Key Points
Question
What is the incidence, type, severity, and preventability of adverse events in long-term care residents transitioning from hospital back to long-term care?
Findings
This prospective cohort study of 555 long-term care residents contributing 762 discharges found that adverse events developed 37.3% of the time. Of these events, 70.4% were preventable or ameliorable, with skin tears, pressure ulcers, and falls being the most common events.
Meaning
Transition back to long-term care is a high-risk period; standardized reporting of events and better coordination and information transfer across settings are potential ways to prevent adverse events in this vulnerable population.
This cohort study evaluates records of 555 residents of 32 New England nursing homes who returned to their nursing homes after hospitalization to measure the frequency, severity, and preventability of adverse events in the 45 days after hospitalization.
Abstract
Importance
Transition from hospital to nursing home is a high-risk period for adverse events in long-term care (LTC) residents. Adverse events include harms from medical care, including failure to provide appropriate care.
Objective
To report the incidence, type, severity, and preventability of adverse events in LTC residents transitioning from hospital back to the same LTC facility.
Design, Setting, and Participants
Prospective cohort study of LTC residents discharged from hospital back to LTC from March 1, 2016, to December 31, 2017, and followed up for 45 days. In a random sample of 32 nursing homes located in 6 New England states, 555 LTC residents were selected, contributing 762 transitions from hospital back to the same LTC facility.
Main Outcomes and Measures
The main outcome was an adverse event within the 45-day period after transition from hospital back to nursing home. Trained nurse abstractors reviewed nursing home records for the period, and then 2 physicians independently reviewed each potential adverse event to determine whether harm occurred and to characterize the type, severity, and preventability of each event. When reviewers disagreed, they met to reach consensus.
Results
Of the 555 individual residents, 365 (65.6%) were female, and the mean (SD) age at the time of discharge was 82.2 (11.5) years. Five hundred twenty (93.7%) were non-Hispanic white, 21 (3.8%) were non-Hispanic black, 9 (1.6%) were Hispanic, and 5 (0.9%) were of other non-Hispanic race/ethnicity. In the cohort, there were 379 adverse events among 762 discharges. One hundred ninety-seven events (52.0%) related to resident care, with pressure ulcers, skin tears, and falls with injury representing the most common types of events in this category. Health care–acquired infections (108 [28.5%]) and adverse drug events (64 [16.9%]) were the next most common. One hundred ninety-eight (52.2%) adverse events were characterized as less serious. However, 145 (38.3%) events were deemed serious, 28 (7.4%) life-threatening, and 8 (2.1%) fatal. In terms of preventability, 267 (70.4%) adverse events were found to be preventable or ameliorable, with less serious events more often considered preventable or ameliorable (146 [73.7%]) compared with more severe events (121 [66.9%]). In addition, resident care–related adverse events such as fall with injury, skin tear, and pressure ulcer were more commonly deemed preventable (173 of 197 [87.8%]) compared with adverse drug events (39 of 64 [60.9%]) or health care–acquired infections (49 of 108 [45.4%]).
Conclusions and Relevance
Adverse events developed in nearly 4 of 10 of discharges from hospital back to LTC. Most were preventable or ameliorable. Standardized reporting of events and better coordination and information transfer across settings are potential ways to prevent adverse events in LTC residents.
Introduction
Adverse events pose serious risk to persons older than 65 years (older adults) in the immediate posthospitalization period. An adverse event is a harm or injury resulting from medical care, including the failure to provide needed care.1 Given their advanced age and coexistent disability (cognitive, physical, or both), residents of long-term care (LTC) facilities are especially at risk for adverse events after hospital discharge.2 While hospitalized, residents often suffer malnutrition through illness and purposeful fasting in preparation for surgery or other procedures. Change in familiar surroundings, disruption of sleep, and receipt of central nervous system–acting agents can lead to delirium. Lack of activity and walking leads to deconditioning. In addition, medication is changed in up to 40% of residents.3 The combination of these factors may increase the risk for adverse events, including falls with injury, pressure ulcers, health care–acquired infections, and adverse drug events, during the posthospitalization period.
Although the risk of adverse events may be substantial in this population, there are limited data on the epidemiologic characteristics of these events in LTC residents transitioning from hospital back to nursing home. A recent report from the Office of Inspector General (OIG) found that 40% of community-dwelling older adults newly admitted to a skilled nursing facility for rehabilitation experienced an adverse event.4 Medication-related events and events related to resident care (eg, falls, exacerbations of preexisting disease, fluid and electrolyte management problems) topped the list of most common adverse events occurring in this population. Almost 60% were deemed preventable by physician reviewers. To our knowledge, no one has measured the frequency, etiology, and preventability of adverse events among hospitalized LTC residents transitioning back to the nursing home.
