ABSTRACT
Introduction: Static‐ and moving 2‐point discrimination (S2PD, M2PD), 10‐g monofilaments‐ and tuning fork are validated outcome measures of clinical manifestations of diabetes‐related neuropathy. No modern statistical techniques have been used to investigate how well these instruments combine to measure sensory loss. Methods: To grade sensory loss at the feet, we fitted parametric forms of Item Response Theory models to the data of these instruments. Results: The fit statistics indicate that the loss of sensation is gradable, with readily available instruments. S2PD and M2PD are lost first, followed by vibration sense, the 10‐g monofilament and the ability to feel a cold stimulus. Conclusions: This test battery appears to provide sound measurement properties in a group of diabetic patients with diverse amounts of sensory loss. This approach may be used in clinical practice to grade sensory loss reliably and quickly, with instruments that are easy to use. Muscle Nerve 58: 559–565, 2018
Keywords: diabetes, diabetic sensory loss, psychometry, sensorimotor polyneuropathy, scale development
Abbreviations
- 2PL
2‐parameter logistic model
- DIF
differential item functioning
- DSP
diabetic sensorimotor polyneuropathy
- ICC
item characteristic curve
- IRT
item response theory
- LTMR
low‐threshold mechanoreceptor
- M2PD
moving 2‐point discrimination
- MNSI
Michigan Neuropathy Screening Instrument
- RDF
Rotterdam Diabetic Foot (Study)
- RDF‐39
Rotterdam Diabetic Foot Study Test Battery (39‐items)
- S1PD
static 1‐point discrimination
- S2PD
static 2‐point discrimination
Sensory loss due to diabetic sensorimotor polyneuropathy (DSP) can be assessed using noninvasive and readily available screening instruments such as a tuning fork, static‐ and moving 2‐point discrimination (S2PD and M2PD) and monofilaments.1, 2, 3, 4, 5, 6, 7
Each instrument tests different somatosensory functions that are progressively lost during diabetes, in the context of DSP.8, 9, 10, 11 S2PD, for example, tests the slowly adapting type I afferent Aß large‐fiber system in the skin, with the Merkel cell‐neurite complex as peripheral receptors. M2PD tests both the quickly adapting type I afferent Aß fiber system, with Meissner corpuscles as mechanoreceptors, as well as the quickly adapting type II afferent Aß fiber system, with Pacinian corpuscles as somatosensory receptors. The latter system is also tested when performing vibratory testing.12, 13 Static 1‐point discrimination (S1PD, e.g., monofilaments) tests the slowly adapting type I Aß system, with the Merkel cell‐neurite complex as mechanoreceptors. To establish the exact temporal sequence in which these sensory functions are lost, thereby grading the degree of sensory loss, measurement properties of each instrument need to be clarified in relation to each other.
To investigate how well instruments combine to measure sensory loss, we applied parametric forms of item response theory (IRT) to the tests used in the Rotterdam Diabetic Foot (RDF) study. IRT is a set of mathematical models (e.g., the Rasch model) that describe the relationship between an individual's “ability” or “trait” and how they respond to test items.14, 15, 16 In short, IRT assumes that patients with a higher ability (less ill) should have a greater chance of obtaining a nonaberrant score on an item. Thus, the probability of a response to an item depends on the differences between the ability of the person and the difficulty of the item. With this information, relative patient disability can be rank ordered on an individual level by measuring patient ability (in this study foot sensation) and comparing this to the ability of each individual test item to differentiate between disability levels. Moreover, parametric IRT models can be used to explore individual ability range, identify fitting and misfitting items to the models and investigate differential item functioning (DIF) between subgroups of patients, which is indicative of item bias.
The purpose of this study was to evaluate the psychometric properties of the 39‐item Rotterdam Diabetic Foot Study Test Battery (RDF‐39), to explore how test instruments and test locations on the foot compare, to create an outcome measure of foot sensation on a continuous scale and investigate in which areas of the trait (i.e., foot sensation) test information is rich or scarce.
MATERIALS AND METHODS
Study Design and Subjects
Between January 2014 and June 2015, a total of 416 subjects with type 1 and type 2 diabetes were assessed prospective for the sensory status of their feet at the outpatient Diabetes Clinic of the Franciscus Gasthuis, Rotterdam, the Netherlands as part of the RDF‐study to investigate the deterioration of sensation in their feet over time. Baseline RDF‐data are used for this cross‐sectional analysis. Inclusion criteria were: patients diagnosed with diabetes mellitus (treated by oral blood glucose lowering drugs and/or insulin), age over 18 years, speaking Dutch or English, and no significant cognitive impairment. Exclusion criteria were: active radicular syndrome and neurological disease interfering with sensation of the feet, as assessed in the interview and screening questionnaire. Demographic data were obtained from the patient file. All subjects provided written informed consent. The institutional review board and the Medical Ethical Committee of Erasmus MC Medical University Center, Rotterdam, the Netherlands approved the study (MEC‐2009‐148).
