Abstract
This chapter describes the use of fluorescence via indocyanine green (ICG) in minimally invasive adrenal surgery (laparoscopic and robotic). ICG is a non-toxic dye that can aid identification of vascular structures and parenchymal tissue planes in real time. The primary utility of ICG fluorescence in adrenal surgery is to help delineate the margins of resection, to guide a more precise operation. In particular, for patients with bilateral adrenal disease or a heredity associated with high risk of recurrence (e.g., VHL, MEN2a) this may facilitate subtotal adrenal resection (e.g., cortical sparing adrenalectomy), obviating the incidence of iatrogenic adrenal insufficiency and its numerous sequelae including lifelong hormone supplementation, osteoporosis and risk of Addisonian crisis.
Keywords: Adrenal surgery, fluorescence angiography, indocyanine green (ICG), near infrared imaging (NIR imaging), laparoscopy
History
Fluorescence is a natural phenomenon known to humans for thousands of years. It was initially used in ophthalmology for retinal angiography (1) but as its utility was further understood, clinical application expanded to include various surgical procedures such as cholecystectomy (2,3), gastrointestinal anastomoses, renal auto-transplantation (4) and nodal dissections (5-10). More recently indocyanine green (ICG) has become available for minimally invasive surgery, with the development of more advanced laparoscopic and robotic platforms that can display ICG-enhanced images on the same screen (11). The utility of ICG is to provide additional visual information regarding tissue perfusion, critical vascular structures and planes of dissection (6,7,11-15).
ICG was first developed by Kodak Laboratories (Rochester, NY, USA) in 1955, for photography using near infrared (NIR) imaging technology. It was approved for medical use by the US FDA in 1959 (6,16) and the technology gradually crept into laparoscopic surgery offering real-time fluorescence angiography and assessment of vascular status of solid and hollow viscera (7,17,18).
The feasibility of ICG fluorescence for adrenal surgery was first described by Dip et al. in 2015 following a case series in pigs (19). They reported that adrenal fluorescence was distinct from the surrounding retroperitoneal tissue in all five animals and persisted for a mean of 4 hours.
The first clinical application of ICG in humans was made by Manny et al. in 2013 (20). In their case series of three patients undergoing robotic partial adrenalectomies, all of the adrenal tumours (phaeochromocytoma, lipoadenoma and follicular lymphoid hyperplasia) were hyperfluorescent. Subsequently, DeLong et al. reported the use of ICG in a series of five patients undergoing laparoscopic adrenal surgery and showed superior identification of adrenal vasculature and demarcation of the tumour from the retroperitoneum in all cases (11).
What is it?
ICG is an amphiphilic (water soluble), tricarbocyanine organic dye (MW 751.4 Da) that exhibits fluorescence when excited by NIR light (wavelength ~800 nm).
Its use in biomedical applications is attributed to the variable concentration of ICG within different tissue types, which is related to their blood supply. Endocrine organs have an abundant blood supply and therefore are ideally suited to the use of ICG (5). In particular the adrenal glands have multiple feeding arteries and collecting veins. The mean organ blood flow of the adrenal gland is 1.87 mL/g/min, which is the third highest among the intra-abdominal organs after the spleen and renal cortex (21).
How does it work?
The mechanism of action of ICG fluorescence is incompletely understood. Upon intravenous administration, ICG becomes strongly bound to plasma proteins (especially lipoproteins) and is confined to the intravascular space (22). Its configuration is not altered by binding, resulting in a relative lack of toxicity. ICG enters the hepatic sinusoids, is uptaken by hepatocytes and ultimately excreted into bile via protein glutathione S-transferase (GST) (6).
When excited by NIR light, tissues are illuminated with light 750–800 nm corresponding to the excitation wavelength of ICG (22,23). Signal intensity is proportional to the relative blood flow to that organ (20). Furthermore, receptor mediated uptake of ICG by different tissues, depends on the differential expression of bilitranslocase, a carrier protein for ICG expressed in normal renal parenchyma (proximal and convoluted tubercles but not glomeruli) (24).
