Abstract
BACKGROUND AND AIMS:
Vaporized nicotine products (VNPs) can vary in important characteristics including size, shape, flavor, and nicotine yield. We examined whether complex interactions among these characteristics could affect smokers’ VNP perceptions and usage patterns.
DESIGN:
A within-subject randomized crossover trial.
SETTING:
Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
PARTICIPANTS:
Eighteen daily cigarette smokers.
MEASUREMENTS:
Participants attended 8 weekly visits during which they sampled six different VNPs (disposable, rechargeable, eGO, mod, e-Cigar, and e-Pipe) with tobacco flavored e-liquid. Prior to device use, participants completed product-ranking questionnaires. Participants completed controlled puffing sessions during each of the six trials, after which satisfaction questionnaires were completed and blood samples were taken.
FINDINGS:
Initial perceptions showed that the smallest device (disposable) was ranked as safer compared with a larger device (e-Pipe) (p<.05). Participants rated the eGO and mod devices higher on satisfaction and enjoyment from use, taste, pleasantness, harshness (“throat hit”), and speed of effect, but lower on perceived health risk and embarrassment from use (p<.05). All devices had a lower Cmax than the combustible cigarette (p<.05), but there were differences among devices (p<.05). The mod, e-Pipe, and eGO provided the highest amount of perceived smoking urge relief, and this correlated strongly with Cmax across all devices (R2=.8614, p=.007). The perceived speed of urge relief was not correlated with Tmax (R2=.0035, p=.911)
CONCLUSIONS:
Daily cigarette smokers testing six types of vaporized nicotine products (VNPs) reported that they varied in taste, amount of withdrawal relief, harshness, embarrassment from use, perceived health risk, and subjective and objective nicotine delivery. The eGO and mod models have properties that may make them most effective for cigarette substitution among smokers who intend to switch to a VNP.
INTRODUCTION
Vaporized nicotine products (VNPs) deliver nicotine through aerosolization instead of combustion, and may be useful in tobacco harm reduction1–3, with use of these devices increasing substantially in recent years. In a study of 28 European Union member states, the prevalence of “ever users” increased from 11.6% in 2014 to 14.6% in 2017.4 In the United States, rates of ever use of VNPs increased significantly from 12.6% in 2014 to 15.3% in 2016. Between 2014 and 2016, those aged 18-44 showed a 3.8% increase in ever use while those aged 45-64 only showed a 2.5% increase in ever use.5 However, despite increases in ever use, current use (use every day or some days) of VNPs decreased from 3.5% in 2015 to 2.8% in 2017.6,7Frequent users of VNPs view these products as equally satisfying and less dangerous than combustible cigarettes,8 and studies demonstrate that the vapor produced by VNPs contains significantly lower (9- to 450-fold less) tobacco-specific toxicants, as compared with combustible cigarettes.9 Several studies have noted that VNPs reduce exposure to toxicants as compared with combustible cigarettes and have quantified potential toxicants associated with VNP use and exposure.9–13 There is substantial evidence that a complete switch from a combustible cigarette to VNP can reduce short term adverse health outcomes in several organ systems.10
In addition to reduced exposure to toxicants, studies also indicate that there are differences in rates of smoking cessation among users of different VNP classes (i.e. cigalike vs. tank).14 Vaporized nicotine products have been shown to differ in nicotine delivery,15−17 reduction of nicotine withdrawal symptoms,18 and type of VNP used differs between daily and non- daily users.19A recent study showed that over one year, 87.0% of smokers did not use a VNP, 6.9% of smokers became experimental VNP users, and only 2.6% became daily VNP users.20 This study also found that cigarette smokers who became daily VNP users were about 8 times more likely than non-users to self-report 30-day cigarette cessation.20 Several studies have examined individual characteristics of VNPs.15–18 Hajek et al. showed that different classes of VNPs deliver differing amounts of nicotine, but subjective effects were not studied.18 Notably, this study was comprised of experienced VNP users, while our study was comprised of daily smokers with little VNP experience. We are unaware of any comprehensive and systematic studies to assess both product and user characteristics that could account for the low percentage of experimenters who transition to daily VNP use. It is important to determine the characteristics of VNPs that could both facilitate and hinder a tobacco cigarette smoker attempting to transition to a VNP for harm reduction purposes.
To further assess factors that could both inhibit and facilitate the use of VNPs, we conducted a randomized control trial that had the following objectives: 1) to measure systemic nicotine delivery from each device; 2) to assess initial perceptions of various types of VNPs; 3) to assess subjective effects after one-week of experimentation with each device; and 4) assess possible interplay between these factors (i.e. interplay between nicotine delivery and subjective effects).
MATERIALS AND METHODS
Subjects
This study, a randomized within-subject crossover design, recruited 18 nicotine dependent, daily, regular adult cigarette smokers between May 2016 and February 2018 using advertisements in local newspapers and websites (e.g. Craigslist, Buffalo Healthy Living magazine). Initial eligibility was assessed via phone screening and final eligibility was confirmed with a physical exam at the screening visit. Eligible subjects were between the ages of 18 and 55, smoked ≥ 10 CPD, had nicotine dependence assessed as > 4 on the FTND,21 were healthy and had no history of alcohol dependence in the past 12 months on the basis of a medical history, and at physical exam had expired CO breath test ≥ 8ppm along with negative urine illicit drug and pregnancy tests.