The purpose of our study was to measure the frequency, severity, and preventability of adverse events occurring during the 45 days after hospitalization for residents of LTC facilities. A better understanding of the frequency, severity, and preventability of these events may help inform future quality and safety efforts for this highly vulnerable population.
Methods
Study Setting
Our study included 32 nursing homes selected from a pool of 762 facilities participating in the New England Nursing Home Quality Care Collaborative.5 To reach our ultimate study sample of facilities, we first excluded homes deemed too far (greater than a 45-mile radius) in distance from the home offices of Qualidigm, the quality improvement organization partnering in this study. Of the remaining 676 nursing homes, we randomly selected 30 for recruitment within strata defined according to state, bed capacity, and Centers for Medicare & Medicaid Service (CMS) Nursing Home Compare Five-Star Quality Rating. When an invited nursing home declined to participate, we randomly selected another nursing home from the same stratum. Through this process we approached 54 nursing homes to arrive at an initial 30 facilities that agreed to participate. After two 3-month segments of data collection, 1 nursing home closed. We retained those quarters for analysis and randomly selected 2 additional homes from the same stratum. Both homes agreed to participate, and each provided a full year of data for the study. Table 1 presents summary descriptive data on the 32 homes that contributed data.6,7,8 In the table, we compare participating facilities with nursing homes across New England and the United States. The University of Massachusetts Medical School institutional review board approved the study and waived informed consent.
Table 1. Comparison of Study Nursing Homes With Nursing Homes in New England Collaborative, New England Region, and United States.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Study Nursing Homes (n = 32) | All New England Nursing Homes in Collaborative (n = 762) | All New England Nursing Homes (n = 941) | All US Nursing Homes (n = 15 640) | |
| Bed capacity, bedsa | ||||
| Mean (SD) | 130 (71.8) | 108 (50.6) | 107 (53.0) | 106 (60.8)b |
| <50 | 0 | 83 (10.9) | 125 (13.3) | 2022 (12.9) |
| 50-99 | 11 (34.4) | 243 (31.9) | 297 (31.6) | 5773 (36.9) |
| 100-199 | 17 (53.1) | 404 (53.0) | 474 (50.4) | 6899 (44.1) |
| ≥200 | 4 (12.5) | 32 (4.2) | 45 (4.8) | 946 (6.0) |
| Type of ownershipa | ||||
| For profit | 24 (75.0) | 552 (72.4) | 674 (71.6) | 10 913 (69.8) |
| Nonprofit | 7 (21.9) | 198 (26.0) | 248 (26.4) | 3756 (24.0) |
| Government | 1 (3.1) | 12 (1.6) | 19 (2.0) | 971 (6.2) |
| Nursing Home Compare Five-Star Quality Ratingc | ||||
| 1 | 1 (3.1) | 58 (7.6) | 72 (7.7) | 1629 (10.5)d |
| 2 | 7 (21.9) | 155 (20.3) | 182 (19.3) | 3226 (20.8) |
| 3 | 6 (18.8) | 137 (18.0) | 167 (17.8) | 2821 (18.2) |
| 4 | 10 (31.3) | 195 (25.6) | 243 (25.8) | 4105 (26.5) |
| 5 | 8 (25.0) | 217 (28.5) | 276 (29.3) | 3731 (24.1) |
| Missing | 0 | 0 | 1 (0.1) | NA |
| Statea | ||||
| Connecticut | 9 (28.1) | 178 (23.4) | 228 (24.2) | 229 (1.5) |
| Maine | 3 (9.4) | 91 (11.9) | 103 (11.0) | 103 (0.7) |
| Massachusetts | 11 (34.4) | 328 (43.0) | 413 (43.9) | 415 (2.7) |
| New Hampshire | 4 (12.5) | 68 (8.9) | 76 (8.1) | 76 (0.5) |
| Rhode Island | 4 (12.5) | 63 (8.3) | 84 (8.9) | 84 (0.5) |
| Vermont | 1 (3.1) | 34 (4.5) | 37 (3.9) | 37 (0.2) |
Abbreviation: NA, not applicable.
We obtained national data on bed capacity, profit status, and states from the Centers for Medicare & Medicaid Services Nursing Home Data Compendium 2015 Edition.6
We obtained national data on mean and standard deviation of bed size from the Centers for Medicare & Medicaid Services Nursing Home Compare data.7
We obtained national data on star ratings from Nursing Home Compare Five-Star Quality Rating System: Year Five Report.8
The denominator for this distribution was 15 512.
Study Participants
From these nursing homes, we identified all LTC residents who were hospitalized and discharged back to the same facility from March 1, 2016, to December 31, 2017, and followed up for 45 days. Long-term care residents are defined as individuals residing in a nursing home for 100 or more days. We followed each of these residents for up to 45 days after return to the nursing home. Previous work has established a suggested review period of 30 to 45 days.4,9,10 To ensure capture of adverse events that may have occurred within 30 days but were not noted until several days later and to identify cascade events, we selected a 45-day review period. We permitted a long-term care resident to contribute information relating to more than 1 hospitalization, counting each hospitalization and discharge back to the facility as an episode of care.
