Abstract
Problem
Several studies have reported the increased risk of preterm birth, premature rupture of membranes, and low birth weight in patients with recurrent pregnancy loss (RPL). There have been a limited number of large population‐based studies examining adverse pregnancy and perinatal outcome after RPL. Multiple‐imputed analyses (MIA) adjusting for biases due to missing data is also lacking.
Method of study
A nationwide birth cohort study known as the “Japan Environment and Children’s Study (JECS)” was conducted by the Ministry of the Environment. The subjects consisted of 104 102 registered children (including fetuses or embryos).
Results
No increased risk of a congenital anomaly, aneuploidy, neonatal asphyxia, or a small for date infant was observed among the children from women with a history of RPL. A novel increased risk of placental adhesion and uterine infection was found. The adjusted ORs using MIA in women with three or more PL were 1.76 (95% CI, 1.04‐2.96) for a stillbirth, 1.68 (1.12‐2.52) for a pregnancy loss, 2.53 (1.17‐5.47) for placental adhesion, 1.87 (1.37‐2.55) and 1.60 (.99‐2.57) for mild and severe hypertensive disorders of pregnancy, respectively, 1.94 (1.06‐3.55) for uterine infection, 1.28 (1.11‐1.47) for caesarean section and .86 (.76‐.98) for a male infant.
Conclusion
MIA better quantified the risk, which could encourage women who might hesitate to attempt a subsequent pregnancy.
Keywords: birth cohort study, multiple imputation analyses, perinatal outcome, pregnancy outcome, propensity score adjustment, recurrent pregnancy loss
1. INTRODUCTION
Miscarriage is the most common pregnancy complication with a frequency of 15%.1, 2 Recurrent pregnancy loss (RPL) is defined as two or more losses at any time during pregnancy.2, 3 Most of these occur before 12 weeks of gestation.
RPL is a heterogenous reproductive problem with multiple etiologies and contributing factors include age, body mass index (BMI), parity, and smoking habit.1, 2 Identifiable causes of RPL include antiphospholipid syndrome (APS), uterine anomalies and parental and embryonic chromosomal abnormalities.1, 2, 3, 4, 5, 6 Endocrine, infectious, and immune inflammatory conditions have been reported to be associated with RPL. Of these, APS is the treatable etiology.1, 2 There has been no randomized control trial to compare the live birth rate between with and without surgery for a uterine anomaly or preimplantation genetic diagnosis for a translocation.
However, the cumulative live birth rate was 84% in non‐genetic carriers and 85.5% in couples with no explanation in the previous studies.5, 7 The live birth rate decreased significantly according to the number of previous miscarriages in both groups.8 Information on the adverse pregnancy and perinatal outcome is available but limited.9, 10 The risk of a preterm birth (PTB) is most frequently examined.9, 10, 11, 12, 13, 14, 15 Other previous studies have shown a significantly increased risk of very PTB.13, 14, 15, 16 Premature rupture of membrane (PROM),12, 14, 17 low birth weight (LBW),11, 13, 15 caesarean section, placenta abruptio, and hypertensive disorders of pregnancy (HDP).17
However, there has been controversy regarding whether RPL increases the risk of a congenital anomaly or neonatal asphyxia. A case‐control study with 18 534 malformed and 17 544 non‐malformed babies indicated that multiple malformations, Down’s syndrome, anencephaly, spinabifida, talipes equinovarus, congenital dislocation of the hip and LBW were associated with previous miscarriage and stillbirth.11 Another study with 638 recurrent miscarriages (RM) patients and 3099 non‐RM patients also reported a risk of congenital anomalies with RM.12 A recent study found no association between RM and congenital anomaly or aneuploidy.10
These studies did not employ a population‐based cohort, but rather case‐control retrospective approaches. The influence of covariates and medical histories were not considered.11, 12 Furthermore, recent concern regarding the treatment of missing data has been raised because the generalizability of findings might be limited by the extent of the missing values.
We have conducted the nationwide population‐based birth cohort study known as the “Japan Environment and Children’s Study (JECS)” planned by the Ministry of the Environment, Government of Japan.18, 19, 20, 21, 22 The study subjects consisted of 104 102 registered pregnancies recruited during the first 3 years of the JECS, and their babies are now being followed up for 13 years mainly to examine the influence of the uterine environment on the fetus.
We determined the adverse pregnancy and perinatal outcome according to the number of previous pregnancy losses (PL) reported by the JECS with the use of multiple imputation analyses (MIA).
2. METHODS
2.1. Study design and participants
Pregnant women were recruited by the JECS between January 31, 2011 and March 31, 2014.
Eligibility criteria for expectant mothers were as follows: that they (i) resided at the time of recruitment in any of the study areas selected by 15 Regional JECS Centers located countrywide, (ii) had an expected delivery date after August 1, 2011, and (iii) were capable of comprehending the Japanese language and completing the self‐administered questionnaire.18, 19, 20, 21, 22 The sample size has been calculated in the JECS protocol by the Ministry of the Environment.23 In principle, pregnant women completed the questionnaire during the second (MT1) and third trimester (MT2). Their medical records were transcribed by doctors or research coordinators at registration (DrT1), just after delivery (Dr0m) and at 1 month after delivery (Dr1m).
The present study was based on the jecs‐ag‐20160424 dataset, which includes 104 102 registered children (including fetuses and embryos), and was released restrictively to all concerned in July, 2016 (Figure 1). A total of 1994 children of mothers with multiple pregnancies were excluded because several outcomes were influenced by multiple pregnancies. Furthermore, 310 fetuses or embryos terminated by induced abortion were also excluded. In addition, a total of 5586 children (fetuses or embryos) whose mothers had participated for the second or the third time were excluded. Finally, 96 212 participants were included in the main analysis. The mean (SD) age at registration was 30.7 (5.1). The mean (SD) gestational weeks at registration was 14.0 (5.7) weeks.
Figure 1.
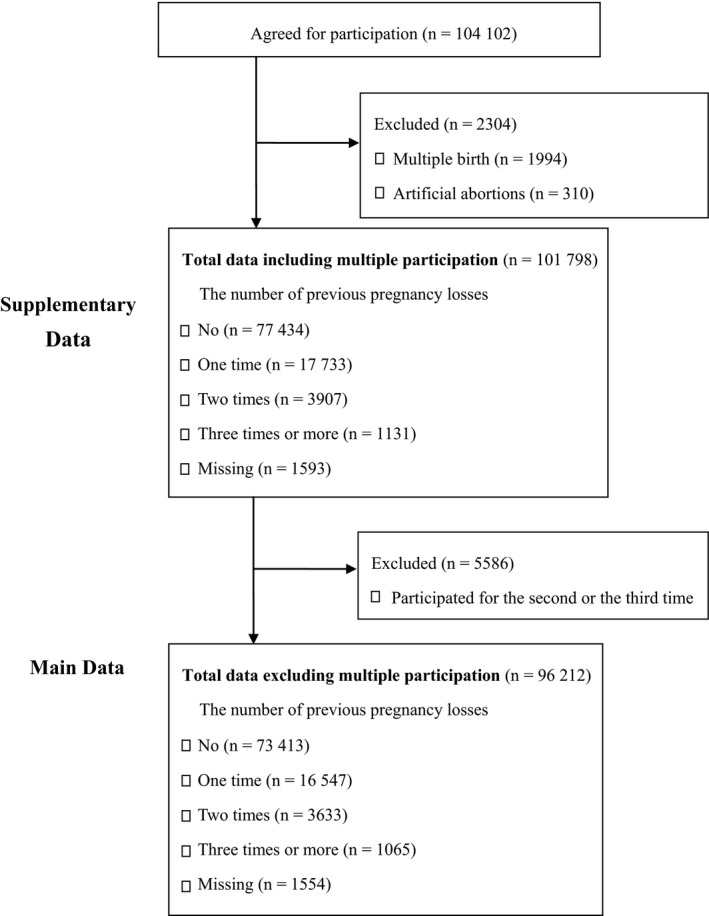
The flow diagram for assessing eligibility
The JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and by the Ethics Committees of all participating institutions. Written informed consent was obtained from all participating women.
