Abstract
Adult mesenchymal stem cells exert immunomodulatory effects that might improve the host response during sepsis. Knowledge on the effect of adipose‐derived mesenchymal stem cells (ASCs) in sepsis is limited. Klebsiella (K.) pneumoniae is a common cause of gram‐negative pneumonia and sepsis. This study sought to determine the effect of human ASCs on the host response during pneumosepsis in mice. Mice were infected with K. pneumoniae via the airways to induce a gradually evolving infection in the lung culminating pneumosepsis. One or 6 hours after infection, mice were infused intravenously with ASCs or vehicle, and euthanized after 16 hours or 48 hours, respectively. The effects of freshly cultured and cryopreserved ASCs were compared, the latter formulation being more clinically relevant. Intravenously administered ASCs were visualized in lung tissue by immunostaining at 1 and 3 hours, but not at 15 hours after infusion. Although early after infection, ASCs did not or only modestly influence bacterial loads, they reduced bacterial burdens in lungs and distant organs at 48 hours. ASCs reduced the lung levels of pro‐inflammatory cytokines and attenuated lung pathology, but did not influence distant organ injury. ASCs strongly modified the lung transcriptome in uninfected mice and especially mice with pneumosepsis. Cryopreserved and cultured ASCs induced largely similar effects on the lung transcriptome. These data indicate that human ASCs induce profound immune modulatory effects in the lungs, resulting in reduced bacterial burdens and lung inflammation during pneumosepsis caused by a common human pathogen, suggesting that ASCs may be an adjunctive therapeutic in this condition. stem cells translational medicine 2019;8:785&796
Keywords: Pneumonia, Sepsis, Mesenchymal stem cells, Immunomodulation
Significance Statement.
With an urgent need for new sepsis therapies, mesenchymal stem cells are of interest as they have immune modulatory effects, which could be beneficial in sepsis. This study used a clinically relevant mouse model of pneumosepsis to evaluate the effects of adipose‐derived mesenchymal stem cells (ASCs), of which knowledge on their effect in sepsis is limited. This study showed that human ASCs induced profound immune modulatory effects in the lungs, resulting in reduced bacterial burdens and lung inflammation, suggesting that ASCs may be an adjunctive therapeutic in sepsis.
Introduction
Sepsis is a major cause of morbidity and mortality, with an estimated worldwide population incidence rate of 437 cases per 100,000 person years 1. Despite the availability of antibiotics, sepsis remains a major health concern and currently is the most common reason for death in intensive care units 2. The majority of sepsis cases are preceded by pneumonia 3 . Klebsiella (K.) pneumoniae is a common case of hospital‐acquired pneumonia and an emerging pathogen in community‐acquired respiratory tract infection 4, 5.
Sepsis is a clinical syndrome characterized by a dysregulated host response to an infection resulting in organ failure 6, 7. With the urgent need for new therapeutics, adult mesenchymal stem cells (MSCs) could potentially be a new approach in the field of immune modulatory therapies in sepsis 8, 9, 10, 11, 12. MSCs have low immunogenicity because of their low expression of major histocompatibility complex (MHC) class I as well as absence of MHC class II and T‐cell costimulatory molecules 13. Furthermore, MSCs possess a large secretive repertoire of anti‐inflammatory, angiogenic, and antibacterial molecules that are able to modulate the immune response in various ways 8, 14, 15, 16.
The therapeutic potential of MSCs has been demonstrated in animal models of sepsis and other infections 8, 12, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26. Notably, the vast majority of these investigations studied the effect of bone marrow‐derived MSC 16, 17, 18, 21, 22, 23, 25, 26. The effects of adipose‐derived mesenchymal stem cells (ASCs) are underreported, even though they have some important advantages over bone marrow‐derived MSC; the human body contains far more ASCs than bone marrow‐derived MSC (adipose tissue contains 100,000 ASCs per gram fat) 27, and isolation of ASCs is less invasive 28, 29.
The aim of this study was to determine the effect of human ASCs treatment on the host response during K. pneumoniae‐evoked pneumosepsis in mice.
Materials and Methods
Mice
Pathogen free 8‐week‐old to 10‐week‐old female C57BL/6 mice were purchased from Charles River (Leiden, The Netherlands). All animals were specific‐pathogen‐free and housed in the Animal Research Institute Amsterdam facility under standard care. All experiments were carried out in accordance with the Dutch Experiment on Animals Act and were approved by the local animal welfare committee of the Academic Medical Center (protocol DIX21CU‐1).
ASC Preparation
ASCs were prepared at TiGenix SAU (Madrid, Spain) as described previously 30. In short, ASCs were obtained from adipose tissue from a healthy donor, and expanded. ASCs fulfilled the International Society Cell and Gene Therapy criteria for MSCs and were thoroughly checked for viability, population doublings, morphology, potency, identity, purity, sterility, and genetic stability, among other quality controls. Cells were kept in liquid nitrogen until used.
For the preparation of freshly cultured ASCs, cells were thawed and recovered in tissue culture flasks with Dulbecco's Modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM l‐glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (37°C at 5% CO2). At the day of administration, cells were trypsinized, washed in phosphate buffered saline (PBS), and resuspended at the required concentration in Ringers lactate. Cryopreserved cells were thawed and directly resuspended at the required concentration in Ringers lactate.
Experimental Study Design
Pneumonia was induced by intranasal inoculation with 104 colony forming units K. pneumoniae serotype 2 (ATCC 43816, Rockville, MD) as described 31, 32, 33. This model is associated with dissemination of the infection from the lungs to distant organs, with systemic inflammatory responses, organ injury, and eventually death 31, 32, 33, thereby meeting the most recent sepsis definition 34. In all experiments mice were intravenously (i.v.) infused with 1 × 106 ASCs at 1 or 6 hours after infection (as indicated); in one experiment mice were administered with 0.4 × 106 ASCs as indicated. ASCs were administered in 200 μl Ringers lactate, which for cryopreserved ASCs was supplemented with 3% dimethylsulfoxide; control groups received matched vehicle. Mice were euthanized 16 or 48 hours after induction of pneumonia. Citrate‐anticoagulated blood and organs were harvested and processed as described previously 31, 35. Each experiment was performed with eight mice per group. Some experiments were repeated and data pooled as indicated in the figure and table legends.
Histology and Immunohistochemistry
Lung, spleen, and liver were fixed in 10% formaldehyde and embedded in paraffin. Four‐micrometer sections of the lung were stained with hematoxylin and eosin (H&E) or Ly‐6G monoclonal antibody (mAb; clone 1A8; Biolegend, San Diego, CA) and scored as described 31. In short, the following parameters were scored on a scale of 0 (absent), 1 (mild), 2 (moderate), 3 (severe), and 4 (very severe): interstitial damage, vasculitis, peribronchitis, oedema, thrombus formation, and pleuritis. In all experiments, the samples were scored by the same pathologist blinded for experimental groups.
To assess the distribution of ASCs, organs were harvested 1, 3, and 15 hours after i.v. ASCs infusion and treated as described above (n = 4 per group). Sections were stained with a human antimitochondrial antibody (clone 113‐1; Millipore, Amsterdam‐Zuidoost, The Netherlands) to show the presence and location of the ASCs.
Flow Cytometry
Single cell suspensions (lung, liver) were prepared after enzymatic digestion. In brief, tissues were digested in a buffer containing 0.2 mg/ml Liberase TM, 0.1 mg/ml DNAse in PBS supplemented with 1.5% fetal calf serum) for 30 minutes at 37°C. Single cell suspensions were obtained by passage through a 19G needle and through a 100 μM cell strainer (BD, Germany). Blood leukocytes were obtained after lysis of erythrocytes with erythrocyte lysis buffer (Qiagen, Hilden, Germany). For visualization of ASCs, cell suspensions were stained with viability dye APC‐Cy7 (BD Biosciences, San Jose, CA), anti‐mouse CD45 mAb (PE‐eFluor610), and anti‐human CD90 mAb (clone Thy‐1; APC; a specific stem cell marker) in the presence of Fc blocker (CD16/CD32, all eBiosciences).