Measurement Instrument
To test the different somatosensory functions at multiple sites of the feet, a battery of tests was used. The RDF‐39 includes both instruments and test sites to measure overall foot sensation and consists of 39 individual items that measure the unidimensional construct of foot sensation.17 This scoring system contains dichotomized items on static‐ and moving 2‐point discrimination (S2PD and M2PD), static 1‐point discrimination (S1PD), vibration sense, cold stimulus tests, Romberg's test, experienced numbness, prior diabetic foot ulcer and prior amputation. S2PD and M2PD were tested with a Disk‐Criminator™ (US Neurologicals, LCC, Poulsbo, WA), with the threshold set at 8 mm (abnormal: > 8 mm), based on previously published normative values.4 S1PD was tested with a 10 g Semmes‐Weinstein monofilament (Baseline® Tactile™, Minneapolis, MN), based on current international standards of medical care in diabetes.2, 3 S2PD, M2PD, and S1PD test sites were chosen in concordance with the nerve territories of the foot: I, plantar hallux (medial plantar nerve [tibial nerve]); II, medial heel (calcaneal nerve [tibial nerve]); III, first dorsal web (deep peroneal nerve); IV, lateral foot (sural nerve) and V, plantar fifth toe (lateral planter nerve [tibial nerve]), see Supplementary Figure SA, which is available online. M2PD was not tested at the fifth toe due to its small surface area.
Vibration sense was tested with a Rydel‐Seiffer tuning fork (Martin, Tuttlingen, Germany) at the medial malleolus and dorsal interphalangeal joint of the hallux and compared with normative threshold data.6 Cold sensation was tested by applying a cold piece of metal to the arch of the foot. Information on numbness was derived from the Michigan Neuropathy Screening Instrument (MNSI), which was administered before the physical examination. Information on prior ulceration and/or amputation, as indicators of severe sensory loss, was derived from the patient interview. Sensory test items consisted of both a sensory test and test location (e.g., S1PD at the lateral foot [S1PD IV], S2PD at the plantar fifth toe [S2PD V]). All 39 individual items were scored with 1 or 0. A score 1 indicated abnormality on a test item (i.e., could not feel the stimulus). The sum score per patient ranged from 0 to 39. In addition to the 39 items on sensory loss, localized tibial nerve compression over the tarsal tunnel was assessed using Tinel's sign.18 Both feet were examined.
Parametric Item Response Theory
We examined the psychometric properties of the RDF‐39 using parametric IRT models. Supplementary Table SA provides definitions of the terms used. A parametric IRT model compares the relative ability of each individual test item to differentiate between patient ability levels. Patients with a lower ability (more ill) have a greater chance of obtaining an aberrant test result, compared with patients with higher ability (less ill). This relationship is visualized by an item characteristic curve (ICC), in which theta (θ) is a variable used to express a patient's underlying ability (or trait) level, measured along the x‐axis. The probability of endorsing an item is given along the y‐axis and measured from 0 to 1. The location of the item (in log‐odd units, logits) on the latent trait (theta, in this study sensation at the feet) represents the estimated difficulty of the item, which is the mean of the threshold location. An example of this relationship is that a patient with a history of diabetic foot ulceration (more ill) is very unlikely to have a nonaberrant S2PD test result at the hallux (less ill). Higher values on theta represent greater levels of loss of foot sensation.
Fit Statistics
Fit statistics estimate how well the data match model expectations. By using chi‐square statistics, observed data were compared with expected model values. Three parametric IRT models were considered: the 1‐parameter logistic (standard and extended Rasch) and 2‐parameter logistic (2PL) models. The standard Rasch model estimates a location parameter for each item and assumes a common discrimination parameter (a) that is fixed at 1. The extended Rasch model, in contrast, estimates the common discrimination parameter (i.e., not fixed at 1). If the assumption of equal discrimination does not hold, the 2PL‐model can be used to estimate both location and discrimination parameters for each item. The fitted models were compared with a likelihood ratio test. Once a model appropriate for the data has been selected, further tests of model and item fit are executed.
Item Parameters
The most important parameters under IRT are item location, discrimination and trait score. In this study, binary RDF‐39 items were considered, so item location (b) (item difficulty) represents the location on the latent trait (theta) where the probability of endorsing an item is 50% (where the item functions best). Items with higher b values represent more severe sensory loss. Item discrimination (a) reflects the ability of an item to discriminate between patients at different levels of the trait and is defined as the slope of the ICC at the item location. A steeper curve is indicative of better discriminatory characteristics, with values closer to zero meaning low discrimination power.
The items of the test battery were analyzed by calculating the negative and positive responses per item. Cronbach's alpha assessed descriptively whether the items measured a common construct (foot sensation, hence foot sensation determines the item scores). To investigate which items fulfill the Rasch assumption (i.e., which items have a discrimination parameter equal to 1), we calculated the 95% confidence intervals for the discrimination parameters and checked for which items the value 1 was included in the interval. The probability of a positive response from the median patient was calculated, i.e. the patient with theta value equal to zero.