2.2. Data collection
The first questionnaire (MT1) included the sociodemographic characteristics, medical histories and the details of all previous pregnancies.
Medical histories included atopic dermatitis, asthma, collagen disease, autoimmune disease, systematic lupus erythematosus (SLE), rheumatic arthritis (RA), insulin‐dependent diabetes mellitus (IDDM), non‐insulin‐dependent DM (NIDDM), gestational diabetes, hyperthyroidism, hypothyroidism, anemia, hypertension, hyperlipidemia, stroke, myocardiac infarction, congenital heart disease, Kawasaki disease, depression, dysautonomia, anxiety disorder, gastroesophageal reflex disease, gastritis, gastric ulcer, duodenal ulcer, irritable colon, Crohn’s disease, ulcerative colitis, fatty liver, chronic nephritis, nephrotic syndrome, menstrual disorder, endometriosis, adenomyosis, uterine fibroids, uterine anomaly, ovarian tumor, and polycystic ovary syndrome (PCOS).
The socioeconomic status was assessed by the education level and annual household income in the second questionnaire (MT2).
The first medical record transcript (DrT1) included maternal age, gestational weeks at registration, maternal body weight, height, conception, and the details of all previous pregnancies (vaginal delivery/caesarian delivery/miscarriage/induced abortion/stillbirth).
The Dr0m included maternal age, gestational weeks at miscarriage and delivery, single/multiple, live birth/stillbirth, miscarriage/induced abortion, male/female, birth weight, vaginal/caesarian delivery, pregnancy complications, and perinatal outcomes.
The third medical record transcription (Dr1m) included questions on the presence/absence of congenital anomalies.
2.3. Outcome, exposure, and covariates
The pregnancy histories provided by the doctors in filling out the DrT1 form were given priority over the participants’ answers with regard to the number of previous PL (categorized as 0, 1, 2, 3 or more).
The obstetric outcomes included stillbirth > 20 weeks’ gestation, late miscarriage, early miscarriage <12 weeks’ gestation, PL, PTB (<37 and <34 weeks’ gestation), PROM, placenta praevia, abruptio placenta, adherent placenta, oligohydramnios, mild and severe HDP, uterine infection and caesarean section. The perinatal outcomes were small‐for‐date of the 10th percentile (SFD), IUFD, sex, Apgar score <7 at 5 minutes, an umbilical artery blood pH <7.1, the presence/absence of congenital anomalies of the head, eyes, ears, face, limbs, lungs, heart, intestine, urogenital organs, skin, and skeleton and chromosome aneuploidy. The presence of chromosomal aneuploidy as indicated by the doctors in filling out the Dr1m was given priority over the Dr0m.
Potential covariates were maternal age at registration, BMI, marital status, the presence/absence of IVF‐ET, previous live birth, smoking, education, and income.
2.4. Statistical analysis
The associations between the number of previous PL and covariates were tested using chi‐squared tests for categorical variables. Associations between each outcome and the covariates were examined using Fisher’s exact test.
The prevalence of each outcome including the crude odds ratio (OR) and the 95% confidence interval (CI) were calculated for 0 (reference), 1, 2, and 3 or more PL (as categorized). We conducted both complete‐case analysis and MIA using logistic regression to estimate the risks of previous PL affecting the adverse pregnancy and perinatal outcome. In the complete‐case analysis, the unadjusted model (Model I) was used to estimate the independent effects of the PL history, and the adjusted model (Model II) included potential confounders in the model, adjusting for the propensity score including all covariates with P values <.05.24, 25 In general, it has been found that propensity score adjustment has the advantages of being less biased, providing more robust estimates than those of the traditional logistic regression. An unlimited number of covariates can be theoretically included in propensity score estimation so that propensity score adjustment has been employed when adjusting for a large number of covariates and widely used in recent observational research.
Adjusted Model III in accord with Model II included MIA. Since the percentage of missing values in Model II across all variables ranged from 5.2% to 21.1%, prior to conducting logistic regression analyses using propensity score adjustment, MIA was applied to handle missing data. Five imputed datasets were created for each outcome, and all variables that were used in Model IIs were included in each imputation model using the propensity score. Use of MIA is more efficient in most settings and widely recommended for improving biases within complete‐case analyses.26 Model IV additionally adjusted for covariates related to the gynecological history in Model III. It was speculated that women with PL could have a greater chance of being diagnosed with a gynecological disease as a result of frequent ultrasound sonography. Thus, we performed MIA with and without covariates for gynecological history.
All calculations were carried out using SPSS version 23 and 24 (IBM Corp., Japan).
3. RESULTS
Histories of previous PL were available for 94 658 participants out of 96 212. Among these women, 77.6% (73 413) had no history of PL, 17.5% (16 547) had experienced one PL, 3.8% (3633) experienced two losses, and 1.1% (1065) experienced three or more losses (Table 1). Age, BMI, IVF‐ET and previous live births were positively associated with the number of previous PL. The rates of marital status, smoking, education background, and income were influenced by the number of previous PL. All the listed variables were significantly associated with an increasing number of previous PL (Table 1, P < .0001 for all covariates). In the following analyses, women with no PL were regarded as the reference category.
Table 1.