Protein Assays
Interleukin (IL)‐1β, IL‐6, tumor necrosis factor (TNF)‐α (both Ebioscience, San Diego, CA), macrophage inflammatory protein (MIP)‐2, myeloperoxidase (MPO), E‐selectin, vascular cell adhesion molecule 1 (VCAM‐1, all R&D systems, Minneapolis, MN), and thrombin–antithrombin complexes (TATc; Affinity Biologicals, Ancaster, Ontario, Canada) were measured by enzyme‐linked immunosorbent assay. In plasma, monocyte chemotactic protein 1 (MCP‐1), IL‐6, and TNF‐α were determined using a cytometric bead array multiplex assay (BD Biosciences). Alanine aminotransferase (ALT) was measured in plasma using a c702 Roche Diagnostics (Roche Diagnostics BV, Almere, The Netherlands).
RNA Preparation and Microarrays
RNA was isolated from lung homogenate using the Nucleospin RNA isolation kit (Macherey‐Nagel, Düren, Germany) as described by the manufacturer. RNA samples with integrity number >6 (Agilent Bioanalyzer) were included for microarrays. RNA was hybridized to the mouse Clariom S Assay HT chip (ThermoFisher Scientific, Eindhoven, the Netherlands) and scanned at the Cologne Center for Genomics, Cologne, Germany, as per manufacturer's instructions. Preprocessing and quality control of the scans were performed by using the oligo method (version 1.44) 36 and probes were annotated using the platform design info for Affymetrix Clariom_S_Mouse_HT available through bioconductor 37. Array data were background corrected by robust multiarray average and quantiles‐normalized. Microarray quality control was performed by means of the array quality metrics method (version 3.36.0) 38. The occurrence of nonexperimental chip effects was evaluated by the surrogate variable analysis method (version 3.28.0) 39 and corrected using the combat method 40. Probes were filtered by means of a 0.5 variance cutoff using the genefilter method (version 1.62.0) 41, resulting in 14,564 expressed transcripts. Comparison between groups was performed by moderated t statistics implemented in the empirical Bayesian linear models method limma (version 3.36.2) 42, 43, 44, 45. Throughout, Benjamini–Hochberg multiple comparison adjusted probabilities 46 (adjusted p < .05) defined significance. All analyses were performed in the R statistical computing environment (version 3.5.0). Pathway analysis was performed by Ingenuity pathway analysis (Qiagen Bioinformatics) specifying the Ingenuity knowledgebase as reference set and human species. All other parameters were default. Significance was evaluated by Fisher's exact test and Benjamini–Hochberg adjusted p‐values (adjusted p < .05). Normalized and non‐normalized array data are accessible through the Gene Expression Omnibus with accession number GSE121970.
Statistical Analysis
Data are expressed as box‐and‐whisker plots, or as median with interquartile range (IQR) as indicated. Comparisons between multiple groups were first performed using Kruskal–Wallis analysis of variance test, and Mann–Whitney U test where appropriate. Analysis were done using GraphPad Prism version 6.0 (Graphpad Software, San Diego, CA). p‐Values <.05 were considered statistically significant.
Results
Cryopreserved and Cultured ASCs Reduce Pulmonary Outgrowth and Dissemination of K. pneumoniae During the Later Phase of Infection
To study the effect of ASCs in K. pneumoniae‐evoked pneumosepsis, our first aim was to assess if ASCs treatment affected bacterial growth and dissemination during an early and later phase of the infection. In addition, we compared the effect of freshly cultured ASCs with that of cryopreserved ASCs, with the former being the preferable formulation for treatment of human sepsis. Mice were infected with K. pneumoniae via the airways, infused i.v. with 106 freshly cultured or cryopreserved ASCs (or vehicle), 1 or 6 hours after initiation of infection, and sacrificed at 16 or 48 hours after infection. Infusion of cultured ASCs 1 hour after infection did not affect bacterial loads at 16 hours (Fig. 1A–1D). Infusion of cultured ASCs 6 hours after infection was associated with lower bacterial burdens in lungs and distant organs at 48 hours after infection when compared with the vehicle control group (Fig. 1A–1D). Infusion of cryopreserved ASCs according to the same treatment schedule did lower bacterial loads in lungs (but not in distant organs) at 16 hours after infection (Fig. 1E–1H). Akin to the effect of cultured ASCs, cryopreserved ASCs reduced bacterial burdens in lungs and distant organs at 48 hours after infection when compared with control (Fig. 1E–1H). Thus, both cultured and cryopreserved ASCs were able to reduce bacterial growth and spreading during K. pneumoniae induced pneumosepsis, with cryopreserved cells exerting a more rapid effect at the primary site of infection.
Figure 1.
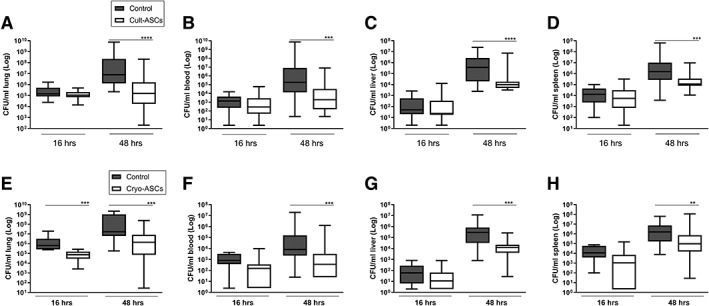
Infusion of cultured or cryopreserved adipose‐derived mesenchymal stem cells (ASCs) reduce bacterial burdens during pneumosepsis. Bacterial loads (colony‐forming units) in the lung, blood, liver, and spleen 16 and 48 hours after infection with Klebsiella pneumoniae via the airways in mice treated with 1 × 106 freshly cultured (Cult‐ASCs, A–D) or cryopreserved (Cryo‐ASCs, E–H) ASCs intravenously either 1 hour after bacterial inoculation (for measurements at 16 hours after infection) or 6 hours after infection (for measurements at 48 hours after infection). Data are expressed as box‐and‐whisker diagrams of eight mice per group at each time point, and a representative result of at least two (three for 48 hours) independent repeated experiments. **, p < .01; ***, p < .001; ****, p < .0001 versus the control group.
ASCs Reduce Lung Inflammation Evoked by K. pneumoniae Infection
Infusion of either cultured or cryopreserved ASCs resulted in significant reductions of lung TNF‐α, IL‐1β, IL‐6, and MIP‐2 concentrations at 16 and 48 hours after infection with K. pneumoniae (Table 1). Considering the greater clinical relevance of cryopreserved ASCs, we studied their effect on lung inflammation in more detail. To determine the impact of cryopreserved ASCs on lung pathology, H&E stained lung sections were semiquantitatively scored with reference to histological features characteristic of pneumonia 31. Total pathology scores were reduced at 48 hours upon treatment with cryopreserved ASC (p < .01 versus vehicle control, Fig. 2), together with diminished confluent lung inflammation (p < .05, Fig. 2). The effect of ASCs on neutrophil influx and activity into the lung was assessed by measuring the number of Ly‐6G‐positive cells in lung tissue slides and by determining MPO levels in lung homogenates. The number of Ly‐6G‐positive cells was not altered upon treatment with cryopreserved ASCs (Fig. 2), whereas lung MPO levels were slightly reduced at 16 hours (p < .01, Fig. 2). In conclusion, cryopreserved ASCs reduced lung cytokine levels and the extent of lung pathology induced by K. pneumoniae, but had minimal impact on neutrophil influx into the lung. Notably, as reported earlier by others 47, i.v. injection of either freshly cultured or cryopreserved ASCs was associated with transient formation of multiple micro thrombi in the lungs, which was clearly present at 16 hours after infection and almost completely resolved at 48 hours (Supporting Information Fig. S1).
Table 1.
