Abstract
Scope
The Mediterranean (MED) diet has been associated with a decreased risk of cardiovascular diseases. It is unclear whether this health effect can be mainly contributed to high intakes of monounsaturated fatty acids (MUFA), characteristic for the MED diet, or whether other components of a MED diet also play an important role.
Methods and Results
A randomized fully controlled parallel trial is performed to examine the effects of the consumption of a saturated fatty acid rich diet, a MUFA‐rich diet, or a MED diet for 8 weeks on metabolite profiles, in 47 subjects at risk of the metabolic syndrome. A total of 162 serum metabolites are assessed before and after the intervention by using a targeted NMR platform. Fifty‐two metabolites are changed during the intervention (false discovery rate [FDR] p < 0.05). Both the MUFA and MED diet decrease exactly the same fractions of LDL, including particle number, lipid, phospholipid, and free cholesterol fraction (FDR p < 0.05). The MED diet additionally decreases the larger subclasses of very‐low‐density lipoprotein (VLDL), related VLDL fractions, VLDL‐triglycerides, and serum‐triglycerides (FDR p < 0.05).
Conclusion
The findings clearly demonstrate that the MUFA component is responsible for reducing LDL subclasses and fractions, and therefore causes an antiatherogenic lipid profile. Interestingly, consumption of the other components in the MED diet show additional health effects.
Keywords: circulating biomarkers, clinical trial, humans, lipoprotein composition, metabolomics
1. Introduction
Cardiovascular diseases (CVDs) are the main cause of death worldwide.1 The development of CVD and other related metabolic disorders, are known to be affected by diet and especially by dietary fatty acids.2, 3 High intakes of saturated fatty acids (SFA) have been widely recognized to have detrimental health effects.1 Randomized controlled trials have demonstrated that a reduction in SFA intake can lead to a reduction in CVD events.4 In particular, replacing part of the saturated fatty acids (SFA) in the diet by monounsaturated fatty acids (MUFA) has led to a decrease in inflammation,5 a reduction in triglycerides, total cholesterol, and low‐density lipoprotein (LDL)‐cholesterol,3, 6 and an improvement in insulin sensitivity7, 8; thereby positively affecting cardiovascular health. Furthermore, a higher intake of MUFA, when consumed in the form of olive oil, has been associated with a reduction in risk of all‐cause mortality, cardiovascular mortality, and stroke.9
The Mediterranean (MED) diet is characterized by a high consumption of MUFA mainly derived from olive oil.10 The consumption of this diet has been associated with a decreased risk of the metabolic syndrome and CVD.11 Additionally, the consumption of a MED diet has been shown to affect several risk factors of CVD, such as reductions in total cholesterol, LDL cholesterol, triglycerides, blood pressure, endothelial dysfunction, and insulin concentrations, and an increase in serum high‐density lipoprotein (HDL) cholesterol concentration.1, 3, 11, 12, 13, 14 The question remains whether these observed health effects are caused by the MUFA component in this diet only, or whether other components of the MED diet have additional health effects.
Therefore, the objective of this study was to investigate the effects of partly replacing SFA by MUFA from olive oil in a Western type diet and the effects of a MED‐type diet equally high in MUFA on concentrations of circulating lipids, lipoprotein particles, lipoprotein composition, and low‐molecular‐weight metabolites, including amino acids, in a parallel fully controlled‐feeding trial in both men and women at risk of the metabolic syndrome. Since the MED‐type diet is characterized not only by a high consumption of MUFA, but also by other potent dietary components such as nuts, legumes, fruits, fish, red wine, and unrefined cereals,3, 15, 16, 17, 18 we hypothesized that the effect of a MED‐type diet on the measured metabolites is more pronounced than the effect of partly replacing SFA by MUFA alone.
2. Experimental Section
2.1. Subjects
In total, 60 men and women participated in this study. The recruitment of subjects, inclusion and exclusion criteria, the study design, composition of the diets, and the primary outcome variable have previously been reported in more detail.3 In short, subjects ranged in age from 45‐ to 60‐year‐olds, and were included if they were at a risk of developing the metabolic syndrome. The latter was defined as having a body mass index of ≥25 kg m−2 or a waist circumference of ≥80 cm for women and ≥94 cm for men. Subjects were excluded if they had fasting total cholesterol ≥ 8 mmol L−1, if they used antihypertensive or cholesterol‐lowering medication, or if they had treated or non‐treated diabetes. Subjects were tested for non‐treated diabetes by an oral glucose‐tolerance test.19 The power calculation was based on the primary outcome of the study, which was detecting a difference of at least 8.5 pmol L−1 in insulin concentration between the intervention groups.
2.2. Study Design
The study was a randomized fully controlled parallel dietary intervention trial (Figure S1, Supporting Information). All subjects consumed a Western‐type diet high in SFA (19% of total energy intake [en‐%]) for a 2‐week run‐in period, thereby standardizing the dietary conditions. After the run‐in period, subjects were randomly allocated to one of the three intervention diets, which they had to consume for 8 weeks.
2.3. Diets
During these 8 weeks, subjects continued on the Western‐type diet high in SFA (SFA diet), received a Western‐type diet in which part of the SFA was replaced by MUFA (MUFA diet, 20 en‐% MUFA), or received a Mediterranean‐type diet with a similar amount of MUFA (MED diet, 21 en‐% MUFA) as the MUFA diet. The MED diet was higher in fatty fish, legumes, nuts, unrefined grain products, and red wine, and lower in dairy products and meat, compared with the other two intervention diets. During the study, 90% of the energy needs of the subjects were provided. The remaining 10% of the energy needs was chosen by the subjects from a list of low‐fat and low‐fiber products. All these choices were recorded in a food diary. Body weight was measured twice a week and the energy intake was adjusted if the subject gained or lost weight. The Medical Ethical Committee of Wageningen University, Netherlands, approved the study, and all subjects gave written informed consent. This trial was registered at clinicaltrials.gov as NCT00405197.