Case-Finding Approach
We used a 2-stage review process to identify events. The initial stage was a retrospective review of nursing home records in 3-month segments performed by trained nurse abstractors who searched for possible adverse events using a trigger tool methodology adapted from tools developed by OIG and the Institute for Healthcare Improvement.4,11,12 For each trigger (eg, abnormal laboratory result, phone call to family) that could be linked to a suggestion of possible harm to a resident, abstractors completed a detailed harm event form for later review and adjudication by pairs of physician reviewers (A.K., S.H., K.F., and J.H.G.) as described below.
Seven nurses completed chart abstractions for the study. Each participated in extensive training including an all-day, in-person session and 2 webinar-based sessions. After familiarization with the trigger tool, nurse abstractors completed 3 practice nursing home records which were compared with abstraction by an experienced, criterion standard, nurse reviewer (A.S.). Nurse abstractors then talked through any discrepancies and clarified any questions they had. Throughout the course of data collection, abstractors attended periodic webinars in which physician reviewers responded to questions about exemplar cases and provided feedback. To minimize the chance of missing a potential harm, we instructed nurse abstractors to report all potential harms to residents. Physician reviewers made the final decision about the presence of an adverse event using the approach detailed below.
Outcome Measures
Each possible harm reported by nurse abstractors was evaluated by pairs of physician-reviewers (A.K., S.H., K.F., and J.H.G.) who independently classified incidents according to a review process that has been used in previous studies of adverse events across several clinical settings.13,14,15,16,17 Nurse abstractors reported possible harm events, providing detailed text summaries of each case. Reviewers judged each reported incident for the presence of an adverse event and then further characterized these events as described below employing structured implicit review. Previous researchers have suggested that “implicit review reflects peer judgment and as such has intrinsic validity.”18 Because it relies on expert judgment rather than preset standards, it automatically reflects advances in standard of care. To provide some additional guidance for physicians to conduct reviews, we created a manual adapted from the 2014 OIG report4 and examples in earlier literature.10,13,14
Presence, Type, and Severity of Adverse Event
Reviewers determined if an event identified by a nurse abstractor represented an adverse event using the definition that comes from the Harvard Medical Practice Study1 which has been adopted by the Agency for Healthcare Research and Quality (AHRQ).19 For those that were deemed an adverse event, reviewers then assigned them to 1 of 4 categories: health care acquired infections; events related to resident care, events related to medication, and events related to procedures. Reviewers also categorized the severity of events as less than serious, serious, life-threatening, or fatal.10,13,14 Examples of less than serious events included rashes and skin tears. Examples of serious events included pressure ulcers, health care acquired pneumonia, and gastrointestinal bleeding. Life-threatening events included sepsis and opioid overdose (eTable in the Supplement).
Preventability and Ameliorability of Adverse Events
Reviewers judged the preventability and ameliorability of events if the events were preventable by any currently available means following previous examples in the patient safety literature.10,13 Specifically, they categorized preventability into definitely preventable, probably preventable, probably not preventable, or definitely not preventable. We then collapsed these assessments into categories of preventable (definitely preventable and probably preventable) and nonpreventable (probably not preventable and definitely not preventable). Reviewers could also characterize an event as ameliorable if it could not have been prevented, but the harm could have been moderated or alleviated. Reviewers also made determinations about whether discharge occurred prematurely based on their clinical experience.
Present on Admission
Reviewers determined whether events were present on admission (ie, on return to the nursing home) or occurred after return to the nursing home. We included these events in our total count of adverse events.
Physician Consensus
When physician reviewers disagreed on the presence, preventability, or severity of an adverse event, they met to reach consensus. In all cases, reviewers achieved consensus. To examine the level of agreement based on the initial assessment, we calculated a kappa statistic (κ). A κ between 0.60 and 0.80 reflects substantial agreement, and a score between 0.41 and 0.60 represents moderate agreement.20 In the current study, we found that the κ for presence of an adverse event was 0.61 (95% CI, 0.55-0.66), the κ for severity of the event was 0.42 (95% CI, 0.35-0.49), and the κ for preventability was 0.35 (95% CI, 0.26-0.45).
Statistical Analysis
To assess representativeness of our population, we compared the 32 sampled facilities to all facilities in the New England Nursing Home Quality Care Collaborative, to all New England facilities, and to all United States facilities with respect to bed size, ownership, quality rating, and state using χ2 tests. To calculate frequency of adverse events, we divided the number of events by the total number of discharges permitting individual patients to contribute multiple discharges. Because some residents did not remain in the LTC facility for observation through the entire 45-day follow-up period (such as in the case of transfer back to the hospital or death), we also calculated separate incidence rates for all adverse events and for preventable adverse events. Specifically, we calculated the incidence rate as the number of all adverse events and number of preventable adverse events, divided by the number of days we observed residents. For each of these estimates, we calculated 95% CIs. We considered 2-sided P < .05 significant. We performed all analyses using SAS statistical software, version 9.4 (SAS Institute Inc).
