This cohort study investigates the association of prediagnostic serum immune markers with the subsequent diagnosis of stable monoclonal gammopathy of undetermined significance vs progression to multiple myeloma.
Key Points
Question
Are changes in serum immune marker results associated with disease progression from monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma?
Findings
In this cohort study of 685 individuals with a diagnosis of progressing or stable MGUS, in longitudinal analysis of individuals with serial samples prior to progression, 23 of 43 (53%) had high-risk MGUS before progression, and 16 of these 23 (70%) experienced conversion from low-risk or intermediate-risk MGUS to multiple myeloma within 5 years; similar results were found for light-chain MGUS. Evolving monoclonal proteins, serum-free light chains, and immunosuppression were associated with disease progression.
Meaning
These findings support annual blood testing and risk assessment for all individuals with MGUS or light-chain MGUS.
Abstract
Importance
Multiple myeloma is consistently preceded by monoclonal gammopathy of undetermined significance (MGUS). Risk models that estimate the risk of progression from MGUS to multiple myeloma use data from a single time point, usually the initial workup.
Objective
To longitudinally investigate the alterations of serum immune markers with stable vs progressive MGUS.
Design, Setting, and Participants
This prospective cross-sectional cohort study included 77 469 adult participants aged 55 to 74 years in the screening arm of the National Cancer Institute Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial who had a diagnosis of progressing MGUS (n = 187) or stable MGUS (n = 498), including light-chain subtype, from November 1993, through December 2011. For each participant, all available serially stored prediagnostic serum samples (N = 3266) were obtained. Data analysis was performed from April 2018, to December 2018.
Main Outcomes and Measures
Serum protein and monoclonal immunoglobulin levels, serum free light chains, and serum light chains within each immunoglobulin class were measured.
Results
Of 685 individuals included in the study, 461 (67.3%) were men; the mean (SD) age was 69.1 (5.6) years. In cross-sectional modeling, risk factors associated with progressive MGUS were IgA isotype (adjusted odds ratio [OR], 1.80; 95% CI, 1.03-3.13; P = .04), 15 g/L or more monoclonal spike (adjusted OR, 23.5; 95% CI, 8.9-61.9; P < .001), skewed (<0.1 or >10) serum free light chains ratio (adjusted OR, 46.4; 95% CI, 18.4-117.0; P < .001), and severe immunoparesis (≥2 suppressed uninvolved immunoglobulins) (adjusted OR, 19.1; 95% Cl, 7.5-48.3; P < .001). Risk factors associated with progressive light-chain MGUS were skewed serum free light chains ratio (adjusted OR, 44.0; 95% CI, 14.2-136.3; P < .001) and severe immunoparesis (adjusted OR, 48.6; 95% CI, 9.5-248.2; P < .001). In longitudinal analysis of participants with serial samples prior to progression, 23 of 43 participants (53%) had high-risk MGUS before progression; 16 of these 23 (70%) experienced conversion from low-risk or intermediate-risk MGUS within 5 years. Similar results were found for light-chain MGUS.
Conclusions and Relevance
The findings of evolving risk patterns support annual blood testing and risk assessment for patients with MGUS or light-chain MGUS.
Introduction
Worldwide more than 120 000 individuals per year are diagnosed with multiple myeloma.1 This cancer is characterized by the proliferation of abnormal monoclonal plasma cells derived from B cells in the bone marrow. Frequently, there is invasion of the adjacent bone, which destroys skeletal structures and causes bone pain and fractures in more than 80% of newly diagnosed patients.2 The abnormal plasma cells can produce monoclonal (M) proteins and circulating free light chains (FLCs) detectable in peripheral blood. Approximately 15% to 20% of newly diagnosed patients with multiple myeloma secrete FLCs only.2 Renal impairment, commonly caused by FLCs or hyperviscosity from excessive amounts of M protein in the blood, affects up to 40% of newly diagnosed patients.3 Multiple myeloma is one of the few cancers with a defined precursor condition, monoclonal gammopathy of undetermined significance (MGUS), which is detectable in peripheral blood.4 Similarly, the light-chain subtype of multiple myeloma is preceded by light-chain MGUS.4
Recent population-based screening studies have shown that MGUS can start at age 30 years5,6 and is found in 2.3% of individuals who are 50 years or older.6 Because there is no established screening for MGUS in the general population to date, it is typically diagnosed incidentally during medical workups for other reasons (such as infectious, neurologic, or rheumatologic symptoms or as part of blood screening for life insurance).7 Long-term retrospective follow-up studies7,8,9 of individuals diagnosed with MGUS have shown 0.5% to 1.0% annual risk of progressing to multiple myeloma. Risk models using data from a single time point (usually the initial workup) have been proposed to estimate the risk of progression from MGUS to multiple myeloma.7,9 This modeling has led to development of clinical consensus guidelines recommending annual peripheral blood monitoring of serum protein markers and other assays for patients with intermediate-risk and high-risk MGUS.10 Smaller retrospective studies11,12,13 suggest that evolving changes in M protein levels may be associated with progression; however, in clinical practice, most patients are counseled based on their risk profile at initial workup. Limited information is available regarding the risk of progression from light-chain MGUS to light-chain multiple myeloma, and clinical guidelines for this condition are lacking.14
We conducted, to our knowledge, the first prospective study to longitudinally investigate the dynamics of serum immune markers in individuals with myeloma precursor disease. The primary outcome of the analysis was progression from MGUS to multiple myeloma or from light-chain MGUS to light-chain multiple myeloma.