ICCs and Item Information Curves
For all items, ICCs were drawn that describe the relationship between a patient's underlying trait and how that patient responds to a dichotomous item, together with corresponding item information curves. An item information curve can be drawn for each item to see in which range of theta values the specific item provides information (y‐axis) and to reveal how precision varies across different levels of the underlying trait (x‐axis). The amount of test information per item is defined as Ii (θ) at an ability level of theta. In IRT, the concept of information reflects how precisely an item or scale can measure the underlying ability (trait). Greater measurement precision is associated with greater information and is inversely related to the standard error of the estimate (theta). Thus, with more information a smaller standard error of the estimated theta score is obtained. Generally, more items provide more information. To quantify the available information from all RDF‐39 items, we calculated the area under the total test information curve in specific intervals of theta.
Theta Estimates
The trait score can be calculated after a patient has responded to the items of the battery, to estimate their position along the underlying trait (ability). Findings in healthy volunteers without known neuropathy have been published separately and are presented in this study for comparative purposes.4
DIF
To investigate whether the items function differently (so called item bias) in patients with respect to gender, type of diabetes, age, duration of diabetes, complaints and signs of tibial nerve compression at the tarsal tunnel, we tested for DIF using the General Mantel‐Haenszel method. This method examines whether the odds of a symptomatic response within each group on the measure is the same across subgroups, after controlling for ability. Assessing DIF is important for valid comparisons between subgroups of patients. Patients were arbitrarily categorized, aiming for a logical and/or approximately equal distribution between the constructed groups. Four age groups were identified by age (18–50 years, 51–60 years, 61–70 years, and >70 years); 4 by duration of diabetes (0–10 years, 11–20 years, 21–30 years, > 30 years); and 3 by the total score on the MNSI questionnaire as an indicator of severity of experienced complaints of DSP (0 < 4: minor, 4 ≤ 7: intermediate, > 7 severe).
Statistics
Rasch and 2PL‐model analyses were conducted using the R packages “ltm” and “difR”.19, 20 Missing data were handled as part of the modeling process, but were rare (mean per item: 2.0%, range: 0–5.8%), resulting in minimal bias. Unidimensionality was assessed through principal components analysis of the residuals. Other statistical analysis was carried out using IBM's SPSS Statistics 22.0 (IBM Corp., New York, NY). Kolmogorov‐Smirnov tests were used to assess normality. Differences in continuous variables with skewed distributions (theta) between 2 independent groups were assessed with Mann‐Whitney U tests. P‐values < 0.05 (2‐sided) were considered statistically significant.
RESULTS
General Characteristics
A total of 416 RDF‐study participants with diabetes were included. Fifty‐two patients had a history of diabetic foot ulceration, for which 13 underwent lower extremity amputations. Further baseline characteristics are given in Table 1.
Table 1.
Demographic data
| Gender (M/F) | 241/175 |
| Age (median (y), IQR) | 64.0 (15.4) |
| Height (median (cm), IQR) | 173.0 (15.8) |
| Weight (median (kg), IQR) | 87.8 (26.5) |
| BMI (median (kg/m2), IQR) | 29.4 (7.6) |
| Duration of diabetes (median (y), IQR) | 16.0 (16) |
| Type of diabetes (n (%)) | |
| Type 1 | 93 (22.4%) |
| Type 2 | 323 (77.6%) |
| Drugs (n (%)) | |
| Lipid lowering drugs | 267 (64.2%) |
| Oral blood glucose lowering drugs | 229 (55.0%) |
| Insulin | 351 (84.4%) |
| HbA1c (median (mmol/L), IQR) | 60.0 (17.0) |
| MDRD (median ml/min/1.73 m, IQR) | 77.9 (36.0) |
| Total cholesterol (median (mmol/L), IQR) | 4.0 (1.3) |
| LDL‐C (median (mmol/L), IQR) | 1.8 (1.1) |
| HDL‐C (median (mmol/L), IQR) | 1.3 (0.5) |
| TG (median (median mmol/L), IQR) | 1.6 (1.4) |
| Systolic blood pressure (median mmHg, IQR) | 136.0 (23) |
| Diastolic blood pressure (median mmHg, IQR) | 77.0 (13) |
| Diabetic sensory polyneuropathy (n (%) | |
| MNSI score ≥ 4 | 148 (35.6%) |
| Retinopathy (n (%)) | 59 (14.2%) |
BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high density lipoprotein; IQR, interquartile range; LDL, low density lipoprotein; MDRD, Modification of Diet in Renal Disease; M/F, male/female; TG, triglycerides.
Fit Statistics
Excellent internal consistency of the 39 items was observed (Cronbach's alpha > 0.938). The likelihood ratio test between the estimated and fixed values of the common discrimination parameter across items was (χ 2 (df = 1) = 390.93; P < 0.001), suggesting that the extended Rasch model, which does not fix the discrimination parameter to 1, provides a better fit for the data. However, the likelihood ratio test between the extended Rasch model and the 2PL‐model was (χ 2 (df = 38) = 267.47; P < 0.001), suggesting that the 2PL‐model fitted the data better than the extended Rasch model. Hence, for the remainder of this analysis we continued with the 2PL‐model.