Products
The types of VNPs tested in this study were: 1) disposable; 2) rechargeable; 3) eGO; 4) mod; 5) e-Cigar; and 6) e-Pipe. Products 1 and 2 are early generation “cigalike” devices, products 3 and 4 are later generation devices, and products 5 and 6 are more niche devices. These devices were chosen to capture the wide range of products available on the market. The selected devices differed in many design characteristics such as shape, weight, color, location of mouthpiece, manner in which users hold and puff. Photos of all devices can be seen in Figure S2. In order to select VNP brands for the clinical trial, we performed laboratory testing of the five most popular brands (done by screening web resources and marketing reports of the US market as of 2016, in 2016 the JUUL was not on the market) from each category of VNPEach device was tested using a smoking machine (Borgwaldt LX-1) and a puffing protocol (55mL puff volume, 3 sec puff duration, 30 sec interval between puffs) to determine an average nicotine yield in 20 puffs of each device.22 Variations in nicotine content across brands and from various batches of the same brand were measured and products with the lowest within-brand variability were chosen. Device brands, models, and characteristics are presented in Table 1. The disposable, rechargeable, and e-Cigar were pre-filled by the manufacturer with tobacco flavored nicotine solution, and the highest available nicotine concentration was purchased for each of these devices. The eGO, mod, and e-Pipe were filled with House of Vapor (Buffalo, NY) classic tobacco flavor e-liquid (density 1.062, corresponding to an approximately 80:20 PG:VG ratio) . The Mod was set to its highest setting (listed power 15W, coil resistance 2.6Ω) and the participant was unable alter this during the study sessions. Participants were also asked to bring and smoke their usual brand of tobacco cigarettes (reference product).
Table 1.
Characteristics of Vaporized Nicotine Products (VNPs) used in study.
| Type | Brand | Model | Coil Resistance (Ω) | Power (W) | Battery Output Voltage (V) | Nicotine Concentration in Refill Solution (mg/ml) | pH of Refill Solution | Nicotine Yield Delivered with 20 Puffs (mg)* | Length (cm) | Weight of prefilled device (g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Labeled | Measured | ||||||||||
| Disposable | v2 | Fine Electric Disposable Cigarette | 2.90 | Listed: none Calculated: 5.41 | 3.96 | 18 | 11.7 | 8.69 | 0.64 | 10.6 | 10.2 |
| Rechargeable | Green Smoke | Rechargeable E-Cigarettes Express Kit | 3.35 | Listed: none Calculated: 4.31 | 3.80 | 24 | 19.4 | 7.59 | 1.53 | 11.6 | 17.6 |
| eGO | v2 | Pro Series 3-in-1 vaporizer | 3.30 | Listed: none Calculated: 5.19 | 4.14 | 24 | 29.9 | 9.86 | 2.54 | 14.3 | 56.1 |
| Mod | iTazte | VTR vaporizer | 2.60 | Listed: 15.0 Calculated: 14.1 | 6.06 | 24 | 29.9 | 9.86 | 9.11 | 14.2 | 380 |
| e-Cigar | Cuvana | e-Cigar | --** | --** | --** | 18 | 15.5 | 9.22 | 1.00 | 15.1 | 45.4 |
| e-Pipe | Smoktech | Guardian e-Pipe Mod II | 2.50 | Listed: 15.0 Calculated: 14.4 | 6.01 | 24 | 29.9 | 9.86 | 1.00 | 13.3 | 275 |
Laboratory determined using a smoking machine (Borgwaldt LX-1)
unable to test e-Cigar battery output voltage, coil resistance, and power due to product design
Study Design
Participants attended 8 sessions (visits), each one week apart. At visit 1, participants provided informed consent and eligibility was determined. A product ranking questionnaire was administered at visit 1 and participants were randomized to the order of the 6 devices by a study statistician using the Williams Design, a special case orthogonal latin squares design, generated using SAS v9.3 (Cary, NC).23 Neither participant nor study staff were blinded to condition.
At visit 2, participants were asked to smoke their preferred brand of tobacco cigarette ad lib (reference). Throughout this visit, participants completed questionnaires pertaining to the subjective effects of nicotine delivered by the tobacco cigarette, and blood samples were collected to assess the individual’s nicotine pharmacokinetic profiles. At the end of this visit, participants were asked to practice using the VNP device during the upcoming week and were scheduled to return the following week.
During visits 3 to 7, participants underwent a controlled puffing session using the assigned device for 10 mins, puffing every 30 sec (total of 20 puffs). Throughout this visit, participants completed the subjective effect questionnaires and blood samples were taken. A device satisfaction questionnaire was administered during this visit, following the puffing session. At visit 8, participants completed the same procedure as visits 3 to 7, but an additional product ranking questionnaire was administered.
During visits 2 through 8, participants were asked to abstain from smoking for at least 8 hours prior to each visit, confirmed by an expired CO < 8ppm to control for withdrawal and craving. Participants were compensated $40 for their first visit with a $10 increase in payment for each subsequent visit. If participants completed all 7 study sessions, they received a $50 bonus. This study was approved by the Roswell Park Comprehensive Cancer Center Institutional Review Board. A complete schema for the study design can be seen in Figure S3.
Pharmacokinetic Analysis
Venous blood samples (4 ml) were collected to measure nicotine using a butterfly needle before and at 2, 4, 5, 6, 8, 10, 13, 15, 20, 30, 45, 60, 90, and 120 mins after the onset of use of each product. Plasma nicotine concentration was determined at the Clinical Pharmacology Laboratory at the University of California, San Francisco, using GC-MS/MS24 which was modified for tandem mass spectrometry for improved sensitivity.25 The limit of quantitation (LOQ) was 1.0 ng/mL. Any time points at which plasma nicotine concentration was below LOQ were replaced with . To analyze changes in plasma nicotine attributable only to study product use, all nicotine concentrations were corrected for baseline using subtraction of a projected level based on log linear decline (Ct=C0e-Kt, K calculated using t1/2=.693/K, t1/2= 120 minutes).26 Pharmacokinetic parameters were estimated using PCModfit for Excel v. 6.0.27 The following parameters were estimated using a non-compartmental model and trapezoidal rule: maximum concentration of nicotine in plasma (Cmax), time to maximum concentration of nicotine in plasma (Tmax), and the area under the plasma concentration - time curve to 10 mins and 120 mins (AUC 0→10 min, AUC0→120 min). AUC0→10 min was measured to assess the rate of nicotine delivery over the 10 minute regulated puffing session. Tmax was determined from the start of the puffing session.