Results
We identified 762 hospital discharges for 555 individual LTC residents. Of the 555 individual residents, 365 (65.6%) were female, and the mean (SD) age at the time of discharge was 82.2 (11.5) years. Five hundred twenty (93.7%) were non-Hispanic white, 21 (3.8%) were non-Hispanic black, 9 (1.6%) were Hispanic, and 5 (0.9%) were of other non-Hispanic race/ethnicity. Of the 555 residents, 417 contributed 1 discharge, 101 contributed 2 discharges, and 37 contributed more than 2 discharges. The length of stay in the preceding hospitalization was 6 or fewer days for 383 (69.0%) patients. Four hundred forty-four patients (80.0%) had a Charlson Comorbidity score of 2 or greater and 402 (72.4%) patients were taking 10 or more medications (which appears higher than the general LTC resident, who takes approximately 7 to 9 medications (Table 2).21 Compared with other New England nursing homes, the 32 study facilities were more likely to have greater mean bed capacities (130 vs 107 beds; P = .02). In terms of type of ownership and Nursing Home Compare Five-Star Quality Rating, study facilities were similar to New England nursing homes, which were similar to all US nursing homes (Table 1).
Table 2. Characteristics of Long-term Care Residents Transitioning From Hospital Back to Nursing Home.
| Characteristic | Frequency, No. (%) (n = 762)a |
|---|---|
| Age group, y | |
| <64 | 78 (10.2) |
| 65-74 | 129 (16.9) |
| 75-84 | 200 (26.3) |
| 85-94 | 285 (37.4) |
| ≥95 | 70 (9.2) |
| Race/ethnicity | |
| Non-Hispanic white | 703 (92.3) |
| Non-Hispanic black | 36 (4.7) |
| Non-Hispanic other | 10 (1.3) |
| Hispanic | 13 (1.7) |
| Sex | |
| Male | 498 (65.4) |
| BMI range | |
| <18.5 | 35 (4.7) |
| 18.5-24.9 | 216 (29.2) |
| 25-29.9 | 240 (32.4) |
| 30-34.9 | 124 (16.7) |
| 35-39.9 | 48 (6.5) |
| ≥40 | 78 (10.5) |
| Length of stay for preceding hospitalization, d | |
| 0-2 | 130 (17.1) |
| 3-4 | 236 (31.0) |
| 5-6 | 160 (21.0) |
| 7-8 | 93 (12.2) |
| ≥9 | 143 (18.8) |
| Charlson Comorbidity score | |
| 0-1 | 142 (18.7) |
| 2-3 | 298 (39.2) |
| 4-5 | 196 (25.8) |
| ≥6 | 125 (16.4) |
| No. of medicationsb | |
| 0-9 | 173 (23.0) |
| 10-13 | 197 (26.2) |
| 14-17 | 182 (24.2) |
| ≥18 | 201 (26.7) |
| Fallsc | 146 (19.3) |
| Pressure ulcersd | 42 (5.6) |
| Comorbid conditionse | |
| History of myocardial infarction | 146 (19.2) |
| Heart failure | 379 (49.8) |
| Peripheral vascular disease | 128 (16.8) |
| Cardiovascular disease | 186 (24.4) |
| Dementia | 445 (58.5) |
| Chronic obstructive pulmonary disease | 268 (35.2) |
| Connective tissue disease | 34 (4.5) |
| Peptic ulcer disease | 38 (5.0) |
| Hemiplegia | 74 (9.7) |
| Renal disease | 73 (9.6) |
| Cancer | |
| Metastatic | 13 (1.7) |
| Nonmetastatic | 67 (8.8) |
| Diabetes | |
| With end-organ damage | 118 (15.5) |
| Mild to moderate | 184 (24.1) |
| Liver disease | |
| Moderate or severe | 14 (1.8) |
| Mild | 22 (2.9) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Table based on 762 discharges; some of the 555 unique residents are reported multiple times.
Medications refers to the number of regularly scheduled medications.
Falls refers to any reported falls on the most recent Minimum Data Set Falls report (falls since admission/entry or reentry or previous assessment) before the resident’s hospitalization.
Pressure ulcers refers to presence of any pressure ulcers reported on the most recent Minimum Data Set Ulcers report before the resident’s hospitalization.
Based on any positive documentation in chart by a provider within 2 years of the date of resident’s return to the nursing home.
