Abstract
We present a dataset obtained by extracting information from an extensive literature search of toxicological experiments using mice and rat animal models to study the effects of exposure to airborne particulate matter (PM). Our dataset covers results reported from 75 research articles considering paper published in 2017 and seminal papers from previous years. The compiled data and normalization were processed with an equation based on a PM dosimetry model. This equation allows the comparison of different toxicological experiments using instillation and inhalation as PM exposure protocols with respect to inhalation rates, concentrations and PM exposure doses of the toxicological experiments performed by different protocols using instillation and inhalation PM as exposure methods. This data complements the discussions and interpretations presented in the research article “Inhale, exhale: why particulate matter exposure in animal models are so acute?” Curbani et al., 2019.
Keywords: Concentration, Dose, Health effects, Inhalation rate, Instillation, Toxicology
Specifications table
| Subject area | Environmental Science |
| More specific subject area | Air quality, particulate matter, toxicology and health |
| Type of data | Table |
| How data was acquired | Data acquired by literature search and normalization of data from different exposure protocols based on a model that calculates concentration, inhalation rates and particulate matter exposure dose. The selection filters applied to build the dataset were: published works and data were published in 2017, in the English language, and were referenced in indexed journals (with editorial board, peer reviewed and included in Clarivate Analytics Journal Citation Reports). Also, the papers selected were based on mice and/or rat models, and the protocol exposed the respiratory tract to PM to study the health effects in one or more specific endpoints (respiratory tract, pulmonary and extra-pulmonary). Given these conditions, a search string was used to query PubMed Particularly, the following keywords were used: (particulate matter) AND (mice or mouse or rats or rat) AND (inhalation or instillation). Apart from the most recent articles published in 2017, we also included seminal papers from previous years. |
| Data format | Calculated and analysed |
| Experimental factors | Data were compiled from experimental design description from 75 different research articles (155 toxicological experiments) |
| Experimental features | We used a model to calculate inhalation rates, concentrations and PM exposure dose from 155 different toxicological experiments |
| Data source location | Vila Velha, Brazil, Universidade Vila Velha |
| Data accessibility | Data is provided with this article |
| Related research article | F. Curbani, F.O. Busato, M.M. Nascimento, D.N. Olivieri, C.E. Tadokoro, Inhale, exhale: why particulate matter exposure in animal models are so acute? Environmental Pollution. In Press [1] |
Value of the data
|
1. Data
The data consists of a list of selected articles where particulate matter (PM) exposure experiments were performed in mice and rats. From each of source article, we extracted the objectives of each study as related by the original authors (Table 1). The compiled data and normalization were processed with an equation based on a PM dosimetry model considering physiological breathing parameters and PM inhaled fraction (inhalability) in mice and rats (Table 2). From the collection of selected articles, the data provides a list of reviewed experiments, methods and characteristics of each exposure protocol, including concentrations, doses and PM inhalation rates normalized by a PM dose model (Table 3), and a summary of the variables from different PM exposure protocols in mice and rats (Table 4).
Table 1.
List of articles from PubMed where PM exposure was performed in mice and rats and the objectives of each study as related by the authors. The dataset includes 30 articles published in 2017 and 45 articles published in previous years.
| Exposure protocols | Objectives | Authors |
|---|---|---|
| CAP inhalation | The objective of this study was to determine whether short-term exposures to concentrated ambient particles alter the morphology of small pulmonary arteries in normal rats and rats with chronic bronchitis (CB). | [15] |
| CAP inhalation | Our objective was to provide experimental plausibility for epidemiological observations by testing the hypothesis that exposure to particulate matter with nominal mean aerodynamic diameters of 2.5 μm or less (PM2.5) during discrete periods of pregnancy results in preterm birth (PTB) and low birth weight (LBW). | [16] |
| CAP inhalation | The objectives of the present study were: 1) to determine whether acute exposure to low levels of particles promotes measurable acute systemic and cardiopulmonary effects; and 2) to assess if the magnitude of the observed alterations is influenced by season. | [17] |
| CAP inhalation | This study investigates the effects of inhaled ammonium sulphate, which is a major compound of inorganic air pollutants in PM2.5, on adult neurogenesis in aged Sprague-Dawley rats. | [18] |
| CAP inhalation | The hypothesis tested was that older animals would exhibit more severe pulmonary inflammation and haematological changes following the CAP exposure when compared to young, normal animals. | [19] |
| CAP inhalation | We evaluated the effects of air pollution on the adrenal cortex using female mice. One group was conditioned daily in a chamber with exposure to particulate matter. | [20] |
| CAP inhalation | Mice were used to investigate the effects of iRhom2 on PM2.5-induced hepatic dyslipidaemia. | [21] |
| CAP inhalation | The present work was designed to: (i) determine whether short-term exposure to concentrated air particles causes pulmonary inflammation in normal rats; (ii) characterize the component(s) of CAP that are significantly associated with the development of the inflammatory reaction; and (iii) define the induction of mediators and other pathophysiological response elements of the lung with CAP exposure. | [22] |
| CAP inhalation | The aim of this study was to investigate the mechanism by which PM2.5 influences the Notch signalling pathway leading to worsening immune disorder and accelerating chronic obstructive pulmonary disease (COPD) development. | [23] |
| CAP inhalation | We investigated the roles of T-helper (Th)1–Th2 cytokines and nasal remodelling after ambient PM2.5 exposure in a rat model of allergic rhinitis. | [13] |
| CAP inhalation | We investigated the in vivo effects of PM2.5 exposure on the inflammatory response, oxidative stress, the enzyme activities of Na + K + -ATPase and Ca2+-ATPase, and the morphology and function of mitochondria in the nasal mucosa of rats. | [24] |
| CAP inhalation | To evaluate the ability of particulate air pollution to promote oxidative stress and tissue damage in vivo, we studied a rat model of short-term exposure to concentrated ambient particles. | [25] |
| CAP inhalation | We conducted a study to compare the inflammatory response of the lung to instilled versus inhaled particles. | [26] |
| CAP inhalation | We have investigated whether long-term inhalation exposure to diesel engine exhaust, a dominant contributor to particulate air pollution in urban environments, can aggravate Alzheimer's Disease (AD)-like effects in female 5X Familial AD (5XFAD) mice and their wild-type female littermates. | [27] |
| CAP inhalation | The aim of this study was to identify the impact of titanium dioxide (TiO2) nanoparticles on inflammasome in a mouse model of allergic asthma. | [28] |
| CAP inhalation | To test the impact of chronic airborne particulate matter exposure on the upper respiratory system in vivo. | [12] |
| CAP inhalation | To determine whether oxidants are implicated in PM-dependent lung inflammation, we tested the ability of N-acetylcysteine (NAC) to prevent lung inflammation in a rat model of short-term exposure to concentrated ambient particles. | [29] |
| CAP inhalation | The objectives of this study were (1) to determine whether short-term exposures to concentrated air particles cause pulmonary inflammation in normal rats and rats with chronic bronchitis (CB); (2) to identify the site within the lung parenchyma where CAP-induced inflammation occurs; and (3) to characterize the component(s) of CAP that is significantly associated with the development of the inflammatory reaction. | [30] |
| CAP inhalation | The objective was to identify and quantify estrogenic receptor-b (ERb), aryl hydrocarbon receptor (AhR), the cytochrome P450 enzymes CYP1A1, 1A2, 1B1, and mucus profile in the nasal epithelium of mice | [31] |
| CAP inhalation | This study evaluated the inflammatory differences in BALB/c mouse males and females in three phases of the estrous cycle that were exposed to ambient air or concentrated ambient particles. | [32] |
| CAP inhalation | The purpose of this study was to determine the respiratory effects of inhaled ultrafine iron particles in rats. | [33] |
| CAP inhalation and intranasal instillation | We compared the physiological consequences of short-term exposure to diesel exhaust via inhalation to those due to exposure to the same diesel exhaust particles suspended in solution and delivered intranasally. | [34] |
| CAP inhalation and intratracheal instillation | The present study was designed to compare intratracheal instillation to inhalation exposure derived health endpoints of acute lung toxicity in the rat that relate to homologous clinical outcomes that have been reported with ambient PM using a well characterized model emission PM, which would have demonstrable and relevant effects at low lung doses by both methods. | [35] |
| CAP inhalation and intratracheal instillation | We used pharmacological strategies to determine whether oxidants are implicated in PM-dependent cardiac dysfunction and whether PM-induced increase in autonomic stimulation on the heart mediates cardiac oxidative stress and toxicity. | [36] |
| EAP inhalation | The aim of this study was to verify the effects of ambient air pollution of São Paulo City on coronary of healthy non-isogenic Swiss mice, chronically exposed since birth until adulthood. | [37] |
| EAP inhalation | We investigated effects of chronic exposure (2 months) to ambient levels of particulate matter on development of protease-induced emphysema and pulmonary remodelling in mice. | [38] |
| EAP inhalation | The aim of the present study was to analyse the effects of air pollution in the city of São Paulo on mouse female fertility. | [39] |
| EAP inhalation | The present study was conducted to a) determine whether short-term exposure to ambient levels of particulate air pollution from vehicles elicits inflammatory responses and lipid peroxidation in rat lungs, and b) determine if intermittent short-term exposures induce some degree of tolerance. | [40] |
| Intranasal instillation | To investigate how the combination of soybean allergens and diesel exhaust particles (DEP) can affect the induction or exacerbation of asthma in a murine model. | [41] |
| Intranasal instillation | We hypothesized that sub-chronic exposure to PM2.5 in HFD-treated mice, susceptible to type 2 diabetes mellitus (T2DM), would also be able to change nutrient metabolism-related tissues (NMRT) cellular antioxidant defense, and the balance between intracellular 70-kDa heat shock proteins (iHSP70) and extracellular 72-kDa heat shock proteins (eHSP72) contents expressed as [eHSP72]/[iHSP70] ratio, predisposing for a major risk of cellular damage and development T2DM. | [42] |