Observation of ICG fluorescence in real time provides different information based on the flow of the dye throughout the tissues. Similar to a phased CT contrast study, the arterial anatomy is first to be delineated. This is followed by the parenchyma and lastly the adrenal vein. Enhancement of the vascular anatomy is especially important when it is altered, as is the case with adrenal tumours (Figure 1).
Figure 1.
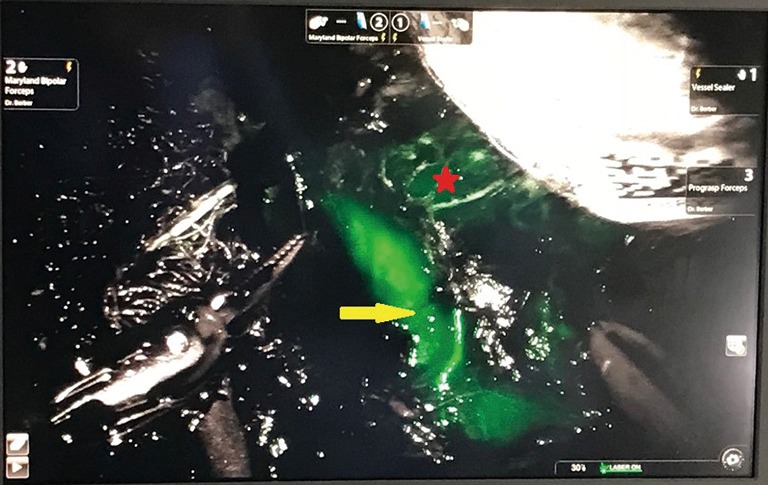
Photograph from robotic platform demonstrating a plane of dissection between the left renal vein (yellow arrow) and left adrenal tumour (red star) following ICG administration (original image).
Uses
Since Gagner et al. reported the first laparoscopic adrenalectomy in 1992, minimally invasive surgery has become the gold standard approach to remove both benign functional and non-functional tumours (25). Compared with conventional open surgery, minimal access surgery is associated with improved optics, decreased pain and wound complications and improved recovery time and cosmesis (26). On the other hand, it has eliminated tactile feedback traditionally used to determine the margin of resection.
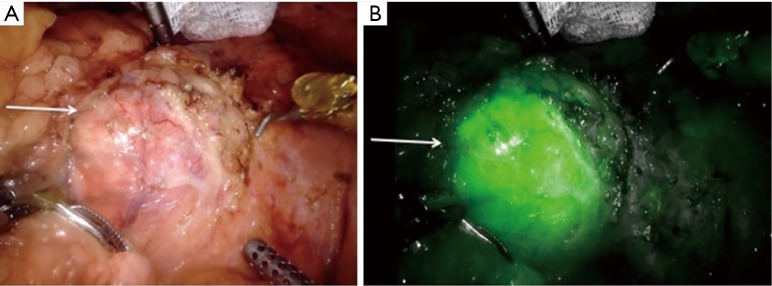
The use of ICG in adrenal surgery is advantageous for two primary reasons. First, it provides contrast distinction between very vascular, hyperfluorescent adrenocortical tissue and less vascular, hypofluorescent retroperitoneal tissue, which helps with dissection (Figure 2). Secondly, it can guide cortical sparing adrenalectomy by demonstrating the borders between normal adrenal tissue and tumour when operating for phaeochromocytomas (medullary lesions). ICG also provides information in real time to the surgical team. It is quick to perform only adding minutes to the procedure. The real time feedback of ICG fluorescence helps to compensate for the lack of tactile feedback in minimally invasive surgery (27).
Figure 2.
Photograph demonstrating the utility of ICG in delineating adrenal cortical tumours (yellow arrow) from the surrounding retroperitoneum, before ICG administration (A) and after ICG administration (B) (original image). ICG, indocyanine green.