2.4. Blood Collection
Overnight fasting venous blood samples were collected at baseline (at the end of the 2‐week run‐in period), and after the 8‐week intervention. All serum samples were kept at −80 °C until further analysis within one run.
2.5. Serum Metabolite Measurements
The serum samples were processed by Nightingale Health's blood biomarker analysis service to obtain the metabolite profiles. This service employs a high‐throughput NMR spectroscopy platform, using a single experimental setup that allows for the simultaneous quantification of 162 metabolic measures that represent a broad molecular signature of the systemic metabolite profile in serum. This platform has been used in multiple large‐scale epidemiologic studies, of which an overview can be found in ref. [20]. Details of the methodology have been described previously.21, 22 This platform quantifies absolute concentration units of routine lipids, total lipid concentrations of 14 lipoprotein subclasses, fatty acid compositions, various glycolysis precursors, ketone bodies, and amino acids. All measured metabolites fall in the range of detection, and numbers on the analytical performance in terms of repeatability (CV%) can be found in the article of Holmes and colleagues.23
2.6. Statistical Analysis
The differences in baseline characteristics between the three diet groups were examined by the analysis of variance (ANOVA) testing, or Chi‐square testing for categorical data. Statistical analysis of the metabolites were performed on log‐transformed data. The main effects of the diet × time interaction with an ANOVA were tested, and linear mixed models were used to assess between‐diet effects. For the effect of the diets, diet, time, and the interaction between diet and time as fixed effects, were included. Subjects with a random intercept were included in the model. Significant metabolites were first selected using the false discovery rate (FDR) adjusted F‐statistic24 p‐value <0.05. Unadjusted p‐values below 0.05 for the between‐diet effects were considered statistically significant within the metabolites that passed the F‐test. Within‐diet effects were tested with a paired t‐test; here FDR adjusted p‐values below 0.05 were considered statistically significant. Using sparse partial least squares discriminant analysis (sPLS‐DA), it was attempted to separate the responses of the metabolites between the three diets. The sPLS‐DA model was made using the caret R library.25 This model was validated using ten times repeated tenfold cross‐validation. The final number of components for the sPLS‐DA model selected by grid search was 4 for the diet‐model. All analyses were done using R (version 3.4.2).26 Heat maps were made in Excel 2016, and the tree‐structure figure was made using Cytoscape (version 3.2.1).27
3. Results
3.1. Subject Characteristics
Of the 60 subjects that entered this study, 57 completed the study. Mean daily intakes of energy and nutrients per diet have been published previously, as well as the baseline characteristics of these 57 subjects.3 For the metabolite profile analysis we had enough serum left for 47 out of the 57 subjects (flowchart: Figure 1). In these subjects, 162 metabolites were determined in serum collected before and after the interventions. Baseline characteristics of the 47 subjects are summarized in Table 1. The baseline characteristics of the 10 subjects with missing data were not significantly different from the 47 included subjects (data not shown). Ages differed significantly between the three intervention groups (p = 0.006). Age of the subjects in the SFA group was significantly lower compared to the other two diet groups.
Figure 1.
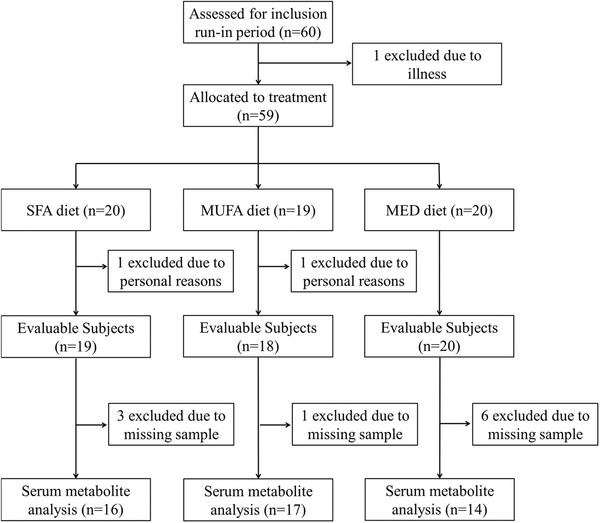
Flowchart of the subjects included for metabolite profile analysis in serum. Abbreviations: Mediterranean (MED), monounsaturated fatty acid (MUFA), saturated fatty acid (SFA).
Table 1.