Demographic characteristics of women according to the number of previous pregnancy losses (n = 94 658)
| Variables | No miscarriage (n = 73 413) | One (n = 16 547) | Two (n = 3633) | Three or more (n = 1065) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years), % (n) | <0.0001 | ||||||||
| <20 | 1.5 | (1026) | 0.3 | (52) | 0.2 | (7) | 0.3 | (3) | |
| 20‐29 | 42.8 | (30 278) | 29.9 | (4,758) | 21.6 | (759) | 15.5 | (158) | |
| 30‐39 | 52.9 | (37 392) | 64.4 | (10,264) | 68.1 | (2,388) | 67.9 | (694) | |
| >40 | 2.8 | (1971) | 5.4 | (859) | 10.1 | (354) | 16.3 | (167) | |
| Missing, % (n) | 3.7 | (2746) | 3.7 | (614) | 3.4 | (125) | 4.0 | (43) | |
| BMI, % (n) | <0.0001 | ||||||||
| <18.5 | 16.7 | (12 264) | 14.8 | (2,442) | 14.3 | (519) | 14.5 | (154) | |
| 18.5‐25.0 | 72.9 | (53 368) | 74.0 | (12,212) | 73.1 | (2,651) | 72.7 | (773) | |
| ≥25.0 | 10.4 | (7587) | 11.2 | (1,852) | 12.6 | (455) | 12.9 | (137) | |
| Missing, % (n) | 0.3 | (194) | 0.2 | (41) | 0.2 | (8) | 0.1 | (1) | |
| Marital status, % (n) | <0.0001 | ||||||||
| Married | 95.0 | (67 516) | 97.1 | (15,677) | 97.5 | (3,457) | 96.4 | (1,004) | |
| Single | 4.3 | (3068) | 1.7 | (282) | 1.0 | (34) | 0.9 | (9) | |
| Divorced | 0.7 | (518) | 1.2 | (190) | 1.6 | (55) | 2.7 | (28) | |
| Missing, % (n) | 3.1 | (2311) | 2.4 | (398) | 2.4 | (87) | 2.3 | (24) | |
| IVF‐ET, % (n) | |||||||||
| Carried out | 2.7 | (1965) | 4.2 | (702) | 5.2 | (188) | 9.4 | (100) | |
| Missing, % (n) | 0.0 | (20) | 0.0 | (4) | 0.0 | (1) | 0.0 | (0) | |
| Previous live birth, % (n) | |||||||||
| Yes | 52.1 | (38 258) | 68.8 | (11,354) | 76.7 | (2,781) | 79.0 | (840) | <0.0001 |
| Missing, % (n) | 0.0 | (18) | 0.3 | (49) | 0.2 | (8) | 0.2 | (2) | |
| Smoking, % (n) | <0.0001 | ||||||||
| Never smoked | 59.4 | (42 128) | 55.6 | (8,952) | 53.0 | (1,878) | 52.4 | (543) | |
| Quit smoking before pregnancy | 22.3 | (15 798) | 26.8 | (4,311) | 26.3 | (931) | 29.7 | (308) | |
| Quit smoking during early pregnancy | 13.8 | (9774) | 12.2 | (1,969) | 13.5 | (478) | 11.1 | (115) | |
| Current smoker | 4.5 | (3198) | 5.3 | (860) | 7.2 | (254) | 6.8 | (71) | |
| Missing, % (n) | 3.4 | (2515) | 2.7 | (455) | 2.5 | (92) | 2.6 | (28) | |
| Educational background (years), % (n) | <0.0001 | ||||||||
| Junior high/ High school | 35.7 | (24 974) | 36.7 | (5,809) | 39.2 | (1,356) | 42.1 | (426) | |
| College/Junior college/Technology college | 41.8 | (29 258) | 43.1 | (6,829) | 42.3 | (1,465) | 41.8 | (423) | |
| University | 20.9 | (14 613) | 18.8 | (2,981) | 17.5 | (607) | 15.0 | (152) | |
| Graduate school | 1.5 | (1079) | 1.4 | (214) | 1.0 | (35) | 1.0 | (10) | |
| Missing, % (n) | 4.8 | (3489) | 4.3 | (714) | 4.7 | (170) | 5.1 | (54) | |
| Income (JPY), % (n) | <0.0001 | ||||||||
| <200 | 5.7 | (3720) | 5.4 | (802) | 5.2 | (168) | 6.7 | (64) | |
| 200‐< 400 | 34.9 | (22 736) | 33.2 | (4,943) | 31.9 | (1,031) | 30.5 | (292) | |
| 400‐< 600 | 32.8 | (21 382) | 33.7 | (5,016) | 32.9 | (1,065) | 33.9 | (324) | |
| 600‐< 800 | 15.8 | (10 269) | 16.4 | (2,446) | 18.1 | (586) | 16.4 | (157) | |
| 800‐< 1,000 | 6.6 | (4295) | 6.6 | (984) | 7.0 | (228) | 8.3 | (79) | |
| ≥1,000 | 4.2 | (2728) | 4.6 | (684) | 4.9 | (158) | 4.3 | (41) | |
| Missing, % (n) | 11.3 | (8283) | 10.1 | (1,672) | 10.9 | (397) | 10.1 | (108) | |
BMI: Body Mass Index, IVF‐ET: In Vitro Fertilization‐Embryo Transfer, JPY: ×10 000 Japanese yen, 1US$ = 103JPY, as of September, 2016, P‐value: Fisher’s exact test.
Histories of pregnancy losses were not available for 1554 participants out of 96 212.
3.1. Model I (crude analysis)
Crude analysis showed a significant association with stillbirth, PL, PTB (both <37 and <34 weeks’ gestation), placental adhesion, HDP, caesarean section and a male infant with three or more PL (Model I in Tables 2 and 3). There was no risk associated with PROM, placenta praevia, oligohydramnios, abruptio placenta, or uterine infection. No association with SFD, IUFD, LBW, low Apgar score, low pH, congenital anomaly, or aneuploidy was observed.
Table 2.
Obstetric outcome of women according to the number of previous pregnancy losses
| Number of pregnancy losses | Prevalence %, (n) | OR, 95%CI | Pooled OR, 95%CI | Covariates included in propensity score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model I | Model II | Multiple imputation model III | Multiple imputation model Ⅳ | |||||||||
| Late miscarriage and stillbirth> 20 weeks gestation | No | 0.7 | (534/71708) | 1 | 1 | 1 | 1 | Age, BMI, Smoking, ART, Previous live birth, NIDDM, Gastroesophageal reflux disease, Gastric ulcer, Adenomyosis | ||||
| One | 1.0 | (158/16 233) | 1.31 | 1.10‐1.57 | 1.27 | 1.03‐1.57 | 1.18 | 0.99‐1.42 | 1.18 | 0.99‐1.41 | ||
| Two | 1.2 | (42/3564) | 1.59 | 1.16‐2.18 | 1.49 | 1.03‐2.15 | 1.34 | 0.97‐1.84 | 1.33 | 0.97‐1.82 | ||
| Three | 1.6 | (17/1034) | 2.23 | 1.37‐3.63 | 2.12 | 1.23‐3.65 | 1.76 | 1.04‐2.96 | 1.72 | 1.05‐2.83 | ||
| Missing, % (n) | 3.8 | (3673) | 9.7 | (9332) | 2.8 | (2646) | 2.8 | (2646) | ||||
| Early miscarriage<12 weeks gestation | No | 0.3 | (238/71 412) | 1 | 1 | 1 | 1 | Age, Ulcerative colitis | ||||
| One | 0.5 | (76/16 151) | 1.41 | 1.09‐1.83 | 2.09 | 1.34‐3.23 | 1.38 | 1.05‐1.81 | 1.38 | 1.05‐1.81 | ||
| Two | 0.3 | (9/3531) | 0.76 | 0.39‐1.49 | 1.11 | 0.40‐3.08 | 0.68 | 0.35‐1.33 | 0.68 | 0.35‐1.33 | ||
| Three | 0.7 | (7/1024) | 2.06 | 0.97‐4.38 | 2.63 | 0.81‐8.50 | 1.89 | 0.89‐4.02 | 1.89 | 0.89‐4.02 | ||