Item Parameters (2PL‐Model)
Estimated difficulty and discrimination parameters from the 2PL‐model are given per item in Table 2. In the first column, items are ordered according to the difficulty parameter (from the item with the highest chance of a positive response to the item with the lowest probability of a positive response, as indicated in the second column). Thus, item difficulty ranged from S2PD and M2PD, ordered from those most likely representing milder disease severity to items on severe sensory loss. Specifically, items on S2PD, M2PD, vibration sense, and S1PD were clustered, suggesting that for all test sites the loss of S2PD and M2PD precedes the loss of a protective sensation, as assessed with a 10‐g monofilament. Of interest, complaints of numbness are reported when S2PD, M2PD, vibration sense and plantar S1PD items are already abnormal, suggesting that the patient is unaware of the loss of these sensory modalities.
Table 2.
Estimated difficulty and discrimination parameters from the 2PL‐model.
| Item | Difficulty parameter value | P (x=1 | z=0) | Discrimination parameter value | 95% confidence interval | DIF due to |
|---|---|---|---|---|---|
| S2PD II left | −1.076 | .893 | 1.97 | 1.46 – 2.34 | Neuropathy complaints |
| S2PD II right | −1.040 | .916 | 2.30 | 1.74 – 2.86 | Type of diabetes |
| S2PD I left | −0.984 | .867 | 2.57 | 1.93 – 3.21 | |
| S2PD III left | −0.929 | .837 | 1.76 | 1.35 – 2.17 | |
| S2PD IV right | −0.915 | .881 | 2.18 | 1.68 – 2.69 | |
| S2PD V left | −0.901 | .897 | 2.40 | 1.85 – 2.96 | |
| S2PD V right | −0.877 | .943 | 3.21 | 2.42 – 4.00 | |
| S2PD I right | −0.862 | .900 | 2.55 | 1.97 – 3.13 | |
| S2PD IV left | −0.844 | .894 | 2.53 | 1.96 – 3.10 | |
| S2PD III right | −0.839 | .859 | 2.16 | 1.66 – 2.65 | |
| M2PD II left | −0.657 | .760 | 1.76 | 1.35 – 2.16 | Gender, Tinel |
| M2PD III left | −0.618 | .757 | 1.84 | 1.42 – 2.26 | Gender, type of diabetes, age |
| M2PD III right | −0.580 | .758 | 1.97 | 1.53 – 2.40 | Gender |
| M2PD II right | −0.416 | .711 | 2.16 | 1.69 – 2.64 | |
| M2PD IV left | −0.392 | .665 | 1.75 | 1.36 – 2.15 | Tinel |
| M2PD IV right | −0.368 | .683 | 2.09 | 1.62 – 2.56 | Duration of diabetes |
| M2PD I left | −0.279 | .673 | 2.59 | 2.01 – 3.17 | Gender, type of diabetes, age, neuropathy complaints |
| M2PD I right | −0.260 | .648 | 2.34 | 1.81 – 2.87 | |
| Vibration sense IP lefta | 0.250 | .429 | 1.14 | 0.86 – 1.42 | Gender, age |
| Vibration sense IP righta | 0.250 | .429 | 1.14 | 0.86 – 1.42 | Gender, age |
| Vibration sense MM righta | 0.498 | .384 | 0.95 | 0.70 – 1.20 | Age |
| Vibration sense MM lefta | 0.601 | .346 | 1.06 | 0.79 – 1.33 | Age |
| S1PD I left | 0.781 | .094 | 2.90 | 2.17 – 3.64 | |
| S1PD I right | 0.835 | .117 | 2.43 | 1.83 – 3.02 | Neuropathy complaints |
| S1PD V left | 0.958 | .079 | 2.57 | 1.93 – 3.21 | Neuropathy complaints, Tinel |
| Numbnessa | 0.960 | .234 | 1.24 | 0.93 – 1.54 | Type of diabetes, neuropathy complaints, Tinel |
| S1PD V right | 0.966 | .070 | 2.67 | 1.99 – 3.36 | Age, neuropathy complaints |
| S1PD II right | 1.012 | .145 | 1.75 | 1.33 – 2.18 | |
| S1PD II left | 1.018 | .144 | 1.75 | 1.33 – 2.17 | |
| S1PD III left | 1.371 | .012 | 3.23 | 2.33 – 4.13 | Type of diabetes, neuropathy complaints |
| S1PD III right | 1.405 | .022 | 2.71 | 1.98 – 3.43 | |
| S1PD IV left | 1.532 | .022 | 2.46 | 1.80 – 3.13 | |
| S1PD IV right | 1.578 | .026 | 2.29 | 1.68 – 2.90 | Neuropathy complaints |
| Prior ulcer | 1.696 | .057 | 1.65 | 1.23 – 2.08 | Neuropathy complaints |
| Cold stimulus lefta | 1.812 | .097 | 1.23 | 0.89 – 1.57 | Gender |
| Cold stimulus righta | 1.902 | .091 | 1.21 | 0.88 – 1.54 | Duration of diabetes |
| Romberg testa | 2.157 | .143 | 0.83 | 0.56 – 1.10 | Gender, type of diabetes, age |
| Amputation lefta | 3.159 | .001 | 2.16 | 0.79 – 3.53 | |
| Amputation righta | 3.322 | .004 | 1.64 | 0.79 – 2.49 |
*Roman capitals are indicatives of test locations: I, hallux; II, medial heel; III, first dorsal web; IV, lateral foot; V, fifth toe. IP, interphalangeal joint; MM, medial malleolus. For details on statistical information on DIF, see Supplementary Table SB.