Baseline characteristics of the 47 subjects included in this study. Data is presented as mean ± standard deviation
| SFA diet [n = 16] | MUFA diet [n = 17] | MED diet [n = 14] | Differences at baseline p‐valuea | |
|---|---|---|---|---|
| Sex [m/f] | 8/8 | 8/9 | 4/10 | 0.444 |
| Age [years] | 51.4 ± 7.8A | 58.1 ± 5.2B | 57.4 ± 5.1B | 0.006 |
| Body weight [kg] | 82.2 ± 12.5 | 77.3 ± 11.9 | 84.4 ± 14.5 | 0.297 |
| BMI [kg m−2] | 26.4 ± 2.9 | 27.2 ± 5.3 | 28.9 ± 6.5 | 0.425 |
| Waist circumference [cm] | ||||
| Men | 103.1 ± 8.1 | 99.7 ± 7.8 | 101.5 ± 6.3 | 0.679 |
| Women | 89.0 ± 3.9 | 94.6 ± 17.4 | 98.5 ± 18.4 | 0.435 |
| Heart rate [bpm] | 67.8 ± 9.6 | 69.9 ± 10.1 | 67.2 ± 10.9 | 0.744 |
| Systolic blood pressure [mmHg] | 115.9 ± 12.5 | 119.7 ± 18.4 | 117.9 ± 9.5 | 0.744 |
| Diastolic blood pressure [mmHg] | 71.8 ± 10.0 | 75.9 ± 14.4 | 74.6 ± 9.5 | 0.597 |
| Total cholesterol [mmol L−1] | 5.69 ± 1.18 | 5.75 ± 0.57 | 5.74 ± 0.81 | 0.979 |
| LDL cholesterol [mmol L−1] | 4.05 ± 1.02 | 3.87 ± 0.62 | 3.90 ± 0.74 | 0.806 |
| HDL cholesterol [mmol L−1] | 1.27 ± 0.27 | 1.42 ± 0.39 | 1.36 ± 0.52 | 0.553 |
| Triglycerides [mmol L−1] | 1.06 ± 0.51 | 1.23 ± 0.46 | 1.28 ± 0.63 | 0.475 |
Means were compared using ANOVA (or chi‐square for categorical values) and corresponding p‐values are shown. In case of a significant overall p‐value: Bonferroni post‐hoc test was performed and values with different superscript letters in the row are significantly different, p < 0.05
Abbreviations: body mass index (BMI), high‐density lipoprotein (HDL), low density lipoprotein (LDL), Mediterranean (MED), monounsaturated fatty acid (MUFA), saturated fatty acid (SFA)
3.2. Diet Effect on Metabolites
No differences in baseline concentration of the 162 metabolites were observed between the three diets after correcting for multiple testing (data not shown). Fifty‐two of the 162 metabolic parameters were significantly changed between the three diet groups during the intervention (FDR p < 0.05) (Table S1, Supporting Information). Details on the number of significantly changed metabolites between the diets and within each diet group are summarized in Figure 2.
Figure 2.
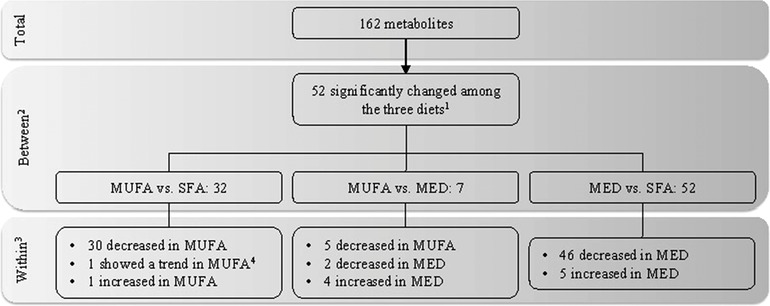
Significantly changed metabolites upon the three intervention diets. This flow diagram shows the number of metabolites of which the change in concentration was significantly different among the three diet groups. Main effects of the diet × time interaction were tested with an 1ANOVA with an FDR correction (FDR p < 0.05). 2Linear mixed model on the differential changes between the diet groups (p < 0.05), on the metabolites that passed the F‐test. 3Results of the within‐diet group changes were tested with a paired t‐test (FDR p < 0.05). 4FDR p‐value = 0.052 in the within‐diet test. Abbreviations: Mediterranean (MED), saturated fatty acid (SFA).
The main subgroup of metabolites that was affected by the diets were the lipids and lipoproteins. In Figure 3 (and in more detail in Figure S2, Supporting Information) the effects of consumption of the MUFA and MED diet are visualized. Briefly, the MUFA diet mainly decreased the LDL related fractions and a subset of the cholesterol fractions including serum cholesterol, very‐low‐density lipoprotein (VLDL)‐cholesterol, free cholesterol, and remnant cholesterol. The MED diet decreased exactly the same LDL and cholesterol fractions as the MUFA diet, however the MED diet additionally decreased multiple VLDL related fractions; mainly in the XL‐, L‐, and M‐VLDL subclasses, plus total VLDL‐TG concentration, and total triglyceride (TG) concentration.
Figure 3.
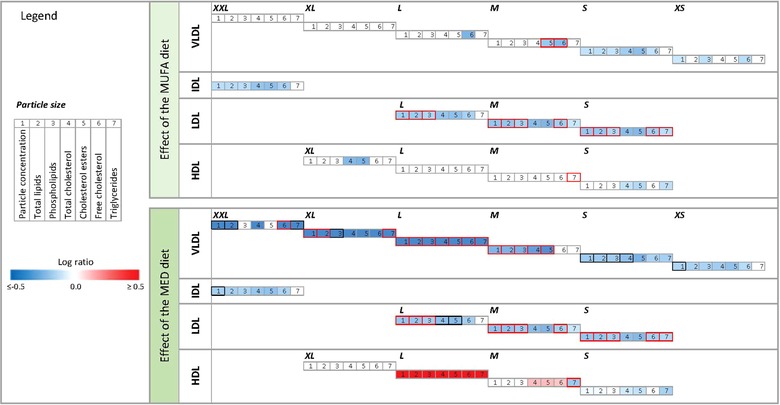
The effects of diets on lipoproteins and the subfractions. On the left of the figure the legend is presented with the log ratio scale and an explanation of the meaning of the numbers. On top of the figure the effect of the MUFA diet is visualized. At the bottom of the figure the effect of the MED diet is visualized. The color of the edge of a box represents the between treatment effect. If the edge is red, the ANOVA FDR p < 0.05, and between diet effect, tested with linear mixed models was p < 0.05. If the color is black the ANOVA FDR p < 0.05, and the between diet effect showed a trend (0.05 < p ≤ 0.06). The color inside a box represents the changes within the MUFA diet (top) or MED diet (bottom), ranging from dark blue (log ratio (LR) ≤ −0.5) to dark red (LR ≥ 0.5), the inside is only colored if the paired t‐test FDR p < 0.05.
Changes in apolipoprotein B (ApoB) and the ApoB to ApoA1 ratio were also significantly different between the three diets, FDR p = 0.022 and FDR p = 0.025, respectively (Table S1, Supporting Information). ApoB decreased significantly in the MUFA diet group (−0.110 ± 0.090 g L−1, p < 0.001), and the MED diet group (−0.151 ± 0.122 g L−1, p = 0.003), compared to the SFA diet group. The ApoB to ApoA1 ratio decreased significantly in the MUFA (−0.059 ± 0.46, p < 0.001) and MED diet group (−0.107 ± 0.081, p = 0.003), compared to SFA diet group.