By contrast laparoscopic ultrasound which is a widely adopted intra-operative adjunct, requires interruption of dissection for scanning and might not be possible if there is not a good contact plane between the piezoelectric probe surface and the tissue.
In 2018 Kahramangil et al. characterized patterns of fluorescence exhibited by different adrenal pathological conditions in order to define the best clinical indications for the use of ICG (28). They reported that the adrenal, liver and retroperitoneal tissues all fluoresced after administration of ICG. Fluorescence of retroperitoneal tissue was transient. The liver and adrenal fluorescence persisted throughout the duration of the procedure and in particular, healthy adrenal cortical tissue was always hyperfluorescent (Figure 3). From 100 patients, 74% were hyperfluorescent and 26% were not. Exhibition of fluorescence was dependent on the histological origin (medullary vs. cortical). On multivariate analysis, adrenal cortical tissue origin was the only predictor of hyperfluorescence following ICG administration: 95%, 33% and 50% for tumours of adrenocortical, medullary and other tissue origins respectively. Of note, adrenal cortical cancer (ACC) manifested higher intensity of fluorescence than differentiated tumours. The weaker fluorescence from phaeochromocytomas compared with other adrenal tumours has been related to the lack of expression of bilitranslocase (20).
Figure 3.
Photograph demonstrating hyper-fluorescence of adrenal cortical tumours (white arrow) before (A) and after ICG administration (B) (original image). ICG, indocyanine green.
It should be anticipated that the utility of ICG in the resection of right sided tumours and or tumours of medullary origin will be inferior (27). The exception to this is cortical sparing resection of phaeochromocytomas where there is sufficient tissue distinction between the hyperfluorescent healthy adrenal cortex and the hypofluorescent medullary tumour.
Dosing/administration
Owing to its pharmacokinetics, the specifics of intraoperative ICG administration change with the organ system it is being used in. For adrenal surgery, our group prepares a solution by mixing 25 mg of ICG (Akron Inc., Lake Forrest, IL, USA) in 10 mL of distilled water, making a preparation with final concentration of 2.5 mg/mL (Figure 4). Doses less than 5 mg do not provide sufficient contrast discrimination between tissue types, whereas doses greater than 5 mg are associated with too much fluorescence and all the tissues are overexposed (29).
Figure 4.

Photograph of ICG vial and sterile (distilled) water required for constitution prior to intravenous administration (original image). ICG, indocyanine green.
A single dose of this concentration (5 mg or 2 mL) is administered by the anesthetist, via a peripheral intravenous line. The timing is critical and nominated by the primary surgeon. ICG is most effective if it is administered after exposure of the retroperitoneum but before dissection of the adrenal gland. Typically, fluorescence of the adrenal gland and retroperitoneal tissues occurs with 30–60 s of ICG administration. Optimal contrast distinction between tissues is achieved at 5 minutes, when the retroperitoneal fat releases the ICG but the adrenocortical tissue still retains the molecule. Adrenal fluorescence can persist for up to 20 min (29,30).
Repeat doses of ICG can be administered to maintain contrast distinction if required.
The median lethal dose for ICG is relatively high (LD50 of 50–80 mg/kg) (6). In our experience most patients require an average of three doses (15 mg), irrespective of BMI and tumour size over the course of their adrenal resection (5,29).
ICG fluorescence systems are available for laparoscopic and conventional open surgery. There are multiple commercially available products. The ideal product fuses the laparoscopic or robotic images with the fluoresced images, avoiding the need to switch back and forth between platforms.
Limitations
Following administration, ICG circulates to the liver and is rapidly uptaken by hepatocytes. Hepatic fluorescence is bright and remains present for several hours, often the duration of the procedure. This can interfere with tissue plane discrimination for tumours on the right-hand side, as the hyperfluorescent liver obscures visualization of adjacent tissue (Figures 5,6). This is most problematic if the surgical approach is posterior because there is limited room for retraction of the liver in the smaller retroperitoneal space. A practical strategy to minimize this effect, is to zoom in on the adrenal gland to reduce the fraction of liver visualized in the NIR image. However, in general for right sided adrenal tumours, the lateral/anterior approach is preferable when using ICG.