Cultured and cryopreserved adipose‐derived mesenchymal stem cells reduce lung cytokine levels during pneumosepsis
| 16 hours | 48 hours | |||
|---|---|---|---|---|
| Control | ASCs | Control | ASCs | |
| Cultured ASCs (pg/ml) | ||||
| TNF‐α | 2,154 (1,718–3,411) | 1,384 (1,061–1,655)**** | 2,173 (1,184–3,312) | 894 (642–1,792)*** |
| IL‐1β | 1,066 (480–1,944) | 570 (440–789)* | 767 (460–1,598) | 310 (157–534)*** |
| IL‐6 | 3,003 (1,598–3,858) | 1,405 (1,136–2,658)* | 874 (366–1,646) | 361 (276–675)** |
| MIP‐2 | 15,684 (13,972–17,587) | 12,865 (9,323–15,261)* | 17,086 (12,279–26,432) | 12,233 (9,458–14,011)*** |
| Cryopreserved ASCs (pg/ml) | ||||
| TNF‐α | 2,512 (2,381–2,967) | 1,540 (1,156–1,982)* | 1,674 (1,242–2,644) | 910 (738–2,180)* |
| IL‐1β | 1,267 (1,193–1,454) | 690 (373–936)** | 665 (363–1,207) | 341 (208–696)* |
| IL‐6 | 4,084 (3,812–5,423) | 1,951 (1,410–3,174)** | 2,322 (777–5,531) | 1,733 (912–2,534) |
| MIP‐2 | 20,874 (19,257–22,821) | 17,383 (16,809–18,654)** | 18,331 (13,574–32,766) | 12,928 (9,946–19,983)* |
Mice were treated with 1 × 106 ASCs intravenously either 1 or 6 hours after infection with Klebsiella pneumoniae via the airways and lungs were harvested 16 or 48 hours after infection, respectively. Data are median (interquartile range) of 8–24 mice per group.
p < .05.
p < .01.
p < .001.
p < .0001 versus control.
Abbreviations: ASCs, adipose‐derived mesenchymal stem cells; TNF, tumor necrosis factor; IL, interleukin; MIP, macrophage inflammatory protein.
Figure 2.
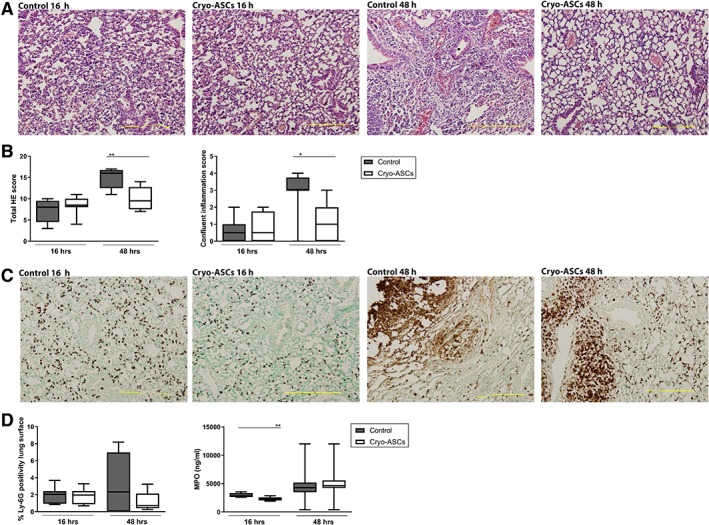
Infusion of cryopreserved adipose‐derived mesenchymal stem cells (ASCs) reduces lung inflammation during pneumonia derived sepsis. Mice were treated with 1 × 106 cryopreserved ASCs intravenously either 1 or 6 hours after infection with Klebsiella pneumoniae via the airways and lungs were harvested 16 or 48 hours after infection, respectively. (A): Representative photographs of H&E‐stained tissue sections of infected lungs at 16 and 48 hours, treated with ASCs or vehicle (control), (B) the extent of inflammation scored on H&E tissue sections as total HE score and confluent inflammation score, (C) neutrophil accumulation in lung tissue measured by Ly‐6G stainings, and (D) quantification of Ly‐6G staining of lung tissue and myeloperoxidase concentrations in lung homogenates. Original magnification ×4 for panels (A) and (C). Data are expressed as box‐and‐whisker diagrams for panels (B) and (D). n = 8 mice per group. *, p < .05; **, p < .01 versus the control group.
Intravenous infusion of a lower dose of cryopreserved ASCs (0.4 × 106 cells) 6 hours after infection with Klebsiella did not alter bacterial loads in lungs or distant organs, or lung inflammatory responses at 48 hours (Supporting Information Figs. S2 and S3). These results indicate that the effects of cryopreserved ASCs are dose‐dependent.
The Effect of ASCs Treatment on Plasma Cytokine Levels, Endothelial Cell Activation, and Organ Injury During K. pneumoniae Pneumosepsis
To study the effect of ASCs infusion on systemic host responses during K. pneumoniae induced pneumosepsis, plasma cytokine levels, markers for endothelial cell activation (soluble E‐selectin, soluble VCAM‐1), and liver damage (ALT) were measured. Cryopreserved ASCs treatment was associated with reduced plasma TNF‐α, IL‐6, and MCP‐1 concentrations at 48 hours after infection, whereas the plasma levels of soluble E‐selectin, soluble VCAM‐1, and ALT were not altered (Table 2). Likewise, cultured ASCs diminished plasma cytokine levels without affecting soluble E‐selectin, soluble VCAM‐1, or ALT concentrations (Supporting Information Table S1). Together, these results indicate that ASCs attenuate systemic cytokine release, but have little impact on endothelial cell activation or liver injury during K. pneumoniae‐evoked pneumosepsis.
Table 2.
Cryopreserved adipose‐derived mesenchymal stem cells reduce plasma cytokine levels during pneumosepsis
| 16 hours | 48 hours | |||
|---|---|---|---|---|
| Control | ASCs | Control | ASCs | |
| TNF‐α (pg/ml) | 11 (6–92) | 5 (5–5)* | 25 (15–44) | 22 (9–28) |
| IL‐6 (pg/ml) | 80 (53–175) | 33 (17–41)** | 155 (75–358) | 62 (23–137)** |
| MCP‐1 (pg/ml) | 204 (65–1,797) | 34 (31–58)* | 375 (159–537) | 189 (64–15–254)* |
| E‐selectin (ng/ml) | 605 (401–965) | 645 (525–864) | 370 (306–404) | 396 (278–434) |
| VCAM‐1 (pg/ml) | 1,201 (1,075–1,517) | 1,202 (1,032–1,403) | 1,290 (1,034–1,925) | 1,575 (1,315–1,862) |
| ALT (pg/ml) | n.d. | n.d. | 62 (13–198) | 34 (11–72) |
Mice were treated with 1 × 106 cryopreserved ASCs intravenously either 1 or 6 hours after infection with Klebsiella pneumoniae via the airways and lungs were harvested 16 or 48 hours after infection, respectively. Data are medians (interquartile range) of 8–24 mice per group.
p < .05.
p < .01 versus control.
Abbreviation: ASCs, adipose‐derived mesenchymal stem cells; TNF, tumor necrosis factor; IL, interleukin; MCP, monocyte chemotactic protein; VCAM, vascular cell adhesion molecule; ALT, alanine aminotransferase; n.d., not determined.
ASCs Accumulate in the Lung Directly After Intravenous Infusion
To better understand the mobilization of ASCs into specific tissue, we used flow cytometry to determine the presence of ASCs in the lung, blood, liver, and spleen 1, 3, and 15 hours after i.v. injection of 106 cryopreserved ASCs in mice with K. pneumoniae induced pneumonia (n = 4 per group). As a control, naïve mice were sacrificed 1 hour after injection with cryopreserved ASCs.