| Missing, % (n) | 4.3 | (4094) | 9.3 | (8927) | 3.2 | (3052) | 3.2 | (3052) | ||||
| Pregnancy loss | No | 1.1 | (772/71946) | 1 | 1 | 1 | 1 | Age, BMI, Smoking, ART, Previous live birth, NIDDM, Adenomyosis | ||||
| One | 1.4 | (234/16 309) | 1.34 | 1.16‐1.56 | 1.38 | 1.14‐1.67 | 1.21 | 1.04‐1.41 | 1.21 | 1.05‐1.40 | ||
| Two | 1.4 | (51/3573) | 1.34 | 1.00‐1.78 | 1.43 | 1.01‐2.02 | 1.09 | 0.82‐1.46 | 1.08 | 0.81‐1.44 | ||
| Three | 2.3 | (24/1041) | 2.18 | 1.44‐3.28 | 2.20 | 1.34‐3.60 | 1.68 | 1.12‐2.52 | 1.73 | 1.14‐2.62 | ||
| Missing, % (n) | 3.5 | (3343) | 9.6 | (9238) | 2.4 | (2282) | 2.4 | (2282) | ||||
| Preterm birth<37 weeks gestation | No | 4.8 | (3419/71 356) | 1 | 1 | 1 | 1 | Age, Marital status, BMI, Smoking, Education, (Household income), ART, Atopic Dermatitis, Collagen disease, SLE, RA, IDDM, NIDDM, Gestational diabetes, High blood pressure, Congenital heart disease, Depression, Dysautonomia, Anxiety disorders, Gastric ulcer, Chronic nephritis, Endometriosis, Uterine fibroids, Adenomyosis, Congenital uterine anomalies | ||||
| One | 5.0 | (810/16 126) | 1.05 | 0.97‐1.14 | 0.96 | 0.88‐1.05 | 0.98 | 0.90‐1.06 | 0.97 | 0.90‐1.05 | ||
| Two | 5.8 | (206/3532) | 1.23 | 1.07‐1.42 | 1.01 | 0.85‐1.19 | 1.09 | 0.94‐1.27 | 1.07 | 0.93‐1.24 | ||
| Three | 7.1 | (73/1025) | 1.52 | 1.20‐1.94 | 1.23 | 0.94‐1.61 | 1.29 | 1.01‐1.65 | 1.27 | 0.99‐1.62 | ||
| Missing, % (n) | 4.3 | (4173) | 17.5 | (16 866) | 3.3 | (3164) | 3.3 | (3164) | ||||
| Preterm birth <34 weeks gestation | No | 1.1 | (793/71 356) | 1 | 1 | 1 | 1 | Age, Marital status, BMI, Smoking, Education, ART, SLE, IDDM, NIDDM, Gestational diabetes, High blood pressure, Gastric ulcer, Fatty liver, Chronic nephritis, Endometriosis, Uterine fibroids, Adenomyosis, PCOS | ||||
| One | 1.3 | (207/16 126) | 1.16 | 1.00‐1.36 | 1.06 | 0.89‐1.27 | 1.05 | 0.90‐1.23 | 1.05 | 0.90‐1.23 | ||
| Two | 1.6 | (57/3532) | 1.46 | 1.11‐1.91 | 1.12 | 0.81‐1.56 | 1.22 | 0.93‐1.61 | 1.21 | 0.92‐1.60 | ||
| Three | 2.0 | (21/1025) | 1.86 | 1.20‐2.88 | 1.17 | 0.67‐2.05 | 1.43 | 0.92‐2.23 | 1.41 | 0.90‐2.21 | ||
| Missing, % (n) | 4.3 | (4173) | 11.8 | (11 313) | 3.3 | (3164) | 3.3 | (3 164) | ||||
| Premature rupture of membrane | No | 9.3 | (6622/71 392) | 1 | 1 | 1 | 1 | Age, Marital status, Smoking, Education, (Household income), ART, Previous live birth, SLE, IDDM, Anemia, Congenital heart disease, Uterine fibroids, Adenomyosis | ||||
| One | 8.2 | (1315/16 131) | 0.87 | 0.82‐0.92 | 0.95 | 0.89‐1.02 | 0.96 | 0.90‐1.02 | 0.96 | 0.90‐1.02 | ||
| Two | 8.2 | (291/3533) | 0.88 | 0.78‐0.99 | 1.06 | 0.93‐1.21 | 1.03 | 0.91‐1.17 | 1.03 | 0.91‐1.17 | ||
| Three | 8.4 | (86/1025) | 0.90 | 0.71‐1.10 | 1.07 | 0.84‐1.36 | 1.11 | 0.89‐1.39 | 1.12 | 0.89‐1.41 | ||
| Missing, % (n) | 4.3 | (4131) | 17.5 | (16 828) | 3.2 | (3120) | 3.2 | (3120) | ||||
| Placenta praevia | No | 0.6 | (438/71 392) | 1 | 1 | 1 | 1 | Age, Household income, ART, Gestational diabetes, Endometriosis, Uterine fibroids, Ovarian tumors | ||||
| One | 0.8 | (137/16 131) | 1.39 | 1.14‐1.68 | 1.25 | 1.02‐1.53 | 1.26 | 1.04‐1.53 | 1.24 | 1.02‐1.51 | ||
| Two | 1.1 | (40/3533) | 1.86 | 1.34‐2.57 | 1.44 | 1.01‐2.06 | 1.56 | 1.12‐2.17 | 1.50 | 1.08‐2.09 | ||
| Three | 1.1 | (11/1025) | 1.76 | 0.96‐3.21 | 1.30 | 0.69‐2.46 | 1.34 | 0.73‐2.46 | 1.27 | 0.70‐2.34 | ||
| Missing, % (n) | 4.3 | (4131) | 16.4 | (15 814) | 3.2 | (3120) | 3.2 | (3120) | ||||
| Oligohydramnios | No | 1.3 | (944/71 392) | 1 | 1 | 1 | 1 | Marital status, BMI, (Household income), ART, Previous live birth, Gestational diabetes, Depression, Bronchial asthma | ||||
| One | 1.3 | (207/16 131) | 0.97 | 0.83‐1.13 | 1.06 | 0.90‐1.25 | 1.08 | 0.93‐1.26 | 1.08 | 0.93‐1.26 | ||
| Two | 1.4 | (50/3533) | 1.07 | 0.80‐1.43 | 1.29 | 0.95‐1.75 | 1.27 | 0.95‐1.69 | 1.27 | 0.95‐1.69 | ||
| Three | 1.6 | (16/1025) | 1.18 | 0.72‐1.95 | 1.22 | 0.69‐2.18 | 1.48 | 0.90‐2.44 | 1.48 | 0.90‐2.44 | ||
| Missing, % (n) | 4.3 | (4131) | 13.8 | (13 249) | 3.2 | (3120) | 3.2 | (3120) | ||||
| Abruptio placentae | No | 0.4 | (292/71 392) | 1 | 1 | 1 | 1 | Age, Smoking, ART, Adenomyosis | ||||
| One | 0.5 | (84/16 131) | 1.28 | 1.00‐1.63 | 1.15 | 0.89‐1.49 | 1.21 | 0.95‐1.55 | 1.19 | 0.93‐1.53 | ||
| Two | 0.5 | (18/3533) | 1.25 | 0.77‐2.01 | 1.05 | 0.63‐1.75 | 1.13 | 0.70‐1.83 | 1.13 | 0.70‐1.83 | ||
| Three | 0.7 | (7/1025) | 1.67 | 0.79‐3.55 | 1.30 | 0.57‐2.93 | 1.47 | 0.69‐3.14 | 1.41 | 0.66‐3.00 | ||
| Missing, % (n) | 4.3 | (4131) | 9.8 | (9419) | 3.2 | (3120) | 3.2 | (3120) | ||||
| Placental adhesion | No | 0.2 | (150/71 392) | 1 | 1 | 1 | 1 | Age, ART, Hyperthyroidism, Endometriosis, Adenomyosis, Congenital uterine anomalies, PCOS | ||||
| One | 0.3 | (45/16 131) | 1.33 | 0.95‐1.86 | 1.20 | 0.84‐1.70 | 1.23 | 0.88‐1.72 | 1.17 | 0.84‐1.64 | ||
| Two | 0.4 | (15/3533) | 2.03 | 1.19‐3.45 | 1.83 | 1.07‐3.14 | 1.74 | 1.01‐2.98 | 1.64 | 0.95‐2.82 | ||
| Three | 0.7 | (7/1025) | 3.27 | 1.53‐6.99 | 2.69 | 1.24‐5.82 | 2.53 | 1.17‐5.47 | 2.33 | 1.08‐5.04 | ||
| Missing, % (n) | 4.3 | (4131) | 9.1 | (8767) | 3.2 | (3120) | 3.2 | (3120) | ||||
| Hypertensive disorders of pregnancy (mild) | No | 2.3 | (1659/71 392) | 1 | 1 | 1 | 1 | Age, BMI, Smoking, Education, (Household income), ART, Previous live birth, Bronchial asthma, IDDM, NIDDM, Gestational diabetes, Hyperthyroidism, Anemia, High blood pressure, Fatty liver, Chronic nephritis, Uterine fibroids, Adenomyosis | ||||
| One | 2.2 | (354/16 131) | 0.94 | 0.84‐1.06 | 0.95 | 0.84‐1.07 | 0.94 | 0.84‐1.06 | 0.95 | 0.84‐1.06 | ||
