Abstract
Steroid hormones regulate a variety of physiological processes, including reproductive function, and are widely used in hormonal therapy. Synthetic progestogens, or progestins, were designed to mimic progesterone (P4) for use in contraception and hormonal replacement therapy in women. Medroxyprogesterone acetate (MPA) and norethisterone (NET) are the most widely used injectable contraceptives in the developing world, while other progestins such as levonorgestrel (LNG), etonogestrel (ETG) and nestorone (NES) are used in or being developed for other forms of contraception. As concerns remain about the most appropriate choice of progestin and dosage, and the associated side-effects, the mechanisms and biological effects of progestins are frequently investigated in various in vitro mammalian cell line and tissue models. However, whether progestogens are differentially metabolised in different cell types in vivo or in vitro is unknown. For nine mammalian cell lines commonly used to investigate progestogen mechanisms of action, we developed and validated an ultra-high performance supercritical fluid chromatography-tandem mass spectrometry (UHPSFC-MS/MS) protocol for simultaneously quantifying the metabolism of the above-mentioned steroids. We show for the first time that, while 50–100% of P4 was metabolised within 24 hours in all cell lines, the metabolism of the progestins is progestin-and cell line-specific. We also show that MPA and NET are significantly metabolised in human cervical tissue, but to a lesser extent than P4. Taken together, our findings suggest that differential progestogen metabolism may play a role in cell-specific therapeutic and side-effects. Relative affinities for binding to steroid receptors as well as potencies, efficacies and biocharacters for transcriptional activity of progestins, relative to P4, are most frequently determined using some of the cell lines investigated. Our results, however, suggest that differential metabolism of progestins and P4 may confound these results. In particular, metabolism may under-estimate the receptor-mediated intrinsic in vitro binding and dose-response values and predicted endogenous physiological effects of P4.
Keywords: contraceptives, metabolism, progesterone, progestins, steroids, UHPSFC-MS/MS
1. Introduction
Choice of hormonal contraception and hormone replacement therapy (HRT) in women is an important public health issue, especially regarding possible side-effects relevant to cancer, metabolic disorders, cardiovascular complications, bone mineral density and susceptibility to infectious diseases [1,2,3]. Synthetic steroids are commonly utilised in contraceptive treatments and HRT. These synthetic steroids, known as progestins or synthetic progestogens, are intended to mimic the actions of the endogenous hormone P4 [1–3] and are classified into two groups. The first class of progestins, which include medroxyprogesterone acetate (MPA) and nestorone (NES), is structurally related to P4, while the second class is structurally related to testosterone (T), and includes norethisterone (NET), etonogestrel (ETG) and levonorgestrel (LNG) [2,3]. Injectable progestins, which are especially popular in the developing world as contraceptives due to their discreet nature, include MPA and NET, the latter administered in its enanthate form (NET-EN) [2,3]. Other progestins such as LNG and ETG are widely used in combined oral contraceptives and implants and together with NES, are currently being investigated for use intravaginally or in multipurpose prevention technologies [3].
Progestin research relies extensively on model systems using well-established laboratory cell lines [2–6] or in vitro experiments with primary cells, tissue or tissue extracts [7–12]. In such experiments, specific concentrations of the steroids are used and these concentrations are assumed to remain constant over the incubation period. Differences in activity between steroids is thought to be due to their different biocharacters, and metabolism is not taken into account. Differential metabolism may confound the results of concentration-dependent experiments such as dose-response analyses and binding studies [2–4]. It is well established that progestins act intracellularly via binding to and activating the progesterone receptor (PR) [2,3], which is a ligand-activated transcription factor. Evidence is emerging that some of the side-effects of progestins may occur by off-target effects via binding to and activating steroid receptors other than the PR [3,5]. However, very little is known about the metabolism of progestins, in particular whether this is cell-specific, which metabolites are produced, what the role is of metabolites and whether metabolism may confound interpretation of the results when investigating relative biological activities.
The aim of this work was therefore to investigate the metabolism of P4 and selected progestins in nine commonly used laboratory cell lines, and to validate select findings in endocervical tissue. To this end, we developed and validated an ultra-high-performance supercritical fluid chromatography-tandem mass spectrometry (UHPSFC-MS/MS) method for the separation and quantification of these progestogens in the nanomolar range, as detected in the serum of women. We included the synthetic glucocorticoid dexamethasone (DEX) in our panel of steroids, since the activity of progestins is often investigated in parallel with DEX, given the established glucocorticoid activity of MPA [10, 13–14]. Results showed that P4 was substantially metabolised in all cells lines and the endocervical tissue after 24 hours, while cell line-and steroid-specific metabolism were observed for the different progestins.
2. Materials and Methods
2.1. Steroids and solvents
LNG was obtained from the United Stated Pharmacopoeia (USP, Rockville, MD, USA) and Sigma Aldrich (South Africa). P4, MPA, NES, DEX, NET, ETG, T, UHPLC-grade methanol, absolute ethanol, formic acid and methyl tert-butyl ether (MTBE) were all purchased from Sigma-Aldrich (South Africa).
2.2. Cell lines and endocervical tissue
Human embryonic kidney cells (HEK293T), human epithelial cervical cancer cells (HeLa), human endocervical cells (END-1), human bone osteosarcoma epithelial cells (U2OS) and monkey kidney fibroblast cells (COS-1) were purchased from American Type Culture Collection (ATCC, USA). Human cervical cells (TZM-bl) were procured from the NIH AIDS Reagent Program, Division of AIDSNIAID, NIH, from Dr John C. Kappes, Dr Xiaoyun Wu and Tranzyme Inc. (ARP, NIH, USA). The human MDA-MB-231 breast cancer cell line was originally acquired from ATCC, but were received from Prof Adrienne Edkins at Rhodes University, South Africa, while Prof Ana Soto at Tufts University, Boston, USA provided the human MCF-7 BUS breast cancer cells. The human T47D breast cancer cell line was donated by Prof Iqbal Parker at the University of Cape Town, South Africa.
Endocervical tissue was obtained after informed consent from HIV-1 negative, post-menopausal women undergoing hysterectomies for benign reasons. Ethical permission was obtained from the Human Research Ethics Committee (University of Cape Town) for the duration of this study (HREC 258/2017). Fresh tissue was supplied from two sites in the Western Cape, South Africa; namely, Groote Schuur and Tygerberg Hospitals.