One hour after injection 33% (median; IQR 32–44) of the injected ASCs could be detected in uninfected lungs, and 15% (median; IQR 9–34) in infected lungs (difference between uninfected and infected lungs was not significant). Three hours after injection, 9% (median; IQR 8–12) of injected ASCs were identified in lungs of infected mice. ASCs were not detectable at 15 hours after injection, from any other body site at any time point (data not shown). To assess the location of the ASCs in the lung, we stained ASCs in lung tissue slides using a species specific anti‐human mitochondrial DNA marker (Fig. 3). ASCs were located near the vasculature at 1 and 3 hours after injection, whereas not visible anymore at 15 hours. Overall, these results show that ASCs accumulate in the lung near blood vessels directly after infusion, rapidly disappearing thereafter.
Figure 3.
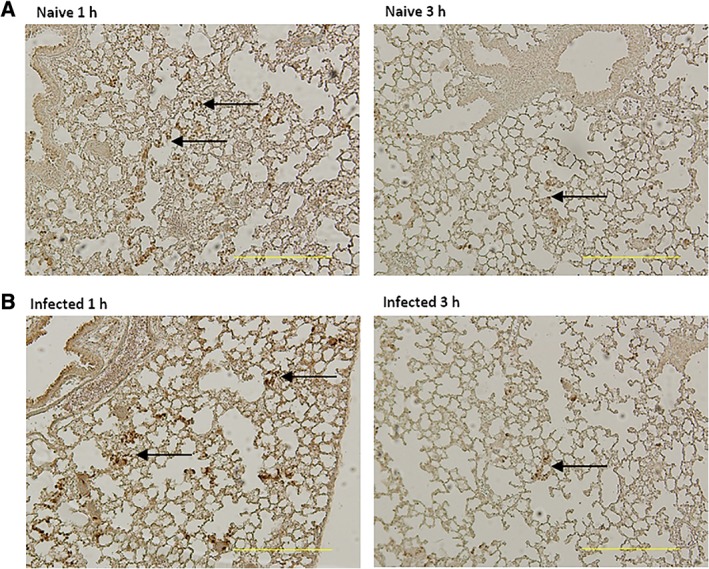
Adipose‐derived mesenchymal stem cells (ASCs) accumulate in lungs near the vasculature early after intravenous infusion. Mice were treated with 1 × 106 cryopreserved ASCs intravenously 1 hour after infection with Klebsiella pneumoniae via the airways; uninfected mice were used as control. Lungs were harvested 1 or 3 hours after infection. Representative photographs of antimitochondrial DNA‐stained tissue sections of lungs of naïve uninfected (A) and infected mice (B). Arrows indicate ASCs. Original magnification ×4.
ASCs Modify the Lung Transcriptome in Uninfected Mice and Mice with Pneumosepsis
We performed genome‐wide scans of pulmonary gene expression in uninfected and infected mice treated with ASCs. For this analysis, we selected the 16 hour time point to avoid confounding effects by differences in bacterial burden observed at later time points. Infusion of cryopreserved ASCs in uninfected mice induced a strong transcriptomic response in the lungs (Fig. 4A). Considering multiple comparison adjusted probabilities (adjusted p < .05) 1,141 and 845 transcripts were significantly elevated and reduced in ASCs infused mice, respectively (Fig. 4B). Pathway analysis of high expression transcripts revealed a significant association to various stress response (e.g., unfolded protein response and protein ubiquitination), granulocyte mobility (e.g., granulocyte adhesion and diapedesis), and fibrosis pathways (e.g., hepatic fibrosis/hepatic stellate cell activation; Fig. 4C). Low expression transcripts were significantly associated with predominantly metabolic pathways that included nicotine degradation, thymine degradation, and uracil degradation pathways (Fig. 4C). The granulocyte adhesion and diapedesis pathway was enriched with transcripts encoding matrix metalloproteinases Mmp14, Mmp12, Mmp3, Mmp27, Mmp19, Mmp10, as well as antimicrobial genes Cxcl2, Cxcl10, Cxcl9, Cxcl14, and IL‐1 receptor family member Il1r1 (Fig. 4D). Transcripts encoding heat shock proteins Hspa5, Hspa9, Hspa1l, and Hsph1 as well as transcription factors Atf4, Xbp, Cebpb, and Cepbd were prominent in the unfolded protein response pathway (Fig. 4D). The nicotine degradation pathway was enriched for transcripts encoding flavin containing monooxygenases Fmo2, Fmo1, and Fmo5 (Fig. 4D). Infusion of cultured ASCs into uninfected mice elicited changes in the lung transcriptome that were highly similar to those altered by cryopreserved ASCs (Supporting Information Table S2). K. pneumoniae‐induced pneumosepsis was associated with a substantial transcriptomic response in the lungs, with 2,010 elevated transcripts and 925 reduced transcripts when compared with uninfected mice (Supporting Information Fig. S4A). Pathway analysis revealed significant over‐representation of pathways associated with granulocyte mobility pathways (e.g., granulocyte adhesion and diapedesis and agranulocyte adhesion and diapedesis), IL‐10 signaling and acute phase response signaling (Supporting Information Fig. S4B). Treatment with cryopreserved ASCs resulted in a significant shift in the lung transcriptome of K. pneumoniae infected mice relative to untreated (placebo) control infected mice (Fig. 5A). Considering adjusted p‐values <.05, 936 elevated transcripts and 1,286 reduced transcripts were detected in ASC treated mice relative to placebo controls (Fig. 5B). Pathway analysis revealed high expression transcripts were associated with fibrosis pathways (e.g., hepatic fibrosis/hepatic stellate cell activation), coagulation (e.g., role of tissue factor in cancer) and metabolic pathways (e.g., mTOR signaling). Low expression transcripts in ASC treated mice were associated with granulocyte mobility pathways (e.g., granulocyte adhesion and diapedesis), innate immune pathways (e.g., LPS/IL‐1 mediated inhibition of RXR function, communication between innate and adaptive immune cells, and role of hypercytokinemia/hyperchemokinemia in the pathogenesis of influenza), and adaptive immune pathways (e.g., Th1 and Th2 activation pathway; Fig. 5C). The fibrosis pathway was particularly enriched for transcripts encoding collagens, including Col1a1 and Col1a2, and growth factors that included Ctgf, Pgf, and Pdgfb (Fig. 5D). Transcripts encoding genes that are central to cellular metabolism, growth and proliferation, including Hif1a, Nras, and Hras, were prominent in the mTOR signaling pathway (Fig. 5D). Low expression transcripts in granulocyte adhesion and diapedesis were enriched for key innate immune cytokines Tnf, Il1b, Il1a, Il1rn, as well as matrix metalloproteinases (e.g., Mmp2 and Mmp15) and cell junction factors including Cldn15, Cldn10, and Cldn3 (Fig. 5D). Furthermore, the Th1 and Th2 activation pathways was enriched for T‐cell surface membrane proteins Cd4, Cd3e, and Cd3g, interferons and related molecules (e.g., Ifng and Irf1) as well as transcription factors including Tbx21 and Stat1 (Fig. 5D). Altogether, these findings suggest that in the absence of infection ASCs induce transcriptomic alterations attuned to tissue stress and damage; albeit, in the context of infection ASCs reduce, at least in part, the pulmonary host immune response concomitant with an increase in fibrosis, coagulation, and metabolic signaling.
Figure 4.
