Abstract
Background
Certolizumab pegol, an Fc‐free, PEGylated, anti‐tumour necrosis factor (TNF) biologic, has demonstrated favourable results in three ongoing, phase 3, randomized, double‐blinded, placebo‐controlled trials in adults with psoriasis.
Objective
Data were pooled from the ongoing trials to investigate efficacy in selected subgroups and add precision to estimates of treatment effects during the initial 16 weeks of treatment.
Methods
In each trial, patients ≥18 years with moderate‐to‐severe chronic plaque psoriasis for ≥6 months were randomized to receive certolizumab 400 mg, certolizumab 200 mg or placebo every 2 weeks for 16 weeks. Coprimary endpoints for the pooled analysis were responder rates at Week 16, defined as ≥75% reduction in psoriasis area and severity index (PASI 75) and physician global assessment (PGA) of 0/1 (‘clear’/‘almost clear’ with ≥2‐category improvement). Safety was assessed by treatment‐emergent adverse events.
Results
A total of 850 patients treated with certolizumab 400 mg (N = 342), certolizumab 200 mg (N = 351) or placebo (N = 157) were included in the pooled analysis. At Week 16, PASI 75 and PGA 0/1 responder rates were 80.1% and 63.7% in the certolizumab 400 mg group, 74.5% and 54.6% in the certolizumab 200 mg group, and 7.5% and 2.8% in the placebo group (P < 0.0001 for each dose versus placebo). In patients with and without prior biologic therapy, both doses of certolizumab resulted in substantially higher responder rates versus placebo. The incidence of adverse events was generally similar between the 400 mg and placebo groups, and somewhat lower in the 200 mg group versus placebo. No new safety signals were identified.
Conclusion
Certolizumab pegol 400 mg or 200 mg every 2 weeks for 16 weeks was associated with statistically significant and clinically meaningful improvements in signs and symptoms of psoriasis in patients with and without prior biologic therapy, and a safety profile consistent with the anti‐TNF class in psoriasis.
Introduction
Psoriasis is a chronic inflammatory disease that affects ~3% of adults in the United States1, 2 and ~2–6% of adults in Europe.3 Treatments for moderate‐to‐severe psoriasis resistant to topical therapies include phototherapy and systemic medications; unfortunately, therapeutics for psoriasis, including biologics, may lose efficacy over time, and prior biologic therapy may negatively affect outcomes with subsequent treatments.4, 5, 6
Certolizumab pegol (CZP), an Fc‐free, PEGylated, anti‐tumour necrosis factor (TNF) biologic, does not bind the neonatal Fc receptor for IgG (FcRn) and consequently shows minimal placental transfer from mothers to infants.7 Favourable results through 48 weeks of treatment have been reported for each of three ongoing, phase 3, randomized, double‐blinded, placebo‐controlled clinical trials in adults with moderate‐to‐severe chronic plaque psoriasis (CIMPASI‐1,8 CIMPASI‐28 and CIMPACT9). Several prespecified pooled analyses of the CIMPASI‐1, CIMPASI‐2 and CIMPACT trials were performed to investigate efficacy in selected subgroups, and to add precision to estimates of treatment effects. Pooled efficacy and safety results from the first 16 weeks of these ongoing 144‐week trials are reported here, including analysis of response to treatment in patients with and without prior exposure to biologic therapy.
Materials and methods
CIMPASI‐1 (NCT02326298), CIMPASI‐2 (NCT02326272) and CIMPACT (NCT02346240) are ongoing phase 3, multicentre, double‐blinded, placebo‐controlled (and active comparator‐controlled for CIMPACT) trials, conducted in outpatient clinics in North America and Europe. The CIMPASI trials commenced in December 2014, and CIMPACT commenced in February 2015. Study protocols were approved by local institutional review boards or independent ethics committees, and were carried out in accordance with Good Clinical Practice requirements10 and the Declaration of Helsinki.11 Informed consent was acquired from study participants.
