Abstract
Aerosols are dynamic systems, responding to variations in the surrounding environmental conditions by changing in size, composition and phase. Although, widely used in inhalation therapies, details of the processes occurring on aerosol generation and during inhalation have received little attention. Instead, research has focused on improvements to the formulation of the drug prior to aerosolization and the resulting clinical efficacy of the treatment. Here, we highlight the processes that occur during aerosol generation and inhalation, affecting aerosol disposition when deposited and, potentially, impacting total and regional doses. In particular, we examine the response of aerosol particles to the humid environment of the respiratory tract, considering both the capacity of particles to grow by absorbing moisture and the timescale for condensation to occur.
Keywords: : aerosol microphysics, condensation, drug delivery to the lungs, inhalation, moisture
Therapeutic aerosols & the analytical challenge
Aerosols are widely used in the treatment of respiratory diseases, particularly asthma and chronic obstructive pulmonary disease. Affording direct delivery to the disease site, rapid clinical response and requiring a small fraction of a typical dose required in oral or subcutaneous administration routes [1], aerosols are increasingly viewed as an appropriate route to deliver other therapeutic agents, including the delivery of insulin, and to treat systemic diseases [2]. However, studies of aerosol therapeutics largely focus on the formulation of the drug and the device used to generate and deliver the aerosol, or the resulting deposition pattern in the lung, the pharmacokinetics of the inhaled medicine and the efficacy of the treatment; the processes occurring between the points of aerosol generation and deposition are rarely considered. Despite this, improving disease outcomes are likely dependent on targeting and improving lung deposition fraction in specific regions in the respiratory tract, inherently requiring knowledge of the aerosol processing that occurs [3]. Indeed, the key barriers to progress in inhalation therapies that have been identified include a poor understanding of the relationships between the physicochemical characteristics of the formulation and the performance in the humid environment of the respiratory tract, and an incomplete understanding of drug disposition in the lung after deposition [4].
The formulation of the drug (excipient, active pharmaceutical ingredient, propellant, co-solvent) and delivery device (pressurized metered dose inhaler, dry powder inhaler, nebulizer) must be considered in conjunction when considering the aerosol route for drug delivery [5–8]. Aerosol particles are generated as either solid crystalline, amorphous or liquid droplet form, the latter as suspensions or solutions, undergoing rapid evolution in their size and composition in the aerosol phase, shown schematically in Figure 1 [6,7,9]. The response in size can be driven by the loss in volatile propellants or co-solvents, or by the condensation of water in the humid environment of the lung, effecting the phase state and morphology of particles [10,11]. Deposition patterns are critically dependent on particle size and may affect clinical efficacy, although these processes in the aerosol phase lead to continual variation in size [10,12]. Coarse large particles (>5 μm) penetrate very little into the respiratory tract when inhaled at typical flow rates with high losses due to impaction and sedimentation, especially in upper and larger airways; the fraction of fine particles is seen as crucial for increasing deposition in the small peripheral airways with diffusional losses only becoming significant for particles <200 nm in diameter [13,14]. Indeed, the transition from chlorofluorocarbon propellants to hydrofluoroalkanes (HFAs), a consequence of the Montreal protocol, led to reports of improved clinical efficacy at lower dose [15,16]. This observation has been attributed to the smaller particle sizes resulting from HFA formulations when compared with chlorofluorocarbon, which are able to penetrate deeper into the respiratory tract and show improved total lung deposition [17]. Once deposited on the airway epithelial layer, the physical state of the particles may be important for determining the pharmacokinetic profile and drug release rate [2].
Figure 1. . Aerosols can be formulated for a number of delivery modalities including metered dose inhalers and dry powder inhalers, producing solutions, suspensions or dry powders.
The outcome of the therapy can be dependent on the formulation. Aerosolization and aerosol processing are key intermediate steps that are often ignored when considering the delivery of drugs to the lungs but result in rapid changes in particle size, phase, morphology and composition, thereby affecting deposition pattern, disposition and, possibly, clinical efficacy.
The dynamic processes occurring in the aerosol phase following aerosolization and prior to deposition, occur on timescales of milliseconds to seconds or longer [10,11], commensurate with the timescales of inhalation and exhalation [18]. Particle sizes are usually measured as residual dry particle sizes using instruments such as the Anderson cascade impactor, or under conditions of a certain relative humidity (RH) [12]. Although, these techniques can facilitate assessments of quality and reproducibility of product formulation, we suggest that they are unable to provide any more than a qualitative assessment of the aerosol particle size distribution and phase state once inhaled. New analytical approaches are required to provide time-dependent information on the phase state, morphology, size and moisture content of a particle prior to and up to the point of deposition, if in silico deposition models are to be refined [18,19], the significance of measurements of deposited dose [13,14] and lung images [20] interpreted, and the efficacy of aerosol therapies improved [2]. Our purpose here is to highlight new techniques for probing the aerosol dynamics that occur on inhalation, helping to provide information on a key step in the process of drug delivery to the lungs.