Glycolysis related metabolites, amino acids, proteins, and glycoprotein acetyls were also determined. However, no differences between the diet groups were observed (Table S1, Supporting Information) except for albumin (FDR p = 0.048), which decreased within the MUFA diet (−0.002 ± 0.003 mmol L−1, p = 0.014) and the MED diet (−0.002 ± 0.003 mmol L−1, p = 0.048), compared to the SFA diet group.
3.3. Dietary Exposure Markers
Dietary exposure markers were included in the metabolomics measurement in the serum samples (Table S1, Supporting Information). The following metabolites were significantly differently changed among the three diets: docosahexaenoic acid (DHA), DHA to total fatty acids (FA) ratio, omega‐3 fatty acids (FAω3), FAω3 to FA ratio, conjugated linoleic acid (CLA), CLA to FA ratio, and MUFA to FA ratio, all FDR p < 0.001, except MUFA to FA ratio (FRD p = 0.002). Comparisons between the diets showed that DHA, and DHA to FA ratio were significantly increased in the MED diet group versus the SFA group (0.024 ± 0.035 mmol L−1, FDR p = 0.039, and 0.363 ± 0.274%, FDR p = 0.003, respectively). Furthermore, FAω3 was significantly decreased in the MUFA group (−0.094 ± 0.048 mmol L−1, FDR p < 0.001) and increased in the MED diet group (0.041 ± 0.077 mmol L−1, FDR p = 0.131) compared to the SFA group, though within the MED diet group this increase was not significant. FAω3 to FA ratio decreased in the MUFA group (−0.583 ± 0.251%, FDR p < 0.001), but increased in the MED group (0.769 ± 0.632%, FDR p = 0.003) compared to the SFA group. CLA, and CLA to FA ratio decreased upon both the MUFA diet (−0.028 ± 0.013 mmol L−1, FDR p < 0.001, and −0.222 ± 0.092%, FDR p < 0.001, respectively) and the MED diet (−0.022 ± 0.015 mmol L−1, FDR p = 0.003, −0.176 ± 0.130%, FDR p = 0.006) versus the SFA diet group. Last, MUFA to FA ratio increased significantly in both the MUFA diet group (3.344 ± 2.124%, FDR p < 0.001), and the MED diet group (2.200 ± 1.509%, FDR p = 0.002).
To examine the differences in effect between the three diets we performed a sparse partial least squares discriminant analysis (sPLS‐DA) model. The best fitting sPLS‐DA model had four components, a Cohen's kappa of 0.77, an eta of 0.9, and an accuracy of 85.4% to place the right subject in the right diet group. The variables most important for the separation between the three intervention diets were: CLA, CLA/FA, DHA/FA, FAω3/FA, and MUFA/FA; all exposure markers for the diets the subjects consumed. The changes in these five variables upon the three intervention diets are displayed in Figure 4.
Figure 4.
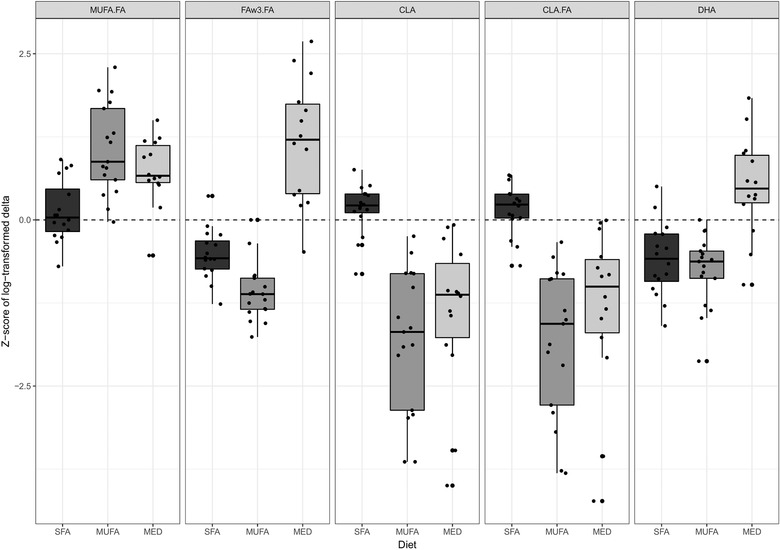
Boxplots of the changes upon the intervention in the five metabolites most important for the separation between the three intervention diets, as determined by sparse partial least squares discriminant analysis. Abbreviations: conjugated linoleic acid (CLA), ratio of conjugated linoleic acid to total fatty acids (CLA.FA), 22:6 docosahexaenoic acid (DHA), ratio of omega‐3 fatty acids to total fatty acids (FAω3.FA), Mediterranean (MED), ratio of monounsaturated fatty acid to total fatty acids (MUFA.FA), saturated fatty acid (SFA).
3.4. Individual Changes
To visualize the individual changes of the 52 metabolites that were significantly different among the three diets a heat map was created (Figure 5). The figure shows that for the dietary exposure biomarkers, each individual within a diet group is affected in the same direction. Furthermore, the figure shows that the individual changes in the lipoproteins are robust within each lipoprotein subclass; however, variations in response between subjects are present.
Figure 5.