Items included based on discrimination parameter value 1.
The third column gives the probability of a positive response from the median diabetic patient. Patients experiencing more sensory loss (i.e., having a higher sum score) have a higher chance of scoring positive on items with lower difficulty parameter values (e.g., patients with a history of diabetic foot ulceration or amputation). The fourth column displays the discrimination parameter of each item that quantifies how well the corresponding item can discriminate between patients, together with the 95% confidence interval of the discrimination parameter (fifth column). By checking which items were included based on value 1, we investigated which items fulfilled the Rasch assumption (equal discrimination across items). Ten items met this criterion (Table 2).
Item Information Curves and Theta Estimates
The total item information of all 39 items is given in a plot (Fig. 1). The area under this curve was divided into specific intervals on theta, to see from which level item information is scarce. High logits indicate more severe sensory loss. From ‐4 < theta < ‐3 the available information was 0.5%, from ‐3 < theta < ‐2 3.1%, from ‐2 < theta < ‐1 15.9%, from ‐1 < theta < 0 26.5%, from 0 < theta < 1 21.9%, from 1 < theta < 2 19.4%, from 2 < theta < 3 7.5% and from 3 < theta < 4 3.5%. Most information is provided in the interval of ‐2 < theta < 2. This may help to determine where information from tests in areas of the trait is scarce.
Figure 1.
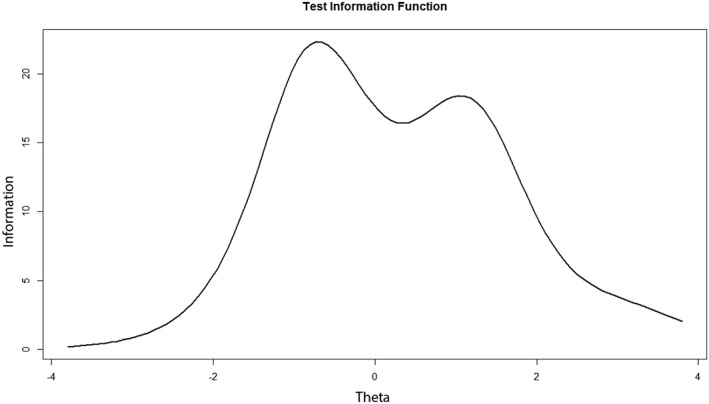
The total information curve of all items (n = 39) shows the total test information along the trait (with Y = Ii (θ) * the number of items in the test) and provides data on where test information is scarce.
The patient who scored nonaberrant on all items (equal to zero) had a theta estimate of ‐2.17, whereas the patient who scored all items aberrant had a theta estimate of 3.21. Most patients fell in the ability range of theta where much of items are situated, indicating that the risk of floor and ceiling effects is low (Fig. 2).
Figure 2.
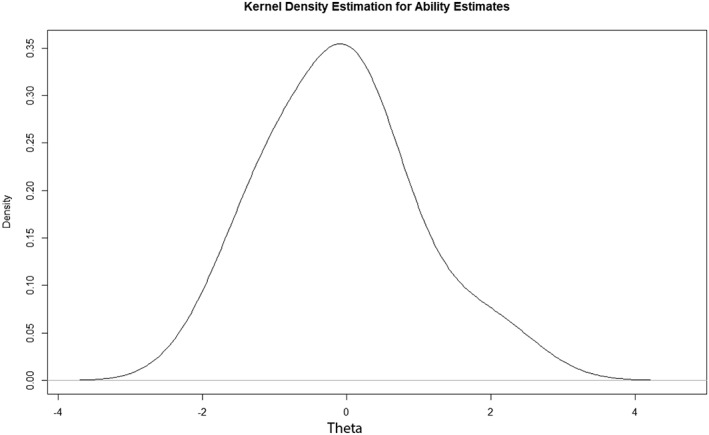
The distribution of patients along theta (in logits).
Figure 3 shows the theta distribution of diabetic RDF‐study participants compared with controls without known neuropathy.
Figure 3.
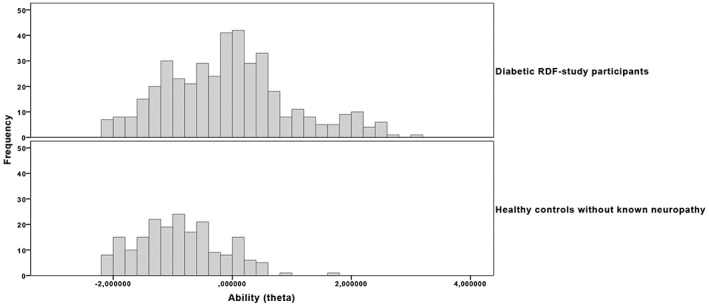
Comparison between diabetic subjects and controls. Histograms of theta estimates in both groups show that median theta scores (interquartile range) differed significantly between individuals without known neuropathy (‐0.924 [0.94]) and subjects with diabetes (‐0.088 [1.37]), P < 0.0005. Control subjects may experience some aberrancy in S2PD and M2PD items, but generally do have intact vibration sensation and protective sensation, compared with diabetic subjects.