2.3. Cell line culture
HEK293T, HeLa, U2OS, TZM-bl, T47D and COS-1 were all cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, South Africa) supplemented with 1 mM sodium pyruvate (Sigma-Aldrich, South Africa), 44 mM sodium bicarbonate (Sigma-Aldrich, South Africa), 10% (v/v) fetal bovine serum (FBS) (Thermo Scientific, South Africa), 100 IU/mL penicillin and 100 µg/mL streptomycin (Sigma-Aldrich, South Africa). Culture medium for MDA-MB-231 cells was as described above, with the addition of 2 mM L-glutamine (Sigma-Aldrich, South Africa). Culture medium for MCF-7 BUS cells was as described above, except that 5% heat inactivated FBS was used. END-1 cells were maintained in keratinocyte serum-free (KSF) medium (Sigma-Aldrich, South Africa) supplemented with the provided keratinocyte growth supplement, 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco Invitrogen). Cells were maintained at 37°C in a water-jacketed incubator (90% humidity and 5% CO2). All cells were routinely tested and found to be mycoplasma-free.
2.4. Cervical tissue experiments
Cervical tissue was processed as previously described by Fletcher et al. (i.e. between one to three hours post-operation) [15]. Excess underlying stromal tissue was removed from the epithelial layer of the endocervical tissue. The epithelial layer was then diced into 3 mm3 explant pieces that were randomly placed into separate wells of 96-well round-bottomed plates. Non-polarised explants were cultured in 200 μL Roswell Park Memorial Institute medium (RPMI) (Lonza, Switzerland) supplemented with 10% (v/v) charcoal stripped FBS (Thermo Scientific, USA), 2 mM L-glutamine (Sigma-Aldrich, South Africa), 10 μg/mL Fungizone (Sigma-Aldrich, South Africa), 10 U/mL interleukin-2, 100 IU/mL penicillin and 100 mg/mL streptomycin (Sigma-Aldrich, South Africa). Cervical tissue explants were incubated in quadruplicate with steroids in RPMI and incubated at 37°C in a water-jacketed incubator (90% humidity and 5% CO2) for 24 hours.
2.5. Cell line and tissue incubations with steroids
Cells were seeded at 5 × 104 cells per well (T47D, MCF-7 BUS, MDA-MB-231) or 1× 105 cells per well (END-1, U2OS, TZM-bl, HEK293T, COS-1 and HeLa) in full phenol red-containing media in a 24-well Greiner Bio-One CELLSTAR tissue culture plate. Tissue was processed and plated as described above. Following a 24-hour incubation period T47D, MCF-7 BUS and MDA-MB-231 cell media was replaced with phenol red-free media. For analysis of extent of metabolism, the cells and no-cell controls were washed with pre-warmed media then treated with 100 nM steroid or vehicle (0.1% v/v ethanol) in serum-free media. The U2OS, TZM-bl, HEK293T, COS-1, END-1 and HeLa cells were treated in phenol red-containing DMEM, while T47D, MCF-7 BUS and MDA-MB-231 cells were treated in phenol red-free DMEM. Upon treatment, 500 µL of the steroid-and vehicle-containing media was aliquoted into a glass tube and stored at −20°C; this served as the T0 control. After 24 hours, 500 µL aliquots of media were removed from the cells (or no-cell control) and transferred into clean glass tubes and stored at −20°C prior to extraction
2.6. Preparation of standards and samples
Individual stock solutions of the seven steroids (P4, MPA, NES, NET, LNG, ETG and DEX) plus internal standard T were prepared in absolute ethanol (1 mg/mL) and stored at −20°C until use. These individual stock solutions were later used to prepare two standard master mixes (1 000 ng/mL and 1 ng/mL) containing all of the above-mentioned steroids in ethanol. These standard master mixes were subsequently used to prepare standards (1 mL, 0.01–100 ng/mL) by the addition of the appropriate volume of the standard master mix to either (i) DMEM containing 1% penicillin-streptomycin and 10% FBS (“supplemented DMEM”), (ii) DMEM without penicillin-streptomycin or FBS (“unsupplemented DMEM”), (iii) KSF without penicillin-streptomycin or FBS, (iv) RPMI 1640 without penicillin-streptomycin or FBS or (v) 50% methanol (no matrix). Samples used for method validation (1 mL) were prepared by spiking the matrix with the appropriate volume of the master mixes. 100 µL of internal standard prepared in distilled water to a final concentration of 1 ng/mL was added to all samples and standards.
2.7. Steroid extractions
Samples and standards were extracted using a 1:3 ratio of sample to MTBE (v/v). The samples were shaken at 1 000 rpm for 15 minutes before being placed at −80°C for an hour to allow the aqueous phase to freeze. The MTBE layer containing steroids was transferred to a pyrolyzed glass test tube and the MTBE was evaporated at room temperature in a fume hood overnight, or under a stream of nitrogen gas. Samples were subsequently reconstituted in 150 µL 50% methanol and stored at −20°C prior to analysis.
2.8. Instruments and chromatographic conditions for UHPSFC-MS/MS
Steroids were separated using an Acquity Ultra High Performance Convergence Chromatography (UPC2) system (Waters Corporation, Milford, USA) with an Acquity UPC2 Ethylene Bridged Hybrid (BEH) column (3 mm x 100 mm, 1.7 µm particle size). The mobile phase consisted of liquid CO2 (Mobile phase A) and methanol [Mobile phase B (MPB)]. A 2.5-minute gradient inlet method was used to separate the steroids using a constant flow rate of 1.9 mL/min according to the following protocol: 4% MPB from 0–1 min; 10% MPB from 1–1.5 min; 25% MPB from 1.5–2.5 min and back to 4% MPB at 2.5 min for re-equilibration. The column temperature and automated back pressure regulator were set to 60°C and 1700 pounds-force per square inch (psi), respectively. The injection volume was 2.0 µL. Quantitative mass spectrometric detection was carried out using a Xevo TQ-S triple quadrupole mass spectrometer (Waters, Milford, USA). A make-up pump fed 1% formic acid in methanol into the mixer preceding the MS line at a constant flow rate of 0.2 mL/min. All steroids were analysed in multiple reaction monitoring (MRM) mode using an electrospray probe in the positive ionisation mode (ESI+). The following settings were used: capillary voltage of 3.8 kV, desolvation temperature 350°C, desolvation gas 900 L/h and cone gas 150 L/h. MRM transitions are included in Supporting Table 1. Data collection and analysis were performed using MassLynx 4.1 (Waters Corporation).