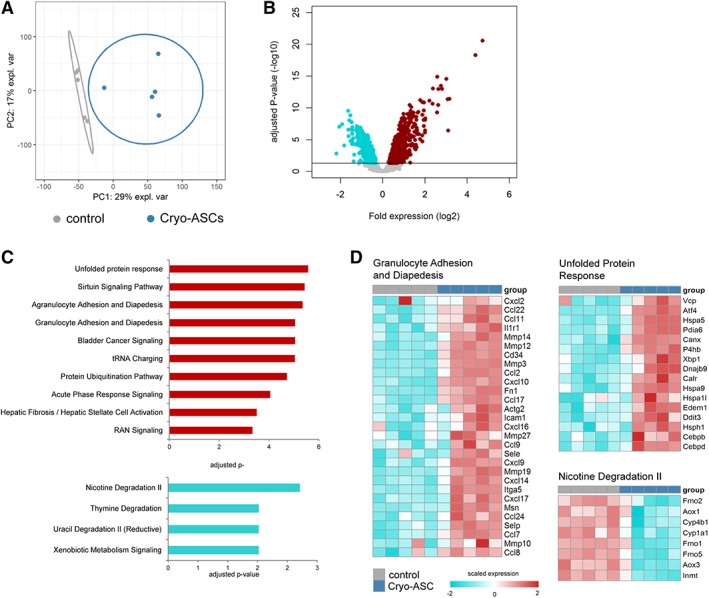
Infusion of cryopreserved adipose‐derived mesenchymal stem cells (ASCs) induce transcriptomic responses in the lungs of healthy mice. (A): Principal component plot of highly expressed transcripts (n = 10.218) grouped as control (vehicle) or Cry‐ASCs (treated with cryopreserved adipose‐derived stem cells) mice. (B): Volcano plots (integrating adjusted p‐values and fold expression [log2 fold change]) depicting the global alteration in gene expression after treatment with cryopreserved ASCs relative to controls. Horizontal line indicates Benjamini–Hochberg (BH) adjusted p < .05. Red dots denote overexpressed genes; turquoise dots indicate underexpressed genes. (C): Ingenuity pathway analysis of elevated transcripts (red bars) and reduced transcripts (turquoise bars) with BH adjusted Fisher's p < .01 demarcating significance. (D): Heatmap representation of transcript expression (rows) grouped as Ingenuity canonical signaling pathways in control and Cryo‐ASC treated animals. Red, high expression; turquoise, low expression.
Figure 5.
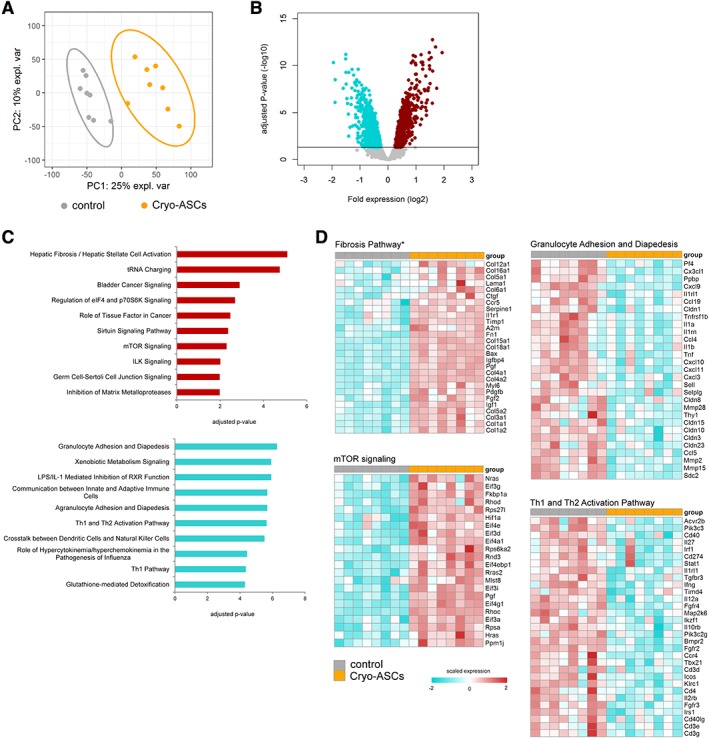
Cryopreserved adipose‐derived mesenchymal stem cells (ASCs) treatment was associated with a profound transcriptomic response in the lungs of infected mice. (A): Principal component plot of highly expressed transcripts (n = 10.218) grouped as control (vehicle) or Cry‐ASCs (treated with cryopreserved adipose‐derived stem cells) mice infected with Klebsiella pneumoniae. (B): Volcano plots (integrating adjusted p‐values and fold expression [log2 fold change]) depicting the global alteration in gene expression after treatment with cryopreserved ASCs relative to infected controls. Horizontal line indicates Benjamini–Hochberg (BH) adjusted p < .05. Red dots denote overexpressed genes; turquoise dots indicate underexpressed genes. (C): Ingenuity pathway analysis of elevated transcripts (red bars) and reduced transcripts (turquoise bars) with BH adjusted Fisher's p < .01 demarcating significance. (D): Heatmap representation of transcript expression (rows) grouped as Ingenuity canonical signaling pathways in control and Cryo‐ASC treated animals infected with K. pneumoniae. Red, high expression; turquoise, low expression.
Discussion
Various reports have described the potential of MSCs as adjunctive therapy in sepsis and other infections. MSCs have shown to be able to inhibit pro‐inflammatory cytokine release, to reduce bacterial outgrowth and to improve survival 17, 25, 26, 48, 49, 50. Although the vast majority of investigations examined the effects of bone marrow‐derived MSCs, limited information is available regarding the function of ASCs in sepsis. We here studied the effect of ASCs in K. pneumoniae induced pneumosepsis. This model resembles a frequent clinical scenario of sepsis, in which infection is initiated by a low inoculum of a common human sepsis pathogen administered via the airways, resulting in pneumonia with subsequent dissemination of bacteria, organ failure, and systemic inflammation. We investigated the effects of both freshly cultured and cryopreserved ASCs, the latter formulation bearing a greater translational value. Our results show that both freshly cultured and cryopreserved ASCs reduced bacterial growth and inhibited inflammatory responses in the lung during K. pneumoniae‐induced pneumosepsis.
Earlier studies have documented beneficial effects of MSCs derived from bone marrow or umbilical cord tissue in bacterial pneumonia models 18, 21, 23, 51, 52, 53, 54. Most studies used Escherichia (E.) coli to infect mice or rats via the airways, reporting reduced pulmonary edema and alveolar epithelial permeability, attenuated proinflammatory cytokine release, and enhanced bacterial clearance after i.v. or intratracheal administration of MSCs 18, 23, 51, 52, 53. The E. coli model relates more to acute lung injury than pneumonia and strongly differs from the K. pneumoniae model used here: whereas the E. coli model is associated with acute onset severe lung inflammation and clearance of bacteria, our model is associated with a steadily growing bacterial load and a gradually evolving inflammatory response eventually resulting in sepsis, thereby better resembling the clinical scenario of bacterial pneumonia. Intratracheal administration of bone marrow derived MSCs reduced alveolitis and protein leakage in experimental pneumonia induced by a K. pneumoniae strain different from the one used here, while not influencing bacterial loads 21. We here evaluated the therapeutic potential of MSCs derived from adipose tissue, which more recently has become an attractive source of MSCs due to its higher content of stem cells 27 and because ASCs may be acquired through procedures less invasive than bone marrow aspiration 28, 29. One previous study addressed the effects of ASCs (derived from mouse adipose tissue) in bacterial pneumonia: in experimental respiratory tract infection caused by Pseudomonas aeruginosa, which like the E. coli model was associated with acute lung injury and clearance of bacteria from the airways. Intratracheal administration of ASCs reduced bacterial burden, protein leak, the extent of lung injury, inhibited chemokine release, and neutrophil influx 24. ASCs have also been reported to exert anti‐inflammatory effects in models of noninfectious acute lung injury 55. The present data are in line with and extend on these previous studies, suggesting that both freshly cultured and cryopreserved ASCs administered via a clinically relevant route (i.v.) influence host defense in a model of bacterial pneumonia that resembles human infection.
Although MSCs harbor great therapeutic potential for several diseases, their use in acute indications such as pneumonia and sepsis is hampered by the fact that critically ill patients need to receive their treatments immediately which makes the use of allogeneic freshly cultured MSCs logistically challenging, being the use of bed‐side cryopreserved MSCs the preferred option. Cryopreservation is currently the only method to preserve ASCs with retained functional properties and low concentrations of dimethyl sulphoxide have been identified as an ideal cryoprotective agent for efficient long‐term cryopreservation of human ASCs 56.To confirm that thawed ASCs share the therapeutic properties of cultured ASCs in this indication, we here compared the effects of freshly cultured and cryopreserved ASCs side by side, showing that cryopreserved ASCs exert comparable immune modulatory effects as freshly cultured ASCs, thereby enhancing the translational value of our findings. In accordance, cryopreserved human bone marrow or umbilical cord derived MSCs attenuated acute lung injury caused by E. coli 23, 53. Likewise, cryopreserved MSCs maintained their potency in a retinal ischemia/reperfusion injury model 57 and cryopreserved ASCs remained effective in renal ischemia/reperfusion injury 58.