Methods
Study Population
The prospective National Cancer Institute Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial has previously been described in detail.15 The PLCO Cancer Screening Trial was conducted according to a protocol approved by the institutional review boards of the National Cancer Institute and the 10 screening centers, and the current investigation was approved by the National Cancer Institute Special Studies institutional review board. Participants provided written informed consent in accordance with the Declaration of Helsinki16 at the trial baseline.
The PLCO Cancer Screening Trial included 77 469 individuals aged 55 to 74 years who had a diagnosis of progressing MGUS (n = 187) or stable MGUS (n = 498), including light-chain subtype, from November 1993, through December 2011. The selection of screening-arm participants for the current investigation is summarized in the eMethods in the Supplement. In brief, serial prediagnostic serum samples from 208 patients with multiple myeloma diagnosed during follow-up were selected with samples from the latest available study year for 5916 persons who never received a diagnosis of multiple myeloma or another hematologic malignancy. In the latter group, individuals with non-IgM MGUS or light-chain MGUS that did not progress to multiple myeloma or light-chain multiple myeloma were identified, and all earlier available serum samples from these individuals were selected.
Laboratory Tests
For all participants, prediagnostic serum samples (0.5 mL) stored at –70 °C were thawed, blindly processed, and analyzed in an identical fashion. All assays were conducted at The Binding Site (Birmingham, United Kingdom). Serum protein electrophoresis and immunofixation electrophoresis were performed on agarose gel (Hydrasys and Hydragel; Sebia). Levels of serum FLCs were determined in all study samples using Freelite assays (The Binding Site), and serum light chains within each immunoglobulin class (ie, IgGκ, IgGλ, IgAκ, IgAλ, IgMκ, and IgMλ) were measured using Hevylite assays (The Binding Site); these assays were performed on a SPAPLUS turbidimeter (The Binding Site).
Statistical Analysis
Cross-sectional Marker Analysis
First, the risk of progression in association with each marker was analyzed using the prediagnostic measurements from the time point most proximal to the multiple myeloma diagnosis date for cases or the selection date for MGUS without progression and light-chain MGUS controls. Serum immune markers proposed in previous studies were included.7,9 Odds ratios (ORs) and 95% CIs for associations were estimated with case status using logistic regression models with and without adjustment for sex, race/ethnicity, study center, and age and calendar year at the most proximal blood sample obtainment.
Patterns of Serum Markers
Using the results from the cross-sectional analyses, a scoring system was developed based on accumulated points. For MGUS, the following variables were defined as risk factors for progression: M spike with IgA isotype (1 point), M spike concentration of 15 g/L or more (to convert to grams per deciliters, divide by 10.0) (1 point), serum FLC ratio less than 0.1 or more than 10 (1 point), and immunoparesis (≥1 uninvolved immunoglobulins below lower level of normal; 1-2 points). In the scoring system for light-chain MGUS, serum FLC ratio less than 0.1 or more than 10 (1 point) and immunoparesis (1, 2, or 3 points) were used as risk factors. The score was defined as the total number of points assigned to risk factors for each individual blood sample. Scores for select individuals are as follows as shown in Table 1: MGUS (0-1, low-risk and low-risk light chain; 2, intermediate-risk and intermediate-risk light chain; ≥3, high-risk and high-risk light chain).