Subjective Measures
At the screening visit and at the final visit, participants completed a product-ranking questionnaire to assess anticipated enjoyment, satisfaction, and perceived danger to health. This allows for a comparison of devices prior to any use and following use of all devices.
During visit 2 to 8, nicotine withdrawal symptoms were assessed using an adapted Minnesota Nicotine Withdrawal Scale (MNWS)28 before product use, and at 3, 15, and 110 mins after product use. This measure was given before product use to control for baseline withdrawal symptoms. This assesses 5 DSM-IV criteria for tobacco withdrawal (e.g. “Depressed,” “Irritable,” “Restless,” “Hungry,” and “Poor Concentration”) on a 5-point Likert scale from “Not at all” to “Extremely.” Total score is assessed for this scale.29
Craving to smoke was measured using the Questionnaire of Smoking Urges-Brief (QSU-B) at 3, 15, and 110 mins after the regulated puffing session. This is a 10-item measure in which participants are asked to score each statement from 0 (strongly agree) to 100 (strongly disagree) (e.g. “I have a desire for a cigarette right now”). This measure is used as a reliable global measure of craving.30
Subjective responses to the effects of nicotine were measured using the Drug Effect Questionnaire (DEQ-5) at 1, 3, 5, 7, 10, 15, 20, 35, 50, 80, and 110 mins after the regulated puffing session. This is a 5-item measure in which participants are asked to indicate responses to questions by placing a mark between the response anchors (“Not at all” to “Extremely”). Each item is analyzed as a distinct construct. It has been shown that each DEQ item reflects pharmacological substance-induced effects.31 These three measures were used to assess comparison between the VNPs and the participant’s preferred brand tobacco cigarette.
A 12-item adapted product evaluation scale32 was used to assess satisfaction with and helpfulness of each VNP following product use. Validity of this scale was supported by a significant relationship between subjective responses and product choice.32 Participants were asked to rate VNPs on a scale from 0-100 on how satisfying, enjoyable, and dangerous to health the product was compared to a tobacco cigarette. On this scale, a score of 50 indicated that the device was the same as the participant’s tobacco cigarette. Additionally, participants were asked to rate each product on a Likert scale on measures such as how good the product tasted, how much the product tasted like a tobacco cigarette, pleasantness, harshness, urge relief, speed of urge relief, and embarrassment from using the product. These measures compared device satisfaction to the participant’s preferred brand tobacco cigarette. All measures can be seen in supplemental Tables S2–S6.
Statistical Analysis
Sample size was estimated to detect an AUC difference at a two-sided alpha of .05, if the true difference between the dosing regiments is 700 ng/mL × min, with the assumption that the within-patient standard deviation of response is 500 ng/mL × min.33 All analyses were performed using Stata 14.2 (Stata Corporation, College Station, TX). Descriptive statistics and frequencies were used to characterize the study sample. The Kruskal-Wallis H test was used to evaluate the three ranking measures, satisfaction, MNWS, QSU, and DEQ, as well as pharmacokinetic data parameters, across all devices. The Wilcoxon signed rank test was used for a pairwise comparison between each device on each of these measures. Additionally, the Krukal-Wallis H test was used to evaluate differences between participants who had ever used a VNP and those who had never used a VNP (in this analysis, ever use was operationalized as a binary variable). Due to the small sample size and exploratory nature of this study, all p-values presented reflect pairwise comparisons, but Hochberg34 adjusted values (to control for familywise error) can be seen in supplementary material Tables S7b–S25b.
RESULTS
Participant Demographics
As shown in Figure 1, 35 subjects were consented, of whom 18 completed all visits; there were no significant differences in demographic characteristics between those who did or did not complete the trial (p>.05). A flow diagram documenting how the study sample size was attained is shown in Figure 1. This figure also shows the main reasons for participant drop out. The final completing sample consisted of 9 male and 9 female smokers with an average age of 41.3±9.7 years, FTND of 6.5, and CPD of 16.6. At baseline, the average CO level was 13.7±6.9 ppm. Of the 18 participants who completed the study, 11 had prior experience with a VNP. For those who had used a VNP in the past, 7 last used a VNP more than a year ago, 3 used a VNP “a few months ago”, and 1 last used a VNP “about a month ago”. There were no significant differences on measures between ever users of VNP and never users of VNP. Strict inclusion and exclusion criteria resulted in a high number of screened individuals and low number of completers. Demographics for those who consented, but did not complete the study and those who completed the study did not differ.
Figure 1.
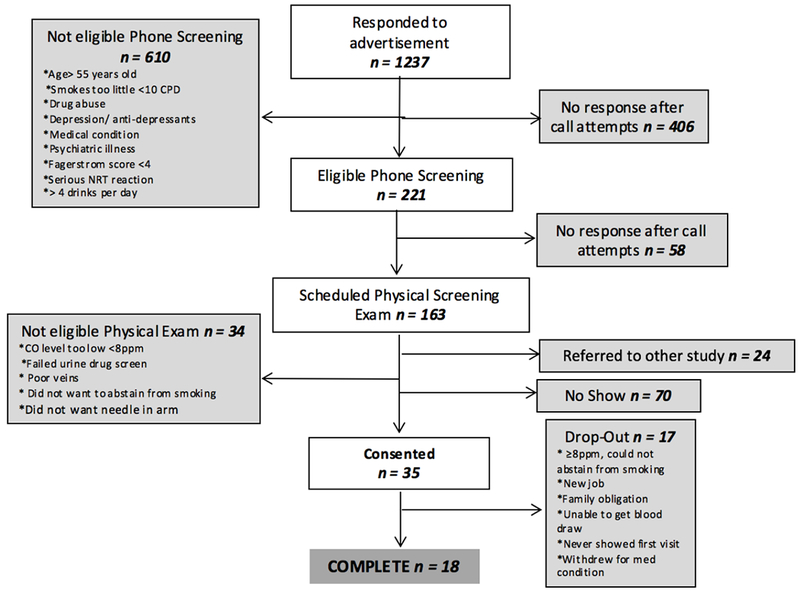
Participant flow-chart.