| Intranasal instillation | We assessed the effects of Bufei Huoxue (BFHX) capsules on PM2.5-induced pulmonary inflammation and the underlying mechanisms of action. | [43] |
| Intranasal instillation | This study aimed to investigate the effects of winter and spring particulate matter on airway inflammation and allergies in a mouse asthma model. | [44] |
| Intranasal instillation | This study aimed to investigate the effects of AD on the early stage of antigen sensitization using a mouse model of asthma, as well as the role of leukotrienes (LTs) in antigen-induced airway inflammation potentiated by AD particles. | [45] |
| Intranasal instillation | In order to assess the relationship between PM2.5 exposure and autism spectrum disorder, neonatal male Sprague–Dawley rats were chosen and exposed to PM2.5 by intranasal instillation. | [46] |
| Intranasal instillation | The aim of this work was to evaluate the time changes of systemic markers of oxidative stress and inflammation, after an acute exposure to Residual Oil Fly Ash (ROFA). | [47] |
| Intranasal instillation | Our objective was to analyse air PM from downtown Buenos Aires (UAP-BA) and evaluate its biological impact on normal airways. We studied the inflammatory response to intranasal instillation of UAP-BA in a short-term-exposure mouse model. | [48] |
| Intranasal instillation | We studied lung responses to low doses of urban air particulate matter from Buenos Aires (UAP-BA), with special emphasis on oxidative balance. | [49] |
| Intranasal instillation | The objective was to verify how these organic compartments respond to increasing concentrations of particles of known elemental composition. | [50] |
| Intranasal instillation | The aim of this study was to analyse in vivo the acute biological impact of two environmental particles, urban air particles from Buenos Aires and Residual Oil Fly Ash, on the cardiorespiratory system of middle-aged mice, evaluating oxidative metabolism and inflammation. | [51] |
| Intranasal instillation | We tested the hypothesis that a single acute exposure to low doses of fine particulate matter (PM2.5) may induce functional and histological lung changes and unchain inflammatory and oxidative stress processes. PM2.5 was collected from the urban area of São Paulo city during 24 h and underwent analysis for elements and polycyclic aromatic hydrocarbon contents. | [52] |
| Intranasal instillation | The aim of this study was to evaluate the effects of subchronic exposure to low doses of diesel exhaust particles (DEP) instilled in the respiratory tract of mice. | [53] |
| Intranasal instillation | The therapeutic effects of stemonine on mice with PM2.5-induced COPD were investigated in the present study. | [54] |
| Intranasal instillation | To test our hypothesis that cardiovascular diseases associated with sulphur dioxide (SO2), nitrogen dioxide (NO2), or PM2.5 exposure are the result of increased heart rate (HR), decreased blood pressure (BP) and enhanced systemic inflammation. | [55] |
| Intratracheal instillation | The aim of the present study was to evaluate the effects of PM10 on electrocardiogram (ECG) parameters, blood pressure, lipid peroxidation (MDA), xanthine oxidase, and antioxidant enzyme in healthy rats and also to examine the protective effects of vanillic acid (VA) in this respect. | [56] |
| Intratracheal instillation | This study aims to observe whether the combined treatment with vitamin E (vit E) and omega-3 polyunsaturated fatty acids (U-3 FA) could prevent the fine particulate matter (PM2.5)-induced cardiovascular injury through alleviating inflammation and oxidative stress. | [57] |
| Intratracheal instillation | The purpose of our study is to investigate PM10 sum effects on lungs and extra pulmonary tissues. The aim of this study is to disclose the pulmonary short-term effects and extra-pulmonary translocation of PM10 sum collected in Milano urban centre. | [58] |
| Intratracheal instillation | To assess susceptibility to lung infection following coexposure to particulate matter. | [59] |
| Intratracheal instillation | In this study, we evaluated the primary oxidative stress produced in the lung by crystalline silica (SiO2) in the early phase after SiO2 exposure. The aim of this study is to understand the crystalline SiO2-induced pulmonary oxidative stress in the early phase. | [60] |
| Intratracheal instillation | This study was conducted to investigate the possible protective effects and mechanisms of aspirin, Vitamin C, Vitamin E, or ozone on fertility in female mice treated with PM2.5. | [61] |
| Intratracheal instillation | We investigated the association of the chemical composition and sources of urban air fine (PM2.5−0.2) and coarse (PM10−2.5) particulate samples with the inflammatory activity in the mouse lung. | [62] |
| Intratracheal instillation | This study was undertaken to clarify the effects of Asian sand dust on lung eosinophilia in mice immunized beforehand by ovalbumin (OVA). | [63] |
| Intratracheal instillation | In the present study, urban PM2.5 and coarse particulate matter (CPM) collected during haze events of Northeast China in the winter season were used. The exacerbating effects of PM2.5 and CPM on OVA-induced allergic inflammation in murine lungs were compared to clarify the role of the chemicals and microbial materials in the two types of PM. | [64] |
| Intratracheal instillation | In order to further understand the roles of microRNAs in regulating the imbalance of T-helper 1 (Th1)/T-helper 2 (Th2) differentiation triggered by PM2.5. | [65] |
| Intratracheal instillation | The current study aimed to evaluate the effects of size-fractioned PM on lung immune responses in healthy BALB/c mice. | [66] |
| Intratracheal instillation | we investigated whether exposure to PM2.5, a PM with an aerodynamic diameter of less than 2.5 mm, enhances inflammation-related toxicity in the human respiratory system through activation of the epidermal growth factor receptor (EGFR) signalling pathway. | [67] |
| Intratracheal instillation | This study investigated the effect of acute respiratory exposure to PM on eyes, as induction of retinal thickening. | [68] |
| Intratracheal instillation | We investigated whether PM instillation in the airway could alter the course of acute lung injury, using a murine model with experimental lung injury induced by intratracheal lipopolysaccharide (LPS) challenge. | [69] |
| Intratracheal instillation | The objective is to investigate the influence of PM2.5 exposure on peripheral blood lymphocyte subsets in pregnant mice and the antagonism of quercetin on adverse effects induced by PM2.5 exposure. | [70] |
| Intratracheal instillation | We intend to investigate the toxic effects of PM2.5 during summer and winter on reproductive cells and tissues and focus on endoplasmic reticulum stress (ERS) to illustrate the possible molecular mechanisms. | [71] |
| Intratracheal instillation | We wished to investigate the impact of PM2.5 on placenta and prenatal outcomes and its related mechanisms in a rat model. | [72] |
| Intratracheal instillation | We assessed the effect of prolonged exposure to diesel exhaust particles (DEP) on chronic renal failure induced by adenine, which is known to involve inflammation and oxidative stress. | [73] |
| Intratracheal instillation | To evaluate the effect of airborne particulate matter 2.5 (PM2.5) in winter on airway inflammation, water-soluble supernatant (Sup) and water-insoluble precipitate (Pre) in PM2.5 were inoculated in NC/Nga mice with high sensitivity to mite allergens. | [74] |
| Intratracheal instillation | To evaluate the allergic effect of airborne particulate matter (PM) on the airway, separated soluble supernatant (Sup) and insoluble precipitate (Pre) in suspended PM were inoculated into NC/Nga mice with a high sensitivity for mite allergens. | [75] |
| Intratracheal instillation | The allergic inflammatory effects of particulate matter PM2.5, collected with the cyclone system in Yokohama city in Japan, were investigated in NC/Nga mice. | [2] |
| Intratracheal instillation | We aimed to explore the toxic mechanisms of cardiovascular injuries induced by ambient fine particulate matter (PM2.5) in atherosclerotic-susceptible ApoE−/− mice. | [76] |
| Intratracheal instillation | We investigated by the optical microscopy some cytological characteristics of the bronchoalveolar lavage fluid cell population 24 h after intratracheal instillation of microscale manganese dioxide (MnO2) and barium chromate (BaCrO4) particles (separately or together at two different doses) into the lungs of Wistar rats. | [77] |
| Intratracheal instillation | The aim of this study is to disclose short-term adverse effects on respiratory and cardiovascular systems induced by winter fine particles exposure. | [78] |
| Intratracheal instillation | The immune cells, including pulmonary macrophages of Sprague–Dawley (SD) rats and Raw 264.7 cells, were applied to further investigate the effect of PM2.5 on cell autophagy of macrophages, thus clarified the possible molecular mechanism of immunotoxicity caused by PM2.5. | [79] |
| Intratracheal instillation | We hypothesized that mechanisms independent of inflammation contribute to accelerated thrombus formation following exposure to diesel exhaust particles (DEP). | [80] |
| Intratracheal instillation | The primary objective of this study was to provide insights on the factors affecting the toxicological potency of exhaust PM emitted from different light-duty vehicles. This study presents different research techniques linked together to improve our understanding of the particulate matter (PM) impacts on health. The study develops conceptual dose–response functions for the different vehicle configurations. | [81] |
| Intratracheal instillation | In order to understand the comprehensive pulmonary response to PM2.5 stress, a non-targeted high-throughput metabolomics strategy was adopted to characterize the overall metabolic changes and relevant toxicological pathways. | [82] |
| Intratracheal instillation | We constructed a rat model to investigate the roles of autophagy in blood-testis barrier (BTB) toxicity induced by PM2.5. Sprague–Dawley rats were developmentally exposed to normal saline (NS) or PM2.5 with the doses via intratracheal instillation. | [83] |
| Intratracheal instillation | Short- and long-term exposure to particulate matter (PM) 2.5 instigates adverse health effect upon the cardiovascular system. We demonstrated that Wuhan PM2.5 exposure induced elevation of systemic Angiotensin II (ANGII) and local angiotensin-converting enzyme (ACE)/ANGII/ANGII type 1 receptor (AT1R) axis activation and the subsequent oxidative stress and proinflammatory responses in the vascular endothelium. | [84] |
| Intratracheal instillation | In order to investigate the mechanisms in PM2.5 toxicity, we explored the endogenous metabolic changes and possible influenced metabolic pathways in rats after intratracheal instillation of PM2.5. | [85] |
| Intratracheal instillation | The aim of this study was to evaluate the inflammatory response to SiO2 nanoparticles using in vivo test systems. | [86] |
EAP: environmental air PM, CAP: concentrated air PM.
