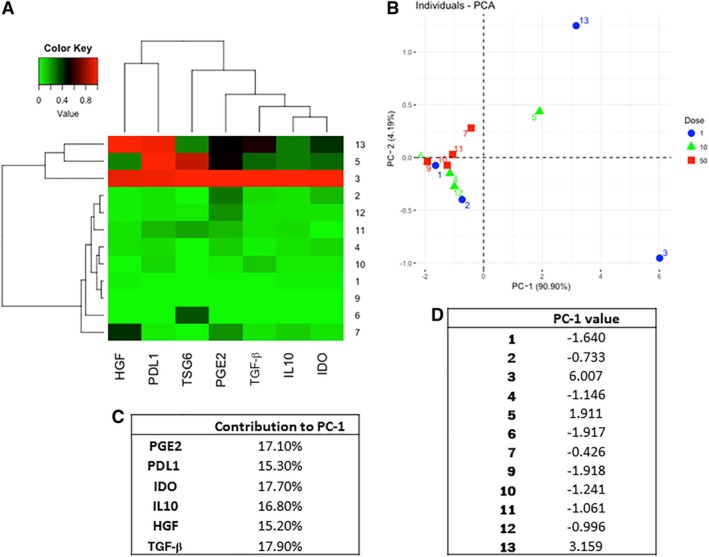
Licensed MSC anti‐inflammatory gene and protein expression is donor‐dependent. (A): Gene expression profile of interferon‐gamma (IFN‐γ) licensed mesenchymal stromal cells (MSCs) and secreted TSG‐6 levels by tumor necrosis factor‐licensed MSCs; markers analyzed indicated under each column and patient number indicated in each row. (B): PCA of IFN‐γ licensed MSC gene expression profile. Plot shows each patient MSC plotted with the two first PCs and identified for the dose at which it was used in the clinical study. Percentage shown next to PC‐1 and PC‐2 on axis labels indicate the contribution of each PC to the variance. (C): Contribution of each gene to PC‐1. (D): PC‐1 of each patient MSC showing high variability between donors. Abbreviations: HGF, hepatocyte growth factor; IDO, indoleamine 2,3 dioxygenase; IL, interleukins; PC, principal component; PCA, principal component analysis; PD‐L1, programmed death‐ligand 1; PGE2, prostaglandin; TGF‐β, transforming growth factor beta; TSG‐6, tumor necrosis factor alpha stimulated gene.
Table 2.
Licensed bone marrow MSC anti‐inflammatory gene expression and TSG‐6 protein profile are significant predictors of improvement in patient‐reported outcome measures (WOMAC and KOOS scores)
| Changes in patient‐reported outcome measures between dose cohorts | Estimate | Nominal p value |
|---|---|---|
| Δmean WOMAC | ||
| MSC PC‐1 | 18.79 | <.001 |
| 1 vs. 10 | −5.30 | .105 |
| 1 vs. 50 | 7.87 | .007 |
| 10 vs. 50 | 13.17 | <.001 |
| Δmean KOOS | ||
| MSC PC‐1 | 18.70 | <.001 |
| 1 vs. 10 | −2.10 | .436 |
| 1 vs. 50 | 8.55 | .005 |
| 10 vs. 50 | 10.66 | <.001 |
| Δmean WOMAC | ||
| TSG‐6 | 19.78 | <.001 |
| 1 vs. 10 | −12.40 | <.001 |
| 1 vs. 50 | 4.12 | .230 |
| 10 vs. 50 | 16.51 | <.001 |
| Δmean KOOS | ||
| TSG‐6 | 17.90 | <.001 |
| 1 vs. 10 | −9.15 | .002 |
| 1 vs. 50 | 4.36 | .181 |
| 10 vs. 50 | 13.51 | <.001 |
Linear model adjusted for bone marrow MSC PC‐1 (gene expression) or TSG‐6 concentrations (normalized to maximum levels detected among patients; i.e., max [TSG‐6] or max [PC‐1] = 1) and dose. Δmean WOMAC = mean WOMAC at baseline‐mean WOMAC at 12 months; Δmean KOOS = mean KOOS at baseline‐mean KOOS at 12 months.