Previous studies have indicated that MSCs administered i.v. are trapped in the lungs and short‐lived, not migrating beyond the lungs 59, 60. We used flow cytometry and immunohistochemistry to trace ASCs in the lungs, up to several hours after injection, and visualized them near the vasculature. ASCs could not be recovered from spleen or liver. The fact that ASCs are only present in the lungs for hours, taken together with immune modulatory effects that occur later and last much longer points toward an indirect effect of ASCs. In accordance, after phagocytosis of MSCs by macrophages, they were able to modulate the function of monocytes/macrophages and neutrophils by polarizing macrophages toward a regulatory and anti‐inflammatory M2 phenotype, having protecting effects against sepsis by releasing IL10 12, 15, 17, 19, 25. Furthermore, MSCs can secrete multiple immune modulatory mediators and can release subcellular particles termed extracellular vesicles that are biologically active 8, 14, 15, 16. Indeed, administration of MSC‐derived microvesicles exerted lung protective effects in models of endotoxin‐induced and E. coli‐induced lung injury 52, 61. The effects of ASC‐derived vesicles in this model remain to be established.
Comprehensive analysis of the lung transcriptome revealed ASCs induce a substantial transcriptomic response in the absence of infection, which was associated with various tissue stress and damage pathways, for example, elevated protein ubiquitination pathway genes, granulocyte adhesion and diapedesis genes, unfolded protein response genes, fibrosis, and acute phase response signaling. In the context of lung infection, ASC treatment further increased tissue stress responses, concomitant with a substantial decrease in innate and adaptive immune responses. The reduction in expression of genes involved in innate and adaptive immune responses is in line with the hypothesis that MSCs, in general, may inhibit the exaggerated host immune response that eventually results in organ damage 11, 62. In addition, we showed that T helper cell activation and granulocyte adhesion and diapedesis pathways were particularly affected by ASCs treatment of K. pneumoniae lung infection. With reference to enhanced expression of genes implicated in fibrotic responses in animals infused with ASCs, it should be noted that the current model is acute and fibrosis cannot be detected at histological level. In more chronic settings, infusion of MSCs have been shown to exert antifibrotic effects in the lungs in several experimental settings, including lung fibrosis induced by bleomycin 63, 64, white smoke 65, silica 66, and chronic allergic airway inflammation 67. These preclinical data led several investigators to suggest infusion of MSCs as a potential novel therapeutic for lung fibrosis in patients 68, 69. Nonetheless, although the acute effect of ASCs on expression of genes involved in fibrosis is less likely to cause fibrosis in the long‐term, this has to be monitored in clinical trials with patients suffering from pneumonia.
Multiple previous studies reported on the effect of MSCs on survival in experimental sepsis (reviewed in reference 70). Our model is less suitable to study survival primarily because of the high virulence of the Klebsiella strain used. In addition, regulations regarding survival studies have become very strict in our country, essentially precluding such experiments in recent years. We report the effects of ASCs administered up to 6 hours after infection and further studies are warranted to determine their effects after more delayed infusion. In preliminary experiments, ASCs infused 30 hours after infection did not impact bacterial growth in this model (data not shown). Of note, results generated in mice cannot be extrapolated to human disease. Indeed, major species differences exist with reference to immunological and inflammatory responses and current mouse models including the one used here do not fully capture the complex syndrome of sepsis in humans. Therefore, results should be interpreted with caution.
Conclusion
This study advanced current knowledge on the therapeutic potential of MSCs in sepsis in several ways. We used a model of pneumosepsis caused by a common human pathogen that entails an early protective innate immune response followed by bacterial growth and dissemination, a harmful inflammatory response and sepsis. We used ASCs, and showed that cryopreserved ASCs modulate the immune response in the lung in a similar manner as cultured ASCs, thereby increasing the translational value of our findings. Finally, we provide detailed information about the effects of ASCs on the lung transcriptome in healthy mice and mice with pneumosepsis, revealing ASCs induce transcriptomic changes related to tissue stress and damage in the absence of infection; whereas in the context of infection ASCs reduce, at least in part, the extent of the host innate and adaptive immune responses concomitant with an increase in tissue fibrosis, coagulation, and metabolic signaling. Together, our results suggest that ASCs may be a valuable adjunctive therapeutic in severe pneumonia and sepsis. At present, human ASCs are evaluated in a phase Ib/IIa clinical trial in patients with severe community‐acquired pneumonia (the SEPCELL trial, NCT03158727).
Author Contributions
D.P., A.F.d.V.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; B.P.S., O.d.l.R: data analysis and interpretation, final approval of manuscript; P.M., P.N.: collection and/or assembly of data, final approval of manuscript; W.D: conception and design, final approval of manuscript; E.L.: conception and design, data analysis and interpretation, final approval of manuscript; T.v.d.P.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
E.L., O.d.l.R, W.D., and P.M. are full time employees of TiGenix. The other authors indicated no potential conflicts of interest. Amsterdam UMC received research funding from TiGenix for mouse studies with stem cells used in the study described in this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting information
Figure S1 Intravenous ASC infusion results in transient thrombi formation in the lungs
Mice were treated with 1 x 106 cryopreserved ASCs intravenously either 1 or 6 hours after infection with K. pneumoniae via the airways and lungs were harvested 16 or 48 hours after infection respectively. (A) Representative photographs of H&E‐stained tissue sections of infected lungs at 16 hoursours, treated with ASCs or vehicle, showing thrombi formation in ASCs treated mice (arrows); (B) H&E‐stained tissue sections of infected lungs at 48 hoursours, treated with ASCs or vehicle; (C) Quantification of thrombi formation (0 = no thrombi, 1 = 1 thrombus, 2 = 2–5 thrombi, 3 = 6–10 thrombi, 4 = > 10 thrombi per microscopic section). Original magnification x4 for panels A & B, unless stated otherwise. Arrows indicate thrombi. Data are expressed as bars for panel C. N = 8 mice per group. Results for cultured ASCs were similar (not shown).
Figure S2 Infusion of 0.4 x 10 6 cryopreserved ASCs has no effect on bacterial growth and dissemination.
Bacterial loads (colony‐forming units [CFU's]) in the lung, blood, liver and spleen 48 hours after infection with K. pneumoniae via the airways in mice treated with 0.4 x 106 cryopreserved ASCs or vehicle 6 hours after infection. Data were expressed box‐and‐whisker diagrams of 8 mice per group at each time point. Differences between groups were not significant.
Figure S3 Infusion of 0.4 x 10 6 cryopreserved ASCs has no effect on lung cytokine or MPO levels
TNF‐α (A), interleukin (IL)‐1β (B), IL‐6 (C), macrophage inflammatory protein (MIP)‐2 (D) and myeloperoxidase (MPO; E) levels in lungs 16 hours after infection with K. pneumoniae via the airways after intravenous administration of 0.4 x 106 cryopreserved ASCs or vehicle 6 hours after infection. Data were expressed box‐and‐whisker diagrams of 8 mice per group at each time point. Differences between groups were not significant.
Figure S4 Cryopreserved ASCs treatment was associated with a profound transcriptomic response in the lungs of infected mice
(A) Volcano plots (integrating adjusted p‐values and fold expression [log2 fold change]) depicting the global alteration in gene expression after treatment with cryopreserved ASCs compared with control in infected mice. Horizontal line indicates Benjamini‐Hochberg (BH) adjusted p < .05. Red dots denote over‐expressed genes; turquoise dots indicate under‐expressed genes. (B) Ingenuity pathway analysis of elevated transcripts (red bars) and reduced transcripts (turquoise bars) with Benjamini‐Hochberg (BH) adjusted Fisher's p < .01 demarcating significance.