Details of the study designs have been reported elsewhere8, 9 and are summarized here. In all three studies, patients were randomized to CZP 400 mg every 2 weeks (Q2W), CZP 200 mg Q2W following a 400 mg loading dose at Weeks 0, 2 and 4, or placebo Q2W for 16 weeks. Eligible patients were ≥18 years of age with moderate‐to‐severe chronic plaque psoriasis for ≥6 months, psoriasis area and severity index (PASI) ≥12, body surface area affected (BSA) ≥10%, physician's global assessment (PGA) ≥3 on a 5‐point scale, and candidates for systemic psoriasis therapy, phototherapy, and/or photochemotherapy. Patients were excluded if they had previous treatment with CZP (or etanercept in CIMPACT only) or >2 biologics, including anti‐TNF agents. Patients with a history of primary failure to any biologic or secondary failure to >1 biologic, or who had erythrodermic, guttate, or generalized pustular forms of psoriasis also were excluded.
Coprimary endpoints for the pooled analysis were PASI 75 (≥75% reduction in PASI) and PGA 0/1 (‘clear’/‘almost clear’ with ≥2‐category improvement) responder rates at Week 16. PASI 90 (≥90% reduction in PASI) responder rate at Week 16 was a key secondary endpoint. The patient‐reported Dermatology Life Quality Index (DLQI) 0/1 responder rate and change from Baseline in DLQI versus placebo at Week 16 also were assessed. Safety evaluation included assessment of treatment‐emergent adverse events (TEAEs).
PASI 75, PGA 0/1 and PASI 90 responder rates were analysed using a logistic regression model with factors of treatment group, region, study, prior biologic exposure (yes/no), and the interactions study × region and study × prior biologic exposure (yes/no). Markov chain Monte Carlo methodology for multiple imputation was used to account for missing data. Analysis of change from Baseline in DLQI used an analysis of covariance model with treatment group, region, study, prior biologic exposure (yes/no), and the interactions study × region and study × prior biologic exposure (yes/no) as factors and Baseline DLQI as a covariate using last observation carried forward (LOCF) imputation. DLQI 0/1 responder rates were summarized descriptively with counts and percentages, using non‐responder imputation for missing data.
For subgroup analyses of patients by prior treatment, PASI 75, PGA 0/1 and PASI 90 responder rates were summarized descriptively applying non‐responder imputation for missing data. The treatment by prior biologic exposure interaction was analysed using a logistic regression model with factors of treatment group, region, study, prior biologic exposure (yes/no), and the interactions study × region and study × prior biologic exposure (yes/no); for the analysis of subgroup interactions between treatment group and prior treatment with anti‐TNF or anti‐IL‐17, terms for subgroup and treatment × subgroup interaction were added to the model. Where appropriate, Firth's penalized maximum likelihood estimation was performed to reduce bias in the parameter estimates. P‐values were not adjusted for multiplicity.
Results
Of 850 patients randomized to CZP or placebo in CIMPASI‐1, CIMPASI‐2 or CIMPACT, 815 (95.9%) completed 16 weeks of treatment. Patient demographics and Baseline disease characteristics were generally well balanced between treatment groups (Table 1), including Baseline PASI and PGA scores. Across the overall population, 607 (71.4%) patients had received prior systemic therapy, and 253 (29.8%) had received prior biologic therapy, including anti‐TNF (N = 116, 13.6%) and anti‐IL‐17 (N = 110, 12.9%) therapy, among others.
Table 1.