Figure 5.
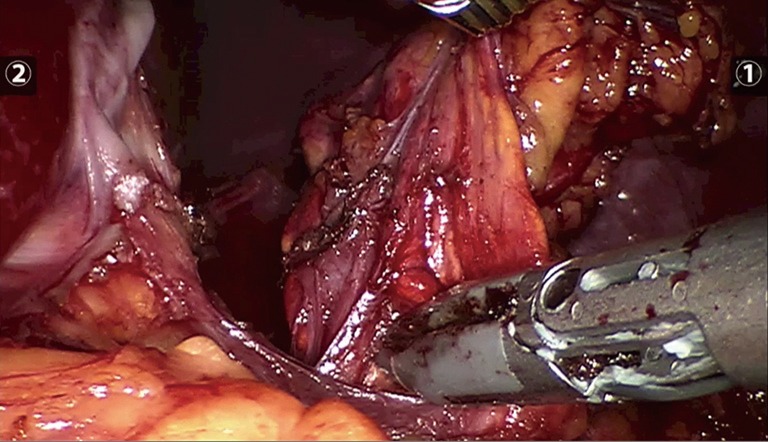
Photograph prior to ICG administration of left adrenal gland (original image). ICG, indocyanine green.
Figure 6.
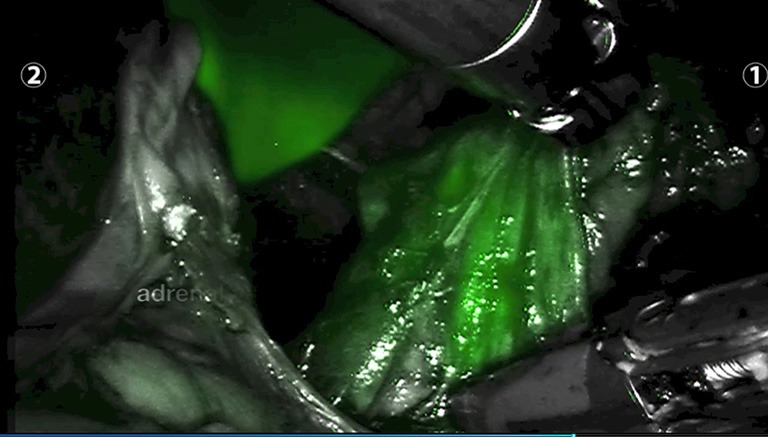
Photograph after ICG administration of left adrenal gland and hyper-fluorescent liver parenchyma in the background) (original image). ICG, indocyanine green.
Current ICG fluorescence systems provide qualitative data only. These need to be interpreted by the operating team and there may not be consensus. Ideally ICG and NIR imaging are used via a platform that integrates with the remote access set up as this enables the visual information to be visualized in real time.
Adverse reactions and contraindications
Contraindications against the use of ICG in adrenal surgery include iodine allergy, previous anaphylaxis to dye injections, renal disease, liver disease and pregnancy (31). There have been no reported mortalities attributed to ICG.
Adverse reactions to ICG have primarily been reported to be allergic and vasovagal in nature. The rate of severe adverse reactions is 0.05% (7). In extremely rare instances (3 out of 240,000 cases in the largest reported case series), ICG has been associated with bronchospasm and cardiac arrest (32) but these were associated with administration of much higher doses (0.5 mg/kg) than is typically required (6).
Conclusions
ICG and NIR imaging are safe and useful adjuncts to remote access adrenal surgery. The intensity of fluorescence is related to differential perfusion of tissue types and expression of bilitranslocase. The pattern of fluorescence is dependent on histological origin of the tumour.