Nicotine Delivery from VNPs
Results of pharmacokinetic analysis can be seen in Table 2. The median Cmax for VNPs ranged from 3.21 ng/ml (delivered by the e-Cigar) to 6.60 ng/ml (delivered by the mod). The Cmax for the tobacco cigarette (median 18.9 ng/ml) was significantly larger than all VNPs (p< .05). The median Tmax for VNPs ranged from 10 mins (immediately after last puff) to 13 mins after initiation of use. The Tmax for the tobacco cigarette was significantly lower than the disposable (p=.0350), rechargeable (p=.0382), mod (p=.0100), and e-Pipe (p=.0359). There were no significant differences between VNPs. The AUC0→120 for the tobacco cigarette and the mod were significantly greater than the AUC0→120 for the disposable (p=.0080, p=.0168), rechargeable (p=.0074, p=.0311), and E-cigar (p=.0198, p=.0020). A graph of the plasma nicotine profiles of each device can be seen in Figure 2. All comparisons can be seen in supplementary Tables S22a–S23a.
Table 2.
Pharmacokinetic data among 18 smokers who used 6 different VNPs. Median value (Range).
| Product Type | Nicotine Yield in 20 Puffs (mg) | Pharmacokinetics |
|||
|---|---|---|---|---|---|
| Cmax (ng/ml) | Tmax (min) | AUC 0→120 (ng/ml/min) | AUC 0→10 (ng/ml/min) | ||
| Disposable | 0.64 | *4.07 (0.08-16.5) | *13.0 (2.0-45.0) | *88.6 (0.78-334.6) | *16.3 (0.20-79.0) |
| Rechargeable | 1.53 | *4.16 (0.71-16.2) | *10.0 (2.0-120.0) | *121.9 (6.96-509.8) | *18.1 (4.40-92.9) |
| eGO | 2.54 | *5.52 (0.16-23.0) | 10.0 (4.0-45.0) | 232.8 (1.48-920.9) | *18.9 (0.20-106.6) |
| Mod | 9.11 | *6.60 (0.06-39.5) | *10.0 (2.0-120.0) | 272.3 (0.44-1271) | *47.3 (0.20-218.8) |
| E-pipe | 1.00 | *5.31 (0.82-33.6) | *10.0 (4.0-45.0) | 113.7 (7.49-1179) | *20.2 (2.01-192.5) |
| E-cigar | 1.00 | *3.21 (0.06-18.6) | 10.0 (5.0-120.0) | *55.9 (0.44-616.8) | *13.1 (0.20-85.4) |
| Tobacco cigarette (Reference) | 1.00-1.50 | 18.9 (3.41-74.4) | 5.50 (2.0-30.0) | 347.5 (17.1-2354) | 126.3 (3.59-396.0) |
Values are averages with SD in parenthesis. Lighter shading of a box indicates the device with the lowest value, and a darker shading indicates the device with the highest value.
Significant difference from the participant’s tobacco cigarette. (p<.05)
Figure 2.
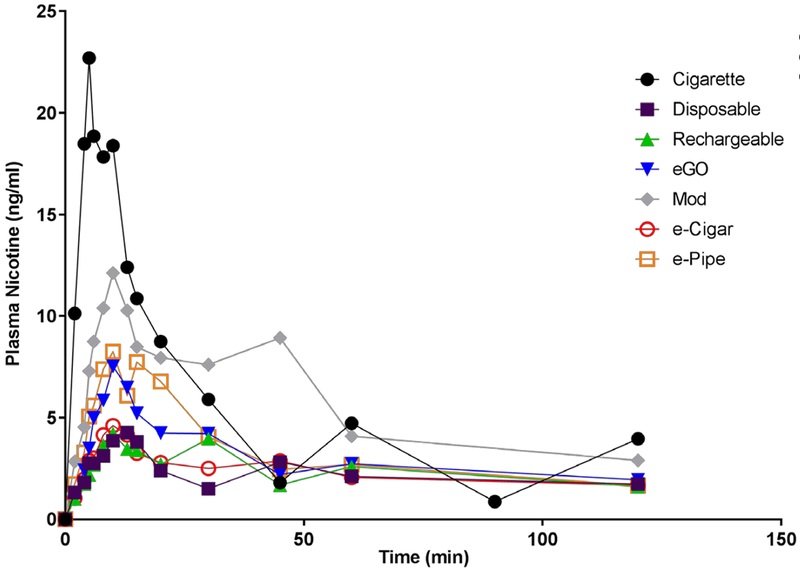
Average plasma nicotine profiles for each device and participants preferred brand tobacco cigarette.
Perception of VNPs Before and After Trial
Prior to use, the e-Pipe was ranked lowest on anticipated satisfaction and enjoyment. After use of all products, the disposable was ranked lowest on satisfaction, while the e-Cigar was ranked lowest on enjoyment. The mod and the eGO were ranked highest on satisfaction and enjoyment both prior to and after use. At the initial and final visits, the disposable was ranked lowest on perceived harm, while the e-Pipe was ranked highest on perceived harm. Interestingly, the disposable was also the smallest device, while the e-Pipe was the second largest device. Before first use, there were fewer significant differences between devices than after all devices were used. The devices were seen by participants as more similar prior to use, and less similar after using each of the devices. Specific values for each comparison can be viewed in supplementary Tables S7a – S9a.