2.9. UHPSFC-MS/MS method validation
Standard curves were generated for each steroid metabolite using standards prepared in either of the four matrices listed above or 50% methanol (no matrix), and included the following concentrations: 0, 0.01, 0.1, 0.25, 0.5, 1.0, 5.0, 10, 25, 50 and 100 ng/mL. The limit of detection (LOD) for each steroid was defined as the lowest concentration at which a signal-to-noise (S/N) ratio greater than three was measured for the quantifier ion. The lower limit of quantification (LLOQ) for each steroid was defined as the lowest concentration for each steroid at which: a S/N ratio greater than ten was measured for the quantifier ion; a S/N ratio greater than three was measured for the qualifier ion; an acceptable precision [% relative standard deviation (% RSD) <20] could be measured. The upper limit of quantification (ULOQ) was defined as the maximum concentration at which the % RSD values did not exceed 20. Precision was defined as the % RSD from the average calculated concentrations following the repeated injection (n=6) of a simple sample. Accuracy was defined as the % RSD from the analysis of independent replicate samples (n=6).
2.10. Statistical analysis
Results were analysed using GraphPad Prism 7 from GraphPad Software, Inc. (La Jolla California, USA). Data are expressed as mean ± SEM. To evaluate whether the metabolism of a steroid within a cell line/tissue was statistically significant, a paired t-test was performed to compare results in the absence and presence of cells. Statistical significance is denoted by the relevant p-value. Multiple paired t-tests were used to compare the metabolism of the seven steroids within a cell line to each other, and to compare the metabolism of a specific steroid across different cell lines. (ANOVA was not used since these experiments were not all performed in parallel.) Where statistical significance was determined, it is denoted by *, **, ***, or **** to indicate p<0.05, p<0.01, p<0.001, or p<0.0001, respectively.
3. Results
3.1. Validation and performance of UHPSFC-MS/MS method
A UHPSFC-MS/MS method was developed for the quantification of six clinically relevant, commercially-available progestogens and DEX. Their chemical structures are depicted in Figure 1. Molecular ion species, MRM mass transitions and retention time for each steroid are given in Supporting Table 1. Comprehensive method validation was performed, the results of which are listed in Table 1.
Figure 1. The chemical structures of the seven commercially-available steroids described in this work, and T.
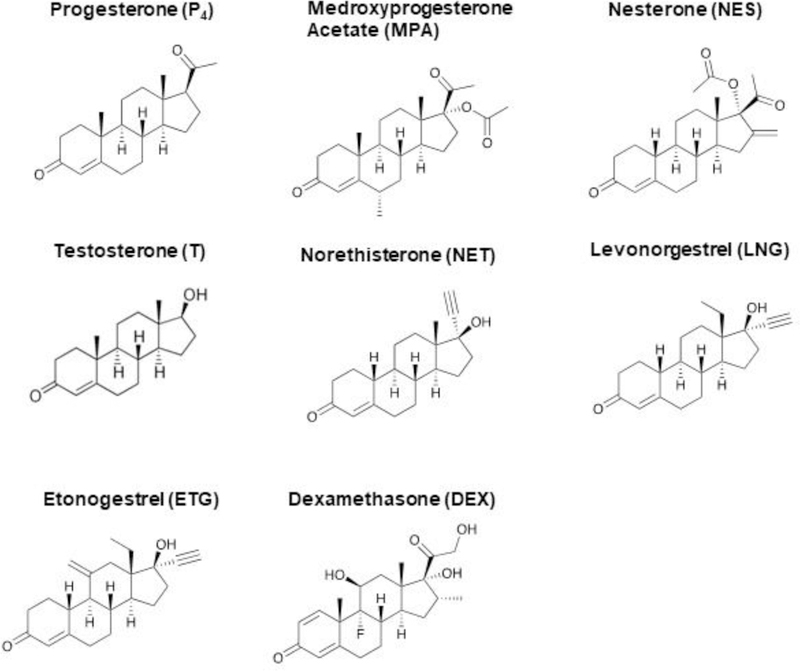
MPA and NES are structurally related to P4; while NET, LNG and ETG are structurally related to T. DEX is the synthetic glucocorticoid often investigated in parallel with progestins.
Table 1.
Comprehensive method validation data: r2, LOD (ng/mL and nM), LLOQ (ng/mL and nM), accuracy (% RSD, n=6) and precision (% RSD, n=6). LOD, LLOQ, accuracy and precision are shown for unsupplemented DMEM only. (−) indicates that the concentration is below the LLOQ for that steroid and is therefore not included.
| Steroid | r2 (Unsupplemented DMEM) | LOD, ng/mL (nM) | LLOQ, ng/mL (nM) | Accuracy % RSD, 1 ng/mL | Accuracy % RSD, 10 ng/mL | Accuracy % RSD, 100 ng/mL | Precision % RSD, 1 ng/mL | Precision % RSD, 10 ng/mL | Precision % RSD, 100 ng/mL |
|---|---|---|---|---|---|---|---|---|---|
| P4 | 0.9928 | 0.01 (0.03) | 0.01 (0.03) | 18.2 | 12.6 | 8.3 | 16.9 | 9.5 | 9.5 |
| MPA | 0.9939 | 0.5 (1.3) | 1 (2.6) | 10.9 | 11.1 | 9.7 | 14.0 | 6.7 | 9.3 |
| NES | 0.9955 | 0.5 (1.3) | 1 (2.7) | 16.5 | 18.9 | 16.9 | 19.0 | 16.1 | 18.8 |
| NET | 0.9951 | 0.5 (1.7) | 1 (3.3) | 14.3 | 11.5 | 9.8 | 14.1 | 4.4 | 9.7 |
| LNG | 0.9739 | 0.01 (0.03) | 5 (16.0) | - | 4.7 | 11.2 | - | 7.4 | 9.7 |
| ETG | 0.9960 | 1 (3.18) | 5 (15.4) | - | 13.1 | 8.0 | - | 10.9 | 8.7 |
| DEX | 0.9951 | 5 (12.7) | 5 (12.7) | - | 12.8 | 16.9 | - | 12.5 | 11.8 |
As most of the cell lines were treated with unsupplemented DMEM, accuracy and precision were determined only for this medium at a minimum of three concentrations within the calibration range of each steroid as shown in Table 1, i.e. 1, 10 and 100 ng/mL. Acceptable % RSDs were obtained for all concentrations for both accuracy and precision, which were less than 20 at concentrations of 1, 10 and 100 ng/mL. Accuracy at low concentrations ranged from 11–18% at 1 ng/mL, 11–18.9% at 10 ng/mL and 8–17% at 100 ng/mL. Precision at low concentrations ranged from 14–19% at 1 ng/mL, 4–16% at 10 ng/mL and 9–19% at 100 ng/mL. LLOQs ranged from 0.01 ng/mL for P4 to 5.00 ng/mL for LNG, ETG and DEX, allowing for the quantification of steroids at levels at the low nanomolar range. For each of unsupplemented DMEM, KSF and RMPI media, the ULOQ was 100 ng/mL (the highest concentration measured), while ULOQ in supplemented DMEM was 50 ng/mL.