Table 2.
Physiological breathing parameters and PM inhaled fraction (inhalability) in mice and rats.
Table 3.
List of experiments where PM exposure was performed in mice and rats, with methods and characteristics of each procedure. The dataset includes 30 articles published in 2017 and 45 articles published in previous years.
| Exposure method | PM size | Animal model | Equivalent atmospheric concentration (μg/m3) | Time of one exposure event (h) | Number of exposure events | Total exposure time (h) | Inhaled dose per event (μg/kg Bw) | PM inhalation rate (μg/kg Bw/h) | Authors |
|---|---|---|---|---|---|---|---|---|---|
| EAP inhalation | PM2.5 | Mouse | 18.1 | 2880 | 1 | 2880 | 12619.2 | 4.4 | [37] |
| PM10 | Rat | 22.0 | 6 | 1 | 6 | 5.0 | 0.8 | [40] | |
| PM10 | Mouse | 33.9 | 24 | 60 | 1440 | 58.9 | 2.5 | [38] | |
| PM10 | Rat | 34.0 | 6 | 1 | 6 | 7.7 | 1.3 | [40] | |
| PM10 | Mouse | 48.9 | 24 | 120 | 2880 | 85.0 | 3.5 | [39] | |
| PM10 | Rat | 99.2 | 5 | 4 | 20 | 18.7 | 3.7 | [40] | |
| PM10 | Rat | 112.4 | 20 | 1 | 20 | 85.0 | 4.2 | [40] | |
| PM10 | Rat | 138.6 | 20 | 1 | 20 | 104.8 | 5.2 | [40] | |
| PM10 | Rat | 224.7 | 6 | 1 | 6 | 51.0 | 8.5 | [40] | |
| CAP inhalation | PM1 | Mouse | 50.0 | 2 | 3 | 6 | 31.9 | 15.9 | [28] |
| PM1 | Rat | 57.0 | 6 | 3 | 18 | 47.6 | 7.9 | [33] | |
| PM1 | Rat | 90.0 | 6 | 3 | 18 | 75.1 | 12.5 | [33] | |
| PM2.5 | Mouse | 60.9 | 6 | 80 | 480 | 88.7 | 14.8 | [12] | |
| PM2.5 | Mouse | 101.5 | 6 | 120 | 720 | 147.8 | 24.6 | [21] | |
| PM2.5 | Mouse | 113.4 | 6 | 17 | 102 | 165.2 | 27.5 | [16] | |
| PM2.5 | Rat | 126.1 | 5 | 3 | 15 | 71.5 | 14.3 | [15] | |
| PM2.5 | Rat | 126.1 | 5 | 3 | 15 | 71.5 | 14.3 | [30] | |
| PM2.5 | Mouse | 163.8 | 6 | 17 | 102 | 238.6 | 39.8 | [16] | |
| PM2.5 | Rat | 170.7 | 5 | 3 | 15 | 96.8 | 19.4 | [15] | |
| PM2.5 | Rat | 170.7 | 5 | 3 | 15 | 96.8 | 19.4 | [30] | |
| PM2.5 | Rat | 187.1 | 5 | 3 | 15 | 106.1 | 21.2 | [15] | |
| PM2.5 | Rat | 187.1 | 5 | 3 | 15 | 106.1 | 21.2 | [30] | |
| PM2.5 | Rat | 200.0 | 3 | 30 | 90 | 68.0 | 22.7 | [24] | |
| PM2.5 | Rat | 200.0 | 3 | 30 | 90 | 68.0 | 22.7 | [13] | |
| PM2.5 | Mouse | 203.0 | 1 | 6 | 6 | 49.3 | 49.3 | [17] | |
| PM2.5 | Mouse | 203.3 | 1 | 6 | 6 | 49.3 | 49.3 | [17] | |
| PM2.5 | Rat | 262.2 | 5 | 3 | 15 | 148.7 | 29.7 | [22] | |
| PM2.5 | Rat | 267.3 | 5 | 3 | 15 | 151.6 | 30.3 | [15] | |
| PM2.5 | Rat | 267.3 | 5 | 3 | 15 | 151.6 | 30.3 | [30] | |
| PM2.5 | Rat | 300.0 | 1 | 1 | 1 | 34.0 | 34.0 | [25] | |
| PM2.5 | Rat | 300.0 | 3 | 1 | 3 | 102.1 | 34.0 | [25] | |
| PM2.5 | Rat | 300.0 | 5 | 1 | 5 | 170.1 | 34.0 | [25] | |
| PM2.5 | Rat | 300.7 | 5 | 3 | 15 | 170.5 | 34.1 | [15] | |
| PM2.5 | Rat | 300.7 | 5 | 3 | 15 | 170.5 | 34.1 | [30] | |
| PM2.5 | Rat | 400.0 | 6 | 3 | 18 | 272.2 | 45.4 | [19] | |
| PM2.5 | Rat | 481.0 | 5 | 3 | 15 | 272.7 | 54.5 | [15] | |
| PM2.5 | Rat | 481.0 | 5 | 3 | 15 | 272.7 | 54.5 | [30] | |
| PM2.5 | Rat | 595.0 | 2 | 28 | 56 | 134.9 | 67.5 | [18] | |
| PM2.5 | Mouse | 600.0 | 1 | 12 | 12 | 145.7 | 145.7 | [32] | |
| PM2.5 | Mouse | 600.0 | 1 | 21 | 21 | 145.7 | 145.7 | [20] | |
| PM2.5 | Mouse | 600.0 | 1 | 12 | 12 | 145.7 | 145.7 | [31] | |
| PM2.5 | Rat | 700.0 | 5 | 1 | 5 | 396.9 | 79.4 | [36] | |
| PM2.5 | Mouse | 770.0 | 1 | 90 | 90 | 186.9 | 186.9 | [23] | |
| PM2.5 | Mouse | 950.0 | 6 | 15 | 90 | 1383.7 | 230.6 | [27] | |
| PM2.5 | Mouse | 950.0 | 6 | 65 | 390 | 1383.7 | 230.6 | [27] | |
| PM2.5 | Rat | 1000.0 | 3 | 30 | 90 | 340.2 | 113.4 | [24] | |
| PM2.5 | Rat | 1000.0 | 3 | 30 | 90 | 340.2 | 113.4 | [13] | |
| PM2.5 | Rat | 1228.0 | 5 | 10 | 50 | 696.3 | 139.3 | [29] | |
| PM2.5 | Rat | 2000.0 | 3 | 30 | 90 | 680.4 | 226.8 | [13] | |
| PM2.5 | Rat | 3000.0 | 3 | 30 | 90 | 1020.6 | 340.2 | [24] | |
| PM2.5 | Rat | 12000.0 | 6 | 1 | 6 | 8164.8 | 1360.8 | [35] | |
| PM2.5 | Mouse | 20000.0 | 2 | 8 | 16 | 9710.1 | 4855.0 | [34] | |
| PM2.5 | Mouse | 30000.0 | 2 | 8 | 16 | 14565.1 | 7282.6 | [34] | |
| PM10 | Rat | 100.0 | 6 | 20 | 120 | 22.7 | 3.8 | [26] | |
| PM10 | Rat | 1000.0 | 6 | 20 | 120 | 226.8 | 37.8 | [26] | |
| PM10 | Rat | 10000.0 | 6 | 20 | 120 | 2268.0 | 378.0 | [26] | |
| Intranasal instillation | PM2.5 | Mouse | 700.3 | 1 | 9 | 9 | 170.0 | 170.0 | [49] |
| PM2.5 | Mouse | 700.3 | 1 | 9 | 9 | 170.0 | 170.0 | [48] | |
| PM2.5 | Mouse | 823.9 | 1 | 1 | 1 | 200.0 | 200.0 | [52] | |
| PM2.5 | Mouse | 823.9 | 1 | 84 | 84 | 200.0 | 200.0 | [42] | |
| PM2.5 | Mouse | 2471.7 | 1 | 1 | 1 | 600.0 | 600.0 | [52] | |
| PM2.5 | Mouse | 4119.4 | 1 | 1 | 1 | 1000.0 | 1000.0 | [51] | |
| PM2.5 | Mouse | 4119.4 | 1 | 1 | 1 | 1000.0 | 1000.0 | [47] | |
| PM2.5 | Mouse | 4119.4 | 1 | 7 | 7 | 1000.0 | 1000.0 | [55] | |
| PM2.5 | Mouse | 4943.3 | 1 | 21 | 21 | 1200.0 | 1200.0 | [53] | |
| PM2.5 | Mouse | 4943.3 | 1 | 42 | 42 | 1200.0 | 1200.0 | [53] | |
| PM2.5 | Mouse | 13841.3 | 1 | 8 | 8 | 3360.0 | 3360.0 | [34] | |
| PM2.5 | Mouse | 20762.0 | 1 | 8 | 8 | 5040.0 | 5040.0 | [34] | |
| PM2.5 | Rat | 17636.7 | 1 | 14 | 14 | 2000.0 | 2000.0 | [46] | |
| PM2.5 | Mouse | 24716.6 | 1 | 9 | 9 | 6000.0 | 6000.0 | [41] | |
| PM2.5 | Mouse | 41194.4 | 1 | 28 | 28 | 10000.0 | 10000.0 | [55] | |
| PM2.5 | Mouse | 164777.4 | 1 | 4 | 4 | 40000.0 | 40000.0 | [43] | |
| PM2.5 | Mouse | 164777.4 | 1 | 7 | 7 | 40000.0 | 40000.0 | [54] | |
| PM2.5 | Rat | 176366.8 | 1 | 14 | 14 | 20000.0 | 20000.0 | [46] | |