DIF
Minimal DIF was found in the items of the RDF‐39 (Table 2). Eight of 39 items showed DIF due to gender. Six items showed DIF due to type of diabetes, 8 due to age, 2 due to a longer duration of diabetes, 9 due to increasing symptoms as indicated by the total MNSI score and 4 due to signs of tibial nerve compression at the tarsal tunnel (Supplementary Table SB). By investigating residual principal component loadings, the RDF‐39 was found to be unidimensional (proportion of variance of first principal component: 94.63%).
DISCUSSION
In this study, we investigated the measurement properties of the RDF‐39 by fitting parametric IRT models to the data. Our analysis showed that it is possible to grade sensory loss of the feet more precisely with a set of simple to use instruments on specific test locations. This grading presumably allows for a more precise risk stratification regarding, for example, foot ulceration, amputation, and falls.
The effects of hyperglycemia and cardiovascular risk factors produce morphological changes in the structures of the peripheral and central nervous system, resulting in dysfunction of the involved tissues.21, 22, 23 Connective tissue and vascular structures undergo changes as well, contributing to the common clinical entity of neuropathy.
Axonal loss occurs along the whole course of the peripheral nerve but is most severe distally. Both myelinated and unmyelinated fibers undergo changes.9, 24, Because the nerve fibers innervating low‐threshold mechanoreceptors (LTMRs) are terminal parts of sensory nerves, the skin of the foot is presumably the best site to assess the functional status of both LTMRs and its afferent fibers, which degenerate more marked distally. Due to neuropathy, a combined loss of sensory receptors and nerve fibers may occur, that both contribute to changed cutaneous threshold and innervation density.11 The results of the current study suggest that as a result of this process the ability to discriminate 2 static points is lost first, followed by the loss of moving 2‐point discrimination, vibration sense, S1PD as assessed with a 10‐g monofilament and cold sensation.
A strength of the RDF study is the broad range of patients with varying degrees of sensory loss, including patients with prior ulcerations and amputations. The RDF‐39 can be used to consecutively assess patient sensory status and measure the incremental sensory loss. Moreover, relative risks of adverse events can also be estimated per consecutive item on the scale, so that patients with absent vibration thresholds have lower odds for ulceration than do patients with prior ulcers or amputations.25 The distribution of patients at the upper and lower ends of the trait makes floor and ceiling effects less likely, as the total information curve suggests.
In many clinical situations, the assumptions of the Rasch model do not hold due to its strict criteria, while more complex models, like 2PL, fit the data better by estimation of item parameters. Our study showed that 10 of 39 RDF items fulfill the requirements for good fit of the Rasch‐model and are indicators of more severe sensory disability in the feet, positioned on the right side of theta. In the 2PL‐model we used, items investigating the loss of S2PD, M2PD, and 10‐g monofilament testing were included. These items provide information on areas of the trait where Rasch model fit items were not present and, therefore, provide information on earlier stages of sensory loss. Several checks, as part of the 2PL‐modeling process, found that no significant deviations between abilities and difficulties were present, resulting in a model fit of all 39 items.
Minimal DIF was found in the 39 items, which allows us to make valid comparisons between subgroups. However, DIF indicates that comparisons between subgroups should be made cautiously. Items showing DIF due to age were most profound in vibration sense items, which is explained by the prior dichotomization of these items using normative values for age.6 The type of diabetes resulted in differences observed, among others, in abnormal Romberg's test and reported negative symptoms, mainly attributable to the higher prevalence of type 2 diabetes at an older age. Only 2 nonclinical significant differences were observed in items related to the duration of diabetes (“M2PD IV right” and “Cold stimulus right”). Patients with Tinel's sign at the tarsal tunnel more frequently report signs of numbness at the feet, a more frequent loss of protective sensation at the left fifth toe and more abnormal scoring of M2PD at the left heel and M2PD at the left lateral foot. While it is ideal to only retain non–DIF‐items, it is possible to account and model for items with DIF to preserve those items in a questionnaire or test battery. Further research on the predictive value of the included items on conditions such as falls and ulcerations will probably show that item reduction is possible, because items on S2PD and M2PD are clustered and highly correlated.
Nerve entrapment is frequently observed in patients with diabetes, in both the upper and lower extremity, and might be partially responsible for sensory disturbances, on top of the metabolic DSP.26, 27, 28 In our cohort, 44.5% of patients have a Tinel's sign at either the left or right tarsal tunnel. However, nerve entrapment is difficult to diagnose with nerve conduction studies in patients with symptoms of DSP.18, 28, 29 The fact that RDF‐39 sensory tests in patients with and without signs of nerve compression at the tarsal tunnel do not exhibit abundant DIF suggests that the loss of sensation due to DSP and concomitant compression neuropathy is virtually the same. Sensitivity to touch is an effective psychophysical measure of peripheral nerve function and impairment. The instruments used in the RDF‐39 might be able to detect earlier nerve dysfunction and complement nerve conduction studies, which may be of importance in candidate selection for decompression of entrapped tibial nerves at the tarsal tunnel.18, 30, 31 Presumably, patients without axonal loss have a better chance of a beneficial surgical outcome.18, 28, 32, 33 However, further clinimetric properties of the RDF‐39, such as responsiveness after this type of surgery, should be the subject of future studies.