3.2. Effects of adsorption and hydrophobicity
Following the development and validation of the UHPSFC-MS/MS method we first considered the potential effects of adsorption of the steroids to the cell culture plates, before measuring the metabolism of the steroids. To do this we assessed the differences in steroid concentration between the T0 and media from no-cell control plate incubations for all the steroids in the different experiments (Supporting Figure 1). There was 0%−40% adsorption of the steroids to the cell culture plates across experiments (Figure 2 and Supporting Figures 1). We further investigated whether there was a correlation between hydrophobicity and adsorption of steroids. Results showed a positive correlation suggesting that the adsorption of steroids to the cell culture plates increases with increasing hydrophobicity (Figure 2). We investigated whether retention of the steroids occurred within the cell pellets. We found that this was negligible (Supporting figure 2) and hence was not taken into account when calculating percentage metabolism. Based on these results metabolism (Section 3.3–3.5) was calculated relative to a no-cell control parallel incubation in cell culture plates and hence independent of adsorption to the cell culture plates.
Figure 2. Predicted steroid hydrophobicity correlates with adsorption.

Regression analysis was performed to determine whether there is a correlation between the adsorption and hydrophobicity of the seven clinically-relevant steroids. The percentage adsorption of each steroid to the tissue culture plates was determined from the difference between the time-zero (T0) measurement and its corresponding no-cell control. CLogP values were predicted using ChemDraw Professional Version 16.0.1.4 (PerkinElmer Informatics, Inc.). Non-linear regression using GraphPad Prism Version 7 (GraphPad Software, Inc.) revealed a correlation with r2 = 0.5394.
3.3. Differential metabolism of steroids between cell lines
Next, we incubated nine common laboratory cell lines, along with a parallel plate without any cells (“no-cell control”), with 100 nM of each steroid for 24 hours. We subsequently measured the concentration of steroid present in the cell and no-cell supernatants. The percentage metabolism of each individual steroid in a cell line was calculated as the difference between the steroid remaining in the absence (no-cell) and presence of cells. Where percentage metabolism was less than zero, the percentage is represented as zero. It should be noted that steroid incubations were performed in the absence of serum for 24 hours as is frequently done for experiments in cell lines incubated with steroids [16–18]. It is possible that the presence of serum may affect metabolism.
When comparing metabolism effects, it was noted that the error bars were in general much greater for some cells than for others, as well as between some progestins for a particular cell line. Assessment of no cell samples indicate that this reflects variations in technical error during experiments. Only differences that were found to be statistically significant are highlighted and discussed below. It should, however, be noted that there may be other differences that are significant, but beyond the statistical power of the experiments. The most striking result was that P4 was extensively metabolised by all the cell lines, although the extent of metabolism varied from 50–97%, showing some cell line-specific effects (Figures 3 and 4). Although the metabolism of the progestins and DEX was also cell line-specific, it ranged from 0–50%, with the rank order for most to least metabolised steroid different for each cell line. These did not appear to be related to the anatomical source or type of cell line, as shown in Figure 4. However, some trends were apparent (Figures 3 and 4). Of the cervical cell lines, HeLa exhibited a higher percentage of significant metabolism for most progestogens than observed in TZM-bl and END-1 cells, except for NET and ETG where END-1 cells exhibited more metabolism. The T47D cell line exhibited the highest significant metabolism within the breast cancer cell lines, except for NES where MCF-7 BUS showed greater significant metabolism. END-1, T47D, MCF-7 BUS and COS-1 cells were the most metabolically diverse cells in the panel, with all four of these cell lines displaying significant metabolism of P4 and ETG; and three of these four cell lines showing metabolism of DEX (Figure 3). Interestingly, COS-1 cells displayed a similar high degree of significant metabolism for both P4 (60%) and DEX (53%). These cell lines were followed by U2OS cells, which significantly metabolised three steroids. TZM-bl and MDA-MB-231 cells had significant metabolism of P4 and one other steroid, namely, MPA and ETG respectively. HeLa and HEK293T cells displayed the lowest metabolic activity of progestogens with significant metabolism observed only for P4 (Figure 3). Taken together, there was no significant difference in the overall metabolism of the progestins structurally related to P4 (MPA and NES) than those structurally related to T (NET, LNG and ETG) (Figure 4), with an average of four times more metabolism observed for P4 than the other steroids.
Figure 3. Differential metabolism of seven clinically-relevant steroids following incubation at 100 nM for 24 hours in nine different cell lines.