Patient demographics and baseline disease characteristics (randomized set)
| Placebo (N = 157) | CZP 200 mg Q2W † (N = 351) | CZP 400 mg Q2W (N = 342) | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 46.0 ± 13.3 | 46.1 ± 13.4 | 45.2 ± 12.6 |
| Male, n (%) | 95 (60.5) | 238 (67.8) | 210 (61.4) |
| White, n (%) | 146 (93.0) | 331 (94.3) | 322 (94.2) |
| Geographic region, n (%) | |||
| North America | 71 (45.2) | 136 (38.7) | 133 (38.9) |
| Europe | 86 (54.8) | 215 (61.3) | 209 (61.1) |
| Weight (kg), mean ± SD | 92.2 ± 25.8 | 92.6 ± 22.3 | 89.2 ± 22.7 |
| BMI (kg/m2), mean ± SD | 31.2 ± 7.8 | 31.0 ± 7.1 | 30.1 ± 7.1 |
| Baseline disease characteristics | |||
| Duration of psoriasis at screening (years), mean ± SD | 17.7 ± 12.7 | 18.5 ± 13.1 | 18.2 ± 12.0 |
| Concurrent psoriatic arthritis (self‐reported), n (%) | 25 (15.9) | 59 (16.8) | 65 (19.0) |
| PASI, mean ± SD | 18.8 ± 6.8 | 20.3 ± 8.1 | 20.2 ± 7.5 |
| DLQI ‡ mean ± SD | 13.4 ± 7.7 | 13.6 ± 7.2 | 14.5 ± 7.1 |
| BSA (%), mean ± SD | 23.5 ± 13.6 | 25.6 ± 15.9 | 25.5 ± 14.9 |
| PGA, n (%) | |||
| 3: moderate | 112 (71.3) | 242 (68.9) | 239 (69.9) |
| 4: severe | 45 (28.7) | 109 (31.1) | 103 (30.1) |
| Any prior systemic psoriasis treatment, n (%) | 111 (70.7) | 249 (70.9) | 247 (72.2) |
| Prior biologic use § n (%) | 40 (25.5) | 106 (30.2) | 107 (31.3) |
| Anti‐TNF | 24 (15.3) | 49 (14.0) | 43 (12.6) |
| Anti‐IL‐17 | 13 (8.3) | 54 (15.4) | 43 (12.6) |
†CZP 200 mg Q2W patients received loading dose of CZP 400 mg at Weeks 0, 2 and 4. ‡For DLQI: Placebo, N = 154; CZP 200 mg Q2W, N = 346; CZP 400 mg Q2W, N = 340. §Patients may have had exposure to >1 prior biologic but ≤2 per exclusion criteria; one patient in the CZP 400 mg Q2W group in CIMPASI‐2 had prior exposure to three biologics, which was a protocol violation.
BMI, body mass index; BSA, body surface area; CZP, certolizumab pegol; DLQI, Dermatology Life Quality Index; IL, interleukin; PASI, psoriasis area and severity index; PGA, physician's global assessment; Q2W, every 2 weeks; TNF, tumour necrosis factor.
At Week 16, PASI 75 and PGA 0/1 responder rates were significantly greater in the pooled CZP 400 mg and pooled CZP 200 mg groups versus placebo, with clinically meaningful differences observed as early as Week 4 for both CZP doses versus placebo (Fig. 1a,b). Similarly, Week 16 PASI 90 responder rates were significantly greater in the CZP 400 mg and CZP 200 mg groups versus placebo, with clinically meaningful differences observed as early as Week 8 for both CZP doses versus placebo (Fig. 1c). Responder rates in patients previously treated with biologics were similar to those in treatment‐naïve patients, and both doses of CZP resulted in substantially higher responder rates versus placebo (Fig. 2).
Figure 1.
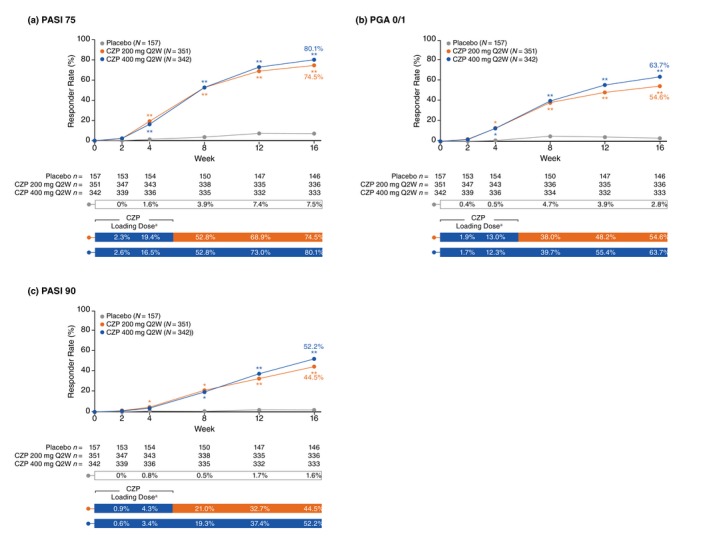
Pooled responder rates from baseline to week 16 (randomized set). *P < 0.05, **P < 0.0001 versus placebo (not adjusted for multiplicity). aCertolizumab pegol (CZP) 200 mg Q2W patients received loading dose of CZP 400 mg at Weeks 0, 2 and 4. Randomized set includes all randomized patients. Responder rates were analysed using a logistic regression model with factors of treatment, region, study, prior biologic exposure (yes/no), and the interactions study × region and study × prior biologic exposure (yes/no); Markov chain Monte Carlo methodology for multiple imputation was used to account for missing data. CZP, certolizumab pegol; PASI 75, ≥75% reduction in psoriasis area and severity index; PASI 90, ≥90% reduction in psoriasis area and severity index; PGA 0/1, ‘clear’/‘almost clear’ with ≥2‐category improvement in physician's global assessment; Q2W, every 2 weeks.