| PM10 | Mouse | 55.2 | 1 | 1 | 1 | 4.0 | 4.0 | [50] | |
| PM10 | Mouse | 552.0 | 1 | 1 | 1 | 40.0 | 40.0 | [50] | |
| PM10 | Mouse | 5520.0 | 1 | 1 | 1 | 400.0 | 400.0 | [50] | |
| PM10 | Mouse | 55200.4 | 1 | 5 | 5 | 4000.0 | 4000.0 | [44] | |
| PM10 | Mouse | 55200.4 | 1 | 5 | 5 | 4000.0 | 4000.0 | [45] | |
| Intratracheal instillation | PM1 | Mouse | 690.0 | 1 | 1 | 1 | 220.0 | 220.0 | [81] |
| PM1 | Mouse | 1254.6 | 1 | 1 | 1 | 400.0 | 400.0 | [81] | |
| PM1 | Mouse | 1380.0 | 1 | 1 | 1 | 440.0 | 440.0 | [81] | |
| PM1 | Mouse | 1505.5 | 1 | 1 | 1 | 480.0 | 480.0 | [81] | |
| PM1 | Mouse | 1568.2 | 1 | 7 | 7 | 500.0 | 500.0 | [73] | |
| PM1 | Mouse | 2509.1 | 1 | 1 | 1 | 800.0 | 800.0 | [81] | |
| PM1 | Mouse | 3010.9 | 1 | 1 | 1 | 960.0 | 960.0 | [81] | |
| PM1 | Mouse | 4390.9 | 1 | 2 | 2 | 1400.0 | 1400.0 | [66] | |
| PM1 | Mouse | 12545.6 | 1 | 2 | 2 | 4000.0 | 4000.0 | [66] | |
| PM1 | Mouse | 31363.9 | 1 | 2 | 2 | 10000.0 | 10000.0 | [66] | |
| PM1 | Rat | 35944.3 | 1 | 1 | 1 | 5000.0 | 5000.0 | [86] | |
| PM2.5 | Rat | 278.3 | 1 | 1 | 1 | 31.6 | 31.6 | [59] | |
| PM2.5 | Rat | 705.5 | 1 | 1 | 1 | 80.0 | 80.0 | [68] | |
| PM2.5 | Rat | 1763.7 | 1 | 1 | 1 | 200.0 | 200.0 | [59] | |
| PM2.5 | Rat | 1763.7 | 1 | 1 | 1 | 200.0 | 200.0 | [59] | |
| PM2.5 | Rat | 1763.7 | 1 | 20 | 20 | 200.0 | 200.0 | [71] | |
| PM2.5 | Rat | 1763.7 | 1 | 20 | 20 | 200.0 | 200.0 | [79] | |
| PM2.5 | Rat | 2645.5 | 1 | 20 | 20 | 300.0 | 300.0 | [71] | |
| PM2.5 | Rat | 2645.5 | 1 | 20 | 20 | 300.0 | 300.0 | [79] | |
| PM2.5 | Mouse | 3295.5 | 1 | 4 | 4 | 800.0 | 800.0 | [67] | |
| PM2.5 | Mouse | 3295.5 | 1 | 7 | 7 | 800.0 | 800.0 | [69] | |
| PM2.5 | Rat | 3527.3 | 1 | 1 | 1 | 400.0 | 400.0 | [59] | |
| PM2.5 | Rat | 3527.3 | 1 | 1 | 1 | 400.0 | 400.0 | [60] | |
| PM2.5 | Rat | 3880.1 | 1 | 1 | 1 | 440.0 | 440.0 | [35] | |
| PM2.5 | Rat | 5291.0 | 1 | 20 | 20 | 600.0 | 600.0 | [71] | |
| PM2.5 | Rat | 5291.0 | 1 | 20 | 20 | 600.0 | 600.0 | [79] | |
| PM2.5 | Mouse | 5767.2 | 1 | 2 | 2 | 1400.0 | 1400.0 | [66] | |
| PM2.5 | Rat | 8218.7 | 1 | 1 | 1 | 932.0 | 932.0 | [59] | |
| PM2.5 | Rat | 8849.7 | 1 | 1 | 1 | 1003.6 | 1003.6 | [59] | |
| PM2.5 | Rat | 9982.4 | 1 | 1 | 1 | 1132.0 | 1132.0 | [59] | |
| PM2.5 | Mouse | 10298.6 | 1 | 1 | 1 | 2500.0 | 2500.0 | [65] | |
| PM2.5 | Mouse | 12358.3 | 1 | 3 | 3 | 3000.0 | 3000.0 | [76] | |
| PM2.5 | Rat | 13227.5 | 1 | 20 | 20 | 1500.0 | 1500.0 | [71] | |
| PM2.5 | Rat | 13227.5 | 1 | 20 | 20 | 1500.0 | 1500.0 | [79] | |
| PM2.5 | Rat | 13227.5 | 1 | 3 | 3 | 1500.0 | 1500.0 | [84] | |
| PM2.5 | Rat | 15873.0 | 1 | 10 | 10 | 1800.0 | 1800.0 | [85] | |
| PM2.5 | Mouse | 16477.7 | 1 | 4 | 4 | 4000.0 | 4000.0 | [64] | |
| PM2.5 | Mouse | 16477.7 | 1 | 2 | 2 | 4000.0 | 4000.0 | [66] | |
| PM2.5 | Mouse | 16477.7 | 1 | 3 | 3 | 4000.0 | 4000.0 | [78] | |
| PM2.5 | Rat | 17636.7 | 1 | 1 | 1 | 2000.0 | 2000.0 | [80] | |
| PM2.5 | Rat | 23809.5 | 1 | 20 | 20 | 2700.0 | 2700.0 | [71] | |
| PM2.5 | Rat | 23809.5 | 1 | 20 | 20 | 2700.0 | 2700.0 | [79] | |
| PM2.5 | Rat | 26455.0 | 1 | 1 | 1 | 3000.0 | 3000.0 | [36] | |
| PM2.5 | Mouse | 27023.5 | 1 | 4 | 4 | 6560.0 | 6560.0 | [67] | |
| PM2.5 | Mouse | 32955.5 | 1 | 6 | 6 | 8000.0 | 8000.0 | [75] | |
| PM2.5 | Mouse | 32955.5 | 1 | 6 | 6 | 8000.0 | 8000.0 | [2] | |
| PM2.5 | Mouse | 32955.5 | 1 | 6 | 6 | 8000.0 | 8000.0 | [74] | |
| PM2.5 | Mouse | 41194.4 | 1 | 11 | 11 | 10000.0 | 10000.0 | [61] | |
| PM2.5 | Mouse | 41194.4 | 1 | 1 | 1 | 10000.0 | 10000.0 | [62] | |
| PM2.5 | Mouse | 41194.4 | 1 | 1 | 1 | 10000.0 | 10000.0 | [65] | |
| PM2.5 | Mouse | 41194.4 | 1 | 2 | 2 | 10000.0 | 10000.0 | [66] | |
| PM2.5 | Mouse | 41194.4 | 1 | 3 | 3 | 10000.0 | 10000.0 | [76] | |
| PM2.5 | Rat | 47619.0 | 1 | 10 | 10 | 5400.0 | 5400.0 | [85] | |
| PM2.5 | Mouse | 61791.5 | 1 | 5 | 5 | 15000.0 | 15000.0 | [70] | |
| PM2.5 | Rat | 79365.1 | 1 | 49 | 49 | 9000.0 | 9000.0 | [83] | |
| PM2.5 | Mouse | 82388.7 | 1 | 1 | 1 | 20000.0 | 20000.0 | [65] | |
| PM2.5 | Rat | 88183.4 | 1 | 1 | 1 | 10000.0 | 10000.0 | [57] | |
| PM2.5 | Rat | 88183.4 | 1 | 1 | 1 | 10000.0 | 10000.0 | [77] | |
| PM2.5 | Mouse | 123583.1 | 1 | 3 | 3 | 30000.0 | 30000.0 | [76] | |
| PM2.5 | Rat | 132275.1 | 1 | 2 | 2 | 15000.0 | 15000.0 | [72] | |
| PM2.5 | Rat | 142857.1 | 1 | 10 | 10 | 16200.0 | 16200.0 | [85] | |
| PM2.5 | Rat | 176366.8 | 1 | 1 | 1 | 20000.0 | 20000.0 | [77] | |
| PM2.5 | Rat | 211640.2 | 1 | 49 | 49 | 24000.0 | 24000.0 | [83] | |
| PM2.5 | Rat | 220458.6 | 1 | 12 | 12 | 25000.0 | 25000.0 | [82] | |
| PM10 | Rat | 5291.0 | 1 | 1 | 1 | 200.0 | 200.0 | [26] | |
| PM10 | Rat | 13227.5 | 1 | 1 | 1 | 500.0 | 500.0 | [56] | |
| PM10 | Mouse | 19320.2 | 1 | 2 | 2 | 1400.0 | 1400.0 | [66] | |
| PM10 | Rat | 21164.0 | 1 | 1 | 1 | 800.0 | 800.0 | [26] | |
| PM10 | Mouse | 55200.4 | 1 | 1 | 1 | 4000.0 | 4000.0 | [58] | |
| PM10 | Mouse | 55200.4 | 1 | 2 | 2 | 4000.0 | 4000.0 | [66] | |
| PM10 | Rat | 66137.6 | 1 | 1 | 1 | 2500.0 | 2500.0 | [56] | |
| PM10 | Rat | 79365.1 | 1 | 1 | 1 | 3000.0 | 3000.0 | [26] | |
| PM10 | Mouse | 110400.9 | 1 | 1 | 8000.0 | 8000.0 | [63] | ||
| PM10 | Rat | 132275.1 | 1 | 1 | 1 | 5000.0 | 5000.0 | [56] | |
| PM10 | Mouse | 138001.1 | 1 | 1 | 1 | 10000.0 | 10000.0 | [62] | |
| PM10 | Mouse | 138001.1 | 1 | 2 | 2 | 10000.0 | 10000.0 | [66] |
EAP: environmental air PM, CAP: concentrated air PM.