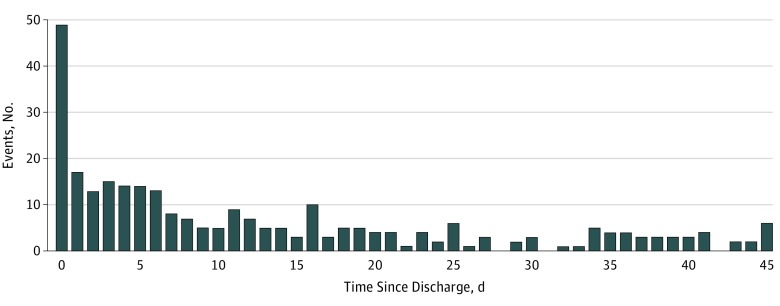
Nurse abstractors identified 716 possible adverse events occurring during the 45 days after hospital discharge for the 555 LTC residents. Physician reviewers judged 379 of the 716 possible events (52.9%) to be adverse events. One hundred forty-six (52.3%) of the first events occurred within the first 7 days after discharge. Another 45 (16.0%) occurred during the following week (Figure). Among the 379 adverse events, 283 (75.7%) represented first events during the 45-day span, with 77 (20.3%) representing second or third events; 19 (5.0%) were either fourth or fifth events. The incidence rate of adverse events was 13.6 (95% CI, 12.2-15.0) per 1000 resident days with an incidence rate of preventable adverse events of 9.5 (95% CI, 8.4-10.8) per 1000 resident days. Of the 762 discharges, 479 (62.8%) were associated with no event, 210 (27.6%) with 1 event, 57 (7.5%) with 2 events, 10 (1.3%) with 3 events, 5 (0.7%) with 4 events, and 1 (0.1%) with 5 events.
Figure. Frequency of Adverse Events by the Number of Days Elapsed Following Hospital Discharge.
Most of the 379 adverse events were related to resident care (197 [52.0%]), with pressure ulcers, skin tears, and falls with injury representing the most common types in this category. Health care–acquired infections (108 [28.5%]) and events related to medication (64 [16.9%]) were the next most common categories of adverse events. Respiratory infections and delirium were the most frequent type of events in these respective categories. Of the 379 events, 10 (2.6%) were related to procedures (Table 3).
Table 3. Frequency of Events Grouped by Type of Event.
| Event | Overall (n = 379) | Preventable/Ameliorable (n = 267) |
|---|---|---|
| Health care–acquired infections, frequency (%) | 108 (28.5) | 49 (18.4) |
| Catheter-associated urinary tract infection | 22 | 5 |
| Respiratory infection | 45 | 13 |
| Clostridium difficile infection | 14 | 3 |
| Surgical/procedural site infection | 8 | 7 |
| Blood stream infection/sepsis | 5 | 3 |
| Other | 14 | 11 |
| Event related to resident care, frequency (%) | 197 (52.0) | 173 (64.8) |
| Fall with injury | 38 | 34 |
| Skin tear, abrasion, or breakdown | 40 | 37 |
| Pressure ulcer | 56 | 53 |
| Cardiogenic volume overload | 8 | 3 |
| Respiratory distress/failure | 7 | 4 |
| Dehydration/AKI | 5 | 4 |
| Confusion/delirium | 8 | 7 |
| Delay in diagnosis or treatment | 8 | 8 |
| Other (eg, bruise, bowel obstruction, UTI, VTE, incontinence, resident self-harm) | 27 | 23 |
| Event related to medication, frequency (%)a | 64 (16.9) | 39 (14.6) |
| Allergic reaction | 7 | 1 |
| Delirium or other change in mental status | 8 | 7 |
| Gastrointestinal | 10 | 7 |
| Hemorrhagic | 8 | 2 |
| Infection | 10 | 2 |
| Other (eg, AKI, hematologic, hypoglycemic events, hypotension) | 29 | 22 |
| Event related to procedures, frequency (%) | 10 (2.6) | 6 (2.2) |
Abbreviations: AKI, acute kidney injury; UTI, urinary tract infection; VTE, venous thromboembolism.
Physician reviewers may have assigned more than 1 type of event related to medication; therefore, the categories were not mutually exclusive.
With regard to severity, 198 of the 379 adverse events (52.2%) were deemed less than serious, followed by 145 (38.3%) that were serious, 28 (7.4%) that were life-threatening, and 8 (2.1%) that were fatal. The duration of harm was greater than 1 day for 294 adverse events (77.6%). Fourteen events (3.7%) resulted in permanent disability (Table 4).