| Two | 2.5 | (90/3533) | 1.10 | 0.89‐1.36 | 1.09 | 0.86‐1.37 | 1.10 | 0.88‐1.36 | 1.10 | 0.88‐1.36 | ||
| Three | 4.3 | (44/1025) | 1.89 | 1.39‐2.56 | 2.02 | 1.46‐2.78 | 1.87 | 1.37‐2.55 | 1.88 | 1.38‐2.56 | ||
| Missing, % (n) | 4.3 | (4131) | 17.3 | (16 626) | 3.2 | (3120) | 3.2 | (3120) | ||||
| Hypertensive disorders of pregnancy (severe) | No | 1.0 | (695/71 392) | 1 | 1 | 1 | 1 | Age, BMI, ART, Previous live birth, NIDDM, Anemia, High blood pressure, High cholesterol, Fatty liver, Chronic nephritis, Uterine fibroids, Congenital uterine anomalies, PCOS | ||||
| One | 1.1 | (171/16 131) | 1.09 | 0.92‐1.29 | 0.96 | 0.80‐1.14 | 1.04 | 0.88‐1.23 | 1.03 | 0.87‐1.22 | ||
| Two | 0.9 | (32/3533) | 0.93 | 0.65‐1.33 | 0.75 | 0.53‐1.08 | 0.84 | 0.59‐1.21 | 0.85 | 0.60‐1.22 | ||
| Three | 1.8 | (18/1025) | 1.82 | 1.13‐2.92 | 1.29 | 0.80‐2.08 | 1.60 | 0.99‐2.57 | 1.63 | 1.00‐2.64 | ||
| Missing, % (n) | 4.3 | (4131) | 9.2 | (8892) | 3.2 | (3120) | 3.2 | (3120) | ||||
| Uterine infection | No | 0.7 | (535/71 980) | 1 | 1 | 1 | 1 | Marital status, BMI, Education, ART, Previous live birth, Endometriosis | ||||
| One | 0.7 | (114/16 312) | 0.94 | 0.77‐1.15 | 1.06 | 0.85‐1.33 | 1.11 | 0.90‐1.36 | 1.11 | 0.90‐1.36 | ||
| Two | 0.8 | (29/3574) | 1.09 | 0.75‐1.59 | 1.26 | 0.83‐1.93 | 1.42 | 0.97‐2.07 | 1.40 | 0.96‐2.04 | ||
| Three | 1.1 | (11/1041) | 1.43 | 0.78‐2.60 | 1.62 | 0.80‐3.28 | 1.94 | 1.06‐3.55 | 1.96 | 1.07‐3.59 | ||
| Missing, % (n) | 3.4 | (3305) | 7.7 | (7405) | 2.3 | (2242) | 2.3 | (2242) | ||||
| Caesarean section | No | 18.2 | (12 978/71 126) | 1 | 1 | 1 | 1 | Age, Marital status, BMI, Smoking, Education, (Household income), ART, Atopic Dermatitis, Bronchial asthma, Collagen disease, SLE, RA, IDDM, NIDDM, Gestational diabetes, Hypothyroidism, High blood pressure, Stroke, Congenital heart disease, Depression, Anxiety disorders, Gastroesophageal reflux disease, Gastritis, Gastric ulcer, Duodenal ulcer, Fatty liver, Chronic nephritis, Endometriosis, Uterine fibroids, Adenomyosis, Congenital uterine anomalies,Ovarian tumors | ||||
| One | 20.4 | (3278/16 081) | 1.15 | 1.10‐1.20 | 1.04 | 0.99‐1.09 | 1.03 | 0.99‐1.08 | 1.03 | 0.98‐1.07 | ||
| Two | 23.8 | (840/3524) | 1.40 | 1.30‐1.52 | 1.13 | 1.04‐1.24 | 1.17 | 1.08‐1.27 | 1.15 | 1.06‐1.25 | ||
| Three | 27.2 | (277/1019) | 1.67 | 1.46‐1.92 | 1.31 | 1.13‐1.52 | 1.28 | 1.11‐1.47 | 1.25 | 1.08‐1.44 | ||
| Missing, % (n) | 4.6 | (4462) | 17.8 | (17 089) | 3.6 | (3460) | 3.6 | (3460) | ||||
Model I Unadjusted model.
Model II Adjusted model using propensity scores based on complete case analysis.
Model III Adjusted model using propensity scores based on multiple imputation model.
Model IV Covariates about gynecological history (underlined covariates) were added to the model III.
Values in boldface indicated a statistical significance level of 0.05.
OR: Odds Ratio, 95%CI: 95% Confidence Interval.
Household income ( ) was not included in the multiple‐imputation model.
Disorders in italic were excluded from Model III and Model IV.
Table 3.
Perinatal outcome of children according to the number of previous pregnancy losses
| Number of pregnancy losses | Prevalence %, (n) | OR, 95%CI | Pooled OR, 95%CI | Covariates included in propensity score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model I | Model II | Multiple imputation model III | Multiple imputation model Ⅳ | ||||||||||
| SFD | No | 10.3 | (7,370/71,271) | 1 | 1 | 1 | 1 | Age, Marital status, BMI, Smoking, Previous live birth, Collagen disease, SLE, IDDM, Anemia, High blood pressure, Congenital heart disease, Adenomyosis, PCOS | |||||
| One | 9.2 | (1,486/16,106) | 0.88 | 0.83‐0.93 | 0.97 | 0.91‐1.03 | 0.96 | 0.91‐1.02 | 0.96 | 0.91‐1.02 | |||
| Two | 8.7 | (306/3,528) | 0.82 | 0.73‐0.93 | 0.95 | 0.84‐1.07 | 0.96 | 0.85‐1.08 | 0.96 | 0.85‐1.09 | |||
| Three | 8.6 | (88/1,025) | 0.81 | 0.65‐1.01 | 0.99 | 0.78‐1.24 | 0.98 | 0.79‐1.23 | 1.01 | 0.81‐1.26 | |||
| Missing, % (n) | 4.5 | (4,282) | 10.5 | (10,124) | 3.4 | (3,273) | 3.4 | (3,273) | |||||
| IUFD | No | 0.4 | (294/71,980) | 1 | 1 | 1 | 1 | Age, BMI, Adenomyosis | |||||
| One | 0.5 | (80/16,312) | 1.20 | 0.94‐1.54 | 1.11 | 0.86‐1.43 | 1.10 | 0.86‐1.42 | 1.11 | 0.86‐1.42 | |||
| Two | 0.4 | (16/3,574) | 1.10 | 0.66‐1.82 | 0.95 | 0.57‐1.58 | 0.94 | 0.56‐1.56 | 0.92 | 0.55‐1.53 | |||
| Three | 0.8 | (8/1,041) | 1.89 | 0.93‐3.82 | 1.53 | 0.75‐3.12 | 1.55 | 0.76‐3.16 | 1.53 | 0.76‐3.11 | |||
| Missing, % (n) | 3.4 | (3,305) | 7.2 | (6,965) | 2.3 | (2,242) | 2.3 | (2,242) | |||||
| Low birth weight<2500g | No | 8.5 | (6,051/71,300) | 1 | 1 | 1 | 1 | Age, Marital status, BMI, Smoking, Education, ART, Previous live birth, Collagen disease, SLE, RA, NIDDM, High blood pressure, Congenital heart disease, Depression, Anxiety disorders, Chronic nephritis, Nephrotic syndrome, Endometriosis, Uterine fibroids, Adenomyosis, Congenital uterine anomalies | |||||
| One | 7.9 | (1,274/16,115) | 0.93 | 0.87‐0.99 | 0.92 | 0.86‐0.98 | 0.93 | 0.87‐0.99 | 0.92 | 0.86‐0.98 | |||
| Two | 9.1 | (321/3,529) | 1.08 | 0.96‐1.21 | 1.04 | 0.91‐1.18 | 1.09 | 0.97‐1.23 | 1.06 | 0.95‐1.20 | |||
| Three | 10.0 | (102/1,024) | 1.19 | 0.97‐1.47 | 1.18 | 0.95‐1.47 | 1.20 | 0.98‐1.47 | 1.17 | 0.95‐1.45 | |||
| Missing, % (n) | 4.4 | (4,244) | 11.9 | (11,438) | 3.4 | (3,236) | 3.4 | (3,236) | |||||
| Male | No | 51.3 | (36,804/71,700) | 1 | 1 | 1 | 1 | Gestational diabetes, Dysautonomia | |||||