Table 4.
Summary of exposure characteristics from different PM exposure protocols in mice and rats.
| Exposure Method | PM size | n | Concentration (μg/m3) |
PM inhalation rate (μg/kg Bw/h) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Range | Mean | SEM | Range | |||
| EAP inhalation | PM2.5 | 1 | 1.8 × 101 | 4.4 × 100 | ||||
| PM10 | 8 | 8.9 × 101 | 2.5 × 101 | 2.2 × 101–2.2 × 102 | 3.7 × 100 | 9.0 × 101 | 8.0 × 10−1 - 8.5 × 100 | |
| CAP inhalation | PM1 | 3 | 6.6 × 101 | 1.2 × 101 | 5.0 × 101–9.0 × 101 | 1.2 × 101 | 2.3 × 100 | 7.9 × 100–1.6 × 101 |
| PM2.5 | 41 | 2.0 × 103 | 8.9 × 102 | 6.1 × 101–3.0 × 104 | 4.0 × 102 | 2.1 × 102 | 1.4 × 101–7.3 × 103 | |
| PM10 | 3 | 3.7 × 103 | 3.2 × 103 | 1.0 × 102–1.0 × 104 | 1.4 × 102 | 1.2 × 102 | 3.8 × 100–3.8 × 102 | |
| Intranasal instillation | PM2.5 | 18 | 3.6 × 104 | 1.5 × 104 | 7.0 × 102–1.8 × 105 | 7.8 × 103 | 3.0 × 103 | 1.7 × 102–4.0 × 104 |
| PM10 | 5 | 2.3 × 104 | 1.3 × 104 | 5.5 × 101–5.5 × 104 | 1.7 × 103 | 9.5 × 102 | 4.0 × 100–4.0 × 103 | |
| Intratracheal instillation | PM1 | 11 | 8.7 × 103 | 3.9 × 103 | 6.9 × 102–3.6 × 104 | 2.2 × 103 | 9.2 × 102 | 2.2 × 102–1.0 × 104 |
| PM2.5 | 53 | 3.9 × 104 | 7.3 × 103 | 2.8 × 102–2.2 × 105 | 6.1 × 103 | 1.0 × 103 | 3.2 × 101–3.0 × 103 | |
| PM10 | 12 | 6.9 × 104 | 1.4 × 104 | 5.3 × 103–1.4 × 105 | 4.1 × 103 | 1.0 × 103 | 2.0 × 102–1.0 × 104 | |
2. Experimental design, materials, and methods
2.1. Criteria for paper selection
The published works included in our dataset were selected using the following criteria: papers were published in English, they were referenced in indexed journals (with editorial board, peer reviewed and included in Clarivate Analytics Journal Citation Reports), and they were published recently (in 2017). The selected papers were based on mice and/or rat models, and the protocol exposed the respiratory tract to PM in order to study the health effects at one or more specific endpoints (respiratory tract, pulmonary and extra-pulmonary). With these criteria, a search query was constructed for PubMed. Searches were performed using the following keywords: (particulate matter) AND (mice or mouse or rats or rat) AND (inhalation or instillation). Apart from the most recent articles published in 2017, we also included seminal papers from previous years.
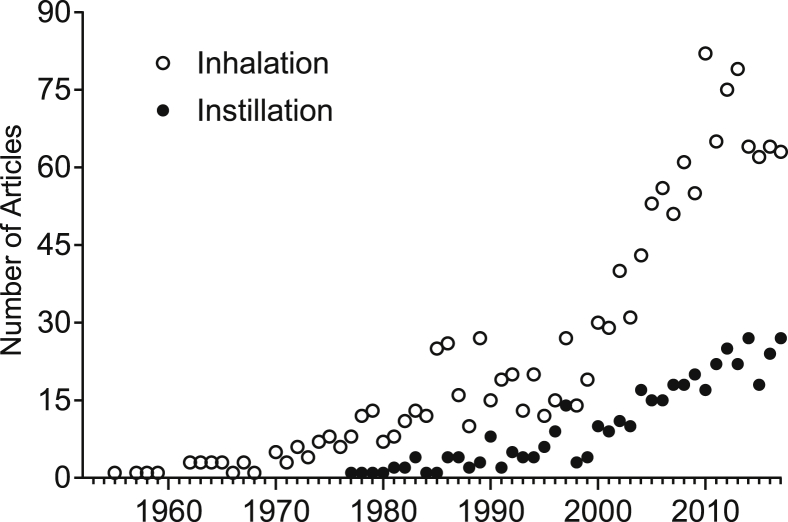
From the PubMed timeline of the selected papers (Fig. 1), an increase can be observed in the number of publications indexed by the keywords “inhalation” and “instillation” with “inhalation” cited in more articles than “instillation”. Such behaviour is similar over other years.
Fig. 1.
PubMed timelines of two datasets considering the number of articles indexed by “inhalation” and “instillation” as keywords in combination with (particulate matter) AND (rat or rats or mouse or mice). The timeline begins at 1955 (first “inhalation” article found) and ends at 2017.
From the resulting group of articles, only those papers containing experimental impacts of environmental PM effects, as described in their material and methods sections, were included. Experiments that used total suspended particles or settleable particulate matter (six articles) and experiments carried out by oropharyngeal aspiration (four articles) were excluded. After applying the filtering criteria to the PubMed query results, 75 articles were selected, containing 155 different experiments.
Normalization of different experimental results to allow comparisons among doses and results.
One difficulty in comparing studies that use different exposure protocols is the way as PM amounts are reported. Studies using instillation protocols report the mass of administered PM (dose) at each exposure event and studies using inhalation, report PM concentration and exposure time. To compare data and results for methods having such intrinsic differences, we used an equation based on PM dosimetry models [3], [4], [5]. This equation normalizes the administered PM dose (D), taking into account PM inhaled fraction (inhalability) as a function of the aerodynamic diameter (da) [3], [4], [5]. Thus, the following formula was defined:
| (1) |
The parameters used in this formula are described below:
D = dose according to da (μg)
C = PM concentration (μg/m3)
Q = air inhalation flow (m3/min)
t = exposure time (min)
I = inhalability according to da (%)
The exposure dose varies according to the respiratory physiologic parameters of each species (Table 1), which are experimentally obtained or calculated by allometric models [4], [6], [7]. The air inhalation flow (Q) is calculated from the ratio between the tidal volume (VT) and the inhalation time (ti) and indicates the inhaled air volume per unit of time [8]. Assuming a ratio of 0.4/0.6 for inhalation versus exhalation times in mice and rats [9], [10], it is possible to estimate the ti according to the respiration frequency (f) in mice and rats. We defined the PM inhaled dose as the PM mass that reaches the respiratory tract, i.e., it is the dose that can be inhaled, even if part of PM were deposited in the upper respiratory tract (URT). This definition is consistent with the concept of delivered dose, the amount of PM inhaled by the animal [11].
This equation is not only useful for calculating the administered PM doses, but can also be used to normalize other quantities and to calculate the equivalent PM concentration (C), according to the instillation protocols (C = D/(Q·t·I)). For example, the PM inhalation rate (IR, μg/h) can be determined if we disregard exposure time (t). Moreover, both D and IR can be expressed in terms of the experimental animal body mass, and in these cases, two other indices are determined: specific dose (DBW) and specific inhalation rate (IRBW).
Since we have different exposure protocols, some considerations about the application of equation (1) are necessary:
(i) Equation (1) was used to establish the equivalence between protocols performed by instillation and inhalation. The protocols by instillation are performed after anaesthetics administration, which can change the physiological breathing parameters of the animals. However, equation (1), does not consider the effect of anaesthesia. Thus, the variations of the breathing parameters that could be caused by anaesthesia are not included in applying equation (1), designed to calculate the delivered dose by inhalation.
(ii) Physiological breathing parameters described in Table 1 may present variations according to the different references. These parameters can vary between species, strains and even between individuals. However, within the limits of application of equation (1), we define the data acquisition referenced in recent publications developed by specialist researchers. At present, order of magnitude parameter estimation is the most relevant way to study different methods because the variations of PM exposure between methods are quite large and exceed orders of magnitude. Thus, variations in the physiological breathing parameters within the same order of magnitude can be assimilated without changing the conclusions of this study.
(iii) In instillation protocols, the PM is administered in a liquid media, while in the inhalation methods, the PM is dispersed in air. The distribution and deposition of PM in the respiratory tract is different when administered in liquid or in the air. It is known that intranasal instillation (INI), even in a liquid media, must pass the URT before reaching the LRT. However, considering the definition of inhaled dose previously mentioned, in this study, even if the deposition occurs in URT, we consider that the respiratory tract had contact with PM. Some studies included in our dataset, examined extra-pulmonary and systemic PM effects, including allergic responses in URT [12], [13]. In addition, instillation protocols introduce into airways types and quantities of particles that would not naturally reach there by inhalation. This may be one of the most relevant differences between exposure methods. Thus, inhalability was used as an important variable to calculate the equivalence between instillation and inhalation methods. As such, inhalability considers the difficulty of reaching the airways imposed by aerodynamic restrictions related to particle size [3], [14].