The capacity of aerosol particles to absorb moisture on inhalation
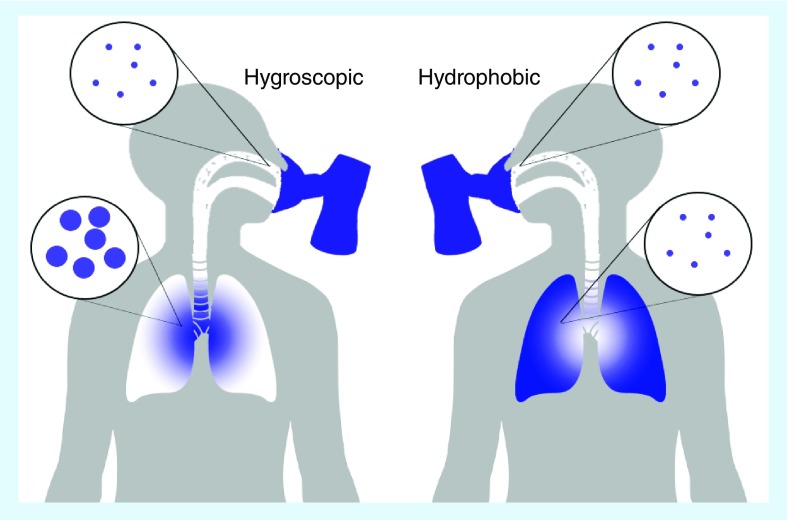
The consequences of the high humidity of the lungs on inhaled particles will depend on the hygroscopic properties of the particles, the aerodynamic characteristics of the particles, the concentration of the aerosol phase, and the respiratory parameters. There is a balance between the response rate of the aerosol particles under the prevailing RH, the timeframe over which the particles are exposed to the high RH conditions, and competing deposition mechanism (e.g., inertial impaction removing particles from the airstream). The RH and air temperature reach 99.5% and 37°C by approximately the fourth dichotomous branch point of the pulmonary tract [21,22]. The humidity is therefore close to saturation for aerosol particles which avoid inertial impaction in the uppermost airways. Under normal tidal breathing conditions, air residence times range between 2 and 5 s [23,24] depending on whether the individual is sedentary or undertaking heavy exercise. Although, near-tidal breathing is achieved during nebulization, breathing parameters differ widely for those using dry powder inhalers and metered dose inhalers (MDI). For example, shorter, rapid inhalation rates are required for dry powder inhalers, which enhances impaction deposition in the upper airway, minimizing the fraction of the aerosol phase subject to the high-RH lung environment. However, patients are usually advised to employ a breath hold period of up to 10 s in duration (or at least as long as is comfortable), and such a period would provide sufficient opportunity for particles that penetrate into the deep lung to undergo growth under the physiological conditions of the airways.
Indeed, control over the hygroscopic growth of submicrometer particles on inhalation has been suggested as a route to achieve low deposition in the extrathoracic region and high deposition in the lung after they have grown [25–27]. The hygroscopic response of particles on inhalation is dependent on the particle size and RH immediately prior to inhalation, and on the nature of the chemical component forming the aerosol [13,28]. Less hygroscopic particles are capable of absorbing less water than more hygroscopic particles and changes in the size dependent dose are observed in the case of the latter [14,29,30]. Not only is the capacity of the aerosol particles to absorb gaseous water important in governing response, but the timescale of the condensation process is important [31]. We will consider these two factors separately.
The capacity of an aerosol particle to absorb water is termed the aerosol hygroscopicity. Typically, the capacity for growth by water condensation is represented as a mass (GFm) or diameter (GFd) growth factor as a function of gas phase RH:
 |
(1) |
 |
(2) |
In both cases, the particle mass (or diameter) at a particular RH is referenced to the particle mass (or diameter) in the absence of moisture, equivalent to a perfectly dry particle that just contains all of the solutes, excipients and active pharmaceutical ingredients (APIs). This provides a convenient way of representing the uniform scaling in particle size with RH observed across all dry particle sizes larger than ∼100 nm in diameter, a lower size limit above which the Kelvin effect has minimal impact on the vapor pressure of a liquid droplet [32].
The capacity of aerosol particles of a particular composition to absorb water can be measured directly in the aerosol phase using an array of conventional techniques [32], such as a hygroscopicity tandem differential mobility analyzer to measure the response of a particle population or an electrodynamic balance (EDB) for single particle measurements. Using these techniques, it is possible to make accurate measurements of hygroscopic growth up to ∼90% RH for pharmaceutical aerosol [33]. However, only recently has it become possible to measure the growth of aerosol up close to the saturation RH of 100%, typical of the respiratory tract, using a refined EDB approach [10,34]. This is a particularly important regime to make measurements in for respiratory aerosol: APIs can often have low hygroscopicity and solubility in water and growth may be only very minor (if it is even measurable) at RHs below 90%.