| One | 51.8 | (8,402/16,219) | 1.02 | 0.98‐1.05 | 1.02 | 0.98‐1.05 | 1.02 | 0.99‐1.06 | 1.02 | 0.99‐1.06 | |||
| Two | 51.8 | (1,844/3,557) | 1.02 | 0.95‐1.09 | 1.02 | 0.95‐1.09 | 1.02 | 0.95‐1.09 | 1.02 | 0.95‐1.09 | |||
| Three | 47.7 | (493/1,034) | 0.86 | 0.76‐0.98 | 0.87 | 0.77‐0.98 | 0.86 | 0.76‐0.98 | 0.86 | 0.76‐0.98 | |||
| Missing, % (n) | 3.8 | (3,702) | 5.2 | (4,987) | 2.8 | (2,676) | 2.8 | (2,676) | |||||
| Apgar score <7 at 5 minute | No | 0.6 | (419/67,588) | 1 | 1 | 1 | 1 | Age, BMI, Smoking, ART, Previous live birth, Atopic Dermatitis, IDDM, NIDDM, High blood pressure, Crohn's disease, Uterine fibroids, Adenomyosis | |||||
| One | 0.8 | (117/15,337) | 1.23 | 1.00‐1.51 | 1.24 | 1.00‐1.54 | 1.20 | 0.98‐1.49 | 1.19 | 0.97‐1.47 | |||
| Two | 0.7 | (22/3,364) | 1.06 | 0.69‐1.62 | 1.05 | 0.67‐1.64 | 1.00 | 0.64‐1.54 | 1.00 | 0.65‐1.55 | |||
| Three | 0.5 | (5/978) | 0.82 | 0.34‐1.99 | 0.51 | 0.16‐1.59 | 0.76 | 0.31‐1.84 | 0.74 | 0.31‐1.81 | |||
| Missing, % (n) | 9.3 | (8,945) | 14.7 | (14,184) | 8.3 | (8,015) | 8.3 | (8,015) | |||||
| pH<7.1 | No | 1.2 | (697/59,523) | 1 | 1 | 1 | 1 | ART, Previous live birth, NIDDM, Anemia, Crohn's disease | |||||
| One | 1.2 | (166/13,545) | 1.05 | 0.88‐1.24 | 1.16 | 0.98‐1.38 | 1.16 | 0.98‐1.39 | 1.16 | 0.98‐1.39 | |||
| Two | 1.2 | (36/2,947) | 1.04 | 0.75‐1.46 | 1.22 | 0.86‐1.72 | 1.22 | 0.87‐1.71 | 1.22 | 0.87‐1.71 | |||
| Three | 1.1 | (10/871) | 0.98 | 0.52‐1.84 | 1.10 | 0.57‐2.13 | 1.20 | 0.64‐2.27 | 1.20 | 0.64‐2.27 | |||
| Missing, % (n) | 20.1 | (19,326) | 21.1 | (20,337) | 19.3 | (18,587) | 19.3 | (18,587) | |||||
| Congenital anomaly | No | 12.2 | (8,607/70,321) | 1 | 1 | 1 | 1 | Age, BMI, Education, (Household income), ART, Autoimmune disease, IDDM, Gestational diabetes, Hyperthyroidism, Hypothyroidism, High blood pressure, Congenital heart disease, Depression, Gastritis, Irritable bowel syndrome, Uterine fibroids, Adenomyosis | |||||
| One | 12.6 | (2,004/15,899) | 1.03 | 0.98‐1.09 | 1.01 | 0.95‐1.06 | 1.01 | 0.96‐1.06 | 1.01 | 0.96‐1.06 | |||
| Two | 13.2 | (461/3,492) | 1.09 | 0.99‐1.21 | 1.01 | 0.91‐1.13 | 1.04 | 0.94‐1.15 | 1.04 | 0.94‐1.15 | |||
| Three | 12.7 | (129/1,015) | 1.04 | 0.87‐1.26 | 0.97 | 0.80‐1.18 | 0.96 | 0.80‐1.16 | 0.96 | 0.80‐1.16 | |||
| Missing, % (n) | 5.7 | (5,485) | 17.8 | (17,142) | 4.7 | (4,505) | 4.7 | (4,505) | |||||
| Aneuploidy | No | 0.2 | (140/71,424) | 1 | 1 | 1 | 1 | Age, Smoking, Previous live birth, Gestational diabetes, Depression, Uterine fibroids, Adenomyosis | |||||
| One | 0.2 | (38/16,136) | 1.20 | 0.84‐1.72 | 0.94 | 0.65‐1.37 | 0.99 | 0.69‐1.42 | 0.97 | 0.67‐1.39 | |||
| Two | 0.2 | (8/3,533) | 1.16 | 0.57‐2.36 | 0.73 | 0.34‐1.58 | 0.80 | 0.39‐1.64 | 0.82 | 0.40‐1.68 | |||
| Three | 0.2 | (2/1,025) | 1.00 | 0.25‐4.03 | 0.63 | 0.16‐2.58 | 0.61 | 0.15‐2.46 | 0.59 | 0.15‐2.31 | |||
| Missing, % (n) | 4.3 | (4,094) | 9.8 | (9,451) | 3.2 | (3,083) | 3.2 | (3,083) | |||||
Model I Unadjusted model.
Model II Adjusted model using propensity scores based on complete case analysis.
Model III Adjusted model using propensity scores based on multiple imputation model.
Model IV Covariates about gynecological history (underlined covariates) were added to the model III.
Values in boldface indicated a statistical significance level of 0.05.
OR, Odds Ratio; 95%CI, 95% Confidence Interval; SFD, Small for date; IUFD, Intrauterine fetal death.
Household income ( ) was not included in the multiple‐imputation model.
3.2. Model II (the adjustment of multiple covariates)
After the adjustment of multiple covariates with significance for each outcome, stillbirth, PL, placental adhesion, HDP, caesarean section and a male infant remained significantly associated with three or more pregnancy losses (Model II in Tables 2 and 3). The significance of PTB (both at <37 and <34 weeks’ gestation) disappeared after the adjustment.
3.3. Model III (the adjustment with MIA)
After the adjustment with MIA, a novel increased risk of placental adhesion and uterine infection was found (Model III in Table 2). ORs of placental adhesion increased significantly according to the number of previous PL. ORs of uterine infection tended to increase with the number of pregnancy losses and statistical significance was found in the ORs of three pregnancy losses. No increased risk of adverse perinatal outcomes such as a congenital anomaly, aneuploidy, neonatal asphyxia or SFD was observed (Model III in Tables 3). Adjusted OR with MIA were as follows: 1.76 (95% CI, 1.04‐2.96) for stillbirth, 1.68 (1.12‐2.52) for PL, 1.29 (1.01‐1.65) for PTB <37 weeks, 2.53 (1.17‐5.47) for placental adhesion, 1.87 (1.37‐2.57) and 1.60 (.99‐2.57) for mild and severe HDP, 1.94 (1.06‐3.55) for uterine infection, 1.28 (1.11‐1.47) for caesarean section, and .86 (.76‐.98) for a male infant in women with a history of three or more PL (Model III, Tables 2 and 3). Regarding stillbirth, ORs tended to be increased and was significantly higher with three pregnancy losses. With regard to HDP and caesarean section, the prevalence for women with three pregnancy losses was significantly higher. Marginally increased risks were 1.18 (.96‐1.46) for LBW. There was no risk of a very PTB <34 weeks, PROM, placenta praevia, oligohydramnios or abruptio placentae after adjustment. As for placenta praevia, the prevalence tended to increase, but that of women with three pregnancy losses was not significant.