(iv) Finally, in some cases, few assumptions should be pre-determined to allow the calculation of D and C. In studies based on instillation, the amount of time spent to perform this procedure was 1 h. We assumed this time duration to calculate the dosage used. In fact, this duration is smaller than 1 h, probably minutes, but this would raise D and C to very excessive doses when compared with environmental concentrations measured around the world. In addition, we used a 1 h dose duration because most air quality monitoring stations use 1 h as the shortest interval for recording measurements. Thus, based on this average time, we could compare the concentrations used in the experiments and the measured environmental concentrations. In fact, the instillation protocols try to mimic in minutes (or less time) the exposure that would occur in hours, days, even months in environmental conditions.
Acknowledgments
This work was partially supported by a grant from Instituto Capixaba de Ciências e Administração (ICCA), grant #007/2017.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Curbani F., de Oliveira Busato F., do Nascimento M.M., Olivieri D.N., Tadokoro C.E. Inhale, exhale: why particulate matter exposure in animal models are so acute? Environ. Pollut. 2019;251:230–237. doi: 10.1016/j.envpol.2019.04.084. [DOI] [PubMed] [Google Scholar]
- 2.Ogino K., Nagaoka K., Okuda T., Oka A., Kubo M., Eguchi E., Fujikura Y. PM2.5-induced airway inflammation and hyperresponsiveness in NC/Nga mice. Environ. Toxicol. 2017;32:1047–1054. doi: 10.1002/tox.22303. [DOI] [PubMed] [Google Scholar]
- 3.Millage K.K., Bergman J., Asgharian B., McClellan G. A review of inhalability fraction models: discussion and recommendations. Inhal. Toxicol. 2010;22:151–159. doi: 10.3109/08958370903025973. [DOI] [PubMed] [Google Scholar]
- 4.Asgharian B., Price O.T., Oldham M., Chen L.-C., Saunders E.L., Gordon T., Mikheev V.B., Minard K.R., Teeguarden J.G. Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal. Toxicol. 2014;26:829–842. doi: 10.3109/08958378.2014.935535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong B.A. Inhalation exposure systems: design, methods and operation. Toxicol. Pathol. 2007;35:3–14. doi: 10.1080/01926230601060017. [DOI] [PubMed] [Google Scholar]
- 6.Bide R.W., Armour S.J., Yee E. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J. Appl. Toxicol. 2000;20:273–290. doi: 10.1002/1099-1263(200007/08)20:4<273::aid-jat657>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Miller F.J., Asgharian B., Schroeter J.D., Price O. Improvements and additions to the multiple path particle dosimetry model. J. Aerosol Sci. 2016;99:14–26. [Google Scholar]
- 8.Lim R., Zavou M.J., Milton P.-L., Chan S.T., Tan J.L., Dickinson H., Murphy S.V., Jenkin G., Wallace E.M. Measuring respiratory function in mice using unrestrained whole-body plethysmography. J. Vis. Exp. 2014:1–11. doi: 10.3791/51755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler-Heil R., Hofmann W. Modeling particle deposition in the Balb/c mouse respiratory tract. Inhal. Toxicol. 2016;28:180–191. doi: 10.3109/08958378.2016.1148801. [DOI] [PubMed] [Google Scholar]
- 10.DeLorme M.P., Moss O.R. Pulmonary function assessment by whole-body plethysmography in restrained versus unrestrained mice. J. Pharmacol. Toxicol. Methods. 2002;47:1–10. doi: 10.1016/s1056-8719(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 11.Phillips J.E. Inhaled efficacious dose translation from rodent to human: a retrospective analysis of clinical standards for respiratory diseases. Pharmacol. Therapeut. 2017;178:141–147. doi: 10.1016/j.pharmthera.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Ramanathan M., London N.R., Tharakan A., Surya N., Sussan T.E., Rao X., Lin S.Y., Toskala E., Rajagopalan S., Biswal S. Airborne particulate matter induces nonallergic eosinophilic sinonasal inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2017;57:59–65. doi: 10.1165/rcmb.2016-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z.-Q., Dong W.Y., Xu J., Hong Z.C., Zhao R.W., Deng C.R., Zhuang G.S., Zhang R.X. T-helper type 1-T-helper type 2 shift and nasal remodeling after fine particulate matter exposure in a rat model of allergic rhinitis. American Journal of Rhinology and Allergy. 2017;31:148–155. doi: 10.2500/ajra.2017.31.4437. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy N.J., Hinds W.C. Inhalability of large solid particles. J. Aerosol Sci. 2002;33:237–255. [Google Scholar]
- 15.Batalha J.R.F., Saldiva P.H.N., Clarke R.W., Coull B.A., Stearns R.C., Lawrence J., Murthy G.G.K., Koutrakis P., Godleski J.J. Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ. Health Perspect. 2002;110:1191–1197. doi: 10.1289/ehp.021101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blum J.L., Chen L.C., Zelikoff J.T. Exposure to ambient particulate matter during specific gestational periods produces adverse obstetric consequences in mice. Environ. Health Perspect. 2017;125:1–13. doi: 10.1289/EHP1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brito J.M., Macchione M., Yoshizaki K., Toledo-Arruda A.C., Saraiva-Romanholo B.M., Andrade M. de F., Mauad T., Rivero D.H.R.F., Saldiva P.H.N. Acute cardiopulmonary effects induced by the inhalation of concentrated ambient particles during seasonal variation in the city of São Paulo. J. Appl. Physiol. 2014;117:492–499. doi: 10.1152/japplphysiol.00156.2014. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L., Lau W.K.W., Fung T.K.H., Lau B.W.M., Chau B.K.H., Liang Y., Wang Z., So K.F., Wang T., Chan C.C.H., Lee T.M.C. PM2.5 exposure suppresses dendritic maturation in subgranular zone in aged rats. Neurotox. Res. 2017;32:50–57. doi: 10.1007/s12640-017-9710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke R.W., Catalano P., Coull B., Koutrakis P., Murthy G.G.K., Rice T., Godleski J.J. Age-related responses in rats to concentrated urban air particles (CAPs) Inhal. Toxicol. 2000;12:73–84. [Google Scholar]
- 20.Fuchs L.F.P., Veras M.M., Saldiva P.H.N., Sasso G.R. da S., Carvalho K.C., Simões M. de J., Soares J.M., Baracat E.C. Ambient levels of concentrated PM2.5 affects cell kinetics in adrenal glands: an experimental study in mice. Gynecol. Endocrinol. 2017;33:490–495. doi: 10.1080/09513590.2017.1291617. [DOI] [PubMed] [Google Scholar]
- 21.Ge C.X., Qin Y.T., Lou D.S., Li Q., Li Y.Y., Wang Z.M., Yang W.W., Wang M., Liu N., Wang Z., Zhang P.X., Tu Y.Y., Tan J., Xu M.X. iRhom2 deficiency relieves TNF-α associated hepatic dyslipidemia in long-term PM2.5-exposed mice. Biochem. Biophys. Res. Commun. 2017;493:1402–1409. doi: 10.1016/j.bbrc.2017.09.152. [DOI] [PubMed] [Google Scholar]
- 22.Godleski J.J., Clarke R.W., Coull B.A., Saldiva P.H.N., Jiang N.F., Lawrence J., Koutrakis P. Composition of inhaled urban air particles determines acute pulmonary responses. Ann. Occup. Hyg. 2002;46:419–424. [Google Scholar]
- 23.yu Gu X., Chu X., Zeng X.L., Bao H.R., Liu X.J. Effects of PM2.5 exposure on the Notch signaling pathway and immune imbalance in chronic obstructive pulmonary disease. Environ. Pollut. 2017;226:163–173. doi: 10.1016/j.envpol.2017.03.070. [DOI] [PubMed] [Google Scholar]
- 24.Guo Z.-Q., Hong Z., Dong W., Deng C., Zhao R., Xu J., Zhuang G., Zhang R. PM2.5-induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. Int. J. Environ. Res. Public Health. 2017;14 doi: 10.3390/ijerph14020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurgueira S.A., Lawrence J., Coull B., Krishna Murthy G.G., González-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson R.F., Driscoll K.E., Harkema J.R., Lindenschmidt R.C., Chang L.Y., Barr E.B. A comparison of the inflammatory response of the lung to inhaled versus instilled particles in F344 rats. Toxicol. Sci. 1995;24:183–197. doi: 10.1006/faat.1995.1022. [DOI] [PubMed] [Google Scholar]
- 27.Hullmann M., Albrecht C., van Berlo D., Gerlofs-Nijland M.E., Wahle T., Boots A.W., Krutmann J., Cassee F.R., Bayer T.A., Schins R.P.F. Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer's disease. Part. Fibre Toxicol. 2017;14:1–14. doi: 10.1186/s12989-017-0213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim B.-G., Lee P.-H., Lee S.-H., Park M.-K., Jang A.-S. Effect of TiO2 nanoparticles on inflammasome-mediated airway inflammation and responsiveness. Allergy, Asthma & Immunology Research. 2017;9:257. doi: 10.4168/aair.2017.9.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhoden C.R., Lawrence J., Godleski J.J., González-Flecha B. N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol. Sci. 2004;79:296–303. doi: 10.1093/toxsci/kfh122. [DOI] [PubMed] [Google Scholar]