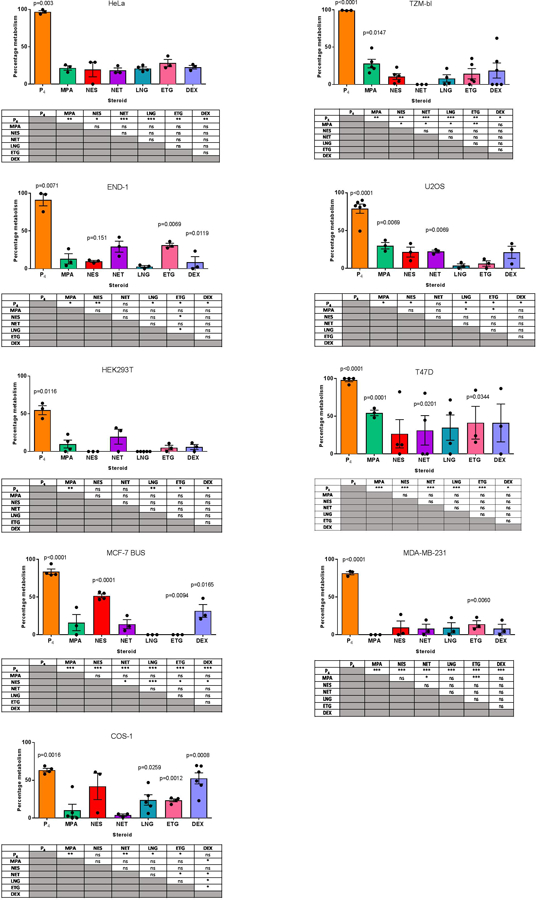
Medium containing the steroids (no cells) was added to a 12-well plate as a negative control for metabolism. Steroids were extracted and analysed by UHPSFC-MS/MS. The amount of steroid present in the medium after incubation with the cells was expressed as a % relative to the amount of progestin in the negative control for metabolism, which was set as 100%. These data show the mean ± SEM of a minimum of three independent biological repeats. Statistical analysis via paired t-tests was performed on each steroid and statistically significant differences relative to its no cell control are indicated by the p-value above bar. To quantify whether the relative metabolism of two steroids within a cell line was significantly different, multiple t-tests were performed between steroids. Significant differences in tables below the histograms are indicated by asterisks where *, **, ***, **** represent p<0.05, p<0.01, p<0.001 and p<0.0001, respectively.
Figure 4. Heat map summarising differential metabolism of seven clinically-relevant steroids.
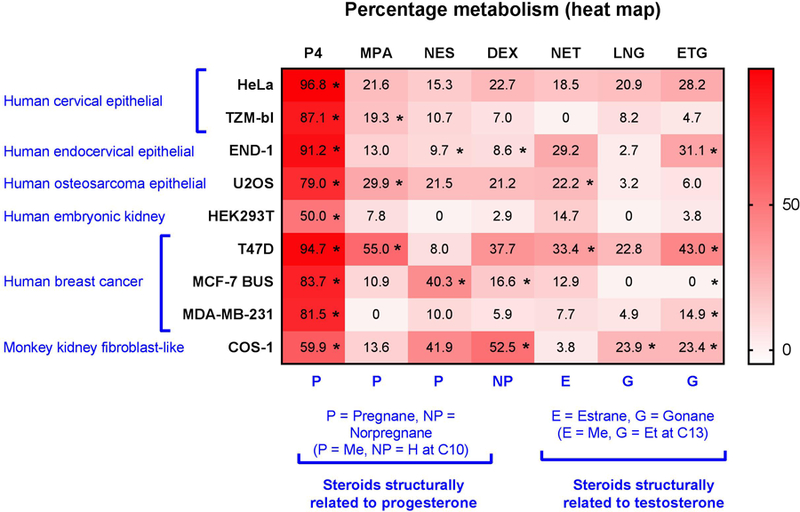
Data are from Figure 3 and cell lines and steroids are grouped according to anatomical and structural similarities, respectively. Pregnanes (P) have a methyl group at C10 position, while norpregnanes (NP) have a hydrogen group at C10; estranes (E) have a methyl group at C13, while gonanes (G) have an ethyl group at C13. Data is represented as % metabolism relative to the no-cell control which was set to 100%. Statistical significance is indicated by *s.
3.4. Differential metabolism of steroids within cell lines
Upon comparison of the metabolism of each steroid within each cell line, it was observed that apart from P4, all steroids were metabolised in a cell-specific manner (Figure 3 and Supporting Figure 3). T47D, END-1 and HeLa cells had the highest percentage metabolism of P4 with over 90% metabolism in all three cell lines. HEK293T and COS-1 cells displayed the least metabolism of P4 with only 50% and 60% metabolism, respectively. P4 demonstrated the greatest differences in metabolism compared to other steroids within each cell line (Figure 4 and Supporting Figure 3).
ETG was the second most metabolised steroid, with significant metabolism in five cell lines ranging between 14% and 43% (Figure 3 and 4). HeLa cells showed a high percentage of metabolism of ETG with 28% metabolism; this, however, was not significant (Figure 4). MPA and DEX were significantly metabolised in 3 cell lines each. Significant metabolism of MPA was observed in U2OS, TZM-bl and T47D cells, ranging between 19% and 55% (Figure 3). While, significant metabolism of DEX was observed in END-1, MCF-7 BUS and COS-1 cells with metabolism ranging from 8% and 52%. As shown in Figure 4, metabolism of DEX in HeLa, T47D and U2OS cells, although not significant, ranged from 21% to 37%. TZM-bl, HEK293T and MDA-MB-231 cells exhibited less than 10% metabolism of DEX. NET and NES were significantly metabolised only in two cell lines each (Figure 3). NET was significantly metabolised only in U2OS and MCF-7 BUS cells (Figure 3). NES was metabolised in END-1 and MCF-7 BUS cell lines by 9% and 40%, respectively (Figure 4). There was 41% metabolism of NES in COS-1 cells, and between 0% and 20% in the remaining cell lines; however, these effects were not statistically significant (Supporting Figure 3). LNG was significantly metabolised only in COS-1 cells with 23% metabolism which was comparable to the metabolism in T47D and HeLa cells which exhibited 22% and 20% metabolism, respectively. However, these latter effects were not statistically significant. The metabolism of LNG in the remaining six cell lines was less than 10% (Supporting Figure 3).
3.5. Metabolism in endocervical tissue
To determine if the metabolism observed in cell lines would be similar in a more physiologically-relevant system, we investigated the metabolism of three progestogens in post-menopausal endocervical tissue explants following a 24-hour incubation. P4 was investigated due to the high rate of metabolism in all cell lines, whilst MPA and NET were chosen as representative of progestins structurally related to P4 and T, respectively.
P4 was completely metabolised in the endocervical tissue explants after 24 hours (Figure 5). The results for P4, but not MPA and NET, are comparable to those observed in the three cervical cell lines (Figure 3). There was between approximately 87% and 96% metabolism of P4 in the cervical cell lines, which is similar to the 100% metabolism in tissue (Figure 5). As depicted in Figure 5, 38% of MPA was metabolised in tissue. This is different from the pattern observed in cervical cell lines where TZM-bl cells exhibited significant, but less, metabolism of MPA, (Figure 3) while no significant metabolism was observed in the other cervical cell lines. While NET was not significantly metabolised in the cervical cell lines, endocervical tissue metabolised 43% of available NET.