Table 4. Characteristics of Adverse Events and the Subpopulation That Are Preventable and Ameliorable in the 45-Day Period After Hospitalization.
| Characteristic | No. (%) | |
|---|---|---|
| Overall (n = 379) | Preventable/ Ameliorable (n = 267) |
|
| Category of severity | ||
| Less serious | 198 (52.2) | 146 (54.7) |
| Serious event | 145 (38.3) | 95 (35.6) |
| Life-threatening event | 28 (7.4) | 19 (7.1) |
| Fatal event | 8 (2.1) | 7 (2.6) |
| Symptoms and outcome of adverse event | ||
| No symptoms, but laboratory abnormality requiring change in therapy | 10 (2.6) | 6 (2.2) |
| Symptoms ≤1 d | 37 (9.8) | 29 (10.9) |
| Symptoms >1 d | 294 (77.6) | 201 (75.3) |
| Nonpermanent disability | 24 (6.3) | 18 (6.7) |
| Permanent disability | 14 (3.7) | 13 (4.9) |
The reviewers judged 66 of the 379 adverse events (17.4%) as present on admission. Of these, 56 (84.8%) were preventable. Of the 56 events, 9 (16.1%) were health care–acquired infections, 39 (69.6%) were related to resident care (mostly pressure ulcers and skin tears), 2 (3.6%) were medication related, and 6 (10.7%) were other types of events. In terms of relative timing and assignment of present on admission for individual adverse event types, 53 (55.2%) of resident care–related items including skin tears and pressure ulcers occurred within the first week of return to the LTC facility, including 35 (36.5%) that were noticed on the day of return. In almost every case, we deemed these events present on admission. By contrast, although 45 (43.7%) of infections occurred within the first week of return to the LTC facility, 14 (13.6%) occurred on the day of return, indicating that these were much less likely to be present on admission.
Of the 379 total adverse events, 267 (70.4%) adverse events were found to be preventable or ameliorable, with less serious events more often considered preventable or ameliorable (146 [73.7%]) compared with more severe events (121 [66.9%]). In addition, resident care–related adverse events such as fall with injury, skin tear, and pressure ulcer were more commonly deemed preventable (173 of 197 [87.8%]) compared with adverse drug events (39 of 64 [60.9%]) or health care–acquired infections (49 of 108 [45.4%]).
Discussion
We determined that adverse events occurred in 37.3% of discharges of long-term care residents from the hospital back to the LTC facility. More than half of these occurred within the first 7 days after return to LTC. Nearly half of events were serious, life-threatening, or fatal. A full 70.4% of these events were preventable or ameliorable.
In 2014, the OIG published a report of Medicare beneficiaries admitted to a hospital from the community and then discharged to skilled nursing facilities for continued treatment and rehabilitation (ie, a short-stay population, in contrast to the LTC population in the present study).4 The OIG reported fewer cases of resident care–related adverse events and more cases of medication-related adverse events than we did. Given that patients with short stays typically do not have the level of dependency in activities of daily living and mobility impairment that LTC residents do, one would expect them to experience fewer cases of pressure ulcers, skin tears, and falls. The fact that they are new to the nursing home perhaps explains the higher rate of medication-related events. Compared with the findings in the OIG report, we also found that a greater percentage of more severe adverse events in our study were preventable (66.9% vs 59%), highlighting the need for greater attention to patient safety in LTC residents being discharged from the hospital.
Data from CMS on its website Nursing Home Compare provide some information that we can use to compare the magnitude of the problem of adverse events in the general LTC population with the LTC population returning after hospitalization.22 More specifically, 4% of LTC residents experienced a fall with injury during 1 calendar quarter (or 0.4 events across 1000 resident days, which is less than the 1.4 rate we found in patients transitioning back to a nursing home after hospitalization). In terms of urinary tract infections, CMS found that 2.9% of these occurred across 30 days (equivalent to 1.0 events per 1000 resident days, which is similar to the 0.8 catheter-associated urinary tract infections we found, although catheter-associated events would be a subset of all urinary tract infections). Differences in age and illness burden among the general LTC resident population and transitioning LTC population limit the ability to draw firm conclusions and suggest the need for further study.
Physician reviewers also classified several events as present on admission. Proportionally more of these events were resident care–related items, compared with other adverse events such as respiratory distress or fluid imbalance. We anticipate that the discrepancy occurred because hospitals typically do not discharge patients until resolution of the latter conditions, but we could not find other studies (including the OIG report), that documented present on admission status to corroborate this statement.
There are several other implications to our findings. There are a substantial number of preventable or ameliorable adverse events occurring each year in the LTC population. By the most recent statistics available, approximately 25% of the 1.4 million LTC residents in the United States are admitted to a hospital each year.23,24 Most of these 350 000 residents will return to LTC, leading to more than 100 000 adverse events, most of which will be preventable based on our estimates. This magnitude of preventable harm requires that more be done to monitor LTC residents closely as they transition from hospital back to LTC. Falls, skin tears, and pressure ulcers were the most common harms, most of which were preventable and attributable to inadequate monitoring or substandard care. The OIG, in their report, also recommended more global measures such as raising awareness of patient safety and requiring nursing homes to report adverse events to patient safety organizations in a manner similar to that required of hospitals.4 Prominent policy analysts have recently suggested separating patient safety–based outcomes including fall and pressure ulcer prevention from the overall clinical quality measure that forms the quality component of the CMS 5-star rating.25 This differentiation would be an additional way to encourage facilities to address these issues preemptively.