- 30.Saldiva P.H.N., Clarke R.W., Coull B.A., Stearns R.C., Lawrence J., Krishna Murthy G.G., Diaz E., Koutrakis P., Suh H., Tsuda A., Godleski J.J. Lung inflammation induced by concentrated ambient air particles is related to particle composition. Am. J. Respir. Crit. Care Med. 2002;165:1610–1617. doi: 10.1164/rccm.2106102. [DOI] [PubMed] [Google Scholar]
- 31.Yoshizaki K., Fuziwara C.S., Brito J.M., Santos T.M.N., Kimura E.T., Correia A.T., Amato-Lourenco L.F., Vasconcellos P., Silva L.F., Brentani M.M., Mauad T., Saldiva P.H.N., Macchione M. The effects of urban particulate matter on the nasal epithelium by gender: an experimental study in mice. Environ. Pollut. 2016;213:359–369. doi: 10.1016/j.envpol.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 32.Yoshizaki K., Brito J.M., Silva L.F., Lino-dos-Santos-Franco A., Frias D.P., Silva R.C.R., Amato-Lourenço L.F., Saldiva P.H.N., Tibério I. de F.L.C., Mauad T., Macchione M. The effects of particulate matter on inflammation of respiratory system: differences between male and female. Sci. Total Environ. 2017;586:284–295. doi: 10.1016/j.scitotenv.2017.01.221. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y., Zhong C., Kennedy I.M., Pinkerton K.E. Pulmonary responses of acute exposure to ultrafine iron particles in healthy adult rats. Environ. Toxicol. 2003;18:227–235. doi: 10.1002/tox.10119. [DOI] [PubMed] [Google Scholar]
- 34.Larcombe A.N., Phan J.A., Kicic A., Perks K.L., Mead-Hunter R., Mullins B.J. Route of exposure alters inflammation and lung function responses to diesel exhaust. Inhal. Toxicol. 2014;26:409–418. doi: 10.3109/08958378.2014.909910. [DOI] [PubMed] [Google Scholar]
- 35.Costa D.L., Lehmann J.R., Winsett D., Richards J., Ledbetter A.D., Dreher K.L. Comparative pulmonary toxicological assessment of oil combustion particles following inhalation or instillation exposure. Toxicol. Sci. 2006;91:237–246. doi: 10.1093/toxsci/kfj123. [DOI] [PubMed] [Google Scholar]
- 36.Rhoden C.R., Wellenius G.A., Ghelfi E., Lawrence J., González-Flecha B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim. Biophys. Acta Gen. Subj. 2005;1725:305–313. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Akinaga L.M.Y., Lichtenfels A.J., Carvalho-Oliveira R., Caldini E.G., Dolhnikoff M., Silva L.F.F., De Siqueira Bueno H.M., Pereira L.A.A., Saldiva P.H.N., Garcia M.L.B. Effects of chronic exposure to air pollution from sao paulo city on coronary of Swiss mice, from birth to adulthood. Toxicol. Pathol. 2009;37:306–314. doi: 10.1177/0192623309332994. [DOI] [PubMed] [Google Scholar]
- 38.Lopes F.D.T.Q.S., Pinto T.S., Arantes-Costa F.M., Moriya H.T., Biselli P.J.C., Ferraz L.F.S., Lichtenfels A.J., Saldiva P.H., Mauad T., Martins M.A. Exposure to ambient levels of particles emitted by traffic worsens emphysema in mice. Environ. Res. 2009;109:544–551. doi: 10.1016/j.envres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Mohallem S.V., Lobo D.J.D.A., Pesquero C.R., Assunção J.V., De Andre P.A., Saldiva P.H.N., Dolhnikoff M. Decreased fertility in mice exposed to environmental air pollution in the city of Sao Paulo. Environ. Res. 2005;98:196–202. doi: 10.1016/j.envres.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Pereira C.E.L., Heck T.G., Saldiva P.H.N., Rhoden C.R. Ambient particulate air pollution from vehicles promotes lipid peroxidation and inflammatory responses in rat lung. Braz. J. Med. Biol. Res. 2007;40:1353–1359. doi: 10.1590/s0100-879x2006005000164. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Simón D., Muñoz X., Gómez-Ollés S., de Homdedeu M., Untoria M.D., Cruz M.J. Effects of diesel exhaust particle exposure on a murine model of asthma due to soybean. PLoS One. 2017;12:1–14. doi: 10.1371/journal.pone.0179569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goettems-Fiorin P.B., Grochanke B.S., Baldissera F.G., dos Santos A.B., Homem de Bittencourt P.I., Ludwig M.S., Rhoden C.R., Heck T.G. Fine particulate matter potentiates type 2 diabetes development in high-fat diet-treated mice: stress response and extracellular to intracellular HSP70 ratio analysis. J. Physiol. Biochem. 2016;72:643–656. doi: 10.1007/s13105-016-0503-7. [DOI] [PubMed] [Google Scholar]
- 43.Jing Y., Zhang H., Cai Z., Zhao Y., Wu Y., Zheng X., Liu Y., Qin Y., Gu M., Jin J. 2017. Bufei Huoxue Capsule Attenuates PM2.5-Induced Pulmonary Inflammation in Mice, Evidence-Based Complementary and Alternative Medicine; p. 12. (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurai J., Watanabe M., Sano H., Hantan D., Shimizu E. The effect of seasonal variations in airborne particulate matter on asthma-related airway inflammation in mice. Int. J. Environ. Res. Public Health. 2016;13:10. doi: 10.3390/ijerph13060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurai J., Watanabe M., Sano H., Hantan D., Tohda Y., Shimizu E. Effects of asian dust particles on the early-stage antigen-induced immune response of asthma in NC/Nga mice. Int. J. Environ. Res. Public Health. 2016;13:12. doi: 10.3390/ijerph13111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li K., Li L., Cui B., Gai Z., Li Q., Wang S., Yan J., Lin B., Tian L., Liu H., Liu X., Xi Z. Early postnatal exposure to airborne fine particulate matter induces autism-like phenotypes in male rats. Toxicol. Sci. 2017;0:1–11. doi: 10.1093/toxsci/kfx240. [DOI] [PubMed] [Google Scholar]
- 47.Marchini T., Magnani N.D., Paz M.L., Vanasco V., Tasat D., González Maglio D.H., Alvarez S., Evelson P.A. Time course of systemic oxidative stress and inflammatory response induced by an acute exposure to Residual Oil Fly Ash. Toxicol. Appl. Pharmacol. 2014;274:274–282. doi: 10.1016/j.taap.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Martin S., Dawidowski L., Mandalunis P., Cereceda-Balic F., Tasat D.R. Characterization and biological effect of Buenos Aires urban air particles on mice lungs. Environ. Res. 2007;105:340–349. doi: 10.1016/j.envres.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Martin S., Fernandez-Alanis E., Delfosse V., Evelson P., Yakisich J.S., Saldiva P.H., Tasat D.R. Low doses of urban air particles from Buenos Aires promote oxidative stress and apoptosis in mice lungs. Inhal. Toxicol. 2010;22:1064–1071. doi: 10.3109/08958378.2010.523030. [DOI] [PubMed] [Google Scholar]
- 50.Medeiros N., Rivero D.H.R.F., Kasahara D.I., Saiki M., Godleski J.J., Koutrakis P., Capelozzi V.L., Saldiva P.H.N., Antonangelo L. Acute pulmonary and hematological effects of two types of particle surrogates are influenced by their elemental composition. Environ. Res. 2004;95:62–70. doi: 10.1016/j.envres.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Orona N.S., Ferraro S.A., Astort F., Morales C., Brites F., Boero L., Tiscornia G., Maglione G.A., Saldiva P.H.N., Yakisich S., Tasat D.R. Acute exposure to Buenos Aires air particles (UAP-BA) induces local and systemic inflammatory response in middle-aged mice: a time course study. Environ. Pollut. 2016;208:261–270. doi: 10.1016/j.envpol.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 52.Riva D.R., Magalhães C.B., Lopes A.A., Lanças T., Mauad T., Malm O., Valença S.S., Saldiva P.H., Faffe D.S., Zin W.A. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal. Toxicol. 2011;23:257–267. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- 53.Yoshizaki K., Brito J.M., Toledo A.C., Nakagawa N.K., Piccin V.S., Junqueira M.S., Negri E.M., Carvalho A.L.N., de Oliveira A.P.L., de Lima W.T., Saldiva P.H.N., Mauad T., Macchione M. Subchronic effects of nasally instilled diesel exhaust particulates on the nasal and airway epithelia in mice. Inhal. Toxicol. 2010;22:610–617. doi: 10.3109/08958371003621633. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J., Li S., Sun L., Chen Y., Zhang L., Zhang Z. Therapeutic effects of stemonine on particulate matter 2.5-induced chronic obstructive pulmonary disease in mice. Exp. Therapeut. Med. 2017;14:4453–4459. doi: 10.3892/etm.2017.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y., Ji X., Ku T., Sang N. Inflammatory response and endothelial dysfunction in the hearts of mice co-exposed to SO2, NO2, and PM2.5. Environ. Toxicol. 2016;31:1996–2005. doi: 10.1002/tox.22200. [DOI] [PubMed] [Google Scholar]
- 56.Dianat M., Radmanesh E., Badavi M., Goudarzi G., Mard S.A. The effects of PM10 on electrocardiogram parameters, blood pressure and oxidative stress in healthy rats: the protective effects of vanillic acid. Environ. Sci. Pollut. Control Ser. 2016;23:19551–19560. doi: 10.1007/s11356-016-7168-1. [DOI] [PubMed] [Google Scholar]