Abbreviations: KOOS, Knee Injury and Osteoarthritis Outcome Score; MSC, mesenchymal stromal cell; PC, principal component; TSG‐6, tumor necrosis factor alpha stimulated gene; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Discussion
The main findings in this study are that autologous BM‐MSCs are safe at all doses tested and are likely improve various dimensions of knee‐specific joint function and quality of life. Using a broad set of analytical tools to quantify imaging, biomarkers, and molecular changes, we probed BM‐MSC‐mediated changes in cartilage morphology, collagen content, cartilage degradation, synovial inflammation, joint microenvironment, and joint immune effectors. We report putative chondroprotective effects and reduced synovial inflammation that is potentially more effective at the 50 million BM‐MSC cell dose. Using a panel of anti‐inflammatory and antifibrotic markers, we were for the first time able to predict clinical efficacy. Taken together, our analytical methodologies provide important insights even in this limited sample size and can be powerful evaluators of MSC efficacy in larger trials.
Our primary findings regarding safety are consistent with what has been reported in the literature. Lalu et al 32 demonstrated no serious adverse events in a systemic review of MSC treatments. In our study, four patients had transient pain and swelling that subsided over a matter of days without intervention. No systemic side effects were observed in our cohort, and we also reported no complications following the bone marrow aspiration.
Data from our 12‐patient autologous BM‐MSC trial show statistically significant improvements in KOOS pain, symptoms, and QOL scores as well as WOMAC stiffness from baseline to 12‐month follow‐up. Our findings support that BM‐MSCs induce analgesic effects and functional improvements, similar to other trials 4, 5, 33, 34.
Early MSC clinical data have also demonstrated improvements in cartilage volume and quality 8, 9. We treated 12 patients with moderate‐to‐late‐stage KOA with autologous BM‐MSCs but did not observe improvements in cartilage morphology at 12 months, based on MR imaging. We also did not report any changes in T2 values or PCI at various doses over a 12‐month interval. Such changes may be indicative of changes in water retention, changes in interaction between collagen and water, or changes in the normal orientation of the collagen fibrils, which is different from the studies from Vega et al. and Orozco et al. 4, 5, possibly because of dosing and donor differences. Another explanation for the lack of regenerative effects may be our inclusion of patients with end‐stage osteoarthritis. It is possible that such regenerative effects are more likely to be observed in earlier‐stage OA.
Interestingly, the cartilage catabolic biomarkers largely remained unchanged over a 12‐month time course in these mid‐to‐late‐stage patients, echoing the MRI results. However, serum C1,2C, urine C2C, and CTX‐II all had significant increases with the 1 million BM‐MSC doses relative to the 50 million and/or 10 million BM‐MSC dose (Table 1), suggestive of a chondroprotective MSC dose effect, as previously reported 10, 35. Increases in serum C1,2C are associated with radiographic KOA 36, whereas levels of urine C2C and CTX‐II predict worsening pain and joint space loss 37, 38. The relatively unchanged levels of these catabolic biomarkers over a 12‐month period, at higher MSC doses, suggest a chondroprotective effect, although we recognize that diurnal variations in these biomarker readings preclude definitive conclusions. We attempted to mitigate these variations by standardizing urine collection time.
There also appears to be a BM‐MSC‐dose‐mediated protective effect with respect to synovial inflammation or synovitis. Synovitis scores, assessing intra‐articular inflammatory changes, showed less of an increase at the 50 million BM‐MSC dose group than the 1 and 10 million BM‐MSC dose groups (Fig. 2B), and there was statistical significance even in the limited sample size between the 1 versus 50 million BM‐MSC dose group (Table 1). Plasma levels of HA, which have been shown to be a sensitive marker of synovial inflammation 39, were also more elevated at lower BM‐MSC doses than in the 50 million BM‐MSC dose (Fig. 3A).
MSCs are immunomodulatory, particularly with their ability to polarize macrophages into inflammation‐resolving subtypes 40. Macrophages are the most prevalent immune cell in OA joints 41 and are elevated in OA versus healthy joints 42, and they contribute to synovitis and fibrosis, hallmarks of OA. We have recently shown an inverse correlation between increased prevalence of pro‐inflammatory monocyte subsets and worsening patient‐reported outcomes (A. Gomez‐Aristizabal, R. Gandhi, N.N. Mahomed et al., manuscript submitted for publication). In our study, we report a decrease in synovial fluid levels of pro‐inflammatory monocytes/macrophages 3 months after MSC infusion, suggestive of a potential mechanism of action. Because of the limited subset of patients presenting synovial fluid both at baseline and 3 months after MSC infusion (n = 5), we do not see statistical significance relative to baseline levels (p = .062), but this downregulation is suggestive of a potential mechanism of action of MSCs in the arthritic joint.