Utilization of fluorescence in adrenal surgery is particularly useful for patients requiring a subtotal resection to minimize the sequelae of adrenal insufficiency and in refractory cases where it is critical to resect all adrenal tissue. Future systems will hopefully enable quantitative interpretation.
Acknowledgments
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Yannuzzi LA, Slakter JS, Sorenson JA, et al. Digital indocyanine green videoangiography and choroidal neovascularization. Retina 1992;12:191-223. 10.1097/00006982-199212030-00003 [DOI] [PubMed] [Google Scholar]
- 2.Ishizawa T, Bandai Y, Kokudo N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch Surg 2009;144:381-2. 10.1001/archsurg.2009.9 [DOI] [PubMed] [Google Scholar]
- 3.Ishizawa T, Bandai Y, Ijichi M, et al. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 2010;97:1369-77. 10.1002/bjs.7125 [DOI] [PubMed] [Google Scholar]
- 4.Tobis S, Knopf JK, Silvers CR, et al. Near infra-red fluorescence imaging after indocyanine green: initial clinical experience with open partial nephrectomy for renal cortical tumours. Urology 2012;79:958-64. 10.1016/j.urology.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 5.Kahramangil B, Berber E. The use of near infra-red fluorescence imaging in endocrine surgical procedures. J Surg Oncol 2017;115:848-55. 10.1002/jso.24583 [DOI] [PubMed] [Google Scholar]
- 6.Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. 10.1155/2012/940585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boni L, David G, Mangano A, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 2015;29:2046-55 10.1007/s00464-014-3895-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krane LS, Manny TB, Hemal AK. Is near infrared fluorescence imaging using indocyanine green dye useful in robotic partial nephrectomy: a prospective comparative study of 94 patients. Urology 2012;80:110-6. 10.1016/j.urology.2012.01.076 [DOI] [PubMed] [Google Scholar]
- 9.Diana M, Noll E, Diemunsch P, et al. Enhanced reality video fluorescence: a real time assessment of intestinal viability. Ann Surg 2014;259:700-7. 10.1097/SLA.0b013e31828d4ab3 [DOI] [PubMed] [Google Scholar]
- 10.Harke N, Schoenberg G, Schiefelbein F, et al. Selective clamping under the use of near-infrared fluorescence imaging with indocyanine green in robot assisted partial nephrectomy: a single surgeon matched pair study. World J Urol 2014;32:1259-65. 10.1007/s00345-013-1202-4 [DOI] [PubMed] [Google Scholar]
- 11.DeLong JC, Chakedis JM, Hosseeini A, et al. Indocyanine Green (ICG) fluorescence guided laparoscopic adrenalectomy. J Surg Oncol 2015;112:650-3. 10.1002/jso.24057 [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H, Zaidi N, Berber E. An initial report on the use of indocyanine green fluorescence imaging in the surgical management of liver tumours. J Surg Oncol 2016;114:625-9. 10.1002/jso.24363 [DOI] [PubMed] [Google Scholar]
- 13.Osayi SN, Wendling MR, Drosdeck JM, et al. Near infra-red fluorescence cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 2015;29:368-75. 10.1007/s00464-014-3677-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafari MD, Lee KH, Halabi WJ, et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted rectal surgery. Surg Endosc 2013;27:3003-8. 10.1007/s00464-013-2832-8 [DOI] [PubMed] [Google Scholar]
- 15.Kudszus S, Rosebel C, Schachtrupp A, et al. Intraoperative laser fluorescence in angiography in colorectal surgery: a non-invasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 2010;395:1025-30. 10.1007/s00423-010-0699-x [DOI] [PubMed] [Google Scholar]