Figure 3 shows how each device scored on multiple subjective measures. There were significant differences between devices on multiple measures of satisfaction. The most satisfying and enjoyable devices were the eGO and mod; both had scores greater than 50 indicating that participants reported more enjoyment and satisfaction compared to their preferred brand of tobacco cigarette. All devices were viewed as less harmful than the preferred brand of tobacco cigarette. The eGO and mod had the fastest perceived urge relief, and both were significantly faster than the e-Cigar (p<.05). The disposable was rated as significantly less harsh than all devices (p<.05) except the rechargeable. No device tasted as good or better than a tobacco cigarette (i.e., no device had an average score at or above 3), and only the mod approached being as pleasant as their preferred brand cigarette. All devices were, on average, seen as less embarrassing than their preferred brand of cigarette, and the mod was seen as the least embarrassing of all devices. Specific values for each comparison can be viewed in supplementary Tables S10a – S18a.
Figure 3.
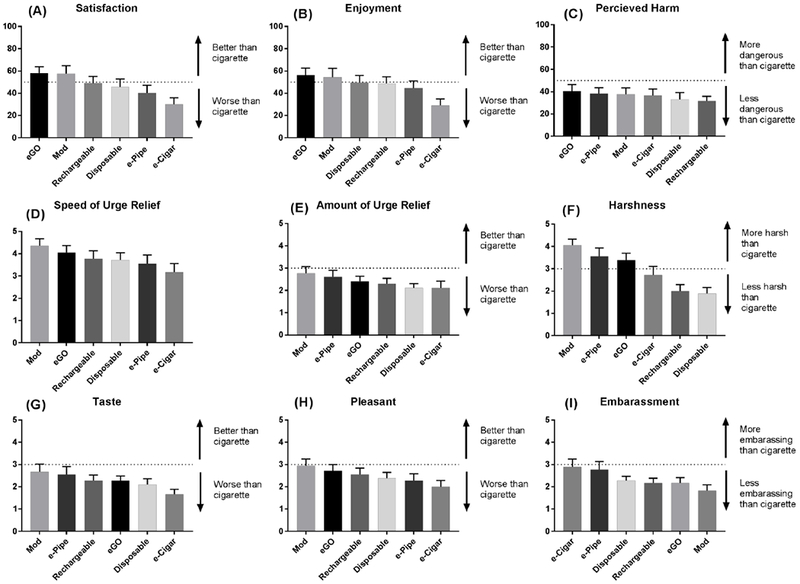
Subjective rating of various VNPs: (A) Satisfaction with VNPs. (B) Perceived harm of VNPs. (C) Enjoyment from using VNPs. (D) Speed of smoking urge relieve after VNP use. (E) Amount of urge to smoke relieved after VNP use. (F) Harshness of inhaled VNP aerosol. (G) Taste of inhaled VNP aerosol. (H) Pleasantness of inhaling VNP aerosol. (I) Embarrassment from using VNPs.
Subjective Effects of VNP Use
Figure 4 illustrates change in DEQ, QSU-B, and MNWS scores over time following VNP use. The first question of the DEQ, which assessed subjective effects of nicotine, was analyzed. At 3 minutes post use, the device that provided a significantly lower average feeling of nicotine effect was the e-Cigar as compared with the tobacco cigarette (p=.0410), eGO (p=.0449) and the mod (p=.0166). The tobacco cigarette provided more nicotine feeling than the disposable (p=.0410) and e-Cigar (p=.0244). There was no clear difference between the tobacco cigarette and the mod or eGO (p>.05) at 3 mins. Withdrawal symptoms were analyzed by subtracting the MNWS total score prior to device use from all other time points. The score prior to use was considered the baseline, and all subsequent scores demonstrate a reduction in withdrawal symptoms from baseline. At 3 minutes post use, withdrawal did not appear to differ significantly among the devices. At 3 minutes post use, there was little evidence of significant differences in smoking urges among the devices, or between VNPs and tobacco cigarette (p>.05). Comprehensive device comparisons for each measure can be seen in supplementary Tables S19a–S21a.
Figure 4.