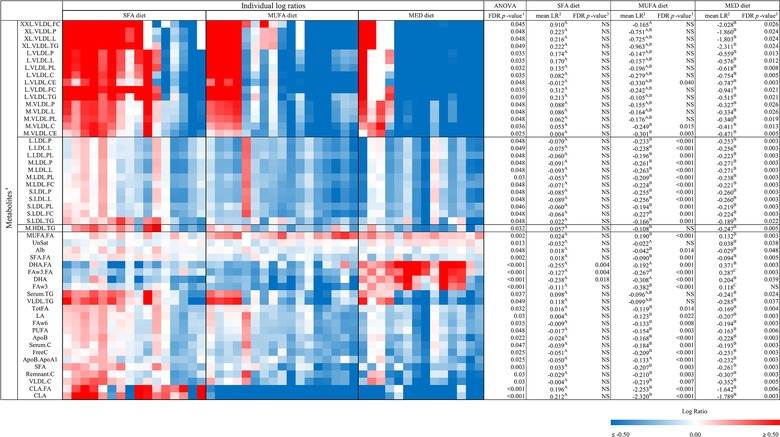
Heat map of the 52 significantly changed metabolic parameters between the three intervention diets, tested with an a) ANOVA with an FDR correction (FDR p < 0.05). Differences between two diet groups (p < 0.05) tested with a linear mixed model, are indicated with a superscript letter in the corresponding row. Individual changes are indicated as individual log ratios (LR) of values after the intervention versus before the intervention, in the SFA diet group (n = 16), MUFA diet group (n = 17), and the MED diet group (n = 14). These changes are presented on a color scale ranging from blue (decreased, LR ≤ −0.50) to red (increased, LR ≥ 0.50). Subjects are clustered within the diets (Euclidian distance and average linkage). b) Mean log ratios per diet group. c) Corresponding FDR‐corrected p‐values, as tested with a paired t‐test. Abbreviations: log ratio (LR), Mediterranean (MED), saturated fatty acids (SFA).
4. Discussion
The present study in healthy men and women at risk of the metabolic syndrome, demonstrates that 8‐week consumption of a MED diet resulted in an additional effect on serum metabolites compared to the effect of replacing SFA by MUFA alone. We observed that the MUFA and the MED diet decreased exactly the same LDL lipoprotein and cholesterol fractions. The MED diet additionally decreased several fractions of the larger VLDL lipoprotein subclasses, TG concentration in total VLDL, and total TG concentration.
This is the first fully controlled dietary intervention study examining not only the effect of a MUFA diet versus the effect of a MED diet on lipids and lipoprotein subclasses, but also the effect on the composition of the different lipoprotein subclasses. Both the MUFA and MED diets were responsible for decreasing the concentration of the large, medium, and small LDL subclasses. Also, within these LDL‐subclasses we observed a decrease in various fractions, amongst which were the total lipids, and the phospholipids. As the MUFA fraction is the same in both diets, and different from the SFA diet, we extrapolate that the MUFA fraction is responsible for these effects on LDL. MUFA has previously been shown to decrease LDL‐cholesterol,3, 28, 29 various LDL‐subclass concentrations, and total LDL particle number.30 We here measured the effect of replacing SFA by MUFA on LDL lipoprotein composition in more detail, and found a reduction in multiple LDL fractions, such as a decrease in the phospholipid and lipid fractions of all three LDL subclasses, and a decrease in free cholesterol in medium and small‐LDL subclass.
Apart from the effect of MUFA on LDL, we observed additional effects in the MED diet group. On top of the effects on LDL, the MED diet decreased larger VLDL subclasses, related subclass composition, total TG concentration in VLDL, and total TG concentration. In the PREDIMED trial a reduction in large VLDL lipoproteins and total serum‐TG concentration was observed in the group that consumed a MED diet supplemented with nuts,30 which was similar to the finding in our MED diet group, which also contained nuts. In our study however, we measured the effect of the MED diet on VLDL lipoproteins in more detail, and we found a reduction in multiple VLDL subclasses and fractions. Moreover, all fractions in the large subclass were reduced, as was the lipid fraction in the extra‐large, large, and medium VLDL subclass. As the MED‐diet induced VLDL reductions were not observed in the MUFA diet, we assume that these effects were not caused by the MUFA component in the diet. Other components of the MED diet are likely responsible for the observed effects. Consumption of omega‐3 polyunsaturated fatty acids has been shown to decrease total TG and TG in VLDL.31, 32, 33, 34, 35 PUFAs are known to activate peroxisome proliferator‐activated receptors (PPARs), which are involved in regulating the expression of genes causing an increase in β‐oxidation, and thereby reduce the bioavailability of lipids for production of VLDL in the liver.32, 36, 37 As n‐3 PUFAs are present in fish and nuts, and both were components of our MED diet and not our MUFA diet, this might be a part of the explanation for the observed decrease in several VLDL fractions upon the MED diet. Another explanation could be that clearance of TG rich particles in the circulation is increased in the MED diet group.37
Except for the triglycerides subfraction in M‐HDL, we did not find significant differences between the MED diet compared to the other two diets on HDL related particles. However, we do observe a shift within the MED diet group from the smaller HDL particles to the larger HDL particles. This shift could have been caused by the consumption of alcohol in the MED diet, as alcohol has been associated with an increase in the larger HDL particles.38, 39 Furthermore, the shift could also have been caused by fatty fish, as a similar shift in HDL particles was observed in the 12‐week intervention study by Lankinen40 in the group with an increased consumption of amongst others, fatty fish. Moreover, Erkkilä et al41 also observed a shift towards the larger HDL particles after an eight‐week intervention study with fatty fish intake four times a week.
4.1. Risk for Diseases
Our study showed that both the MUFA and the MED diet decreased the concentration of the smallest LDL subclass, and the cholesterol and cholesterol ester content of this subclass. Especially the small LDL subclass is associated with an increased risk for CVD,42, 43, 44 indicating a potential favorable role of MUFA consumption on CVD risk. Moreover, the MUFA and MED diet both decreased LDL particle (P) concentration of all the LDL subclasses. A decrease in LDL‐P concentration has been associated with a decrease in atherosclerotic risk, and it has been described to be a better predictor of CVD events compared to the conventionally used LDL‐cholesterol concentrations.43 In addition, both the MUFA and MED diet significantly decreased ApoB concentration, and the ApoB/ApoA1 ratio. An increased ApoB/ApoA1 ratio is a well‐known predictor for e.g. acute myocardial infarction, and acute coronary events.45, 46 In summary, the lowering of small LDL concentration, LDL particle number, and the increase in the ApoB/ApoA1 ratio by both the MUFA and MED diet point toward a potential beneficial effect of a high MUFA intake on risk for cardiovascular health.