Figure 5. Differential metabolism of three clinically-relevant progestogens in post-menopausal endocervical tissue.

Tissue explants were incubated with 100 nM progestogens for 24 hours. The supernatant was removed and extracted before quantification via UHPSFC-MS/MS, as described in methods. Results were normalised to the progestogen concentration detected in a corresponding no-tissue control experiment to account for adsorption loss. The data show the mean ± SEM of three to five independent biological repeats. Statistical analysis via paired t-tests was performed comparing the progestogen concentration in the supernatant of the tissue condition to the no-tissue control, significant results are indicated by the p-value above the bar. To determine whether the relative metabolism of the progestogens was significantly different, an unpaired t-test with Welch’s correction was performed. Statistical significance indicated with * represents p<0.05.
4. Discussion
Previous research into progestogen metabolism has been limited and has typically focused on measuring the serum or urine concentrations of progestogens and/or their metabolites in a clinical setting [19–26]. In this work we investigated, for the first time, the cell-and steroid-specific metabolism of a range of clinically-relevant steroids using in vitro cell line models. These cell line models are widely used to investigate the biocharacter and mechanisms of action of these steroids, which themselves are commonly used in hormonal therapy and steroid receptor-based studies. We developed and validated a UHPSFC-MS/MS method of measuring the concentration of seven steroids in cell culture media, which allowed for the determination of the metabolism of these steroids by these cell lines. Our experimental design corrected for adsorption (up to 40%) of the steroids to the cell culture plates, where we found a positive correlation between adsorption and increasing hydrophobicity. The extent of metabolism could be measured from analysing the medium (supernatant) alone, since we found no significant retention of parent steroid in the cell pellets.
We found that individual progestins and DEX are differentially metabolised within the same cell line, and amongst different cell lines. For example, over 24 hours, some progestins are not significantly metabolised in a particular cell type (<20%), while others display a high degree of metabolism (20–50%) in the same cell type. A particular progestin can be metabolised to a vastly different degree in different cell lines (e.g. MPA metabolised by 55% in T47D and less than 10% metabolism in HEK293T and MDA-MB-231 cells). We detected no correlation between extent of metabolism and whether progestins were structurally related to P4 or T. Taken together, these results may have important physiological and pharmacological implications. Progestins used in hormonal contraception and HRT are known to exert several side-effects, such as effects on bone mineral density, metabolism, cardiovascular effects and reproductive cancers [2–4, 6]. Progestins also exert their contraceptive effects at several levels at different target tissues. If these in vitro effects of metabolism are translated in vivo, this suggests that different progestins may exert very different side-effects and may be more or less efficacious for contraception due not only to their inherent biocharacters, potencies and efficacies, but also due to differential metabolism in different target cells and tissues. This metabolism could both selectively lower their effective concentrations at the target cells in a cell-specific manner and may, also result in different metabolites with different off-target effects. The tissue findings are particularly interesting since they suggest that doses and types of progestins used for intravaginal delivery need to be carefully considered, taking into account that the progestins may be significantly metabolised in the tissues. The results also suggest that different cell types contain different steroid metabolising enzymes which discriminate between the progestins, despite some of their structures being similar. Although, the identification of the enzymes that metabolise DEX and the progestins in our models was beyond the scope of the present study, it would be an interesting avenue to explore in future studies.
Our results with P4 showing the rapid and substantial metabolism of this steroid in all the cells lines and in the cervical tissue, are particularly interesting. These results suggest that enzymes that metabolise P4 are widely expressed in most cell types, including the female genital tract, bones and breast tissue, and that its rapid turnover may be a mechanism required physiologically to fine-control PR responses to endogenous P4. Our results are consistent with those of Arici et al. who reported 90% metabolism of P4 in isolated primary endometrial stromal and gland cells after 24 hours [27]. However, it should be noted that only one time point was assessed and the temporal dynamics of P4 metabolism may differ between cell lines. Moreover, the metabolism of P4 appears to be independent of PR expression since a similar extent of metabolism was seen in all three breast cancer cell lines even though T47D and MCF-7 BUS cells are PR-positive whilst MDA-MB-231 cells are PR-negative [28]. Several researchers have reported that 20α-(S)-hydroxyprogesterone is a major metabolite of P4 via the actions of AKR1C1 [28–30]. Whether AKRIC1 or its isozymes are involved in metabolism of P4 in our model systems remains to be investigated.
Wiebe and Lewis found that breast cancer cell lines express higher levels of SRD5A1 and lower levels of AKR1C enzymes [31]. Therefore, breast cancer cells have a higher conversion of P4 to 5α-pregnane metabolites as opposed to other systemic cells and tissues. This has major implications, as 5α-pregnanes modify the growth of tumour cells within breast tissue. This highlights the importance of examining the metabolism of steroids, as some metabolites are active and may be a confounding factor in receptor-based studies comparing P4 -activity to other progestins [28, 32–33]. HEK293T (human embryonic kidney cells) and COS-1 cells (monkey kidney cells) had the lowest metabolism of P4, which may mean that kidneys have lower turnover of P4.
An important implication of our findings is that the detected differential and rapid metabolism of P4, progestins and DEX may confound the interpretation of results when investigating mechanisms of action and biological responses via steroid receptors using in vitro and preclinical models. This would be particularly relevant to the determination of relative binding affinities and potencies (EC50 values), which are highly relevant to drug efficacy, specificity and design. We have previously proposed that the determination of progestogen binding affinities and potencies via a specific receptor are dependent on a number of factors, including metabolism of the progestogen [4]. Several researchers have investigated binding affinities of one or more progestogens and/or DEX in COS-1 cells or cytosols prepared from MCF-7 cells [16–18, 34–35] or in cytosols prepared from tissue [11, 12]. Given that COS-1 cells show high metabolism of P4 and DEX relative to progestins such as MPA and NET, which show no metabolism in these cells, the reported relative binding affinities may be underestimated for P4 and DEX [16–18]. If metabolising activity is retained in cytosols, our results suggest that relative binding affinities for NET, but not LNG or ETG, from MCF-7 cytosols [34–35] may also be underestimated. Similarly, potencies (EC50s) and/or efficacies (maximal activities) have been reported for transcriptional activity using one or more progestogens and/or DEX in either COS-1, T47D, MCF-7 BUS, END-1, or HEK293 cell lines investigated in this study [11, 16–18, 36–40], or in primary cell models [8–9]. The reported potencies and efficacies may also be underestimated, particularly for P4, compared to some progestins, and to different degrees, depending on the cell model. For example, the relative potency of P4 via the androgen receptor (AR) and PR in HEK293T cells may be greater than that reported relative to LNG or NES [39], while the potency of P4 and DEX may be greater than that reported for other progestins in COS-1 cells for a particular receptor such as the PR, androgen, glucocorticoid and mineralocorticoid receptors [16–18, 39]. Potentially further complicating the interpretation, are different metabolites of these steroids produced in different cells that may also confound the results. Clearly, further investigation into the steroid-and cell-specific effects of metabolism of these clinically-relevant compounds, and the biological activities of their metabolites, is urgently required.