We also analyzed synovial fluid levels of inflammatory markers, lipid mediators, and shed receptors from monocytes/macrophages after MSC injection and report that after MSC injection, there were few changes relative to baseline: significant changes were only seen in VEGF, IL‐12p40, and adiponectin levels. IL12p40 is a pro‐inflammatory cytokine involved in Th1 cell response, and reduction in its levels after MSC injection is reflective of a reduced inflammatory joint microenvironment.
Adiponectin (APN) is a lipid mediator, and its interaction with MSCs is not well characterized. Low systemic levels of adiponectin are associated with insulin resistance and obesity 43, 44. Typically, APN levels are lowered in OA, concomitant with increased prevalence of pro‐inflammatory monocytes/macrophages and increased fibrosis as APN is thought to be anti‐inflammatory and antifibrotic 45. The decreased synovial fluid levels of APN with decreased prevalence of synovial fluid pro‐inflammatory monocytes/macrophages appear to be contradictory to what is known about APN, macrophage polarization, and fibrosis. APN signals through two receptors initiating different signaling pathways 46 and is subject to feedback inhibition as has been shown with macrophage polarization 47. Our findings suggest that the MSC‐modulation of APN in the context of APN receptors on late‐stage arthritic joint‐specific monocytes/macrophages and fibroblasts may be antifibrotic, but this warrants further investigation.
MSCs are known to secrete VEGF, and we report significantly increased levels of VEGF in synovial fluid 3 months after MSC treatment. Interestingly, this local VEGF increase is reported concurrently with significant decreases in patient‐reported pain, although increased VEGF levels are typically associated with synovitis, pain, and OA progression 48. Importantly, our observations are limited to the 3‐month time point. We do not know if these VEGF levels are maintained or change over time.
Taken together, the decrease in IL‐12p40 levels along with a decrease in pro‐inflammatory monocyte/macrophage subsets after BM‐MSC injection is supportive of an anti‐inflammatory and immunomodulatory mechanism of action of BM‐MSCs, which to our knowledge is the first clinical evidence of MSC mechanism of action in OA. The changes in APN and VEGF levels are indicative of a more complex action of MSCs interacting with their microenvironment and other mediators.
We also report for the first time that licensed MSC gene expression and protein profile can correlate with clinical outcomes. This suggests that MSC profiling by both or either of these methods is an important area of research that needs a fully powered study for the improvement of MSC‐based therapies. Although other studies have shown correlation between licensed MSC gene and protein expression, and immunomodulatory properties in vitro 49, and in animal models 50, this is the first instance correlating a panel of MSC gene and protein expression to measured clinical outcomes. Our abbreviated panel of gene and protein marker can be highly predictive of patient‐reported outcomes (Table 2) and can serve as the basis for potency markers to screen allogeneic MSC donors in future OA trials. Taken together, our data suggest that screening allogeneic BM‐MSCs or stratifying autologous BM‐MSC patients may allow for the optimization of clinical outcomes based on the gene expression profiles of donor‐licensed MSCs.
The main limitations of this study are the small sample size and the inclusion of patients with end‐stage OA. The former precluded definitive statements regarding dosing efficacy and rendered many of the analyses in this manuscript underpowered. The latter influenced the microenvironment of the BM‐MSCs wherein late‐ and early‐stage environments may respond differently to BM‐MSCs. Nonetheless, our analytical methods reported important findings relative to MSC mechanisms of action, the joint environment, dosing, and donor heterogeneity even in this limited sample size. These analytical tools provide guidelines for designing and conducting larger, controlled, randomized trials to measure multimodal outcomes in MSC‐mediated treatment of OA. We would like to state that our conclusions are limited by sample size and can be confirmed only in larger, powered trials, although the effect sizes are encouraging, especially in terms of using predictive panels of gene and protein markers to screen for potent MSC donors to enable more efficacious outcomes.