Figure 2.
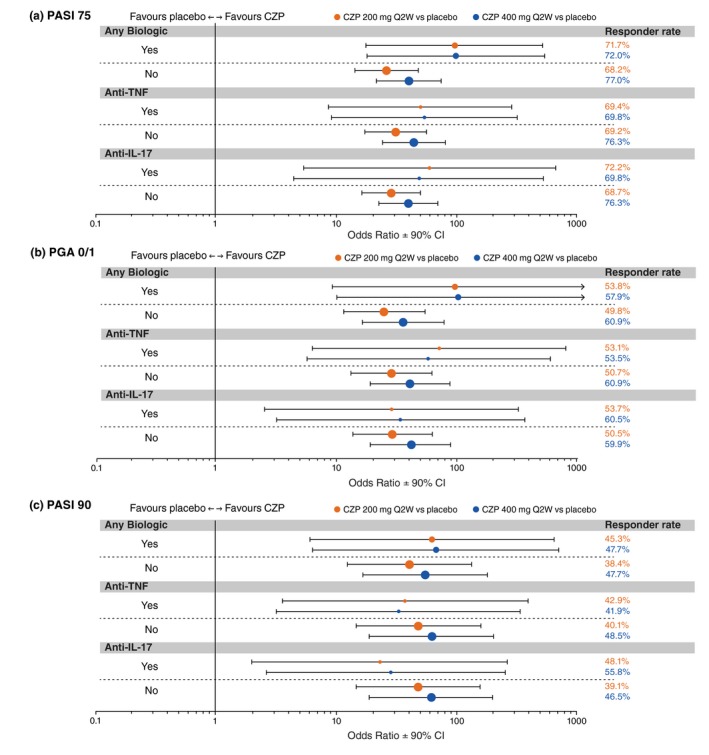
Forest plots of response rate odds ratios at week 16 in patient subgroups by prior biologic exposure (randomized set). Randomized set includes all randomized patients. Point size is proportional to the size of the subgroup. Responder rates were summarized descriptively applying non‐responder imputation for missing data; the treatment by prior biologic exposure interaction was analysed using a logistic regression model with factors of treatment group, region, study, prior biologic exposure (yes/no), and the interactions study × region and study × prior biologic exposure (yes/no); for the analysis of subgroup interactions between treatment group and prior treatment with anti‐TNF or anti‐IL‐17, terms for subgroup and treatment × subgroup interaction were added to the model; Firth's penalized maximum likelihood estimation was performed to reduce bias in the parameter estimates for ‘Anti‐IL‐17’ for PASI 75 response, and for ‘Any Biologic’, ‘Anti‐TNF’, and ‘Anti‐IL‐17’ for PGA 0/1 and PASI 90 response. CI, confidence interval; CZP, certolizumab pegol; IL, interleukin; PASI 75, ≥75% reduction in psoriasis area and severity index; PGA 0/1, ‘clear’/‘almost clear’ with ≥2‐category improvement in physician's global assessment; Q2W, every 2 weeks; TNF, tumour necrosis factor.
Meaningful improvements in quality of life, as measured by change from Baseline in DLQI and DLQI 0/1 responder rates, were observed in CZP‐treated patients versus placebo at Week 16. Least squares mean change from Baseline in DLQI were −10.4, −9.7 and −3.1 for the CZP 400 mg, CZP 200 mg and placebo groups, respectively (P < 0.0001 for each dose versus placebo, not adjusted for multiplicity). DLQI 0/1 responder rates at Week 16 were 47.1%, 42.7% and 8.3% for the CZP 400 mg, CZP 200 mg and placebo groups, respectively.