An abundance of tests have been proposed in the literature as early indicators of sensory loss.9, 34, 35 There is an increasing interest in the quantitative assessment of small fiber damage as an early diagnostic test and for monitoring progression of DSP.36 However, the exact temporal sequence of small and large fiber damage is not fully understood. IRT can help determine the place of tests in the progression of the disease and categorize the consecutive loss accordingly. In scale development, forms of IRT can also be used to develop new unidimensional outcome measures that are free from bias, because of the ideal item response pattern.37, 38 Furthermore, item banks can be constructed so the measurement properties of existing instruments, like nerve conduction studies, can be investigated and compared.14, 16, 37
This study aids clinicians in the wider interdisciplinary field of diabetic foot care in choosing an appropriate instrument to assess loss of sensation in the feet more precisely than by the use of a monofilament alone. By using these quick and simple instruments, a more reliable estimation can be made of the location of the patient in the natural history of loss of sensation. Limitations are that we developed the RDF‐39 from a single‐center population. Therefore, external validity of our findings should be investigated in other settings, such as general practices, academic hospitals and nursing homes. Furthermore, test–retest reliability should be investigated in a group of diabetic subjects with varying degrees of sensory loss, and responsiveness of the RDF‐39 should be assessed during follow‐up of the cohort.
This study establishes how current screening instruments compare and where to apply them on the feet, together with an outcome measure at the interval level.16 Based on this information, a more precise risk stratification may be possible, with subsequent recommendations regarding interventions to prevent complications such as foot ulcers and neurogenic arthropathy.39
We thank Erwin Birnie PhD for critically reviewing the manuscript and useful comments. Ethical Publication Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Products: Semmes‐Weinstein monofilaments (Baseline® TactileTM, USA), Disk‐Criminator™ (US Neurologicals, LCC, WA, USA), Rydel‐Seiffer tuning fork (Martin, Tuttlingen, Germany). Author participation: W.D.R.: study concept and design, acquisition of data. M.H.A.: analysis and interpretation. M.C.C.: acquisition of data, analysis, interpretation and critical revision of the manuscript. D.R.: analysis and interpretation. J.W.v.N.: critical revision of manuscript and study supervision. J.H.C.: study concept and design, critical revision of manuscript and study supervision.
Supporting information
Supplementary Figure A Test locations at the feet
Supplementary Table A
Supplementary Table B
Funding: None.
Conflicts of Interest: The authors declare no conflict of interests regarding commercial associations nor have any financial disclosures.
REFERENCES
- 1. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31:1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes‐‐2014. Diabetes care 2014;37(Suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 3. Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K, International Working Group on the Diabetic Foot . Prevention and management of foot problems in diabetes: a Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab Res Rev 2016;32(Suppl 1):7–15. [DOI] [PubMed] [Google Scholar]
- 4. Rinkel WD, Aziz MH, Van Deelen MJM, Willemsen SP, Castro Cabezas M, Van Neck JW, et al. Normative data for cutaneous threshold and spatial discrimination in the feet. Muscle Nerve 2017;56:399–407. [DOI] [PubMed] [Google Scholar]
- 5. van Nes SI, Faber CG, Hamers RM, Harschnitz O, Bakkers M, Hermans MC, et al. Revising two‐point discrimination assessment in normal aging and in patients with polyneuropathies. J Neurol Neurosurg Psychiatry 2008;79:832–834. [DOI] [PubMed] [Google Scholar]
- 6. Martina IS, van Koningsveld R, Schmitz PI, van der Meche FG, van Doorn PA. Measuring vibration threshold with a graduated tuning fork in normal aging and in patients with polyneuropathy. European Inflammatory Neuropathy Cause and Treatment (INCAT) group. J Neurol Neurosurg Psychiatry 1998;65:743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Res Clin Pract 2001;54:115–128. [DOI] [PubMed] [Google Scholar]
- 8. Dyck PJ, Karnes JL, Daube J, O'Brien P, Service FJ. Clinical and neuropathological criteria for the diagnosis and staging of diabetic polyneuropathy. Brain 1985;108(Pt 4):861–880. [DOI] [PubMed] [Google Scholar]
- 9. Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 2004;127(Pt 7):1593–1605. [DOI] [PubMed] [Google Scholar]
- 10. Dellon AL. Clinical grading of peripheral nerve problems. Neurosurg Clin N Am 2001;12:229–240. [PubMed] [Google Scholar]
- 11. Rinkel WD, Castro Cabezas M, Setyo JH, Van Neck JW, Coert JH. Traditional methods versus quantitative sensory testing of the feet at risk: results from the Rotterdam Diabetic Foot Study. Plast Reconstr Surg 2017;139:752e–763e. [DOI] [PubMed] [Google Scholar]
- 12. Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science 2014;346:950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dellon AL. Evaluation of sensibility and re‐education of sensation in the hand. New York: Williams & Wilkins; 2015. [Google Scholar]
- 14. Draak THP, Vanhoutte EK, van Nes SI, Gorson KC, Van der Pol WL, Notermans NC, et al. Comparing the NIS vs. MRC and INCAT sensory scale through Rasch analyses. J Peripher Nerv Syst 2015;20:277–288. [DOI] [PubMed] [Google Scholar]
- 15. Vanhoutte EK, Faber CG, van Nes SI, Jacobs BC, van Doorn PA, van Koningsveld R, et al. Modifying the Medical Research Council grading system through Rasch analyses. Brain 2012;135(Pt 5):1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanhoutte EK, Hermans MC, Faber CG, Gorson KC, Merkies IS, Thonnard JL, et al. Rasch‐ionale for neurologists. J Peripher Nerv Syst 2015;20:260–268. [DOI] [PubMed] [Google Scholar]
- 17. Rinkel WD, Aziz MH, van Neck JW, Castro Cabezas M, van der Ark LA, Coert JH. Grading the loss of large‐fiber sensation in the distal lower limb of patients with diabetes. A Mokken scale analysis. [submitted]. [Google Scholar]
- 18. Patel AT, Gaines K, Malamut R, Park TA, Toro DR, Holland N, et al. Usefulness of electrodiagnostic techniques in the evaluation of suspected tarsal tunnel syndrome: an evidence‐based review. Muscle Nerve 2005;32:236–240. [DOI] [PubMed] [Google Scholar]
- 19. Rizopoulos D. ltm: an R package for latent variable modeling and item response theory analyses. J Stat Softw 2006;17. [Google Scholar]
- 20. Package ‘difR’. https://cran.r-project.org/web/packages/difR/difR.pdf. Accessed June 19, 2018.