Only the MED diet decreased total VLDL‐TG concentration and serum TG concentration. An increased fasting serum TG concentration has been associated with coronary artery disease, increased mortality risk in coronary heart disease (CHD) patients, and an increased risk for developing both CHD and ischemic heart disease.47, 48, 49, 50 Next to this, higher VLDL lipoprotein concentrations have been associated with ischemic heart disease risk,51, 52, 53, 54 and higher levels of VLDL‐TG have been observed in type 2 diabetes mellitus patients.55 Thus, the effects of the MED diet, for example, the lowering of total VLDL‐TG, and serum TG concentration, may indicate a potential additional beneficial effect on cardiovascular risk on top of the effect of the MUFA content. Furthermore, we observed that the concentrations of XL‐, L‐, and M‐VLDL particles, and triglycerides in XL‐ and L‐VLDL particles were all decreased by the MED diet. These metabolites have been cross‐sectionally and prospectively associated with the development of fatty liver.56, 57 This again indicates an additional favorable effect of the MED diet.
4.2. Exposure Markers
The consistent changes observed in the metabolites: DHA, DHA to FA ratio, FAω3 to FA ratio, MUFA to FA ratio, CLA, and CLA to FA ratio in the serum samples, confirm that these markers are suitable markers to measure dietary exposure. Indicating that they can possibly be used to assess whether an individual had a high consumption of olive oil (reflected by MUFA to FA ratio), fish (reflected by DHA, DHA to FA ratio, and FAω3 to FA ratio), or butter (reflected by CLA, and CLA to FA ratio). Remarkably, we observed interpersonal differences in the diet‐induced effect on other markers. In all three diet groups four to five individuals reacted differently compared to the rest of that diet group. As we found no differences at baseline between any of these measured serum metabolites, this points toward variation in response to the diets. It will be valuable to assess the consistency in such a response to diets, especially with respect to personalized dietary advice in the future.58
4.3. Limitations and Strengths
Even though our sample size was relatively small with 47 subjects, our dietary intervention had a profound effect on various metabolites as 32% of all metabolites were affected by the diets after applying an FDR correction. We hypothesize that this can mainly be explained by the high level of dietary control in this study. In the present study, 90% of the energy needs was provided, and the remaining 10% was chosen by the subjects from a list of low‐fat and low‐fiber products. Other dietary intervention studies with a similar number of subjects33, 41 provided only part of the intended diet, or only gave dietary advice,30, 40, 59, 60 and therefore possibly found smaller effects. Even though body weight was closely monitored, the subjects in the SFA group and the MED group did lose some weight. However, no difference was found in weight loss between the SFA, MUFA, or MED diet group. Last, our study had a duration of 10 weeks in total (including the run‐in period). To determine the long‐term effects of adhering to a diet high in MUFA or a MED diet on changes in lipids, lipoprotein particles, and CVD risk, more long‐term studies are needed.
4.4. Summary and Conclusion
In summary, this 8‐week fully controlled dietary intervention study showed that MUFA in the diet decreased LDL particle concentration in all three subclasses and several related fractions, including a decrease in the small LDL concentration. MUFA were also responsible for an increase in the ApoB/ApoA1 ratio. These are all favorable effects on risk factors for CVD. The MED diet additionally decreased total VLDL‐TG, total serum‐TG concentration, and particle concentration of three larger VLDL subclasses together with several related fractions, including triglycerides in the XL‐ and L‐VLDL particles. Thus, additional favorable effects on other risk factors for CVD.
In conclusion, we were able to disentangle the effect of the MUFA content in the MED diet from the effect of the other components in the MED diet. Our study clearly demonstrates that the MUFA component is responsible for reducing several LDL subclasses and fractions, and therefore causes a more antiatherogenic lipid profile. Interestingly, consumption of the other components in the MED diet show additional health effects, by reducing several other risk factors for CVD.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Acknowledgements
C.C.J.R.M. and R.W.J.H. analyzed the data. C.C.J.R.M. wrote the manuscript, which was critically reviewed and improved by E.J.M.F. and L.A.A. All authors read and approved the final manuscript. This project is part of the FoodBAll project. FoodBAll is a project funded by the BioNH call (grant number 529051002) under the Joint Programming Initiative, “A Healthy Diet for a Healthy Life.” The metabolomics measurement was financially supported by BBMRI‐NL, a research infrastructure financed by the Dutch government (NWO), nr 184033111.
Michielsen C. C. J. R., Hangelbroek R. W., Feskens E. J. M., Afman L. A., Disentangling the Effects of Monounsaturated Fatty Acids from Other Components of a Mediterranean Diet on Serum Metabolite Profiles: A Randomized Fully Controlled Dietary Intervention in Healthy Subjects at Risk of the Metabolic Syndrome. Mol. Nutr. Food Res. 2019, 63, 1801095 10.1002/mnfr.201801095
The copyright line for this article was changed on 11 July 2019 after original online publication.