The incidence of TEAEs was generally similar between the CZP 400 mg and placebo groups, and was somewhat lower in the CZP 200 mg group versus placebo (Table 2). TEAEs infrequently led to study discontinuation (1.2% and 1.1% of patients treated with CZP 400 mg and CZP 200 mg, respectively, versus 0 placebo‐treated patients). Serious TEAEs and serious infections and infestations were infrequent across treatment groups, occurring, respectively, in 4.7% and 0.6% of patients treated with CZP 400 mg, 1.4% and 0% of patients treated with CZP 200 mg, and 4.5% and 0% of patients receiving placebo (Table 2). Two patients reported serious infections in the CZP 400 mg group (infected haematoma and abdominal abscess in 1 patient following a bicycle accident, and pneumonia in 1 patient). No deaths were reported in any of the three studies through Week 16.
Table 2.
Treatment‐emergent adverse events (safety set)
| Placebo (N = 157) | CZP 200 mg Q2W † (N = 350) | CZP 400 mg Q2W (N = 342) | |
|---|---|---|---|
| TEAEs, n (%) [incidence rate ‡ ] | |||
| All | 97 (61.8) [342.6] | 197 (56.3) [292.1] | 217 (63.5) [348.3] |
| Serious | 7 (4.5) [15.4] | 5 (1.4) [4.7] | 16 (4.7) [15.6] |
| Discontinuations due to TEAE, n (%) | 0 | 4 (1.1) [3.8] | 4 (1.2) [3.8] |
| Deaths, n (%) | 0 | 0 | 0 |
| Other TEAEs of interest, n (%) [incidence rate ‡ ] | |||
| Infections and infestations | 53 (33.8) [136.2] | 108 (30.9) [121.6] | 124 (36.3) [146.6] |
| Latent tuberculosis | 0 | 0 | 0 |
| Active tuberculosis | 0 | 0 | 0 |
| Candida infections | 0 | 1 (0.3) [0.9] § | 0 |
| Oral fungal infection | 0 | 0 | 1 (0.3) [1.0] |
| Fungal skin infection | 0 | 0 | 1 (0.3) [1.0] ¶ |
| Nasopharyngitis | 19 (12.1) [43.1] | 42 (12.0) [42.2] | 43 (12.6) [44.1] |
| Upper respiratory tract infection | 11 (7.0) [24.3] | 17 (4.9) [16.4] | 23 (6.7) [22.9] |
| Serious infections | 0 | 0 | 2 (0.6) [1.9]† † |
| Non‐melanoma skin cancer | 0 | 0 | 1 (0.3) [1.0]‡ ‡ |
| Malignancy (excluding non‐melanoma skin cancer) | 0 | 0 | 0 |
| Depression | 0 | 2 (0.6) [1.9] | 2 (0.6) [1.9] |
†CZP 200 mg Q2W patients received loading dose of CZP 400 mg at Weeks 0, 2, and 4. ‡Incidence of new cases per 100 patient‐years. §Vulvovaginal candidiasis. ¶Reported as fungal infection preferred term in the database. ††Haematoma infection and abdominal abscess in 1 patient (bicycle accident), and pneumonia in 1 patient. ‡‡Basal cell carcinoma.
Safety set includes all randomized patients who received ≥1 dose of study medication. TEAEs are summarized descriptively.
CZP, certolizumab pegol; TEAE, treatment‐emergent adverse event; Q2W, every 2 weeks.
Discussion
In this pooled analysis of three placebo‐controlled trials to evaluate certolizumab pegol for the treatment of moderate‐to‐severe chronic plaque psoriasis, CZP 400 mg Q2W and CZP 200 mg Q2W were each associated with statistically significant and clinically meaningful improvements in the signs and symptoms of psoriasis through 16 weeks of treatment, with clinically meaningful differences in PASI 75 and PGA 0/1 responder rates versus placebo observed as early as Week 4. Greater improvement in quality of life, as measured by change from Baseline in DLQI and DLQI 0/1 responder rate, was seen for both CZP dose groups compared with placebo at Week 16.
Clinically meaningful efficacy was observed with both CZP doses compared with placebo in patients with and without prior exposure to systemic therapy, including anti‐TNF and anti‐IL‐17 biologics. This finding is of potential clinical importance, given current recommendations for switching therapies when patients fail to achieve desired treatment goals or are otherwise dissatisfied with current treatment.12
In the phase 3 program, the safety profile for CZP was consistent with the anti‐TNF class in psoriasis,13 and based on the known safety profile of CZP,14 no new safety signals were identified in the pooled analysis. Serious TEAEs were infrequent across treatment groups, and no deaths occurred through 16 weeks of treatment. A limitation to the current analysis is that safety data are reported only for the initial treatment period of 16 weeks. Information regarding CZP's longer term safety in treatment of psoriasis awaits completion of these ongoing, 144‐week trials. With its unique molecular structure, CZP affords a novel biologic treatment option for psoriasis patients, including those previously treated with biologics.