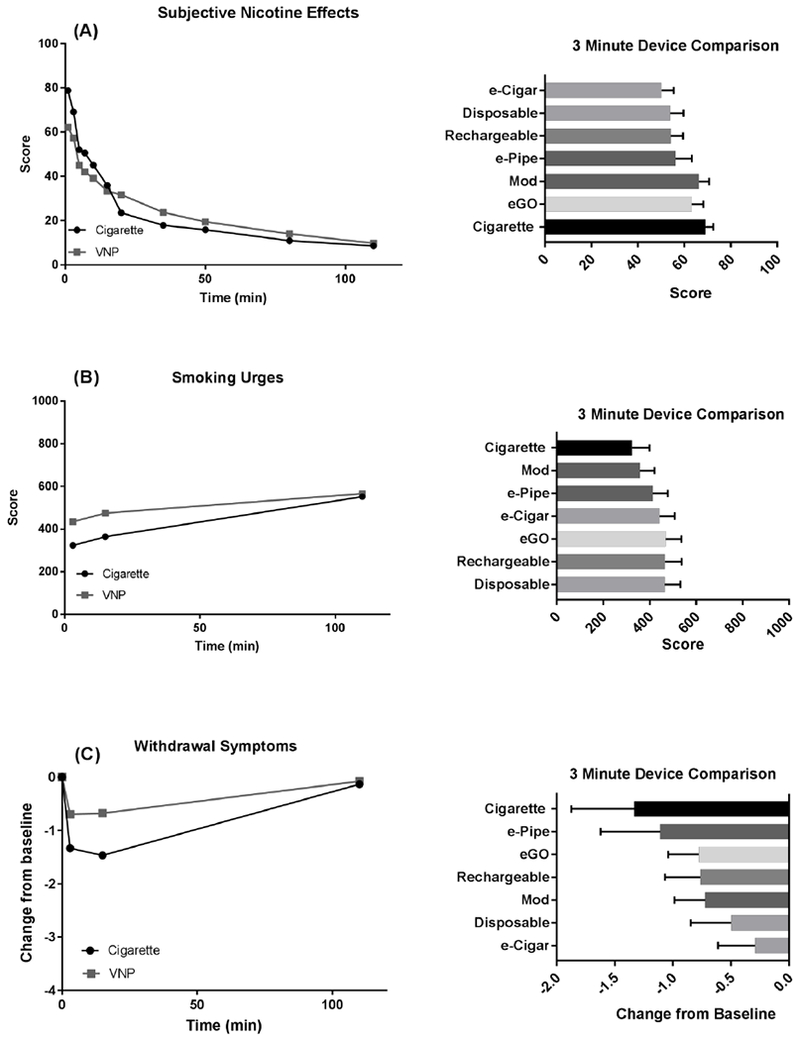
Subjective rating of nicotine effects 3 minutes after use of VNPs: (A) Changes in Questionnaire of Smoking Urges-Brief (QSU-B) scores after VNP use (left) and comparison of VNP effects 3 minutes after use (right). (D). Changes in smoking urges after VNP use (left; corrected for baseline) and comparison of VNP effects 3 minutes after use. (C). Changes in Minnesota Nicotine Withdrawal Scale (MNWS) scores after VNP use (left; corrected for baseline) and comparison of VNP effects 3 minutes after use
Interplay Between Nicotine Delivery and Subjective Effects after VNP Use
Figure 5a illustrates the relationship between mean device Cmax and perceived amount of urge relief (assessed through the satisfaction questionnaire). There was a significant correlation between the two variables (R2=.8614, p=.007). Surprisingly, despite all devices having a similar mean Tmax, they were viewed as providing different subjective speed of urge relief. Figure 5b illustrates the relationship between average device Tmax and perceived speed of urge relief. There is a non-significant correlation between these two variables (R2=.0035, p=.911). Additionally, we noted a correlation between the pH of the e-liquid and the perceived harshness of the device (Figure 5c); as pH increased, the perceived harshness of the devices increased linearly (R2=.7556).
Figure 5.
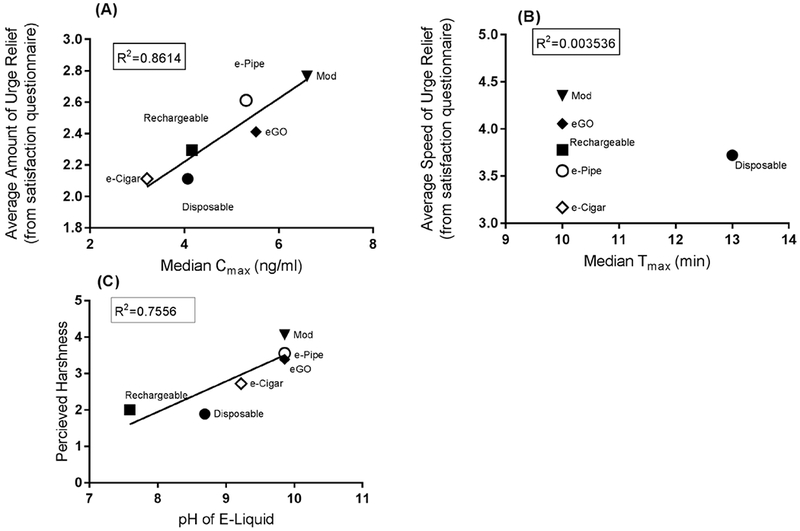
(A) Correlation between median Cmax with subjective urge relief after use of various Vaporized Nicotine Products (VNPs); (B) Correlation between median Tmax with perceived speed of urge relief after VNP use; (C) Correlation between perceived harshness of device and pH of refill solution.
DISCUSSION
In this study of smokers using a variety of VNP devices, the eGO and mod were rated higher than other devices on multiple measures of satisfaction and subjective nicotine effects; the eGO and the mod also delivered a greater amount of nicotine when compared with other devices. Many smokers who use a VNP do so to either cut down or to stop smoking.35–36 Based on our results, the eGO or the mod are likely to be the most effective devices for a tobacco cigarette substitution among smokers who intend to switch to a VNP.
The eGO, mod, and e-Pipe provided the highest Cmax and the highest AUC0→120 and AUC0→10. All devices were significantly different than the tobacco cigarette on Cmax; however, there were fewer significant differences between devices and tobacco cigarettes on Tmax. The difference that were seen between the tobacco cigarette and VNPs could be due to a difference in the time it took participants to smoke their tobacco cigarette ad lib versus the regulated puffing session for VNPs.
The initial perception of a VNP may be an important facilitator or barrier to a tobacco cigarette smoker purchasing or trying a specific VNP type for the first time. At both pre- and post-use, the eGO and mod were ranked higher than other devices on satisfaction and enjoyment. At pre-use, the disposable (the smallest device) was ranked as least harmful, and this ranking continued at post-use. Additionally, we observed a greater number of significant differences among devices at post-use compared to the number of differences at pre-use. This suggests that experience with the VNPs may serve to highlight salient differences among devices.