As the amount of water carried by the gas phase increases (i.e., an increase in RH), an aerosol particle must follow this change in moisture content to remain in a state of thermodynamic equilibrium. For soluble aerosol, for example, saline solutions, the particle has the capacity to absorb a large amount of water growing by as much as an order of magnitude in mass or diameter as the RH surpasses 99.5%. Typical diameter growth factor dependencies on RH are shown in Figure 2. As an example, an aqueous sodium chloride crystal, 1 μm in diameter if represented as a spherical equivalent, will take up enough water to exceed 6 μm in diameter at 99.5% RH. All growth curves rise extremely steeply as the saturation RH is approached (note the change in both scales between the left and right sides of the figure), a point where water condensation on a particle would lead to spontaneous and continuous growth. It should be recognized that the condensation of water leads to a decrease in the mass fraction of solutes within the droplet, in this case sodium chloride, as the proportion of water increases leading to changes in tonicity. As examples, the molality of sodium chloride in an aqueous droplet (moles of sodium chloride per kg of water) decreases from 11.5 mol kg-1 at a typical ambient RH of 50% to 0.6 mol kg-1 at 98% RH. A droplet is isotonic with blood plasma at a RH of only 99.5% with a molality of 0.15 mol kg-1 [34].
Figure 2. . Particles respond to changes in the surrounding relative humidity of the gas phase by absorbing water and growing in size.
Here, the growth in size is represented as a diameter growth factor which reports the change in particle size relative to the dry particle as the relative humidity increases. Growth curves for particles of increasing hygroscopicity parameter (= 0.01, 0.12 and 1.27) are shown as examples of insoluble, low solubility and high solubility, respectively.
In atmospheric aerosol science, it is typical to represent the capacity of an aerosol to grow by condensation by a reduced parameter model, referred to as Köhler theory [35]. Here, the dependence of the diameter growth factor on RH can be calculated from the equation:
 |
(3) |
The sensitivity of hygroscopic growth to the value of the κ parameter is clearly apparent from Figure 2. Even for a compound of low hygroscopicity, a particle may still change size by 10's% at the high humidity in the lung. Necessarily, the water activity in the particle must approach one (an infinite dilution of solute) by an RH of 100% to satisfy equilibrium thermodynamics. In atmospheric science, although the moisture absorbed by particles consisting of very insoluble compounds (with < 0.01) may be only 10's of molecular layers, particles can still exhibit unhindered condensation of water under supersaturated conditions [36], conditions where the water activity of the solution droplet may be >0.999. This is still an area of active research and the moisture content of inhaled particles with low solubility in the respiratory tract has not yet been fully explored [37]. It should be stressed that not only may the hygroscopic growth impact on deposition pattern, but the moisture content acquired in flight will impact on the disposition of the drug particles at the point of deposition [25–27]. Indeed, we have shown recently that the degree of hygroscopic growth of aerosol particles can be very readily controlled by careful consideration of the chemical components used to form the aerosol, for example, by using mixtures of saccharides and amino acids [35] or by adding triblock copolymers [38].
The timescale for aerosol particles to absorb moisture on inhalation
The capacity of aerosol particles to absorb moisture during inhalation is only one part of the challenge in understanding the hygroscopic growth of inhalation therapies: the timescale for a particle to absorb moisture can span from milliseconds to days, depending on particle size, surface composition, and bulk rheological properties (i.e., the viscosity of the particle). The unhindered condensation kinetics of water on a particle depend on the size; particles smaller than 1000 nm in diameter grow in less than 0.1 s, responding to a step change in RH from a dry ambient atmosphere to the RH of the respiratory tract such as would occur on inhalation, as shown in Figure 3. Here, the mechanism is purely governed by the speed of gas phase transport of water from an ‘infinite’ distance to the particle surface, following the gradient in RH [32]. However, for particles of 5 μm size, the eventual fractional change in size is same as smaller particles, but more water must be absorbed by a particle with a lower surface-to-volume ratio, slowing the response and requiring 5–10 s for condensational growth to be achieved (not fully appreciable in Figure 3 as shown) [39].
Figure 3. . Simulations of the response in size of sodium chloride droplets to a step change in the relative humidity surrounding for aerosol particles of varying initial size (note the log diameter axis).
In Figure 4A, we show simulations that illustrate both the capacity of an aerosol particle to grow and the timescale for growth of particles of 1 μm diameter for particles composed of saline, Tobramycin and a Tobramycin-saline mixture. Here, the aerosol particles start at a gas phase RH of 50% and grow in response to a step change in RH up to 99.5%. These simulations are based on generic parameterisations of measurements made for more specific changes in RH and various particle sizes. Equilibration to the high RH is only complete after ∼10 s. The extent of growth is clearly dependent on the composition and hygroscopicity of the aerosol components as well as the level of moisture content in the initial particle prior to experiencing the step change in RH, in other words the initial RH of the ambient environment the aerosol exists in. This illustrates a further point expanded on in Figure 4B: the aerosol particle size is very rarely static but continuously evolving on the timescales of seconds in response to changes in the gas phase environment (RH and temperature). In this example, a solution droplet is prepared under dilute solute conditions (high water activity) and immediately loses water when exposed to a low RH of ∼70%, equilibrated only after ∼10 s. Following a relatively small increase in RH to ∼82%, the particle size responds in ∼5 s. In addition to showing model predictions of the temporal response in the aerosol particle size, we also show a measurement made using the refined EDB approach mentioned above, illustrating the high level of agreement that can be reached in interpreting the size-changing kinetics. Indeed, this new EDB approach can be used in principle to expose a particle to a time-dependent pattern in RH that might be expected for particles following generation and on inhalation [10].