Summary
We report that the use of BM‐MSC is safe and results in improvement in PROMs in our patients with late‐stage knee OA, 12 months after a single MSC injection. We do not report improvements in morphological cartilage scores or a decrease in T2 relaxation values. We do see possible chondroprotective effects based on cartilage catabolic biomarkers at 50 and 10 million BM‐MSC doses. The 50 million dose also shows significant reduction in synovitis scores relative to the 1 million BM‐MSC dose group. Taken together with data suggesting reduced prevalence of synovial fluid pro‐inflammatory monocytes/macrophages, and reduced levels of pro‐inflammatory IL12 cytokine, we surmise that BM‐MSCs may be primarily acting to reduce synovial inflammation. Importantly, we show that BM‐MSCs with elevated levels of anti‐inflammatory and antifibrotic gene and protein markers are likely to have improved clinical efficacy in terms of patient‐reported outcomes, supporting our hypothesis that BM‐MSCs reduce synovial inflammation in OA. Our analytical methodologies provide us with important and complementary insights into putative mechanism of action, dosing, and donor selection and are well poised to be used effectively in future randomized trials that are powered for efficacy.
Author Contributions
J.C.: conception and design, data analysis and interpretation, manuscript writing; A.G.‐A., M.S.S., A.M.N.: data analysis and interpretation, manuscript writing; K.S., M.K.: data analysis and interpretation; S.B., A.C., J.C., A.W.: administrative support, provision of study material; A.F. and J.C.: administrative support, collection and assembly of data; A.K.: conception and design, provision of study material; D.J.O.‐H., K.A.S.: financial support, provision of patients, collection of data; R.G., N.N.M., K.W.M.: financial support, provision of patients; S.V.: conception and design, data analysis and interpretation, manuscript writing, final approval.
Disclosure of Potential Conflicts of Interest
A.G.‐A. declared employment and stock ownership interest with Bluerock Therapeutics. K.A.S. declared advisory role for Sanofi Canada and honoraria from Sanofi Canada. S.V. declared consulting and advising for Stem Cell Network, CellCAN, and OIRM and research funding from NSERC and OIRM (not to support this study). The other authors indicated no potential conflicts of interest.
Supporting information
Supplementary Table 1 Inclusion and exclusion criteria
Supplementary Table 2 Schedule of events
Supplementary Table 3 Final product characterization for BM‐MSCs product
Supplementary Table 4 Primers for panel of gene expression analyses of donor MSCs
Supplementary Table 5 Biomarkers characterized
Supplementary Table 6 Sampling times for blood
Supplementary Table 7 Number of eligible, enrolling and consenting patients
Supplementary Table 8 Patient Demographic and Baseline Characteristics for Each Dose Cohort
Supplementary Table 9 Baseline data for all enrolled participants
Supplementary Table 10 Transformations used for Table 1 within manuscript
Supplementary Figure S1 Bone‐marrow derived mesenchvmal strumal cells (BM‐NISCS) Manufacturing Process
Supplementary Figure S2 Trilineage differentiation of BM‐MSCs. All patients MSCS (except patient 13) were characterized by their ability to undergo trilineage differentiation, after infusion of BM‐MSCs intra‐articularly into consenting patients. Representative staining of trilineage potential is shown here. A) Adipogenic differentiation, B) Chondrogenic differentiation, and C) Osteogenic differentiation from patient 3 is shown.
Acknowledgments
This study was supported by Toronto General & Western Hospital Foundation, Arthritis Program seed funding to S.V., and the Arthritis Society Fellowship grant to A.G.‐A. (Grant TPF‐15‐123). The research on biomarkers was partially funded by The Arthritis Society Young Investigator Operating Grant (TAS‐YIO 15‐321) for S.V. Flow cytometry was performed in the Toronto Western KDT‐UHN Flow Cytometry Facility, with funding from the Canada Foundation for Innovation and Toronto General & Western Hospital Foundation.