3.4. Model IV (the adjustment with MIA including gynecological histories)
Similar results were obtained with MIA including covariates for gynecological history (Model IV, Tables 2 and 3).
The risk of placental adhesion and uterine infection was analyzed according to the number of induced abortions. The results were similar to those in RPL (Table S1).
3.5. Analysis including multiple participants
In another supplementary analysis to assess the risk as it appears in a clinical setting where patients present with a range of pregnancy histories, we incorporated 5586 children whose mothers participated in the JECS for the second or third time. When a total of 101 798 participants were analyzed, 17.7% (17 733) had experienced one PL, 3.9% (3907) experienced two losses, 1.1% (1131) experienced three or more losses and 77.3% (77 434) had no history of PL (100 205 participants whose data about pregnancy loss were available, missing, 1593, Table S2). ORs for rare outcomes displaying marginally significant increases in Tables 2 and 3 yielded statistical significance. Adjusted OR with MIA were as follows: 1.36 (1.08 to 1.71) for PTB <37 weeks’ gestation, 1.61 (1.00‐2.58) for oligohydramnios, 1.99 (1.05‐3.79) for abruptio placenta and 1.23 (1.01‐1.51) for a LBW (Model III, Tables S3 and S4).
4. DISCUSSION
The present study revealed no risk of a congenital anomaly, aneuploidy, neonatal asphyxia, or SFD related to RPL.
The absence of RPL effect on congenital anomalies concurs with the results in RM reported in the PROMISE trial.27 Our results showing the lack of risk of the aneuploidy were not attributable to noninvasive prenatal testing (NIPT) or preimplantation genetic test of aneuploidy (PGT‐A). NIPT is permitted only for research purposes by the Japanese Association of Medical Sciences. RPL does not meet NIPT criteria. In addition, PGT‐A is prohibited by the Japan Society of Obstetrics and Gynecology for ethical reasons.
We found a novel significant association between RPL and uterine infection and placental adhesion. The risk might be due not to RPL pathology but to surgery because the results of analysis according to the number of induced abortions were similar to those in RPL (Table S1) and 79.4% of facilities use curettage at induced abortion in Japan.28 Surgical management of the miscarriage using curettage is mainly selected.
The supplementary analysis including participants for the second or third time revealed increased risk of PTB <37 weeks’ gestation, oligohydramnios, abruptio placenta, and LBW. A recent retrospective study comparing 2030 patients with RM and 28 023 participants with no RM showed an increased risk of PTB and perinatal death after adjustment of covariates but no significantly increased risk of LBW, low Apgar score, congenital anomalies, or aneuploidy.10 A historical study with 732 719 nulliparous women who had a first live birth showed that women with RM were at the greatest risk (adjusted OR 1.73; 95%CI 1.57‐1.90) and the greatest association was with extreme PTB (24‐28 weeks, adjusted OR 3.87; 95%CI, 2.85‐5.26).16
The present study found no association with a PTB <34 weeks or PROM after MIA in contrast with previous studies.12, 13, 14, 15, 16, 17 Further study with the use of MIA is necessary to confirm this association.
HDP and caesarean section were both associated with RPL. This is in line with the results of a population‐based study with 154 294 women, which indicated an increased risk of caesarean section, placenta abruptio, and hypertensive disorder after two or more miscarriages.17 Recently, 472 variants in 187 genes have been reported to be associated with RPL. A meta‐analysis revealed a significant association between RM and 21 genetic variants with ORs .51‐2.37.29 Common risk alleles such as annexin A5 might influence both HDP and RPL.30, 31
Finally, women with three or more PL had a lower tendency to have a male infant on the index pregnancy
A previous study proved that boys were significantly more common than girls among births prior to a secondary RM and the chance of a live birth after RM is lower in those with a firstborn boy compared with a firstborn girl.32 The study also revealed that birth of a girl was a significantly more common outcome of a live birth after a secondary RM, and that the maternal carriage of male‐specific H‐Y‐restricting HLA class II alleles was associated with the reduced birth rate of boys.
The major limitation was that there was no distinction among different etiologies for RPL, nor whether interventions were performed. The prevalence of early miscarriage was only .36% because many of the participants were recruited after 10 weeks’ gestation. Thus, it was one of the limitations that the early miscarriage and pregnancy loss results might not be reliable.
The present study represents the largest nationwide birth cohort study in Japan. The results are reliable because pregnancy and delivery information was drawn from medical records by doctors and research coordinators. The comparisons between complete‐case analyses and MIA allowed for a relevant sensitivity analysis to quantify the risk of a response bias. MIA allowed for a reliable estimation of the results and helped to minimize the risk of bias. ORs tended to be lower after the adjustment with MIA when they were compared using Model II and III. Many of covariates included in propensity score increased the rate of missing data, which might lead to over‐adjustment in Model II. Thus, MIA might insure the stability of the results in Model III, compared with those in Model II. On the other hand, there were no remarkable differences were observed in the results of Model III and Model IV, suggesting few effects from covariates related to the gynecological history. We, therefore, assumed that the results of Model III as the most important and reliable estimation minimizing the risk of bias.
This information, especially the finding that there was no increased risk of a live birth with a congenital anomaly or aneuploidy in women with a history of RPL as compared to women with no history of pregnancy loss, could encourage women who might hesitate to attempt a subsequent pregnancy. Many patients with RPL are afraid that their baby will have an anomaly because an abnormal embryonic karyotype is the most common cause of RPL.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
This study was funded by the Ministry of the Environment, Government of Japan.
M.S‐O. received grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology for conducting other studies on the topic of RPL in the different patient population, and payment for lectures from Kaken Pharmaceutical Co. Ltd., Kissei Pharmaceutical Co., Aska Pharmaceutical Co. Ltd., Sekisui Medical Co. Ltd., Siemens Japan. The remaining authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
The JECS group conducted the nationwide study project. MSO designed the present study, analyzed the data, and wrote the first draft of the manuscript. TE organized the study team and was responsible for obtaining and analyzing the data. MK, a member of the JECS Steering Committee, was responsible for data acquisition and supervision of the study. MK and TO took the initiative in the launch of the Aichi regional subcohort of JECS. NS TM, HK, and TK analyzed the data. YY, TO, MT, MM, and SS were responsible for data acquisition. All authors interpreted the data, contributed to the writing of the manuscript and revised it critically for important intellectual content.
Supporting information
ACKNOWLEDGMENTS
This study was funded by the Ministry of the Environment, Government of Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Ministry of the Environment. The chief members of the Japan Environment and Children’s Study (JECS) as of 2016 are the National Institute for Environmental Studies, Tsukuba (principal investigator, Toshihiro Kawamoto); the National Center for Child Health and Development, Tokyo (Hirohisa Saito); Hokkaido University, Sapporo (Reiko Kishi); Tohoku University, Sendai (Nobuo Yaegashi); Fukushima Medical University, Fukushima (Koichi Hashimoto); Chiba University, Chiba (Chisato Mori); Yokohama City University, Yokohama (Shuichi Ito); University of Yamanashi, Chuo (Zentaro Yamagata); University of Toyama, Toyama (Hidekuni Inadera); Nagoya City University, Nagoya (Michihiro Kamijima); Kyoto University, Kyoto (Toshio Heike); Osaka University, Suita (Hiroyasu Iso); Hyogo College of Medicine, Nishinomiya (Masayuki Shima); Tottori University, Yonago (Yasuaki Kawai); Kochi University, Nankoku (Narufumi Suganuma), University of Occupational and Environmental Health, Kitakyushu (Koichi Kusuhara); Kumamoto University, Kumamoto (Takahiko Katoh).