Figure 4. . Dynamic behavior of pharmaceutical aerosol prior to, and during, inhalation.
(A) A simulation of the time-dependence of the size of particles of varying composition during condensation following a step change in relative humidity. (B) A comparison of the kinetics of particle size change during initial equilibration of an aqueous sodium chloride solution droplet following injection into a gas phase of lower RH than the starting water activity in the droplet, followed by condensation of water once the relative humidity is increased. The measurements were made with an EDB detailed in our previous work [10,34]. The model parameterization includes a quasi-steady analytical treatment of the coupling of heat and mass transport during water evaporation or condensation [34,39,41].
EDB: Electrodynamic balance; RH: Relative humidity.
The timescale for water condensation on a growing particle can depend not only on the particle size and the rate of gas phase moisture transport, but also on the surface and bulk composition of the particle [40]. It was recognized some time ago that control over the surface composition of aerosol particles used in inhalation therapies, for example, by including fatty acids in the formulation, could be exploited to control the size changing dynamics of particles [37]. The presence of an amphiphilic surfactant, a molecule with a hydrophilic head group and a hydrophobic tail that preferentially resides at an aqueous-air surface with the hydrophobic tail pointing into the gas phase, can influence the ‘appearance’ of the droplet surface to a condensing water molecule, in effect changing a hydrophilic surface to a hydrophobic surface. As well as impacting on the evaporation kinetics of water from a droplet by presenting a barrier to water passage through the surface region [41], it has long been considered that a surfactant film can act as a barrier to adsorption of water, delaying water condensation kinetics [32,39]. With inhalation/exhalation cycles typically lasting only a few seconds, slowing the condensation kinetics by including a surfactant in a formulation could readily lead to control over the response timescale of even hygroscopic aerosol on inhalation, particularly for particles larger than 1 μm.
Many of the excipients used in drug delivery to the lungs are saccharides, for example, the disaccharide lactose. Although, sugars are reasonably hygroscopic (e.g., galactose has a value of of 0.13 [35]) they can form amorphous particles under dry conditions, as well as crystalline particles, with some (e.g., sucrose) passing through a moisture driven glass transition at room temperature. From a rheological perspective, this suggests that the viscosity of a dried particle can exceed 1012 Pa s [42], 15 orders of magnitude higher than the viscosity of typical saline solution droplets with viscosities of order 10-3 Pa s. A particle that is so viscous necessarily responds only slowly to changes in moisture content in the gas phase due to the slow transport of water within the particle bulk [43]. Instead of a timescale for the condensation of water that is limited by bulk gas phase transport or transport across the surface (as in the case of a surfactant film), the timescale may be limited by the dissolution kinetics of the particle bulk and the rate at which water can permeate throughout the particle [43]. Indeed, this dissolution process has been measured to extend for timescales longer than >10 s for particles of particular chemical composition [44]. We have shown recently that the dependence of particle viscosity on moisture content can be controlled very readily by choosing appropriate molecular constituents, including mono-, di- and tri-saccharides [45].
Conclusion & future perspective
Aerosol particle size and composition are dynamic, responding on timescales that are comparable to, or longer than, inhalation/exhalation times for particle sizes typical of aerosols used in inhalation therapies. Although often ignored, and leading to additional complexities in treating aerosol-lung interactions, aerosol dynamics could provide a route to exploit particle properties for optimizing delivered does to specific regions in the lung. Particles smaller than 1 μm in diameter can be considered to respond instantaneously to changes in their environment; tuning their hygroscopicity can have a significant impact on the dependence in particle size with RH and, thus, could influence the deposition pattern within the lung [25–27]. In addition, influencing condensation kinetics through using surfactant additives or through controlling the bulk viscosity of the particle can lead to control over timescale, potentially delaying moisture absorption and retaining small particles for longer in the respiratory tract. For particles larger than 1 μm in diameter, timescales for changes in size are much longer than for smaller particles, potentially offsetting any impacts of hygroscopic growth on the size distribution. However, control over particle viscosity and surface composition can have significant effects on the dissolution timescales of particles and could be used to influence the disposition of the aerosol on deposition and the drug release rate.