References
- 1. Bombardier C, Hawker G, Mosher D. The Impact of Arthritis in Canada, Arthritis Alliance of Canada, Toronto, Ontario, Canada: Today and Over 30 Years, 2011. [Google Scholar]
- 2. Wolfstadt JI, Cole BJ, Ogilvie‐Harris DJ et al. Current concepts: The role of mesenchymal stem cells in the management of knee osteoarthritis. Sports Health 2015;7:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Viswanathan S, Keating A, Deans R et al. Soliciting strategies for developing cell‐based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev 2014;23:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vega A, Martin‐Ferrero MA, Del Canto F et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015;99:1681–1690. [DOI] [PubMed] [Google Scholar]
- 5. Orozco L, Munar A, Soler R et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013;95:1535–1541. [DOI] [PubMed] [Google Scholar]
- 6. Gupta PK, Chullikana A, Rengasamy M et al. Efficacy and safety of adult human bone marrow‐derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther 2016;18:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yubo M, Yanyan L, Li L et al. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta‐analysis. PLoS One 2017;12:e0175449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jo CH, Lee YG, Shin WH et al. Intra‐articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof‐of‐concept clinical trial. Stem Cells 2014;32:1254–1266. [DOI] [PubMed] [Google Scholar]
- 9. de Windt TS, Vonk LA, Slaper‐Cortenbach ICM et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single‐stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells 2017;35:256–264. [DOI] [PubMed] [Google Scholar]
- 10. Murphy JM, Fink DJ, Hunziker EB et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 2003;48:3464–3474. [DOI] [PubMed] [Google Scholar]
- 11. Spasovski D, Spasovski V, Baščarević Z et al. Intra‐articular injection of autologous adipose‐derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med 2018;20. [DOI] [PubMed] [Google Scholar]
- 12. Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol 2011;6:457–478. [DOI] [PubMed] [Google Scholar]
- 13. Yagi H, Soto‐Gutierrez A, Parekkadan B et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant 2010;19:667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galipeau J, Krampera M, Barrett J et al. International society for cellular therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 2016;18:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 16. Prockop DJ. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol 2016;51:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Common Terminology Criteria for Adverse Events (CTCAE). Bethesda, Maryland, USA: National Cancer Institute, Department of Health and Human Services, 2010.
- 18. Roos EM, Roos HP, Lohmander LS et al. Knee injury and osteoarthritis outcome score (KOOS)—Development of a self‐administered outcome measure. J Orthop Sports Phys Ther 1998;28:88–96. [DOI] [PubMed] [Google Scholar]
- 19. Western Ontario & McMaster Universities Osteoarthritis Index (WOMUOI) . Available at https://www.rheumatology.org/I‐Am‐A/Rheumatologist/Research/Clinician‐Researchers/Western‐Ontario‐McMaster‐Universities‐Osteoarthritis‐Index‐WOMAC. Accessed July 29, 2018.
- 20. Guermazi A, Roemer FW, Hayashi D et al. Assessment of synovitis with contrast‐enhanced MRI using a whole‐joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis 2011;70:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bae J‐H, Hosseini A, Wang Y et al. Articular cartilage of the knee 3 years after ACL reconstruction. Acta Orthop 2015;86:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abeles RD, MJ MP, Sowter D et al. CD14, CD16 and HLA‐DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA‐DR expression by CD14hi/CD16neg monocytes: Expansion of CD14hi/CD16pos and contraction of CD14lo/CD16pos monocytes in acute liver failure. Cytometry A 2012;81A:823–834. [DOI] [PubMed] [Google Scholar]
- 23. Roos EM, Lohmander LS. The knee injury and osteoarthritis outcome score (KOOS): From joint injury to osteoarthritis. Health Qual Life Outcomes 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sasaki E, Tsuda E, Yamamoto Y et al. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis—A five‐year prospective cohort study. Arthritis Res Ther 2015;17:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Billinghurst RC, Dahlberg L, Ionescu M et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 1997;99:1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poole AR, Ha N, Bourdon S et al. Ability of a urine assay of type II collagen cleavage by collagenases to detect early onset and progression of articular cartilage degeneration: Results from a population‐based cohort study. J Rheumatol 2016;43:1864–1870. [DOI] [PubMed] [Google Scholar]
- 27. Bay‐Jensen AC, Reker D, Kjelgaard‐Petersen CF et al. Osteoarthritis year in review 2015: Soluble biomarkers and the BIPED criteria. Osteoarthr Cartil 2016;24:9–20. [DOI] [PubMed] [Google Scholar]
- 28. Wong KL, Yeap WH, Tai JJY et al. The three human monocyte subsets: Implications for health and disease. Immunol Res 2012;53:41–57. [DOI] [PubMed] [Google Scholar]
- 29. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 30. Krampera M, Galipeau J, Shi Y et al. Immunological characterization of multipotent mesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013;15:1054–1061. [DOI] [PubMed] [Google Scholar]