The eGO and mod both had higher average scores on satisfaction and enjoyment as compared with other devices. These two devices also surpassed the satisfaction and enjoyment that participants felt from their tobacco cigarettes. In a qualitative study of VNP users, Notley et al. found a similar pattern.37 VNPs can replicate the physical, behavioral, and social aspects of smoking37 with the additional benefit of their perception as less harmful, and this interaction may be what leads smokers to find more satisfaction and enjoyment from VNPs. All devices were seen as less harmful to their health than tobacco cigarettes, which aligns with previous research.8,35,38 There was high variability in the perceived harshness between device types with the eGO and mod rated as most harsh. It has been shown that different flavors have the capacity to produce different “throat hit” feelings, and this characteristic is similar to the effect of tobacco cigarette smoke.39 Cigarette smokers have stated a preference for devices that provide this feeling due to more satisfaction and fulfillment.40 This may, in part, explain the results of harshness and satisfaction ratings of different devices. The three harshest devices were the eGO, mod, and e-Pipe, and these ratings could help to explain why the eGO and mod were more satisfying and enjoyable than other devices.
To our knowledge, this study is the first study to look at the complex interplay between factors described above (subjective vs objective responses). Our pharmacokinetic results from smokers align with those found by Hajek et al., for experienced users and we were additionally able to observean association between nicotine delivery and subjective experience of device use. As expected, the VNPs that delivered more nicotine also demonstrated higher mean urge relief; objective nicotine delivery and subjective amount of urge relief are correlated. However, there was no significant correlation between objective speed of nicotine delivery and subjective speed of urge relief. Lastly, a previous study showed that in a sample of smokers who tried VNPs and did not become regular uses, 6.4% gave embarrassment as a reason for non-regular use.41 In our study, the e-Pipe was seen as the second most embarrassing device to use, which could explain the disparity between the high amount of nicotine delivered and the low perceived satisfaction and enjoyment of this device.
This study has several notable strengths. It utilized a comprehensive design that allowed an examination of the complex relationships between device perception, satisfaction, subjective effects and nicotine delivery. This study also randomized the order in which tobacco cigarette users experienced six different VNPs. This novel study is able to distinguish specific VNP device characteristics that influence a tobacco cigarette user’s anticipated and actual satisfaction from these devices. VNP technology is rapidly evolving, thus there may be products with better nicotine delivery on the market today that were not available at the start of this study. Although this study was completed prior to the introduction of JUUL, a very small device with highly effective nicotine delivery,42 our results suggest that such a device could be perceived as less harmful than other VNP devices, and could be more satisfying and enjoyable than other devices used in this study.
Our study has several limitations that should be noted. All devices contained tobacco flavored e-liquid, and several studies have shown that flavoring is an important aspect of device satisfaction for many VNP users.43–44 The products studied varied in nicotine content and in nicotine delivery in aerosol, so it is not possible to disentangle the contributions of individual design elements of the devices and liquids to user evaluations. Due to the primary outcome of the study being nicotine delivery, a controlled puffing session was needed. This may not be naturalistic, and participants may adjust puffing behavior with different devices. However, the user was able to control puff duration volume (we only imposed a regulated number and frequency of puffs). Sensitivity analyses were conducted to see if interactions existed with order. On the primary outcome of nicotine delivery, there was no increase as participants completed more visits. Due to the small sample size, we did not have enough power to come to definite conclusions as to the effect of order on satisfaction. Many comparisons were assessed yet only a small percentage achieved significance. The latter two limitations could be due the exploratory nature and small sample size utilized in this study; therefore, further studies with larger sample sizes are needed.
Our results suggest that these previously unexplored characteristics of VNPs may have clinical importance for both harm reduction and smoking cessation. If daily tobacco cigarette smokers choose a VNP device that is not satisfying, they may return to smoking cigarettes and not sample another device, which may result in no net reduction in harm for that smoker. These findings also contribute to current regulatory science and our understanding of how subjective and objective factors influence use of a particular product.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Trisha Mao for performing analytical chemistry. We thank Danielle Smith for assistance with statistical methodology. This research was supported in part by NIH NIDA grant R01DA037446 and NCI grant R25CA181003. Additional partial funding from P30CA016056 to Roswell Park Comprehensive Cancer Center and lab infrastructure grant to UCSF P30 DA012393 from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST: MLG has received research grant support from Pfizer and served as a member of advisory board of Johnson&Johnson. MCM is on the speakers bureau, has served as a consultant, and has received research support from Pfizer. NLB is a consultant to Pfizer and Achieve Life Sciences and has been a paid expert witness in litigation against tobacco companies. Other authors have no conflicts to declare.