Improvements in our understanding of the microphysical processes occurring in aerosol during inhalation have been inferred from initial aerosol states starting as aqueous solution droplets or amorphous particles and have largely focused on hygroscopic response. Much of this work is immediately relevant to understand the microphysics of aerosols generated from nebulizer formulations [10,38]. However, the processes leading to rapid changes in droplet size, composition and temperature during the initial generation step, particularly from a MDI [5,12], are now accessible using advanced experimental approaches for generating droplets containing high-volatility propellants such as HFAs [11]. It will be instructive to combine these approaches with the refined tools for looking at evolving particle size in humid atmospheres using an EDB [34,41,44,46]. Realistic, high volatility HFA droplets, containing co-solvents and API, could be generated using these approaches and immediately captured in the high RHs attainable in the EDB, facilitating a route to prepare the exact particle types formed by MDIs and to mimic the processes occurring on particles produced from a MDI on inhalation. Alternatively, the evolving composition and size could be probed on timescales of <100 ms by monitoring droplets falling as a droplet chain [47–49].
Aerosol dynamics also play an important role in the production of particles used in dry particle inhalers [7], particularly in processes such as spray drying [50]. Particles of increasing complexity and with tailored properties could be manufactured if the microphysical processes occurring during spray drying were better understood. For example, Vehring and coworkers have developed new tools to probe the detailed evolution in particle size, composition and phase during droplet drying [51–53] and these are already promising new routes to controlling particle synthesis [49,54,55].
All of these avenues of research in therapeutic delivery aerosol modalities require the continual evolution of new analytical tools to provide the detailed level of information essential to improve drug products [32,56]. The development of new tools to fill in the gaps in our knowledge between the points of aerosol generation and deposition will be particularly important as new opportunities in aerosol design move toward particles smaller than 1 m in diameter [57] and as new formulations are explored [3,9,56].
Executive summary.
Aerosol particles respond to changes in the surrounding environmental conditions on timescales comparable to inhalation/exhalation times. In particular, aerosols formed from hygroscopic components (e.g., saline) can grow by as much as an order of magnitude in size when inhaled. By contrast, insoluble drug particles may only absorb molecular layers of water.
Inhaled particles smaller than 1 μm in diameter can be assumed to respond instantaneously to the moisture content of the surrounding environment, except when they contain surfactant molecules or start as crystalline or amorphous glassy particles. In these instances, the absorption of moisture from the vapour phase and the ensuing growth in particle size may be delayed by many seconds or longer.
Inhaled particles larger than 1 μm in diameter absorb water much more slowly, over timescales typically longer than 5–10 s. However, the moisture content acquired can influence the disposition of the particle when deposited.
By selecting the composition of the aerosol particles, the microphysical processes occurring in the aerosol phase can be controlled, delaying the growth of hygroscopic particles or enhancing particle dissolution.
Understanding further microphysical processes, including the rapid processes that occur when propellant and co-solvent evaporate from droplets generated by a MDI or the processes occurring in particle production (e.g., spray drying) could provide further routes to control aerosol dynamics and tailor particle response on inhalation.
Footnotes
Financial & competing interests disclosure
JP Reid and AE Haddrell acknowledge financial support from Chiesi Ltd who contributed partial funding for this work. JP Reid acknowledges financial support from the EPSRC through grant EP/N025245/1. D Lewis and T Church are employees of Chiesi Ltd, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56(6):600–612. doi: 10.1046/j.1365-2125.2003.01893.x. http://doi.wiley.com/10.1046/j.1365–2125.2003.01893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes B, Asgharian B, Dailey LA, et al. Challenges in inhaled product development and opportunities for open innovation. Adv. Drug Deliv. Rev. 2010;63(1–2):69–87. doi: 10.1016/j.addr.2010.11.004. www.ncbi.nlm.nih.gov/pubmed/21144875 [DOI] [PubMed] [Google Scholar]