Supplementary Material
Highlights.
Progestins are differentially metabolised in a cell line-specific manner.
Progesterone is rapidly metabolised by 24 hours in all cell lines investigated.
MPA and NET are significantly metabolised in human cervical tissue ex vivo.
Metabolism may under-estimate effects determined for progesterone in vitro.
Differential metabolism may affect cell-specific therapeutic and side-effects.
Acknowledgements:
We would like to acknowledge Dr Marietjie Stander, Mr Malcolm Taylor, Mr Erick Van Schalkwyk and Mr Jonathan Quanson for their assistance with the UHPSFC-MS/MS. The authors thank the following people for consenting suitable patients and providing cervical tissue: Shane Moore, Lynn Keck, Anne Hoffman and Tony Wu at Groote Schuur Hospital; Hennie Botha, Rudolf Boshoff, and the registrars at Tygerberg Hospital.
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development [R01HD83026] (PI=JH, Subaward to DA), the National Research Foundation (NRF) in South Africa [99114] (DA) and the South African Medical Research Council (DA). Bursaries were funded by the Harry Crossley Research Foundation (SS, MC, MK), University of Cape Town (SS, KE, MK), Poliomyelitis Research Foundation (KE) and the National Research Foundation of South Africa (RLdT, SS, KE, MK).
Abbreviations:
- DEX
dexamethasone
- ETG
etonogestrel
- LNG
levonorgestrel
- MPA
medroxyprogesterone acetate
- NES
nestorone
- NET
norethisterone
- P4
progesterone
- PR
progesterone receptor
- T
testosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
References
- 1.Shchelkunova TA Morozov IA (2015) Molecular Basis and Tissue Specificity of the Progestin Effect. Molekulyarnaya Biologiya 49, 728–748 [DOI] [PubMed] [Google Scholar]
- 2.Africander D, Verhoog N, Hapgood JP (2011) Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 76, 636–652 [DOI] [PubMed] [Google Scholar]
- 3.Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr (2013) Progestogens Used in Postmenopausal Hormone Therapy: Differences in Their Pharmacological Properties, Intracellular Actions, and Clinical Effects. Endocrine Reviews 34, 171–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM (2014) Potency of progestogens used in hormonal therapy: Toward understanding differential actions. J. Steroid Biochem. Mol. Biol 142, 39–47 10.1016/j.jsbmb.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Louw-du Toit R, Perkins MS, Hapgood JP, Africander D (2017) Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy. Biochem Biophys Res Commun 491,140–146. doi: 10.1016/j.bbrc.2017.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hapgood JP, Koubovec D, Louw A, and Africander D (2004) Not all progestins are the same: implications for usage. Trends Pharmacol Sci 25, 554–557 [DOI] [PubMed] [Google Scholar]
- 7.Hapgood JP, Ray RM, Govender Y, Avenant C, and Tomasicchio M (2014) Differential glucocorticoid receptor-mediated effects on immunomodulatory gene expression by progestin contraceptives: implications for HIV-1 pathogenesis. Am J Reprod Immunol 71, 505–512 [DOI] [PubMed] [Google Scholar]
- 8.Kleynhans L, Du Plessis N, Black GF, Loxton AG, Kidd M, van Helden PD, Walzl G, and Ronacher K (2011) Medroxyprogesterone acetate alters mycobacterium bovis BCG-induced cytokine production in peripheral blood mononuclear cells of contraceptive users. in PLoS ONE 6, e24639. doi: 10.1371/journal.pone.0024639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engler JB, Kursawe N, Solano ME, Patas K, Wehrmann S, Heckmann N, Luhder F, Reichardt HM, Arck PC, Gold SM, and Friese MA (2017) Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc Natl Acad Sci U S A 114, E181–E190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maritz M, Ray RM, Bick AJ, Tomasicchio M, Woodland JG, Govender Y, van der Spuy Z, Avenant C, and Hapgood JP (2018) Increased CCR5 Levels Result in Increased R5-HIV Replication by Medroxyprogesterone Acetate, Unlike Norethisterone, via the Glucocorticoid Receptor. AIDS Research and Human Retroviruses 34, Abstract no. OA12.03 [Google Scholar]
- 11.Fuhrmann U, Slater EP, and Fritzemeier KH (1995) Characterization of the novel progestin gestodene by receptor binding studies and transactivation assays. Contraception 51, 45–52 [DOI] [PubMed] [Google Scholar]
- 12.Kasid A, Buckshee K, Hingorani V, and Laumas KR (1978) Interaction of progestins with steroid receptors in human uterus. Biochem J 176, 531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govender Y, Avenant C, Verhoog NJD, Ray RM, Grantham NJ, et al. (2014) The Injectable-Only Contraceptive Medroxyprogesterone Acetate, Unlike Norethisterone Acetate and Progesterone, Regulates Inflammatory Genes in Endocervical Cells via the Glucocorticoid Receptor. PLoS ONE 9, e96497. doi: 10.1371/journal.pone.0096497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louw-du Toit R, Hapgood JP, Africander D (2017) Medroxyprogesterone Acetate Differentially Regulates Interleukin (IL)-12 and IL-10 in a Human Ectocervical Epithelial Cell Line in a Glucocorticoid Receptor (GR)-dependent Manner. J. Biol. Chem 289, 31136–31149. doi: 10.1074/jbc.M114.587311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher P, Kiselyeva Y, Wallace G, Romano J, Griffin G, Margolis L, Shatock R (2005) The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. Journal of Virology, 79, 11179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronacher K, Hadley K, Avenant C, Stringer E, Simons SS Jr, Louw A, and Hapgood JP (2009) Ligand-selective transactivation and transrepression via the glucocorticoid receptor: role of cofactor interaction. in Molecular and cellular endocrinology 299,219–23 [DOI] [PubMed] [Google Scholar]