References
- 1. Sacks F. M., Lichtenstein A. H., Wu J. H., Appel L. J., Creager M. A., Kris‐Etherton P. M., Miller M., Rimm E. B., Rudel L. L., Robinson J. G., Circulation. 2017, 136, e1. [DOI] [PubMed] [Google Scholar]
- 2. Gillingham L. G., Harris‐Janz S., Jones P. J., Lipids. 2011, 46, 209. [DOI] [PubMed] [Google Scholar]
- 3. Bos M., de Vries J., Feskens E., Van Dijk S., Hoelen D., Siebelink E., Heijligenberg R., de Groot L., Nutr., Metab. Cardiovasc. Dis. 2010, 20, 591. [DOI] [PubMed] [Google Scholar]
- 4. Hooper L., Martin N., Abdelhamid A., Davey Smith G., The Cochrane Library. 2015, 6. [DOI] [PubMed] [Google Scholar]
- 5. van Dijk S. J., Feskens E. J., Bos M. B., Hoelen D. W., Heijligenberg R., Bromhaar M. G., de Groot L. C., de Vries J. H., Müller M., Afman L. A., The American Journal of Clinical Nutrition. 2009, 90, 1656. [DOI] [PubMed] [Google Scholar]
- 6. Mensink R. P., Effects of Saturated Fatty acids on Serum Lipids and Lipoproteins: A systematic Review and Regression Analysis, World Health Organisation, Geneva, Switserland: 2016. [Google Scholar]
- 7. Vessby B., Uusitupa M., Hermansen K., Riccardi G., Rivellese A. A., Tapsell L. C., Nälsén C., Berglund L., Louheranta A., Rasmussen B., Diabetologia. 2001, 44, 312. [DOI] [PubMed] [Google Scholar]
- 8. Ros E., The American Journal of Clinical Nutrition. 2003, 78, 617S. [DOI] [PubMed] [Google Scholar]
- 9. Schwingshackl L., Hoffmann G., Nutrients. 2012, 4, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trichopoulou A., Costacou T., Bamia C., Trichopoulos D., N. Engl. J. Med. 2003, 348, 2599. [DOI] [PubMed] [Google Scholar]
- 11. Kastorini C. M., Milionis H. J., Esposito K., Giugliano D., Goudevenos J. A., Panagiotakos D. B., J. Am. Coll. Cardiol. 2011, 57, 1299. [DOI] [PubMed] [Google Scholar]
- 12. Martínez‐González M. A., Salas‐Salvadó J., Estruch R., Corella D., Fitó M., Ros E., Investigators P., Prog. Cardiovasc. Dis. 2015, 58, 50.25940230 [Google Scholar]
- 13. van Dijk S. J., Feskens E. J., Bos M. B., de Groot L. C., de Vries J. H., Müller M., Afman L. A., J. Nutr. 2012, 142, 1219. [DOI] [PubMed] [Google Scholar]
- 14. Esposito K., Marfella R., Ciotola M., Di Palo C., Giugliano F., D'Armiento M., D'Andrea F., Giugliano D., JAMA, J. Am. Med. Assoc. 2004, 292, 1440. [DOI] [PubMed] [Google Scholar]
- 15. Afshin A., Micha R., Khatibzadeh S., Mozaffarian D., The American Journal of Clinical Nutrition. 2014, 100, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rimm E. B., Williams P., Fosher K., Criqui M., Stampfer M. J., BMJ. 1999, 319, 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabaté J., Oda K., Ros E., Arch. Intern. Med. 2010, 170, 821. [DOI] [PubMed] [Google Scholar]
- 18. Lopez‐Garcia E., Rodriguez‐Artalejo F., Li T. Y., Fung T. T., Li S., Willett W. C., Rimm E. B., Hu F. B., The American Journal of Clinical Nutrition. 2014, 99, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alberti K. G. M. M., Zimmet P. f., Diabetic Med. 1998, 15, 539. [DOI] [PubMed] [Google Scholar]
- 20. Würtz P., Kangas A. J., Soininen P., Lawlor D. A., Davey Smith G., Ala‐Korpela M., Am. J. Epidemiol. 2017, 186, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soininen P., Kangas A. J., Würtz P., Suna T., Ala‐Korpela M., Circ. Cardiovasc. Genet. 2015, 8, 192. [DOI] [PubMed] [Google Scholar]
- 22. Soininen P., Kangas A. J., Würtz P., Tukiainen T., Tynkkynen T., Laatikainen R., Järvelin M.‐R., Kähönen M., Lehtimäki T., Viikari J., Analyst. 2009, 134, 1781. [DOI] [PubMed] [Google Scholar]
- 23. Holmes M. V., Millwood I. Y., Kartsonaki C., Hill M. R., Bennett D. A., Boxall R., Guo Y., Xu X., Bian Z., Hu R., Walters R. G., Chen J., Ala‐Korpela M., Parish S., Clarke R. J., Peto R., Collins R., Li L., Chen Z., J. Am. Coll. Cardiol. 2018, 71, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benjamini Y., Hochberg Y., J R Stat Soc Series B. 1995, 289. [Google Scholar]
- 25. Kuhn M., Journal of Statistical Software. 2008, 28, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. R. A language and environment for statistical computing . Vienna, Austria: R Foundation for Statistical Computing; 2017, https://www.R-project.org
- 27. Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T., Genome Res. 2003, 13, 2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berry E. M., Eisenberg S., Friedlander Y., Harats D., Kaufmann N. A., Norman Y., Stein Y., The American Journal of Clinical Nutrition. 1992, 56, 394. [DOI] [PubMed] [Google Scholar]
- 29. Berry E. M., Eisenberg S., Haratz D., Friedlander Y., Norman Y., Kaufmann N. A., Stein Y., The American Journal of Clinical Nutrition. 1991, 53, 899. [DOI] [PubMed] [Google Scholar]