- 3.Ulrik CS, Lange P. Targeting small airways in asthma: improvement in clinical benefit? Clin. Respir. J. 2011;5(3):125–30. doi: 10.1111/j.1752-699X.2010.00235.x. www.ncbi.nlm.nih.gov/pubmed/21106032 [DOI] [PubMed] [Google Scholar]
- 4.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth HDC. Propellant-driven metered-dose inhalers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2005;2(1):53–74. doi: 10.1517/17425247.2.1.53. [DOI] [PubMed] [Google Scholar]
- 6.O'Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax. 1997;52(Suppl. 2):S31–S44. doi: 10.1136/thx.52.2008.s31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gradon L, Sosnowski TR. Formation of particles for dry powder inhalers. Adv. Powder Technol. 2014;25(1):43–55. Epub ahead of print. [Google Scholar]
- 8.Nikander K. Challenges and opportunities in respiratory drug delivery devices. Expert Opin. Drug Deliv. 2010;7(11):1235–8. doi: 10.1517/17425247.2010.525231. www.ncbi.nlm.nih.gov/pubmed/20939686 [DOI] [PubMed] [Google Scholar]
- 9.Rabinowitz JD, Lloyd PM, Munzar P, et al. Ultra-fast absorption of amorphous pure drug aerosols via deep lung inhalation. J. Pharmace. 2006;95:2438–2451. doi: 10.1002/jps.20694. [DOI] [PubMed] [Google Scholar]
- 10.Haddrell AE, Davies JF, Miles REH, Reid JP, Dailey LA, Murnane D. Dynamics of aerosol size during inhalation: hygroscopic growth of commercial nebulizer formulations. Int. J. Pharm. 2014;463(1):50–61. doi: 10.1016/j.ijpharm.2013.12.048. www.ncbi.nlm.nih.gov/pubmed/24406674 [DOI] [PubMed] [Google Scholar]
- 11.Shemirani FM, Church TK, Lewis DA, Finlay WH, Vehring R. Onset of flash atomization in a propellant microjet. J. Fluids Eng. 2015;137(9):91101. http://fluidsengineering.asmedigitalcollection.asme.org/article.aspx?doi=10.1115/1.4030089 [Google Scholar]
- 12.Stein SW. Aiming for a moving target: challenges with impactor measurements of MDI aerosols. Int. J. Pharm. 2008;355(1–2):53–61. doi: 10.1016/j.ijpharm.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Rissler J, Gudmundsson A, Nicklasson H, Swietlicki E, Wollmer P, Löndahl J. Deposition efficiency of inhaled particles (15–5000 nm) related to breathing pattern and lung function: an experimental study in healthy children and adults. Part. Fibre Toxicol. 2017;14(1):10. doi: 10.1186/s12989-017-0190-8. http://particleandfibretoxicology.biomedcentral.com/articles/10.1186/s12989–017–0190–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Löndahl J, Massling A, Pagels J, Swietlicki E, Vaclavik E, Loft S. Size-resolved respiratory-tract deposition of fine and ultrafine hydrophobic and hygroscopic aerosol particles during rest and exercise. Inhal. Toxicol. 2007;19(2):109–16. doi: 10.1080/08958370601051677. www.ncbi.nlm.nih.gov/pubmed/17169858 [DOI] [PubMed] [Google Scholar]
- 15.Aubier M, Wettenger R, Gans SJM. Efficacy of HFA-beclomethasone dipropionate extra-fine aerosol (800 mg day−1) versus HFA-fluticasone propionate (1000 mg day−1) in patients with asthma. Respir. Med. 2001;95(3):212–220. doi: 10.1053/rmed.2000.1025. [DOI] [PubMed] [Google Scholar]
- 16.Fairfax AJ. The relative clinical effectiveness of HFA-BDP and fluticasone propionate in asthma. Respir Med. 2000;94(6):Ds31–6. doi: 10.1016/s0954-6111(00)90122-7. [DOI] [PubMed] [Google Scholar]
- 17.Gentile Da, Skoner DP. New asthma drugs: small molecule inhaled corticosteroids. Curr. Opin. Pharmacol. 2010;10(3):260–5. doi: 10.1016/j.coph.2010.06.001. www.ncbi.nlm.nih.gov/pubmed/20561819 [DOI] [PubMed] [Google Scholar]
- 18.Bates AJ, Doorly DJ, Cetto R, et al. Dynamics of airflow in a short inhalation. J. R. Soc. Interface. 2014;12(102):20140880–20140880. doi: 10.1098/rsif.2014.0880. http://rsif.royalsocietypublishing.org/cgi/doi/10.1098/rsif.2014.0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazaridis M, Broday DM, Hov O, Georgopoulos P. Integrated exposure and dose deposition of inhaled particles in the human respiratory tract. Environ. Sci. Technol. 2007;35(18):3727–3734. doi: 10.1021/es001545w. [DOI] [PubMed] [Google Scholar]
- 20.Conway J, Fleming J, Majoral C, et al. Controlled, parametric, individualized, 2-D and 3-D imaging measurements of aerosol deposition in the respiratory tract of healthy human subjects for model validation. J. Aerosol Sci. 2012;52:1–17. doi: 10.1089/jamp.2014.1191. http://linkinghub.elsevier.com/retrieve/pii/S0021850212000729 [DOI] [PubMed] [Google Scholar]
- 21.Morrow PE. Factors determining hygroscopic aerosol deposition in airways. Physiol. Rev. 1986;66(2):330–376. doi: 10.1152/physrev.1986.66.2.330. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Kim CS, Kleinstreuer C. Water vapor transport and its effects on the deposition of hygroscopic droplets in a human upper airway model. Aerosol Sci. Technol. 2006;40(1):1–16. www.tandfonline.com/doi/abs/10.1080/02786820500461154 [Google Scholar]