Table S1 Plasma cytokine levels, E‐selectin, VCAM and ALT levels after cultured ASC treatment
Table S2: Ingenuity pathway analysis of the common over‐expressed and under‐expressed genes in naïve mice treated with cultured ASC treatment compared with placebo treatment
Acknowledgments
We thank Marieke ten Brink and Joost Daalhuisen for their technical assistance. This work was supported by TiGenix SAU (Madrid, Spain) and the European Union's Horizon 2020 Research and Innovation Programme under grant agreement number 681031.
References
- 1. Fleischmann C, Scherag A, Adhikari NK et al. Assessment of global incidence and mortality of hospital‐treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016;193:259–272. [DOI] [PubMed] [Google Scholar]
- 2. Moskowitz A, Omar Y, Chase M et al. Reasons for death in patients with sepsis and septic shock. J Crit Care 2017;38:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:2063. [DOI] [PubMed] [Google Scholar]
- 4. Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol Mol Biol Rev 2016;80:629–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song JH, Huh K, Chung DR. Community‐acquired pneumonia in the Asia‐Pacific region. Semin Respir Crit Care Med 2016;37:839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long‐term outcome. Immunol Rev 2016;274:330–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Poll T, van de Veerdonk FL, Scicluna BP et al. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 2017;17:407–420. [DOI] [PubMed] [Google Scholar]
- 8. Ho MS, Mei SH, Stewart DJ. The immunomodulatory and therapeutic effects of mesenchymal stromal cells for acute lung injury and sepsis. J Cell Physiol 2015;230:2606–2617. [DOI] [PubMed] [Google Scholar]
- 9. Lombardo E, van der Poll T, DelaRosa O et al. Mesenchymal stem cells as a therapeutic tool to treat sepsis. World J Stem Cells 2015;7:368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matthay MA, Pati S, Lee JW. Concise review: Mesenchymal stem (stromal) cells: Biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells 2017;35:316–324. [DOI] [PubMed] [Google Scholar]
- 11. Kingsley SM, Bhat BV. Could stem cells be the future therapy for sepsis? Blood Rev 2016;30:439–452. [DOI] [PubMed] [Google Scholar]
- 12. Johnson CL, Soeder Y, Dahlke MH. Concise review: Mesenchymal stromal cell‐based approaches for the treatment of acute respiratory distress and sepsis syndromes. Stem Cells Translational Medicine 2017;6:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med 2014;2:1016–1026. [DOI] [PubMed] [Google Scholar]
- 14. Kilroy GE, Foster SJ, Wu X et al. Cytokine profile of human adipose‐derived stem cells: Expression of angiogenic, hematopoietic, and pro‐inflammatory factors. J Cell Physiol 2007;212:702–709. [DOI] [PubMed] [Google Scholar]
- 15. Krasnodembskaya A, Song Y, Fang X et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL‐37. Stem Cells 2010;28:2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mei SH, Haitsma JJ, Dos Santos CC et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 2010;182:1047–1057. [DOI] [PubMed] [Google Scholar]
- 17. Nemeth K, Leelahavanichkul A, Yuen PS et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat Med 2009;15:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta N, Krasnodembskaya A, Kapetanaki M et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012;67:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anderson P, Souza‐Moreira L, Morell M et al. Adipose‐derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 2013;62:1131–1141. [DOI] [PubMed] [Google Scholar]
- 20. Sung PH, Chang CL, Tsai TH et al. Apoptotic adipose‐derived mesenchymal stem cell therapy protects against lung and kidney injury in sepsis syndrome caused by cecal ligation puncture in rats. Stem Cell Res Ther 2013;4:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hackstein H, Lippitsch A, Krug P et al. Prospectively defined murine mesenchymal stem cells inhibit Klebsiella pneumoniae‐induced acute lung injury and improve pneumonia survival. Respir Res 2015;16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asmussen S, Ito H, Traber DL et al. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax 2014;69:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devaney J, Horie S, Masterson C et al. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 2015;70:625–635. [DOI] [PubMed] [Google Scholar]
- 24. Mao YX, Xu JF, Seeley EJ et al. Adipose tissue‐derived mesenchymal stem cells attenuate pulmonary infection caused by Pseudomonas aeruginosa via inhibiting overproduction of prostaglandin E2. Stem Cells 2015;33:2331–2342. [DOI] [PubMed] [Google Scholar]
- 25. Krasnodembskaya A, Samarani G, Song Y et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram‐negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 2012;302:L1003–L1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonzalez‐Rey E, Anderson P, Gonzalez MA et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009;58:929–939. [DOI] [PubMed] [Google Scholar]
- 27. Ra JC, Shin IS, Kim SH et al. Safety of intravenous infusion of human adipose tissue‐derived mesenchymal stem cells in animals and humans. Stem Cells Dev 2011;20:1297–1308. [DOI] [PubMed] [Google Scholar]
- 28. Kern S, Eichler H, Stoeve J et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 29. Zuk PA. The adipose‐derived stem cell: Looking back and looking ahead. Mol Biol Cell 2010;21:1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Álvaro‐Gracia JM, Jover JA, García‐Vicuña R et al. Intravenous administration of expanded allogeneic adipose‐derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single‐blind, placebo‐controlled phase Ib/IIa clinical trial. Ann Rheum Dis 2017;76:196–202. [DOI] [PubMed] [Google Scholar]
- 31. Achouiti A, Vogl T, Urban CF et al. Myeloid‐related protein‐14 contributes to protective immunity in gram‐negative pneumonia derived sepsis. PLoS Pathog 2012;8:e1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Stoppelaar SF, van 't Veer C, Claushuis TA et al. Thrombocytopenia impairs host defense in gram‐negative pneumonia‐derived sepsis in mice. Blood 2014;124:3781–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anas AA, de Vos AF, Hoogendijk AJ et al. Endoplasmic reticulum chaperone gp96 in macrophages is essential for protective immunity during Gram‐negative pneumonia. J Pathol 2016;238:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singer M, Deutschman CS, Seymour CW et al. The third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Stoppelaar SF, Claushuis TA, Jansen MP et al. The role of platelet MyD88 in host response during gram‐negative sepsis. J Thromb Haemost 2015;13:1709–1720. [DOI] [PubMed] [Google Scholar]
- 36. Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010;26:2363–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huber W, Carey VJ, Gentleman R et al. Orchestrating high‐throughput genomic analysis with bioconductor. Nat Methods 2015;12:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics—A bioconductor package for quality assessment of microarray data. Bioinformatics 2009;25:415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leek JT, Storey JD. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet 2007;3:1724–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson W, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007;8:118–127. [DOI] [PubMed] [Google Scholar]
- 41. Bourgon R, Gentleman R, Huber W. Independent filtering increases detection power for high‐throughput experiments. Proc Natl Acad Sci USA 2010;107:9546–9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smyth GK. Limma: Linear models for microarray data In: R G, VJ C, H W. et al., eds. Bioinformatics and Computational Biology Solutions using R. Springer, 2005:397–420. [Google Scholar]
- 43. van Lieshout MH, Scicluna BP, Florquin S et al. NLRP3 and ASC differentially affect the lung transcriptome during pneumococcal pneumonia. Am J Respir Cell Mol Biol 2014;50:699–712. [DOI] [PubMed] [Google Scholar]
- 44. Scicluna BP, van Lieshout MH, Blok DC et al. Modular transcriptional networks of the host pulmonary response during early and late pneumococcal pneumonia. Mol Med 2015;21:430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scicluna BP, Klein Klouwenberg PM, van Vught LA et al. A molecular biomarker to diagnose community‐acquired pneumonia on intensive care unit admission. Am J Respir Crit Care Med 2015;192:826–835. [DOI] [PubMed] [Google Scholar]