- 16.Fox IJ, Wood EH. Indocyanine green: physical and physiologic properties. Proc Staff Meet Mayo Clin 1960;35:732-44. [PubMed] [Google Scholar]
- 17.Smith CD, Weber CJ, Amerson JR. Laparoscopic adrenalectomy: new gold standard. World J Surg 1999;23:389-96. 10.1007/PL00012314 [DOI] [PubMed] [Google Scholar]
- 18.Schaafsma BE, Verbeek FP, Elzevier HW, et al. Optimization of sentinel lymph node mapping in bladder cancer using near-infrared fluorescence imaging. J Surg Oncol 2014;110:845-50. 10.1002/jso.23740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dip FD, Ashbun D, Rosales-Velderrain A, et al. Cost analysis and effectiveness comparing the routine use of Intraoperative fluorescence cholangiography for open and laparoscopic surgery. Surg Endos 2014;28:1076-82. 10.1007/s00464-013-3394-5 [DOI] [PubMed] [Google Scholar]
- 20.Manny TB, Pompeo AS, Hemal AK. Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: the initial clinical experience. Urology 2013;82:738-42. 10.1016/j.urology.2013.03.074 [DOI] [PubMed] [Google Scholar]
- 21.Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intra-abdominal pressure. J Surg Res 1987;43:14-20. 10.1016/0022-4804(87)90041-2 [DOI] [PubMed] [Google Scholar]
- 22.Aoki T, Murakami M, Yasuda D, et al. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Sci 2010;17:590-4. 10.1007/s00534-009-0197-0 [DOI] [PubMed] [Google Scholar]
- 23.Kraft JC, Ho RJ. Interactions of indocyanine green and lipid in enhancing near-infrared fluorescence properties: the basis for near-infrared imaging in vivo. Biochemistry 2014;53:1275-83. 10.1021/bi500021j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golijanin DJ, Marshall J, Cardin A, et al. Bilitranslocase (BTL) is immunolocalised in proximal and distal renal tubules and absent in renal cortical tumours accurately corresponding to intra-operative near infrared fluorescence (NIFR) expression of renal cortical tumours using intravenous indocyanine green (ICG). J Urol 2008;179:abstr 386.
- 25.Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. 10.1056/NEJM199210013271417 [DOI] [PubMed] [Google Scholar]
- 26.Bittner JG, 4th, Gershuni VM, Matthews BD, et al. Risk factors affecting operative approach, conversion, and morbidity for adrenalectomy: a single-institution series of 402 patients. Surg Endosc 2013;27:2342-50. 10.1007/s00464-013-2789-7 [DOI] [PubMed] [Google Scholar]
- 27.Sound S, Okoh AK, Bucak E, et al. Intraoperative tumor localization and tissue distinction during robotic adrenalectomy using indocyanine green fluorescence imaging: a feasibility study. Surg Endosc 2016;30:657-62. 10.1007/s00464-015-4256-0 [DOI] [PubMed] [Google Scholar]
- 28.Kahramangil B, Kose E, Berber E. Characterization of fluorescence patterns exhibited by different adrenal tumours: determining the indications for indocyanine green use in adrenalectomy. Surgery 2018;164:972-7. 10.1016/j.surg.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 29.Colvin J, Zaidi N, Berber E. The Utility of Indocyanine Green Fluorescence Imaging During Robotic Adrenalectomy. J Surg Oncol 2016;114:153-6. 10.1002/jso.24296 [DOI] [PubMed] [Google Scholar]
- 30.Arora E, Bhandarwar A, Wagh A, et al. Role of indo-cyanine green (ICG) fluorescence in laparoscopic adrenalectomy: a retrospective review of 55 Cases. Surg Endosc 2018;32:4649-57. 10.1007/s00464-018-6309-7 [DOI] [PubMed] [Google Scholar]
- 31.Hope-Ross M, Yannuzzi LA., Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529-33. 10.1016/S0161-6420(94)31303-0 [DOI] [PubMed] [Google Scholar]
- 32.Speich R, Saesseli B, Hoffmann U, et al. Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med 1988;109:345-6. 10.7326/0003-4819-109-4-345_2 [DOI] [PubMed] [Google Scholar]