- 23.Broday DM, Georgopoulos PG. Growth and deposition of hygroscopic particulate matter in the human lungs. Aerosol Sci. Technol. 2001;34(1):144–159. [Google Scholar]
- 24.Bates DV, Fish BR, Hatch TF, Mercer TT, Morrow PE. Deposition and retention models for internal dosimetry of the human respiratory tract. Health Phys. 1966;12(2):173–207. [PubMed] [Google Scholar]
- 25.Son YJ, Longest PW, Tian G, Hindle M. Evaluation and modification of commercial dry powder inhalers for the aerosolization of a submicrometer excipient enhanced growth (EEG) formulation. Eur. J. Pharm. Sci. 2013;49(3):390–399. doi: 10.1016/j.ejps.2013.04.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golshahi L, Tian G, Azimi M, et al. The use of condensational growth methods for efficient drug delivery to the lungs during noninvasive ventilation high flow therapy. Pharm. Res. 2013;30(11):2917–2930. doi: 10.1007/s11095-013-1123-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian G, Longest PW, Li X, Hindle M. Targeting aerosol deposition to and within the lung airways using excipient enhanced growth. J. Aerosol Med. Pulm. Drug Deliv. 2013;26(5):248–265. doi: 10.1089/jamp.2012.0997. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Löndahl J, Jakobsson JKF, Broday DM, Aaltonen HL, Wollmer P. Do nanoparticles provide a new opportunity for diagnosis of distal airspace disease? Int. J. Nanomedicine. 2017;12:41–51. doi: 10.2147/IJN.S121369. www.dovepress.com/do-nanoparticles-provide-a-new-opportunity-for-diagnosis-of-distal-air-peer-reviewed-article-IJN [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Löndahl J, Pagels J, Boman C, et al. Deposition of biomass combustion aerosol particles in the human respiratory tract. Inhal. Toxicol. 2008;20(10):923–933. doi: 10.1080/08958370802087124. [DOI] [PubMed] [Google Scholar]
- 30.Lo J, Massling A, Swietlicki E, et al. Experimentally determined human respiratory tract deposition of airborne particles at a busy street. Environ. Sci. Technol. 2009;43:4659–4664. doi: 10.1021/es803029b. [DOI] [PubMed] [Google Scholar]
- 31.Grasmeijer N, Frijlink HW, Hinrichs WLJ. An adaptable model for growth and/or shrinkage of droplets in the respiratory tract during inhalation of aqueous particles. J. Aerosol Sci. 2016;93:21–34. Epub ahead of print. [Google Scholar]
- 32.Krieger UK, Marcolli C, Reid JP. Exploring the complexity of aerosol particle properties and processes using single particle techniques. Chem. Soc. Rev. 2012;41(19):6631–6662. doi: 10.1039/c2cs35082c. www.ncbi.nlm.nih.gov/pubmed/22739756 [DOI] [PubMed] [Google Scholar]
- 33.Peng C, Chow AHL, Chan CK. Study of the hygroscopic properties using single particle levitation. Pharm. Dev. Technol. 2000;17(9):1104–1109. doi: 10.1023/a:1026409813779. [DOI] [PubMed] [Google Scholar]
- 34.Rovelli G, Miles REH, Reid JP, Clegg SL. Accurate measurements of aerosol hygroscopic growth over a wide range in relative humidity. J. Phys. Chem. A. 2016;120:4376–4388. doi: 10.1021/acs.jpca.6b04194. http://pubs.acs.org/doi/abs/10.1021/acs.jpca.6b04194 [DOI] [PubMed] [Google Scholar]
- 35.Marsh A, Miles REH, Rovelli G, et al. Influence of organic compound functionality on aerosol hygroscopicity: dicarboxylic acids, alkyl-substituents, sugars and amino acids. Atmos. Chem. Phys. 2017;17:5583–5599. www.atmos-chem-phys-discuss.net/acp-2016–1051 [Google Scholar]
- 36.Pajunoja A, Lambe AT, Hakala J, et al. Adsorptive uptake of water by semisolid secondary organic aerosols in the atmosphere. Geophys. Res. Lett. 2015;42:3063–3068. [Google Scholar]
- 37.Hickey AJ, Martonen TB. Behavior of hygroscopic pharamceutical aerosols and the influence of hydrophobic additives. Pharm. Res. 1993;10(1):1–7. doi: 10.1023/a:1018952425107. [DOI] [PubMed] [Google Scholar]
- 38.Haddrell AE, Hargreaves G, Davies JF, Reid JP. Control over hygroscopic growth of saline aqueous aerosol using pluronic polymer additives. Int. J. Pharm. 2013;443(1–2):183–92. doi: 10.1016/j.ijpharm.2012.12.039. http://apps.isiknowledge.com/full_record.do?product=UA&search_mode=Refine&qid=3&SID=V2aL3@@6e94GlGMn376&page=1&doc=1 [DOI] [PubMed] [Google Scholar]
- 39.Davies JF, Miles REH, Haddrell AE, Reid JP. Temperature dependence of the vapor pressure and evaporation coefficient of supercooled water. J. Geophys. Res. - Atmos. 2014;119:10,931–10,940. [Google Scholar]