- 17.Africander D, Louw R, and Hapgood JP (2013) Investigating the antimineralocorticoid properties of synthetic progestins used in hormone therapy. Biochemical and biophysical research communications 433, 305–310 [DOI] [PubMed] [Google Scholar]
- 18.Africander DJ, Storbeck KH, and Hapgood JP (2014) A comparative study of the androgenic properties of progesterone and the progestins, medroxyprogesterone acetate (MPA) and norethisterone acetate (NET-A). J Steroid Biochem Mol Biol 143, 404–415 Chu, M. C., Nakhuda, G. S., Zhang, X., Stanczyk, F. Z., Lobo, R. A. (2007) Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 92, 2205–2207. [DOI] [PubMed] [Google Scholar]
- 19.Edelman AB, Cherala G, Stanczyk FZ (2010) Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception 82, 314–323. [DOI] [PubMed] [Google Scholar]
- 20.Helmreich ML, Huseby RA (1962) Identification of a 6,21-dihydroxylated metabolite of medroxyprogesterone acetate in human urine. J Clin Endocrinol Metab 22, 018–1032. [DOI] [PubMed] [Google Scholar]
- 21.Huber J (1998) Pharmacokinetics of Implanon. Contraception 58, 85S–90S. [DOI] [PubMed] [Google Scholar]
- 22.Prasad PV, Bashir M, Sitruk-Ware R, Kumar N (2010) Single-dose pharmacokinetics of nestorone, a potential female-contraceptive. Steroids 75, 252–264. [DOI] [PubMed] [Google Scholar]
- 23.Ravinder P, Shatrugna V, Madhavan K, Sivakumar B (1997) Pharmacokinetics of orally administered norethisterone enanthate in rabbit, monkey, and women. Contraception 55, 373–379. [DOI] [PubMed] [Google Scholar]
- 24.Hümpel M, Wendt H, Pommerenke G, Weiβ C, Speck U (1978) Investigations of pharmacokinetics of levonorgestrel to specific consideration of a possible first-pass effect in women. Contraception 17, 207–220. [DOI] [PubMed] [Google Scholar]
- 25.Jeppsson S, Gershagen S, Johansson ED, Rannevik G (1982) Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera) as a contraceptive agent. Acta. Endocrinol. (Copenh) 99, 339–343. [DOI] [PubMed] [Google Scholar]
- 26.Arici A, Marshburn PB, MacDonald PC, Dombrowsk RA (1999) Progesterone metabolism in adipose cells. Steroids 64, 530–534 [DOI] [PubMed] [Google Scholar]
- 27.Wiebe JP, Zhang G, Welch I, Cadieux-Pitre HT (2013) Progesterone metabolites regulate induction, growth, and suppression of estrogen-and progesterone receptor-negative human breast cell tumors. Breast Cancer Research 15, R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinreih M,. Anko M, Zukunft S, Adamski J, Rizner. (2015) Important roles of the AKR1C2 and SRD5A1 enzymes in progesterone metabolism in endometrial cancer model cell lines. Chemico-Biological Interactions 234, 297–308. 10.1016/j.cbi.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 29.Rizner TL, Penning TM (2014) Role of aldo–keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids 79, 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis J, Wiebe M, Heathcote JG (2004). Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma. BMC cancer 427. doi: 10.1186/1471-2407-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin VC, Jin R, Tan P, Aw S, Woon C, Bay B (2003) Progesterone Induces Cellular Differentiation in MDA-MB-231 Breast Cancer Cells Transfected with Progesterone Receptor Complementary DNA. American Journal of Pathology 162, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diederich S, Hanke B, Oelkers W, Bahr V (1997) Metabolism of Dexamethasone in the Human Kidney:Nicotinamide Adenine Dinucleotide-Dependent 11b-Reduction. Journal of Clinical Endocrinology and Metabolism 82, 1598–602 [DOI] [PubMed] [Google Scholar]
- 33.Schoonen WG, Deckers GH, de Gooijer ME, de Ries R, and Kloosterboer HJ (2000) Hormonal properties of norethisterone, 7alpha-methyl-norethisterone and their derivatives. J Steroid Biochem Mol Biol 74, 213–222 [DOI] [PubMed] [Google Scholar]
- 34.Deckers GH, Schoonen WG, and Kloosterboer HJ (2000) Influence of the substitution of 11-methylene, delta(15), and/or 18-methyl groups in norethisterone on receptor binding, transactivation assays and biological activities in animals. J Steroid Biochem Mol Biol 74, 83–92 [DOI] [PubMed] [Google Scholar]
- 35.Govender Y, Avenant C, Verhoog NJ, Ray RM, Grantham NJ, Africander D, and Hapgood JP (2014) The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS One 9, e96497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar N, Fagart J, Liere P, Mitchell SJ, Knibb AR, Petit-Topin I, Rame M, El-Etr M, Schumacher M, Lambert JJ, Rafestin-Oblin ME, and Sitruk-Ware R (2017) Nestorone(R) as a Novel Progestin for Nonoral Contraception: Structure-Activity Relationships and Brain Metabolism Studies. Endocrinology 158, 170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viswanath G, Halder S, Divya G, Majumder CB, and Roy P (2008) Detection of potential (anti)progestagenic endocrine disruptors using a recombinant human progesterone receptor binding and transactivation assay. Mol Cell Endocrinol 295, 1–9 [DOI] [PubMed] [Google Scholar]
- 38.Sasagawa S, Shimizu Y, Kami H, Takeuchi T, Mita S, Imada K, Kato S, Mizuguchi K (2008) Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. in Steroids 73, 222–231 [DOI] [PubMed] [Google Scholar]
- 39.Courtin A, Communal L, Vilasco M, Cimino D, Mourra N, de Bortoli M, Taverna D, Faussat A-M, Chaouat M, Forgez P, and Gompel A (2012) Glucocorticoid receptor activity discriminates between progesterone and medroxyprogesterone acetate effects in breast cells. in Breast Cancer Res. Treat 131, 49–63. doi: 10.1007/s10549-011-1394-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