Table 1. Adverse Markers for Progression.
| Variablea | Adverse Marker | Points |
|---|---|---|
| MGUS | ||
| M spike isotype | IgA | 1 |
| M spike concentration | ≥15 g/L | 1 |
| Serum FLC ratio (κ:λ) | <0.1 or >10 | 1 |
| Immunoparesis | Uninvolved immunoglobulins below lower level of normal | 1 or 2 |
| Light-Chain MGUS | ||
| Serum FLC ratio (κ:λ) | <0.1 or >10 | 1 |
| Immunoparesis | Uninvolved immunoglobulins below lower level of normal | 1, 2, or 3 |
Abbreviations: FLC, serum free light chain; M spike, monoclonal spike; MGUS, monoclonal gammopathy of undetermined significance.
MGUS risk category for progression to multiple myeloma: 0 to 1, low-risk and low-risk light chain; 2, intermediate-risk and intermediate-risk light chain; 3 or higher, high-risk and high-risk light chain.
Marker changes in a subset of patients were also assessed (see the eMethods in the Supplement for details on the models).17,18 Patients with MGUS that progressed to multiple myeloma (case patients) and patients with MGUS that did not progress (controls) were selected and matched in a 1:1 control-to-case ratio on the basis of age (within 6 years), sex, and race/ethnicity. For each case, the sample most proximal to the multiple myeloma diagnosis was identified and a control of similar age was matched at the blood sample obtainment most proximal to cohort exit. The age at multiple myeloma diagnosis for the case was assigned as the age of selection for the control. Matched controls had to be free of multiple myeloma at the time of the diagnosis of the corresponding case. For 3 cases, no match could be identified, resulting in 159 cases and 156 matched controls. Using the same matching criteria, for each individual with progressing light-chain MGUS (cases), 2 individuals with stable light-chain MGUS (controls) were selected (28 cases, 56 matched controls). For all cases, all samples available before the multiple myeloma diagnosis date were used, and the selection date for the controls was used. Models to the log-transformed marker data that allowed the slopes for time to vary for cases and controls were fit by including interaction terms of case status with time. The 2-tailed P values for these interaction terms were presented as evidence of differences in marker trajectories for cases (individuals with disease progression) and controls (individuals without disease progression). Differences in characteristics between cases and controls were assessed using Fisher exact and χ2 tests. Analysis was performed in SAS, version 9.4 (SAS Institute). Data analysis was performed from April 2018, to December 2018.
Results
Of 685 included in the study, 461 (67.3%) were men; the mean (SD) age was 69.1 (5.6) years. We identified 187 individuals with progression from non-IgM MGUS to multiple myeloma and from light-chain MGUS to light-chain multiple myeloma and 498 individuals whose diagnosis remained non-IgM MGUS without progression and light-chain MGUS without progression to multiple myeloma through 16 years or less of follow-up (eTable 1 in the Supplement). For each participant, we obtained all available serially stored prediagnostic serum samples, collecting 3266 samples in total.
Non-IgM MGUS Risk Factors and Patterns of Progression
Cross-sectional Modeling
Associations with progression from non-IgM MGUS to multiple myeloma by immunoglobulin isotype, concentration of the M spike, skewed serum FLC ratio, and immunoparesis from the time point most proximal to diagnosis or selection are shown in the Table 2. Compared with individuals with IgG isotype, those with IgA isotype had a modest but statistically significant increased risk of progression to multiple myeloma (adjusted OR, 1.80; 95% CI, 1.03-3.13; P = .04). Participants who had an M spike concentration of 15 g/L or more were more than 23 times more likely to develop multiple myeloma compared with those with a lower concentration of the protein (adjusted OR, 23.5; 95% CI, 8.9-61.9; P < .001). We also evaluated risk of progression among participants with altered serum FLC ratios outside the published reference range of 0.26 to 1.65.7,8 Compared with individuals with a serum FLC ratio within the normal reference range, those with a serum FLC ratio less than 0.1 or more than 10 were 46 times more likely to develop multiple myeloma (adjusted OR, 46.4; 95% CI, 18.4-117.0; P < .001). We assessed the risk of progression by severity of the immunoparesis, as defined by the number of suppressed, uninvolved immunoglobulins (IgG, IgA, and/or IgM). Compared with those with no evidence of immunoparesis, individuals with 2 suppressed uninvolved immunoglobulins were more likely to have disease progression to multiple myeloma (adjusted OR, 19.1; 95% Cl, 7.5-48.3; 29% vs 3%; P < .001). As described in the Methods section, we defined a risk score based on the identified risk factors (Table 1). Based on the clinical risk score, we plotted longitudinal patterns of progression to multiple myeloma (eFigure 1 in the Supplement).