- 40.Davies JF, Haddrell AE, Miles REH, Bull CR, Reid JP. Bulk, surface and gas-phase limited water transport in aerosol. J. Phys. Chem. A. 2012;116:10987–98. doi: 10.1021/jp3086667. http://apps.isiknowledge.com/full_record.do?product=UA&search_mode=Refine&qid=3&SID=V2aL3@@6e94GlGMn376&page=1&doc=4 [DOI] [PubMed] [Google Scholar]
- 41.Davies JF, Miles REH, Haddrell AE, Reid JP. Influence of organic films on the evaporation and condensation of water in aerosol. Proc. Natl. Acad. Sci. USA. 2013;110(22):8807–8812. doi: 10.1073/pnas.1305277110. www.ncbi.nlm.nih.gov/pubmed/23674675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Power RM, Simpson SH, Reid JP, Hudson AJ. The transition from liquid to solid-like behavior in ultrahigh viscosity aerosol particles. Chem. Sci. 2013;4(6):2597–2604. http://pubs.rsc.org/en/content/articlehtml/2013/sc/c3sc50682g [Google Scholar]
- 43.Bones DL, Reid JP, Lienhard DM, Krieger UK. Comparing the mechanism of water condensation and evaporation in glassy aerosol. Proc. Natl. Acad. Sci. USA. 2012;109(29):11613–11618. doi: 10.1073/pnas.1200691109. http://apps.isiknowledge.com/full_record.do?product=UA&search_mode=Refine&qid=3&SID=V2aL3@@6e94GlGMn376&page=1&doc=11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marsh A, Rovelli G, Song Y-C, et al. Accurate representations of the physicochemical properties of atmospheric aerosols: when are laboratory measurements of value? Faraday Discuss. 2017;200:639–661. doi: 10.1039/c7fd00008a. http://pubs.rsc.org/en/Content/ArticleLanding/2017/FD/C7FD00008A [DOI] [PubMed] [Google Scholar]
- 45.Song YC, Haddrell AE, Bzdek BR, et al. Measurements and predictions of binary component aerosol particle viscosity. J. Phys. Chem. A. 2016;20:8123–8137. doi: 10.1021/acs.jpca.6b07835. http://pubs.acs.org/doi/abs/10.1021/acs.jpca.6b07835 [DOI] [PubMed] [Google Scholar]
- 46.Davies JF, Haddrell AE, Reid JP. Time-resolved measurements of the evaporation of volatile components from single aerosol droplets. Aerosol Sci. Technol. 2012;46(6):666–677. www.tandfonline.com/doi/abs/10.1080/02786826.2011.652750 [Google Scholar]
- 47.Hopkins RJ, Reid JP. A comparative study of the mass and heat transfer dynamics of evaporating ethanol/water, methanol/water, and 1-propanol/water aerosol droplets. J. Phys. Chem. B. 2006;110(7):3239–3249. doi: 10.1021/jp056523g. [DOI] [PubMed] [Google Scholar]
- 48.Homer CJ, Jiang XM, Ward TL, Brinker CJ, Reid JP. Measurements and simulations of the near-surface composition of evaporating ethanol–water droplets. Phys. Chem. Chem. Phys. 2009;11(36):7780–7791. doi: 10.1039/b904070f. [DOI] [PubMed] [Google Scholar]
- 49.Baldelli A, Boraey M a, Nobes DS, Vehring R. Analysis of the particle formation process of structured microparticles. Mol. Pharm. 2015;12:2562–2573. doi: 10.1021/mp500758s. http://pubs.acs.org/doi/abs/10.1021/mp500758s [DOI] [PubMed] [Google Scholar]
- 50.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008;25(5):999–1022. doi: 10.1007/s11095-007-9475-1. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2292490&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vehring R, Foss WR, Lechuga-Ballesteros D. Particle formation in spray drying. J. Aerosol Sci. 2007;38(7):728–746. [Google Scholar]
- 52.Baldelli A, Power RM, Miles REH, Reid JP, Vehring R. Effect of crystallization kinetics on the properties of spray dried microparticles. Aerosol Sci. Technol. 2016;50(7):693–704. www.tandfonline.com/doi/abs/10.1080/02786826.2016.1177163 [Google Scholar]
- 53.Boraey MA, Vehring R. Diffusion controlled formation of microparticles. J. Aerosol Sci. 2014;67(2014):131–143. http://linkinghub.elsevier.com/retrieve/pii/S0021850213002140 [Google Scholar]
- 54.Leung SSY, Parumasivam T, Gao FG, et al. Production of inhalation phage powders using spray freeze drying and spray drying techniques for treatment of respiratory infections. Pharm. Res. 2016;33(6):1486–1496. doi: 10.1007/s11095-016-1892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoe S, Ivey JW, Boraey MA, et al. Use of a fundamental approach to spray-drying formulation design to facilitate the development of multi-component dry powder aerosols for respiratory drug delivery. Pharm. Res. 2014;31(2):449–465. doi: 10.1007/s11095-013-1174-5. [DOI] [PubMed] [Google Scholar]
- 56.Mansour HM, Hickey AJ. Raman characterization and chemical imaging of biocolloidal self- assemblies, drug delivery systems, and pulmonary inhalation aerosols: a review. Aaps Pharmscitech. 2007:8. doi: 10.1208/pt0803064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhavna, Ahmad FJ, Mittal G, et al. Nano-salbutamol dry powder inhalation: a new approach for treating broncho-constrictive conditions. Eur. J. Pharm. Biopharm. 2009;71(2):282–91. doi: 10.1016/j.ejpb.2008.09.018. www.ncbi.nlm.nih.gov/pubmed/18984050 [DOI] [PubMed] [Google Scholar]