CLINICAL TRIAL REGISTRATION: clinicaltrials.gov #
REFERENCES
- 1.Polosa R, Rodu B, Caponnetto P, Maglia M, Raciti C. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm reduction journal. 2013;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajek P Electronic cigarettes for smoking cessation. The Lancet. 2013;382(9905):1614–6. [DOI] [PubMed] [Google Scholar]
- 3.Sweanor D, Alcabes P, Drucker E. Tobacco harm reduction: how rational public policy could transform a pandemic. Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 4.Laverty AA, Filippidis FT, Vardavas CI. Patterns, trends and determinants of e-cigarette use in 28 European Union Member States 2014-2017. Preventive medicine. 2018. November 1;116:13–8. [DOI] [PubMed] [Google Scholar]
- 5.Bao W, Xu G, Lu J, Snetselaar LG, Wallace RB. Changes in electronic cigarette use among adults in the united states, 2014-2016. JAMA. 2018;319(19):2039–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, Jamal A, Neff L, King BA. Tobacco product use among adults—United States, 2017. Morbidity and Mortality Weekly Report. 2018. November 9;67(44):1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips E, Wang TW, Husten CG, Corey CG, Apelberg BJ, Jamal A, Homa DM, King BA. Tobacco product use among adults—United States, 2015. MMWR. Morbidity and mortality weekly report. 2017. November 10;66(44):1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozlowski LT, Homish DL, Homish GG. Daily users compared to less frequent users find vape as or more satisfying and less dangerous than cigarettes, and are likelier to use non-cig-alike vaping products. Preventive medicine reports. 2017;6:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco control. 2014;23(2):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Academies of Sciences E, Medicine. Public health consequences of e-cigarettes. Washington, DC: The National Academies Press; doi. 2018;10:24952. [PubMed] [Google Scholar]
- 11.Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, et al. Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: a cross-sectional study. Annals of internal medicine. 2017;166(6):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine & Tobacco Research. 2017;19(2):160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine & Tobacco Research. 2014;17(6):704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations between e-cigarette type, frequency of use, and quitting smoking: findings from a longitudinal online panel survey in Great Britain. Nicotine & Tobacco Research. 2015;17(10):1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tobacco control. 2017;26(E1):e23–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Helen G, Havel C, Dempsey DA, Jacob P, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawkins L, Kimber C, Puwanesarasa Y, Soar K. First‐versus second‐generation electronic cigarettes: predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction. 2015;110(4):669–77. [DOI] [PubMed] [Google Scholar]
- 18.Hajek P, Przulj D, Phillips A, Anderson R, McRobbie H. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology. 2017. March 1;234(5):773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor RJ, Fix BV, Goniewicz ML, Travers MB, Heckman BW, Cummings KM, Hitchman S, McNeill A, Borland R, Hammond D, Levy D, Gravely S, Fong GT. Characteristics of vaporized nicotine products used by participants in the ITC Four Country Tobacco and E-Cigarette Project, Wave 1. Addiction. 2018. In Press. [Google Scholar]
- 20.Berry KM, Reynolds LM, Collins JM, Siegel MB, Fetterman JL, Hamburg NM, et al. E-cigarette initiation and associated changes in smoking cessation and reduction: the Population Assessment of Tobacco and Health Study, 2013-2015. Tobacco control. 2018:tobaccocontrol-2017-054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–27. [DOI] [PubMed] [Google Scholar]
- 22.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine Levels in Electronic Cigarettes. Nicotine & Tobacco Research. 2013;15(1):158–66. [DOI] [PubMed] [Google Scholar]
- 23.Wang B-S, Wang X-J, Gong L-K. The construction of a Williams design and randomization in cross-over clinical trials using SAS. J Stat Softw. 2009;29(1):1–10. [Google Scholar]
- 24.Jacob P III, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium‐labeled analogs: absence of an isotope effect in the clearance of (S)‐nicotine‐3′, 3′‐d2 in humans. Biological Mass Spectrometry. 1991;20(5):247–52. [DOI] [PubMed] [Google Scholar]
- 25.Helen GS, Shahid M, Chu S, Benowitz NL. Impact of e-liquid flavors on e-cigarette vaping behavior. Drug and Alcohol Dependence. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers Nicotine psychopharmacology: Springer; 2009. p. 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen GD. MODFIT: A pharmacokinetics computer program. Biopharm Drug Dispos. 1990;11:477–498. [DOI] [PubMed] [Google Scholar]
- 28.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. [DOI] [PubMed] [Google Scholar]
- 29.Etter JF, Hughes JR. A comparison of the psychometric properties of three cigarette withdrawal scales. Addiction. 2006;101(3):362–72. [DOI] [PubMed] [Google Scholar]
- 30.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 31.Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology. 2013;227(1):177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatsukami DK, Zhang Y, O’connor RJ, Severson HH. Subjective responses to oral tobacco products: scale validation. nicotine & tobacco research. 2012;15(7):1259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benowitz NL, Jacob P, Herrera B. Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clinical Pharmacology & Therapeutics. 2006;80(6):703–14. [DOI] [PubMed] [Google Scholar]
- 34.Chen S-Y, Feng Z, Yi X. A general introduction to adjustment for multiple comparisons. Journal of thoracic disease. 2017;9(6):1725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson A, Pearson J, Xiao H, Stalgaitis C, Vallone D. Prevalence, harm perceptions, and reasons for using noncombustible tobacco products among current and former smokers. Am J Public Health. 2014;104(8):1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalkhoran S, Alvarado N, Vijayaraghavan M, Lum PJ, Yuan P, Satterfield JM. Patterns of and reasons for electronic cigarette use in primary care patients. Journal of general internal medicine. 2017;32(10):1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notley C, Ward E, Dawkins L, Holland R. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduction Journal. 2018. December;15(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Zhan Y, Wang L, Leischow SJ, Zeng DD. Analysis of symptoms and their potential associations with e-liquids’ components: a social media study. BMC public health. 2016;16(1):674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbeau AM, Burda J, Siegel M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: a qualitative approach. Addict Sci Clin Pr. 2013;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kralikova E, Novak J, West O, Kmetova A, Hajek P. Do e-Cigarettes Have the Potential to Compete With Conventional Cigarettes?: A Survey of Conventional Cigarette Smokers’ Experiences With e-Cigarettes. Chest. 2013;144(5):1609–14. [DOI] [PubMed] [Google Scholar]
- 42.JUUL Labs Inc. Patents, Trademarks, Copyrights: JUUL Labs, Inc. ; 2018. [Available from: https://www.juul.com/intellectual-property-list (Archived by WebCite® at http://www.webcitation.org/75hDdg6ky)
- 43.Soule EK, Lopez AA, Guy MC, Cobb CO. Reasons for using flavored liquids among electronic cigarette users: A concept mapping study. Drug & Alcohol Dependence. 2016;166:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Litt MD, Duffy V, Oncken C. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tobacco control. 2016:tobaccocontrol-2016-053223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