- 30. Damasceno N. R., Sala‐Vila A., Cofan M., Perez‐Heras A. M., Fito M., Ruiz‐Gutierrez V., Martinez‐Gonzalez M. A., Corella D., Aros F., Estruch R., Ros E., Atherosclerosis. 2013, 230, 347. [DOI] [PubMed] [Google Scholar]
- 31. Mackness M., Bhatnagar D., Durrington P., Prais H., Haynes B., Morgan J., Borthwick L., Eur J Clin Nutr. 1994, 48, 859. [PubMed] [Google Scholar]
- 32. Fernandez M. L., West K. L., J. Nutr. 2005, 135, 2075. [DOI] [PubMed] [Google Scholar]
- 33. Li Z., Lamon‐Fava S., Otvos J., Lichtenstein A. H., Velez‐Carrasco W., McNamara J. R., Ordovas J. M., Schaefer E. J., J. Nutr. 2004, 134, 1724. [DOI] [PubMed] [Google Scholar]
- 34. Bogl L., Pietiläinen K., Rissanen A., Kangas A., Soininen P., Rose R., Ala‐Korpela M., Kaprio J., Nutr., Metab. Cardiovasc. Dis. 2013, 23, 1071. [DOI] [PubMed] [Google Scholar]
- 35. Baumstark M. W., Frey I., Berg A., Keul J., Clin. Biochem. 1992, 25, 338. [DOI] [PubMed] [Google Scholar]
- 36. Harris W. S., Connor W. E., Illingworth D. R., Rothrock D. W., Foster D.M., J Lipid Res. 1990, 31, 1549. [PubMed] [Google Scholar]
- 37. Weintraub M., Zechner R., Brown A., Eisenberg S., Breslow J., J. Clin. Invest. 1988, 82, 1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mukamal K. J., Mackey R. H., Kuller L. H., Tracy R. P., Kronmal R. A., Mittleman M. A., Siscovick D. S., The Journal of Clinical Endocrinology & Metabolism. 2007, 92, 2559. [DOI] [PubMed] [Google Scholar]
- 39. Muth N. D., Laughlin G. A., von Muhlen D., Smith S. C., Barrett‐Connor E., Br. J. Nutr. 2010, 104, 1034. [DOI] [PubMed] [Google Scholar]
- 40. Lankinen M., Kolehmainen M., Jääskeläinen T., Paananen J., Joukamo L., Kangas A. J., Soininen P., Poutanen K., Mykkänen H., Gylling H., PLoS One. 2014, 9, e90352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erkkilä A. T., Schwab U. S., Lehto S., de Mello V. D., Kangas A. J., Soininen P., Ala‐Korpela M., Uusitupa M. I., J. Clin. Lipidol. 2014, 8, 126. [DOI] [PubMed] [Google Scholar]
- 42. Austin M. A., Breslow J. L., Hennekens C. H., Buring J. E., Willett W. C., Krauss R. M., JAMA: The Journal of the American Medical Association. 1988, 260, 1917. [PubMed] [Google Scholar]
- 43. Rizzo M., Berneis K., QJM: An International Journal of Medicine. 2006, 99, 1. [DOI] [PubMed] [Google Scholar]
- 44. Berneis K. K., Krauss R. M., J. Lipid Res. 2002, 43, 1363. [DOI] [PubMed] [Google Scholar]
- 45. McQueen M. J., Hawken S., Wang X., Ounpuu S., Sniderman A., Probstfield J., Steyn K., Sanderson J. E., Hasani M., Volkova E., Lancet. 2008, 372, 224. [DOI] [PubMed] [Google Scholar]
- 46. Dunder K., Lind L., Zethelius B., Berglund L., Lithell H., Am. Heart J. 2004, 148, 596. [DOI] [PubMed] [Google Scholar]
- 47. Haim M., Benderly M., Brunner D., Behar S., Graff E., Reicher‐Reiss H., Goldbourt U., Circulation 1999, 100, 475. [DOI] [PubMed] [Google Scholar]
- 48. Manninen V., Tenkanen L., Koskinen P., Huttunen J. K., Mänttäri M., Heinonen O. P., Frick M. H., Circulation. 1992, 85, 37. [DOI] [PubMed] [Google Scholar]
- 49. Barbir M., Wile D., Trayner I., Aber V. R., Thompson G. R., Heart. 1988, 60, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jeppesen J. ø., Hein H. O., Suadicani P., Gyntelberg F., Circulation. 1998, 97, 1029. [DOI] [PubMed] [Google Scholar]
- 51. Lewis B., Chait A., Oakley C., Wootton I., Krikler D., Onitiri A., Sigurdsson G., February A., BMJ. 1974, 3, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kissebah A. H., Alfarsi S., Adams P. W., Metabolism. 1981, 30, 856. [DOI] [PubMed] [Google Scholar]
- 53. Chan D. C., Barrett H. P., Watts G. F., Am. J. Cardiovasc. Drugs. 2004, 4, 227. [DOI] [PubMed] [Google Scholar]
- 54. Huff M. W., Telford D. E., Arterioscler Thromb Vasc Biol. 1989, 9, 58. [Google Scholar]
- 55. Vrieling F., Ronacher K., Kleynhans L., van den Akker E., Walzl G., Ottenhoff T. H. M., Joosten S. A., EBioMedicine. 2018, 32, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kaikkonen J. E., Würtz P., Suomela E., Lehtovirta M., Kangas A. J., Jula A., Mikkilä V., Viikari J. S. A., Juonala M., Rönnemaa T., Hepatology. 2017, 65, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sliz E., Sebert S., Würtz P., Kangas A. J., Soininen P., Lehtimäki T., Kähönen M., Viikari J., Männikkö M., Ala‐Korpela M., Raitakari O. T., Kettunen J., Hum. Mol. Genet. 2018, 27, 2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fitó M., Konstantinidou V., Nutrients. 2016, 8, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mostad I., Bjerve K., Lydersen S., Grill V., Eur. J. Clin. Nutr. 2008, 62, 419. [DOI] [PubMed] [Google Scholar]
- 60. Rodriguez‐Garcia E., Ruiz‐Nava J., Santamaria‐Fernandez S., Fernandez‐Garcia J. C., Vargas‐Candela A., Yahyaoui R., Tinahones F. J., Bernal‐Lopez M. R., Gomez‐Huelgas R., Medicine. 2017, 96, e7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information