- 21. Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu‐Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. N Engl J mEd 2005;352:341–350. [DOI] [PubMed] [Google Scholar]
- 22. Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ III, O'Brien PC. Risk factors for severity of diabetic polyneuropathy: intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care 1999;22:1479–1486. [DOI] [PubMed] [Google Scholar]
- 23. Tesfaye S, Selvarajah D, Gandhi R, Greig M, Shillo P, Fang F, et al. Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging. Pain 2016;157(Suppl 1):S72–S80. [DOI] [PubMed] [Google Scholar]
- 24. Dyck PJ, Thomas PK. Peripheral neuropathy. Philadelphia: Saunders; 2005. [Google Scholar]
- 25. Crawford F, Cezard G, Chappell FM, Murray GD, Price JF, Sheikh A, et al. A systematic review and individual patient data meta‐analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS). Health Technol Assess 2015;19:1–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vinik A, Mehrabyan A, Colen L, Boulton A. Focal entrapment neuropathies in diabetes. Diabetes Care 2004;27:1783–1788. [DOI] [PubMed] [Google Scholar]
- 27. Tassler PL, Dellon AL. Pressure perception in the normal lower extremity and in the tarsal tunnel syndrome. Muscle Nerve 1996;19:285–289. [DOI] [PubMed] [Google Scholar]
- 28. Siemionow M, Zielinski M, Sari A. Comparison of clinical evaluation and neurosensory testing in the early diagnosis of superimposed entrapment neuropathy in diabetic patients. Ann Plast Surg 2006;57:41–49. [DOI] [PubMed] [Google Scholar]
- 29. Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care 2002;25:565–569. [DOI] [PubMed] [Google Scholar]
- 30. Ahmad M, Tsang K, Mackenney PJ, Adedapo AO. Tarsal tunnel syndrome: a literature review. Foot Ankle Surg 2012;18:149–152. [DOI] [PubMed] [Google Scholar]
- 31. Eryilmaz M, Kocer A, Kocaman G, Dikici S. Two‐point discrimination in diabetic patients. J Diabetes 2013;5:442–448. [DOI] [PubMed] [Google Scholar]
- 32. Bland JD. Do nerve conduction studies predict the outcome of carpal tunnel decompression? Muscle Nerve 2001;24:935–940. [DOI] [PubMed] [Google Scholar]
- 33. Rinkel WD, de Kleijn JL, Macare van Maurik JFM, Coert JH. Optimization of surgical outcome in lower extremity nerve decompression surgery. Plast Reconstr Surg 2018;141:482–496. [DOI] [PubMed] [Google Scholar]
- 34. Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve 2007;35:591–598. [DOI] [PubMed] [Google Scholar]
- 35. Karagoz H, Ozturk S, Siemionow M. Comparison of neurosensory assessment methods in plastic surgery. Ann Plast Surg 2016;77:206–212. [DOI] [PubMed] [Google Scholar]
- 36. Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 2015;38:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Otiv S. The Rasch Model: “Litmus Test” de rigueur for rating scales? Plast Reconstr Surg 2013;131:283e–286e. [DOI] [PubMed] [Google Scholar]
- 38. Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient‐reported outcome measure for breast surgery: the BREAST‐Q. Plast Reconstr Surg 2009;124:345–353. [DOI] [PubMed] [Google Scholar]
- 39. Bus SA, Waaijman R, Arts M, de Haart M, Busch‐Westbroek T, van Baal J, et al. Effect of custom‐made footwear on foot ulcer recurrence in diabetes: a multicenter randomized controlled trial. Diabetes Care 2013;36:4109–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure A Test locations at the feet
Supplementary Table A
Supplementary Table B