We found that nearly one-fourth of events occurred on the day of discharge or the day after, suggesting that the resident was prematurely discharged. There is concern that the hectic hospital environment with focus on decreasing length of stay could increase the risk of adverse events. In addition, posthospitalization syndrome, “a pathophysiologic syndrome of weakness and increased stress” may leave patients more vulnerable to adverse events.26 Development of strategies to better assess readiness for discharge may alleviate some of the transition-related adverse events. Engaging hospitals and their medical staff to better understand the capabilities and challenges confronting LTC facilities would also be important and has been advocated in recent scientific studies.27 The Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) requires that nursing homes and all other postacute care facilities submit standardized data with regard to quality domains including skin integrity, functional status and cognitive function, medication reconciliation, incidence of major falls, and transfer of health information and care preferences when an individual transitions.28 Currently, the IMPACT Act only mandates data collection for the receiving postacute care facilities. To improve care coordination and enhance discharge planning, hospitals also need to collect data and transmit those data in standardized fashion. The IMPACT Act supports the CMS initiative Meaningful Measures, which introduces several priority areas to improve quality in postacute care. The most relevant of these for ensuring safety during transition from hospital to LTC include promoting effective communication and coordination of care; making care safer by reducing harm; and strengthening of person and family engagement as partners in patient care.
Limitations
We acknowledge certain limitations to our study. There is inherently a level of subjectivity in the process of classifying adverse events. Nevertheless, the process of dual physician review of potential adverse events follows a standard protocol exemplified in several prominent examples in the literature.10,15,29 We chose the trigger tool methodology to capture adverse events efficiently and accurately, but recognize that this retrospective review of charts will not capture all events. Researchers and quality experts have demonstrated the value of the trigger tool methodology compared with other surveillance tools in mitigating accuracy concerns.4,30,31,32 We also did not sample from facilities across the country, but did capture a wide swath of nursing homes in the New England region, which were similar to nursing homes in the rest of the country with respect to size, 5-star CMS quality rating, and for-profit status. In addition, although nurses performing medical record review reviewed the discharge summary preceding readmission to LTC facility, they did not have access to the entire hospital record, limiting the ability of physician reviewers to determine preventability of some events. We also did not analyze the association of patient characteristics or personal care guidelines with adverse events. We plan to measure these associations in future work.
Conclusions
Adverse events developed in nearly 4 of 10 of discharges from hospital back to LTC. The majority were preventable or ameliorable. Resident care–related adverse events, including falls with injury, pressure ulcers, and skin tears, were the most frequent types of adverse events. Standardized reporting of events and better coordination and information transfer across settings are potential ways to prevent adverse events in returning LTC residents.
eTable. Selected Examples With Descriptions of Less Serious, Serious, Life-Threatening and Fatal Events
References
- 1.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi: 10.1056/NEJM199102073240604 [DOI] [PubMed] [Google Scholar]
- 2.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. doi: 10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beers MH, Dang J, Hasegawa J, Tamai IY. Influence of hospitalization on drug therapy in the elderly. J Am Geriatr Soc. 1989;37(8):679-683. doi: 10.1111/j.1532-5415.1989.tb02227.x [DOI] [PubMed] [Google Scholar]
- 4.Levinson DR. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Washington, DC: Office of Inspector General, Dept of Health & Human Services; 2014. [Google Scholar]
- 5.New England Quality Innovation Network–Quality Improvement Organization Nursing home quality. https://healthcarefornewengland.org/initiatives/nhquality/. Accessed December 22, 2018.
- 6.Centers for Medicare and Medicaid Services Nursing Home Data Compendium 2015 Edition https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/nursinghomedatacompendium_508-2015.pdf. Accessed May 21, 2018.
- 7.Centers for Medicare and Medicaid Services Provider info. https://data.medicare.gov/Nursing-Home-Compare/Provider-Info/4pq5-n9py/data. Accessed May 22, 2018.
- 8.Centers for Medicare and Medicaid Services Nursing Home Compare Five-Star Quality Rating System: year five report. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/NHC-Year-Five-Report.pdf. Published June 16, 2014. Accessed April 25, 2015.
- 9.Donovan JL, Kanaan AO, Gurwitz JH, et al. A pilot health information technology-based effort to increase the quality of transitions from skilled nursing facility to home: compelling evidence of high rate of adverse outcomes. J Am Med Dir Assoc. 2016;17(4):312-317. doi: 10.1016/j.jamda.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 10.Kanaan AO, Donovan JL, Duchin NP, et al. Adverse drug events after hospital discharge in older adults: types, severity, and involvement of Beers Criteria Medications. J Am Geriatr Soc. 2013;61(11):1894-1899. doi: 10.1111/jgs.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trigger tool for measuring adverse drug events in the nursing home. Institute for Healthcare Improvement. http://www.ihi.org/resources/Pages/Tools/TriggerToolforMeasuringAdverseDrugEvents.aspx. Accessed April 1, 2015.