Sugiura‐Ogasawara M, Ebara T, Yamada Y, et al. Adverse pregnancy and perinatal outcome in patients with recurrent pregnancy loss: Multiple imputation analyses with propensity score adjustment applied to a large‐scale birth cohort of the Japan Environment and Children’s Study. Am J Reprod Immunol. 2019;81:e13072 10.1111/aji.13072
DATA ACCESSIBILITY
Data sharing is not permitted by the JECS due to a government policy restricting the deposition of data containing personal information. See the reference for more details.21
REFERENCES
- 1. Practice Committee of the American Society for Reproductive Medicine . Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 2012;98:1103‐1111. [DOI] [PubMed] [Google Scholar]
- 2. European Society for Human Reproduction and Embryology (ESHRE) early pregnancy guideline development group. Recurrent pregnancy loss guideline of the ESHRE. 2017; 11.
- 3. Practice Committee of American Society for Reproductive Medicine . Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99:63. [DOI] [PubMed] [Google Scholar]
- 4. Sugiura‐Ogasawara M, Ozaki Y, Sato T, Suzumori N, Suzumori K. Poor prognosis of recurrent aborters with either maternal or paternal reciprocal translocation. Fertil Steril. 2004;81:367‐373. [DOI] [PubMed] [Google Scholar]
- 5. Sugiura‐Ogasawara M, Ozaki Y, Kitaori T, Kumagai K, Suzuki S. Midline uterine defect size correlated with miscarriage of euploid embryos in recurrent cases. Fertil Steril. 2010;93:1983‐1988. [DOI] [PubMed] [Google Scholar]
- 6. Sugiura‐Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27:2297‐2303. [DOI] [PubMed] [Google Scholar]
- 7. Franssen MT, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PM, Goddijn ML. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]‐control study. BMJ 2006;332:759‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73:300‐304. [DOI] [PubMed] [Google Scholar]
- 9. van Oppenraaij RH, Jauniaux E, Christiansen OB, et al. Predicting adverse obstetric outcome after early pregnancy events and complications: a review. Hum Reprod Update. 2009;15(4):409‐421. [DOI] [PubMed] [Google Scholar]
- 10. Field K, Murphy DJ. Perinatal outcomes in a subsequent pregnancy among women who have experienced recurrent miscarriage: a retrospective cohort study. Hum Reprod. 2015;30(5):1239‐1245. [DOI] [PubMed] [Google Scholar]
- 11. Paz JE, Otaño L, Gadow EC, Castilla EE. Previous miscarriage and stillbirth as risk factors for other unfavourable outcomes in the next pregnancy. Br J Obstet Gynecol. 1992;99(10):808‐812. [DOI] [PubMed] [Google Scholar]
- 12. Thom DH, Nelson LM, Vaughan TL. Spontaneous abortion and subsequent adverse birth outcomes. Am J Obstet Gynecol. 1992;166:111‐116. [DOI] [PubMed] [Google Scholar]
- 13. Basso O, Olsen J, Christensen K. Risk of preterm delivery, low birthweight and growth retardation following spontaneous abortion: a registry‐based study in Denmark. Int J Epidemiol. 1998;27(4):642‐646. [DOI] [PubMed] [Google Scholar]
- 14. Buchmayer SM, Sparén P, Cnattingius S. Previous pregnancy loss: risks related to severity of preterm delivery. Am J Obstet Gynecol. 2004;191(4):1225‐1231. [DOI] [PubMed] [Google Scholar]
- 15. Bhattacharya S, Townend J, Shetty A, Campbell D, Bhattacharya S. Does miscarriage in an initial pregnancy lead to adverse obstetric and perinatal outcomes in the next continuing pregnancy? BJOG. 2008;115(13):1623‐1629. [DOI] [PubMed] [Google Scholar]
- 16. Oliver‐Williams C, Fleming M, Wood AM, Smith G. Previous miscarriage and the subsequent risk of preterm birth in Scotland, 1980‐2008: a historical cohort study. BJOG. 2015;122(11):1525‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheiner E, Levy A, Katz M, Mazor M. Pregnancy outcome following recurrent spontaneous abortions. Eur J Obstet Gynecol Reprod Biol. 2005;118(1):61‐65. [DOI] [PubMed] [Google Scholar]
- 18. Kawamoto T, Nitta H, Murata K, et al. Rationale and study design of the Japan environment and Children’s Study (JECS). BMC Public Health. 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michikawa T, Nitta H, Nakayama SF, et al. The Japan Environment and Children's Study (JECS). A preliminary report on selected characteristics of approximately 10 000 pregnant women recruited during the first year of the study. J Epidemiol. 2015;25(6):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki K, Shinohara R, Sato M, Otawa S, Yamagata Z. Association between maternal smoking during pregnancy and birth weight: an appropriately adjusted model from the Japan Environment and Children's Study. J Epidemiol. 2016;26(7):371‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Japan Environment and Children’s Study . Tokyo: Ministry of the Environment; Available from: http://www.env.go.jp/en/chemi/hs/jecs/ [updated August 12, 2016; cited August 16, 2016]. [Google Scholar]
- 22. Ishitsuka K, Nakayama SF, Kishi R, et al. Environment and Children’s Study. Backgrounds, activities, and future directions in global perspectives. Environ Health Prev Med 2017. 10.1186/s12199-017-0667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institute for Environmental Studies/National Centre for Japan Environment and Children’s Study, Japan Environment and Children’s Study (JECS) Study Protocol (ver. 1.4). Available from https://www.env.go.jp/chemi/ceh/outline/data/jecs-study_protocol_14_en.pdf [cited January 18, 2017].
- 24. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70(1):41‐55. [Google Scholar]
- 25. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete‐case analysis for missing covariate values. Stat Med. 2010;29(28):2920‐2931. 10.1002/sim.3944. [DOI] [PubMed] [Google Scholar]
- 27. Coomarasamy A, Williams H, Truchanowicz E, et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med. 2015;373(22):2141‐2148. [DOI] [PubMed] [Google Scholar]
- 28. Sekiguchi A, Ikeda T, Okamura K, Nakai A. Safety of induced abortions at less than 12 weeks of pregnancy in Japan. Int J Gynecol Obstet. 2015;129:54‐57. [DOI] [PubMed] [Google Scholar]
- 29. Pereza N, Ostojić S, Kapović M. Systematic review and meta‐analysis of genetic association studies in idiopathic recurrent spontaneous abortion. Fertil Steril. 2016;pii: S0015‐0282(16)62924‐2. [DOI] [PubMed] [Google Scholar]
- 30. Tiscia G, Colaizzo D, Chinni E, et al. Haplotype M2 in the annexin A5 (ANXA5) gene and the occurrence of obstetric complications. Thromb Haemost. 2009;102(2):309‐313. [DOI] [PubMed] [Google Scholar]
- 31. Hayashi Y, Sasaki H, Suzuki S, et al. Genotyping analyses for polymorphisms of ANXA5 gene in patients with recurrent pregnancy loss. Fertil Steril. 2013;100(4):1018‐1024. [DOI] [PubMed] [Google Scholar]
- 32. Nielsen HS, Steffensen R, Lund M, et al. Frequency and impact of obstetric complications prior and subsequent to unexplained secondary recurrent miscarriage. Hum Reprod. 2010;25(6):1543‐1552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not permitted by the JECS due to a government policy restricting the deposition of data containing personal information. See the reference for more details.21