Disclosures/conflicts of interest
Andrew Blauvelt: AbbVie; Aclaris; Akros; Allergan; Almirall; Amgen; Boehringer Ingelheim; Celgene; Dermavant; Dermira; Eli Lilly; Genentech/Roche; GlaxoSmithKline; Janssen; LEO Pharma; Meiji; Merck; Novartis; Pfizer; Purdue Pharma; Regeneron; Sandoz; Sanofi Genzyme; Sienna Pharmaceuticals; Sun Pharma; UCB; Valeant; Vidac. Kristian Reich: AbbVie; Affibody; Almirall; Amgen; Biogen; Boehringer Ingelheim; Celgene; Centocor; Covagen; Eli Lilly; Forward Pharma; Fresenius Medical Care; GlaxoSmithKline; Janssen; Kyowa Kirin; LEO Pharma; Medac; Merck; Novartis; Miltenyi Biotec; Ocean Pharma; Pfizer; Regeneron; Samsung Bioepis; Sanofi Genzyme; Takeda; UCB; Valeant; Xenoport. Mark Lebwohl: AbbVie; Allergan; Amgen; Boehringer Ingelheim; Celgene; Eli Lilly; Janssen; LEO Pharma; Medimmune/AstraZeneca; Novartis; Pfizer; Sun Pharma; UCB; Valeant; Vidac. Daniel Burge and Janice Drew: Employees of Dermira. Catherine Arendt, Luke Peterson, and Robert Rolleri: Employees of UCB Pharma. Alice B. Gottlieb: AbbVie; Allergan; Beiersdorf; Bristol‐Myers Squibb; Celgene; Dermira; Eli Lilly; Incyte; Janssen; Novartis; Reddy Labs; Sun Pharma; UCB; Valeant.
Funding sources
The studies were funded by Dermira, Inc. in collaboration with UCB, Inc. UCB is the regulatory sponsor of certolizumab pegol in psoriasis. Medical writing support for this manuscript was provided by Prescott Medical Communications Group (Chicago, IL). All costs associated with the development of this manuscript were funded by UCB.
The copyright line for this article was changed on 21 July 2019 after original online publication
References
- 1. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014; 70: 512–516. [DOI] [PubMed] [Google Scholar]
- 2. Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003‐2004. J Am Acad Dermatol 2009; 60: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danielsen K, Olsen AO, Wilsgaard T, Furberg AS. Is the prevalence of psoriasis increasing? A 30‐year follow‐up of a population‐based cohort. Br J Dermatol 2013; 168: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 4. Menter A, Papp KA, Gooderham M et al Drug survival of biologic therapy in a large, disease‐based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol 2016; 30: 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long‐term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015; 172: 244–252. [DOI] [PubMed] [Google Scholar]
- 6. Warren RB, Smith CH, Yiu ZZN et al Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015; 135: 2632–2640. [DOI] [PubMed] [Google Scholar]
- 7. Mariette X, Forger F, Abraham B et al Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis 2018; 77: 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gottlieb AB, Blauvelt A, Thaçi D et al Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double‐blinded, placebo‐controlled studies (CIMPASI‐1 and CIMPASI‐2). J Am Acad Dermatol 2018; 79: 302–314.e6. [DOI] [PubMed] [Google Scholar]
- 9. Lebwohl M, Blauvelt A, Paul C et al Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double‐blind, etanercept‐ and placebo‐controlled study (CIMPACT). J Am Acad Dermatol 2018; 79: 266–276.e5. [DOI] [PubMed] [Google Scholar]
- 10. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 11. Kerdel F, Zaiac M. An evolution in switching therapy for psoriasis patients who fail to meet treatment goals. Dermatol Ther 2015; 28: 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pereira R, Lago P, Faria R, Torres T. Safety of anti‐TNF therapies in immune‐mediated inflammatory diseases: focus on infections and malignancy. Drug Dev Res 2015; 76: 419–427. [DOI] [PubMed] [Google Scholar]
- 13. Cimzia (certolizumab pegol) [package insert] UCB, Inc., Smyrna, GA, 2017. [Google Scholar]