- 12.Adler LMJ, Federico F. IHI Skilled Nursing Facility Trigger Tool for Measuring Adverse Events. Cambridge, MA: Institute for Healthcare Improvement; 2015. [Google Scholar]
- 13.Bates DW, Cullen DJ, Laird N, et al. ; ADE Prevention Study Group . Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34. doi: 10.1001/jama.1995.03530010043033 [DOI] [PubMed] [Google Scholar]
- 14.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109(2):87-94. doi: 10.1016/S0002-9343(00)00451-4 [DOI] [PubMed] [Google Scholar]
- 15.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107-1116. doi: 10.1001/jama.289.9.1107 [DOI] [PubMed] [Google Scholar]
- 16.Leape LL, Bates DW, Cullen DJ, et al. ; ADE Prevention Study Group . Systems analysis of adverse drug events. JAMA. 1995;274(1):35-43. doi: 10.1001/jama.1995.03530010049034 [DOI] [PubMed] [Google Scholar]
- 17.Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282(3):267-270. doi: 10.1001/jama.282.3.267 [DOI] [PubMed] [Google Scholar]
- 18.Pearson ML, Lee JL, Chang BL, Elliott M, Kahn KL, Rubenstein LV. Structured implicit review: a new method for monitoring nursing care quality. Med Care. 2000;38(11):1074-1091. doi: 10.1097/00005650-200011000-00003 [DOI] [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality Adverse events, near misses, and errors. https://psnet.ahrq.gov/primers/primer/34/adverse-events-near-misses-and-errors. Accessed December 18, 2018.
- 20.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2nd ed Boston, MA: Little Brown and Co; 1991. [Google Scholar]
- 21.Moore KL, Boscardin WJ, Steinman MA, Schwartz JB. Patterns of chronic co-morbid medical conditions in older residents of U.S. nursing homes: differences between the sexes and across the agespan. J Nutr Health Aging. 2014;18(4):429-436. doi: 10.1007/s12603-014-0001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Medicare and Medicaid Services State US averages. https://data.medicare.gov/d/xcdc-v8bm?category=nursing-home-compare&view_name=state-averages. Accessed April 4, 2019.
- 23.Office of Inspector General Medicare Nursing Home Resident Hospitalization Rates Merit Additional Monitoring. Washington, DC: Dept of Health & Human Services; 2013. [Google Scholar]
- 24.Nursing Home Care FastStats. https://www.cdc.gov/nchs/fastats/nursing-home-care.htm. Updated March 11, 2016. Accessed December 12, 2018.
- 25.Brauner D, Werner RM, Shippee TP, Cursio J, Sharma H, Konetzka RT. Does Nursing Home Compare reflect patient safety in nursing homes? Health Aff (Millwood). 2018;37(11):1770-1778. doi: 10.1377/hlthaff.2018.0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agency for Healthcare Research and Quality Readmissions and Adverse Events after Discharge. https://psnet.ahrq.gov/primers/primer/11/readmissions-and-adverse-events-after-discharge. Accessed April 13, 2019.
- 27.Britton MC, Ouellet GM, Minges KE, Gawel M, Hodshon B, Chaudhry SI. Care transitions between hospitals and skilled nursing facilities: perspectives of sending and receiving providers. Jt Comm J Qual Patient Saf. 2017;43(11):565-572. doi: 10.1016/j.jcjq.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IMPACT Act of 2014 Data Standardization & Cross Setting Measures. Centers for Medicare and Medicaid. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/post-acute-care-quality-initiatives/impact-act-of-2014/impact-act-of-2014-data-standardization-and-cross-setting-measures.html. Accessed January 15, 2019.
- 29.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. doi: 10.7326/0003-4819-138-3-200302040-00007 [DOI] [PubMed] [Google Scholar]
- 30.Adler L, Denham C, McKeever M, et al. Global trigger tool: implementation basics. J Patient Saf. 2008;4:245-249. doi: 10.1097/PTS.0b013e31818e8a87 [DOI] [Google Scholar]
- 31.Handler SM, Hanlon JT. Detecting adverse drug events using a nursing home specific trigger tool. Ann Longterm Care. 2010;18(5):17-22. [PMC free article] [PubMed] [Google Scholar]
- 32.Marcum ZA, Arbogast KL, Behrens MC, et al. Utility of an adverse drug event trigger tool in Veterans Affairs nursing facilities. Consult Pharm. 2013;28(2):99-109. doi: 10.4140/TCP.n.2013.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Selected Examples With Descriptions of Less Serious, Serious, Life-Threatening and Fatal Events