- 46. Benjamini Y, Hochberg Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J R Stat Soc B Methodol 1995;57:289–300. [Google Scholar]
- 47. Tatsumi K, Ohashi K, Matsubara Y et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun 2013;431:203–209. [DOI] [PubMed] [Google Scholar]
- 48. Wu KH, Wu HP, Chao WR et al. Time‐series expression of Toll‐like receptor 4 signaling in septic mice treated with mesenchymal stem cells. Shock 2016;45:634–640. [DOI] [PubMed] [Google Scholar]
- 49. Pedrazza L, Lunardelli A, Luft C et al. Mesenchymal stem cells decrease splenocytes apoptosis in a sepsis experimental model. Inflamm Res 2014;63:719–728. [DOI] [PubMed] [Google Scholar]
- 50. Gupta N, Su X, Popov B et al. Intrapulmonary delivery of bone marrow‐derived mesenchymal stem cells improves survival and attenuates endotoxin‐induced acute lung injury in mice. J Immunol 2007;179:1855–1863. [DOI] [PubMed] [Google Scholar]
- 51. Kim ES, Chang YS, Choi SJ et al. Intratracheal transplantation of human umbilical cord blood‐derived mesenchymal stem cells attenuates Escherichia coli‐induced acute lung injury in mice. Respir Res 2011;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Monsel A, Zhu YG, Gennai S et al. Therapeutic effects of human mesenchymal stem cell‐derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med 2015;192:324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Curley GF, Jerkic M, Dixon S et al. Cryopreserved, xeno‐free human umbilical cord mesenchymal stromal cells reduce lung injury severity and bacterial burden in rodent Escherichia coli‐induced acute respiratory distress syndrome. Crit Care Med 2017;45:e202–e212. [DOI] [PubMed] [Google Scholar]
- 54. Asami T, Ishii M, Namkoong H et al. Anti‐inflammatory roles of mesenchymal stromal cells during acute Streptococcus pneumoniae pulmonary infection in mice. Cytotherapy 2018;20:302–313. [DOI] [PubMed] [Google Scholar]
- 55. Zhang S, Danchuk SD, Imhof KM et al. Comparison of the therapeutic effects of human and mouse adipose‐derived stem cells in a murine model of lipopolysaccharide‐induced acute lung injury. Stem Cell Res Ther 2013;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yong KW, Pingguan‐Murphy B, Xu F et al. Phenotypic and functional characterization of long‐term cryopreserved human adipose‐derived stem cells. Sci Rep 2015;5:9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gramlich OW, Burand AJ, Brown AJ et al. Cryopreserved mesenchymal stromal cells maintain potency in a retinal ischemia/reperfusion injury model: Toward an off‐the‐shelf therapy. Sci Rep 2016;6:26463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feng Z, Ting J, Alfonso Z et al. Fresh and cryopreserved, uncultured adipose tissue‐derived stem and regenerative cells ameliorate ischemia‐reperfusion‐induced acute kidney injury. Nephrol Dial Transplant 2010;25:3874–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eggenhofer E, Benseler V, Kroemer A et al. Mesenchymal stem cells are short‐lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 2012;3:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eggenhofer E, Luk F, Dahlke MH et al. The life and fate of mesenchymal stem cells. Front Immunol 2014;5:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu YG, Feng XM, Abbott J et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells 2014;32:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Laroye C, Gibot S, Reppel L et al. Concise review: Mesenchymal stromal/stem cells: A new treatment for sepsis and septic shock? Stem Cells 2017;35:2331–2339. [DOI] [PubMed] [Google Scholar]
- 63. Rojas M, Xu J, Woods CR et al. Bone marrow‐derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ortiz LA, Gambelli F, McBride C et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cui P, Xin H, Yao Y et al. Human amnion‐derived mesenchymal stem cells alleviate lung injury induced by white smoke inhalation in rats. Stem Cell Res Ther 2018;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen S, Cui G, Peng C et al. Transplantation of adipose‐derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti‐inflammatory and anti‐apoptosis effects in rats. Stem Cell Res Ther 2018;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Royce SG, Rele S, Broughton BRS et al. Intranasal administration of mesenchymoangioblast‐derived mesenchymal stem cells abrogates airway fibrosis and airway hyperresponsiveness associated with chronic allergic airways disease. FASEB J 2017;31:4168–4178. [DOI] [PubMed] [Google Scholar]
- 68. Toonkel RL, Hare JM, Matthay MA et al. Mesenchymal stem cells and idiopathic pulmonary fibrosis. Potential for clinical testing. Am J Respir Crit Care Med 2013;188:133–140. [DOI] [PubMed] [Google Scholar]
- 69. Tzouvelekis A, Antoniadis A, Bouros D. Stem cell therapy in pulmonary fibrosis. Curr Opin Pulm Med 2011;17:368–373. [DOI] [PubMed] [Google Scholar]
- 70. Lalu MM, Sullivan KJ, Mei SH et al. Evaluating mesenchymal stem cell therapy for sepsis with preclinical meta‐analyses prior to initiating a first‐in‐human trial. Elife 2016;5:e17850 10.7554/eLife.17850 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Intravenous ASC infusion results in transient thrombi formation in the lungs
Mice were treated with 1 x 106 cryopreserved ASCs intravenously either 1 or 6 hours after infection with K. pneumoniae via the airways and lungs were harvested 16 or 48 hours after infection respectively. (A) Representative photographs of H&E‐stained tissue sections of infected lungs at 16 hoursours, treated with ASCs or vehicle, showing thrombi formation in ASCs treated mice (arrows); (B) H&E‐stained tissue sections of infected lungs at 48 hoursours, treated with ASCs or vehicle; (C) Quantification of thrombi formation (0 = no thrombi, 1 = 1 thrombus, 2 = 2–5 thrombi, 3 = 6–10 thrombi, 4 = > 10 thrombi per microscopic section). Original magnification x4 for panels A & B, unless stated otherwise. Arrows indicate thrombi. Data are expressed as bars for panel C. N = 8 mice per group. Results for cultured ASCs were similar (not shown).
Figure S2 Infusion of 0.4 x 10 6 cryopreserved ASCs has no effect on bacterial growth and dissemination.
Bacterial loads (colony‐forming units [CFU's]) in the lung, blood, liver and spleen 48 hours after infection with K. pneumoniae via the airways in mice treated with 0.4 x 106 cryopreserved ASCs or vehicle 6 hours after infection. Data were expressed box‐and‐whisker diagrams of 8 mice per group at each time point. Differences between groups were not significant.
Figure S3 Infusion of 0.4 x 10 6 cryopreserved ASCs has no effect on lung cytokine or MPO levels
TNF‐α (A), interleukin (IL)‐1β (B), IL‐6 (C), macrophage inflammatory protein (MIP)‐2 (D) and myeloperoxidase (MPO; E) levels in lungs 16 hours after infection with K. pneumoniae via the airways after intravenous administration of 0.4 x 106 cryopreserved ASCs or vehicle 6 hours after infection. Data were expressed box‐and‐whisker diagrams of 8 mice per group at each time point. Differences between groups were not significant.
Figure S4 Cryopreserved ASCs treatment was associated with a profound transcriptomic response in the lungs of infected mice
(A) Volcano plots (integrating adjusted p‐values and fold expression [log2 fold change]) depicting the global alteration in gene expression after treatment with cryopreserved ASCs compared with control in infected mice. Horizontal line indicates Benjamini‐Hochberg (BH) adjusted p < .05. Red dots denote over‐expressed genes; turquoise dots indicate under‐expressed genes. (B) Ingenuity pathway analysis of elevated transcripts (red bars) and reduced transcripts (turquoise bars) with Benjamini‐Hochberg (BH) adjusted Fisher's p < .01 demarcating significance.
Table S1 Plasma cytokine levels, E‐selectin, VCAM and ALT levels after cultured ASC treatment
Table S2: Ingenuity pathway analysis of the common over‐expressed and under‐expressed genes in naïve mice treated with cultured ASC treatment compared with placebo treatment
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