- 57.Du X., Jiang S., Bo L., Liu J., Zeng X., Xie Y., He Q., Ye X., Song W., Zhao J. Combined effects of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced cardiovascular injury in rats. Chemosphere. 2017;173:14–21. doi: 10.1016/j.chemosphere.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 58.Farina F., Sancini G., Battaglia C., Tinaglia V., Mantecca P., Camatini M., Palestini P. Milano summer particulate matter (PM10) triggers lung inflammation and extra pulmonary adverse events in mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farris B.Y., Antonini J.M., Fedan J.S., Mercer R.R., Roach K.A., Chen B.T., Schwegler-Berry D., Kashon M.L., Barger M.W., Roberts J.R. Pulmonary toxicity following acute coexposures to diesel particulate matter and α-quartz crystalline silica in the Sprague-Dawley rat. Inhal. Toxicol. 2017;29:322–339. doi: 10.1080/08958378.2017.1361487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukui H., Endoh S., Shichiri M., Ishida N., Hagihara Y., Yoshida Y., Iwahashi H., Horie M. The induction of lipid peroxidation during the acute oxidative stress response induced by intratracheal instillation of fine crystalline silica particles in rats. Toxicol. Ind. Health. 2014;32:1430–1437. doi: 10.1177/0748233714564415. [DOI] [PubMed] [Google Scholar]
- 61.Gai H.F., An J.X., Qian X.Y., Wei Y.J., Williams J.P., Gao G.L. Ovarian damages produced by aerosolized fine particulate matter (PM2.5) pollution in mice: possible protective medications and mechanisms. Chin. Med. J. 2017;130:1400–1410. doi: 10.4103/0366-6999.207472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Happo M.S., Hirvonen M.R., Halinen A.I., Jalava P.I., Pennanen A.S., Sillanpaa M., Hillamo R., Salonen R.O. Chemical compositions responsible for inflammation and tissue damage in the mouse lung by coarse and fine particulate samples from contrasting air pollution in Europe. Inhal. Toxicol. 2008;20:1215–1231. doi: 10.1080/08958370802147282. [DOI] [PubMed] [Google Scholar]
- 63.He M., Ichinose T., Yoshida S., Takano H., Nishikawa M., Mori I., Sun G., Shibamoto T. Aggravating effects of Asian sand dust on lung eosinophilia in mice immunized beforehand by ovalbumin. Inhal. Toxicol. 2012;24:751–761. doi: 10.3109/08958378.2012.716870. [DOI] [PubMed] [Google Scholar]
- 64.He M., Ichinose T., Yoshida S., Shiba F., Arashidani K., Takano H., Sun G., Shibamoto T. Differences in allergic inflammatory responses in murine lungs: comparison of PM2.5 and coarse PM collected during the hazy events in a Chinese city. Inhal. Toxicol. 2016;28:706–718. doi: 10.1080/08958378.2016.1260185. [DOI] [PubMed] [Google Scholar]
- 65.Hou T., Liao J., Zhang C., Sun C., Li X., Wang G. Elevated expression of miR-146, miR-139 and miR-340 involved in regulating Th1/Th2 balance with acute exposure of fine particulate matter in mice. Int. Immunopharmacol. 2018;54:68–77. doi: 10.1016/j.intimp.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Huang K.L., Liu S.Y., Chou C.C.K., Lee Y.H., Cheng T.J. The effect of size-segregated ambient particulate matter on Th1/Th2-like immune responses in mice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeong S.-C., Cho Y., Song M.-K., Lee E., Ryu J.-C. Epidermal growth factor receptor (EGFR)-MAPK-nuclear factor(NF)-κB-IL8: a possible mechanism of particulate matter(PM) 2.5-induced lung toxicity. Environ. Toxicol. 2017;32:1628–1636. doi: 10.1002/tox.22390. [DOI] [PubMed] [Google Scholar]
- 68.Kim S., Park H., Park H., Joung B., Kim E. The acute respiratory exposure by intratracheal instillation of Sprague–Dawley rats with diesel particulate matter induces retinal thickening. Cutan. Ocul. Toxicol. 2016;35:275–280. doi: 10.3109/15569527.2015.1104329. [DOI] [PubMed] [Google Scholar]
- 69.Li G., Cao Y., Sun Y., Xu R., Zheng Z., Song H. Ultrafine particles in the airway aggravated experimental lung injury through impairment in Treg function. Biochem. Biophys. Res. Commun. 2016;478:494–500. doi: 10.1016/j.bbrc.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 70.Liu W., Zhang M., Feng J., Fan A., Zhou Y., Xu Y. The influence of quercetin on maternal immunity, oxidative stress, and inflammation in mice with exposure of fine particulate matter during gestation. Int. J. Environ. Res. Public Health. 2017;14 doi: 10.3390/ijerph14060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X., Jin X., Su R., Li Z. The reproductive toxicology of male SD rats after PM2.5 exposure mediated by the stimulation of endoplasmic reticulum stress. Chemosphere. 2017;189:547–555. doi: 10.1016/j.chemosphere.2017.09.082. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y., Wang L., Wang F., Li C. Effect of fine particulate matter (PM2.5) on rat placenta pathology and perinatal outcomes. Med. Sci. Monit. 2016;22:3274–3280. doi: 10.12659/MSM.897808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nemmar A., Karaca T., Beegam S., Yuvaraju P., Yasin J., Hamadi N.K., Ali B.H. Prolonged pulmonary exposure to diesel exhaust particles exacerbates renal oxidative stress, inflammation and DNA damage in mice with adenine-induced chronic renal failure. Cell. Physiol. Biochem. 2016;38:1703–1713. doi: 10.1159/000443109. [DOI] [PubMed] [Google Scholar]
- 74.Ogino K., Takahashi N., Kubo M., Takeuchi A., Nakagiri M., Fujikura Y. Inflammatory airway responses by nasal inoculation of suspended particulate matter in NC/Nga mice. Environ. Toxicol. 2014;29:642–654. doi: 10.1002/tox.21791. [DOI] [PubMed] [Google Scholar]
- 75.Ogino K., Zhang R., Takahashi H., Takemoto K., Kubo M., Murakami I., Wang D.H., Fujikura Y. Allergic airway inflammation by nasal inoculation of particulate matter (PM2.5) in NC/Nga mice. PLoS One. 2014;9:1–9. doi: 10.1371/journal.pone.0092710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pei Y., Jiang R., Zou Y., Wang Y., Zhang S., Wang G., Zhao J., Song W. Effects of fine particulate matter (PM2.5) on systemic oxidative stress and cardiac function in ApoE-/- mice. Int. J. Environ. Res. Public Health. 2016;13 doi: 10.3390/ijerph13050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Privalova L.I., Katsnelson B.A., Varaksin A.N., Panov V.G., Balesin S.L. The pulmonary phagocytosis response to separate and combined impacts of manganese (IV) and chromium (VI) containing particulates. Toxicology. 2016;370:78–85. doi: 10.1016/j.tox.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Sancini G., Farina F., Battaglia C., Cifola I., Mangano E., Mantecca P., Camatini M., Palestini P. Health risk assessment for air pollutants: alterations in lung and cardiac gene expression in mice exposed to milano winter fine particulate matter (PM2.5) PLoS One. 2014;9:e109685. doi: 10.1371/journal.pone.0109685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su R., Jin X., Zhang W., Li Z., Liu X., Ren J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere. 2017;167:444–453. doi: 10.1016/j.chemosphere.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 80.Tabor C.M., Shaw C.A., Robertson S., Miller M.R., Duffin R., Donaldson K., Newby D.E., Hadoke P.W.F. Platelet activation independent of pulmonary inflammation contributes to diesel exhaust particulate-induced promotion of arterial thrombosis. Part. Fibre Toxicol. 2016;13:1–14. doi: 10.1186/s12989-016-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tzamkiozis T., Stoeger T., Cheung K., Ntziachristos L., Sioutas C., Samaras Z. Monitoring the inflammatory potential of exhaust particles from passenger cars in mice. Inhal. Toxicol. 2010;22(Suppl 2):59–69. doi: 10.3109/08958378.2010.519408. [DOI] [PubMed] [Google Scholar]
- 82.Wang X., Jiang S., Liu Y., Du X., Zhang W., Zhang J., Shen H. Comprehensive pulmonary metabolome responses to intratracheal instillation of airborne fine particulate matter in rats. Sci. Total Environ. 2017;592:41–50. doi: 10.1016/j.scitotenv.2017.03.064. [DOI] [PubMed] [Google Scholar]
- 83.Wei Y., Cao X.-N., Tang X.-L., Shen L.-J., Lin T., He D.-W., Wu S.-D., Wei G.-H. Urban fine particulate matter (PM2.5) exposure destroys blood–testis barrier (BTB) integrity through excessive ROS-mediated autophagy. Toxicol. Mech. Methods. 2017;27:1–18. doi: 10.1080/15376516.2017.1410743. [DOI] [PubMed] [Google Scholar]
- 84.Xu X., Qimuge A., Wang H., Xing C., Gu Y., Liu S., Xu H., Hu M., Song L. IRE1α/XBP1s branch of UPR links HIF1α activation to mediate ANGII-dependent endothelial dysfunction under particulate matter (PM) 2.5 exposure. Sci. Rep. 2017;7:1–16. doi: 10.1038/s41598-017-13156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y., Hu H., Shi Y., Yang X., Cao L., Wu J., Asweto C.O., Feng L., Duan J., Sun Z. 1H NMR-based metabolomics study on repeat dose toxicity of fine particulate matter in rats after intratracheal instillation. Sci. Total Environ. 2017;589:212–221. doi: 10.1016/j.scitotenv.2017.02.149. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y., Lin Y., Li X., Zhang L., Pan W., Zhu H., Xi Z., Yang D. Silica dioxide nanoparticles combined with cold exposure induce stronger systemic inflammatory response. Environ. Sci. Pollut. Control Ser. 2017;24:291–298. doi: 10.1007/s11356-016-7649-2. [DOI] [PubMed] [Google Scholar]