- 31. Lalu MM, McIntyre L, Pugliese C et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta‐analysis of clinical trials. PloS One 2012;7:e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pers Y‐M, Rackwitz L, Ferreira R et al. Adipose mesenchymal stromal cell‐based therapy for severe osteoarthritis of the knee: A phase i dose‐escalation trial. Stem Cells Translational Medicine 2016;5:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bastos R, Mathias M, Andrade R et al. Intra‐articular injections of expanded mesenchymal stem cells with and without addition of platelet‐rich plasma are safe and effective for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2018;26:3342–3350. [DOI] [PubMed] [Google Scholar]
- 34. Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol 2013;9:584–594. [DOI] [PubMed] [Google Scholar]
- 35. Kong SY, Stabler TV, Criscione LG et al. Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis. Arthritis Rheum 2006;54:2496–2504. [DOI] [PubMed] [Google Scholar]
- 36. Kumm J, Tamm A, Lintrop M et al. The value of cartilage biomarkers in progressive knee osteoarthritis: Cross‐sectional and 6‐year follow‐up study in middle‐aged subjects. Rheumatol Int 2013;33:903–911. [DOI] [PubMed] [Google Scholar]
- 37. Kraus VB, Collins JE, Hargrove D et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: Data from the FNIH OA biomarkers consortium. Ann Rheum Dis 2017;76:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldberg RL, Huff JP, Lenz ME et al. Elevated plasma levels of hyaluronate in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 1991;34:799–807. [DOI] [PubMed] [Google Scholar]
- 39. Kim J, Hematti P. Mesenchymal stem cell‐educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 2009;37:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laria A, Lurati A, Marrazza M et al. The macrophages in rheumatic diseases. J Inflamm Res 2016;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Brien K, Tailor P, Leonard C et al. Enumeration and localization of mesenchymal progenitor cells and macrophages in synovium from normal individuals and patients with pre‐osteoarthritis or clinically diagnosed osteoarthritis. Int J Mol Sci 2017;18:774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohashi K, Parker JL, Ouchi N et al. Adiponectin promotes macrophage polarization toward an anti‐inflammatory phenotype. J Biol Chem 2010;285:6153–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ouchi N, Kihara S, Arita Y et al. Adipocyte‐derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte‐derived macrophages. Circulation 2001;103:1057–1063. [DOI] [PubMed] [Google Scholar]
- 44. Fang F, Liu L, Yang Y et al. The adipokine adiponectin has potent anti‐fibrotic effects mediated via adenosine monophosphate‐activated protein kinase: Novel target for fibrosis therapy. Arthritis Res Ther 2012;14:R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsuchida A, Yamauchi T, Takekawa S et al. Peroxisome proliferator‐activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity‐related inflammation in adipose tissue: Comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes 2005;54:3358–3370. [DOI] [PubMed] [Google Scholar]
- 46. van Stijn CMW, Kim J, Lusis AJ et al. Macrophage polarization phenotype regulates adiponectin receptor expression and adiponectin anti‐inflammatory response. FASEB J 2015;29:636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaballah A, Hussein NA, Risk M et al. Correlation between synovial vascular endothelial growth factor, clinical, functional and radiological manifestations in knee osteoarthritis. Egypt Rheumatol 2016;38:29–34. [Google Scholar]
- 48. Chinnadurai R, Rajan D, Qayed M et al. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep 2018;22:2504–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee RH, Yu JM, Foskett AM et al. TSG‐6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci USA 2014;111:16766–16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsai AC, Liou M, Simak M et al. On hyperbolic transformations to normality. Comput Stat Data Anal 2017;115:250–266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Inclusion and exclusion criteria
Supplementary Table 2 Schedule of events
Supplementary Table 3 Final product characterization for BM‐MSCs product
Supplementary Table 4 Primers for panel of gene expression analyses of donor MSCs
Supplementary Table 5 Biomarkers characterized
Supplementary Table 6 Sampling times for blood
Supplementary Table 7 Number of eligible, enrolling and consenting patients
Supplementary Table 8 Patient Demographic and Baseline Characteristics for Each Dose Cohort
Supplementary Table 9 Baseline data for all enrolled participants
Supplementary Table 10 Transformations used for Table 1 within manuscript
Supplementary Figure S1 Bone‐marrow derived mesenchvmal strumal cells (BM‐NISCS) Manufacturing Process
Supplementary Figure S2 Trilineage differentiation of BM‐MSCs. All patients MSCS (except patient 13) were characterized by their ability to undergo trilineage differentiation, after infusion of BM‐MSCs intra‐articularly into consenting patients. Representative staining of trilineage potential is shown here. A) Adipogenic differentiation, B) Chondrogenic differentiation, and C) Osteogenic differentiation from patient 3 is shown.