Table 2. Serum Protein Markers Associated With Progression: Cross-sectional Analysis.
| Serum Protein Marker | MGUS | Light-Chain MGUSa | ||||||
|---|---|---|---|---|---|---|---|---|
| Without Progression, No. (%) of Controls (n = 281) | Progression to Multiple Myeloma (n = 159) | Without Progression (n = 217) | Progression to Light-Chain Multiple Myeloma (n = 28) | |||||
| Case, No. (%) | OR (95% CI) | Control, No. (%) | Case, No. (%) | OR (95% CI) | ||||
| Unadjusted | Adjusted | Unadjusted | Adjustedb | |||||
| Immunoglobulin isotype | ||||||||
| IgG | 216 (76.9) | 109 (68.6) | 1 [Reference] | 1 [Reference] | NA | NA | NA | NA |
| IgA | 47 (16.7) | 45 (28.3) | 1.90 (1.2-3.0) | 1.80 (1.0-3.1) | NA | NA | NA | NA |
| Biclonal | 18 (6.4) | 5 (3.1) | 0.55 (0.2-1.5) | 0.49 (0.1-1.6) | NA | NA | NA | NA |
| M spike concentration | ||||||||
| <15 g/L | 178 (96) | 88 (55.3) | 1 [Reference] | 1 [Reference] | NA | NA | NA | NA |
| ≥15 g/L | 8 (4) | 62 (39.0) | 15.68 (7.2-34.2) | 23.50 (8.9-61.9) | NA | NA | NA | NA |
| Serum FLC ratio | ||||||||
| 0.26-1.65 | 185 (65.8) | 37 (23.3) | 1 [Reference] | 1 [Reference] | NA | NA | NA | NA |
| 0.10-0.26 or >1.65 to 10 | 86 (30.6) | 57 (35.8) | 3.31 (2.0-5.4) | 3.45 (1.9-6.7) | 205 (94.5)c | 7 (25.0)c | 1 [Reference]c | 1 [Reference]c |
| <0.10 to >10 | 10 (3.6) | 65 (40.9) | 32.5 (15.3-69.0) | 46.4 (18.4-117.2) | 12 (5.5) | 21 (75.0) | 51.3 (18.2-144.1) | 44.0 (14.2-136.3) |
| Immunoparesisd | ||||||||
| 0 | 211 (75.1) | 64 (40.2) | 1 [Reference] | 1 [Reference] | 190 (87.6) | 13 (46.4) | 1 [Reference] | 1 [Reference] |
| 1 | 45 (16.0) | 45 (28.3) | 3.30 (2.0-5.4) | 3.01 (1.7-5.5) | 22 (10.1) | 5 (17.8) | 3.32 (1.1-10.2) | 3.98 (1.1-14.8) |
| 2 | 7 (2.5) | 45 (28.3) | 21.2 (9.1-49.3) | 19.1 (7.5-48.3) | 5 (2.3) | 10 (35.7) | 23.2 (8.7-98.2) | 48.6 (9.5-248.2) |
Abbreviations: FLC, free light chain (κ:λ); M spike, monoclonal spike; MGUS, monoclonal gammopathy of undetermined significance; NA, not applicable; OR, odds ratio.
SI conversion: To convert M spike to grams per deciliters, divide by 10.0.
Models for light-chain–secreting multiple myeloma and light-chain MGUS cases were not adjusted for race/ethnicity owing to small numbers of light-chain multiple myeloma cases for nonwhite persons. Analysis was performed in SAS, version 9.4 (SAS Institute).
ORs and 95% CIs are adjusted for sex, age at most proximal blood sample obtainment (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, and other [Hispanic, Asian, and Pacific Islander]), calendar year of most proximal blood sample obtainment (1995-1998, 1999-2002, and 2003-2006), and study center (coded as Upper Midwest [Wisconsin and Minnesota], West/South [Colorado, Hawaii, Missouri, Utah, and Alabama], and East [Georgetown, Detroit, and Pittsburgh]).
FLC ratio is 0.1 to 10 with no exclusion.
Immunoparesis is the number of uninvolved immunoglobulins (IgG, IgA, or IgM) below the lower level of normal.
Changes in Immune Marker Values Over Time
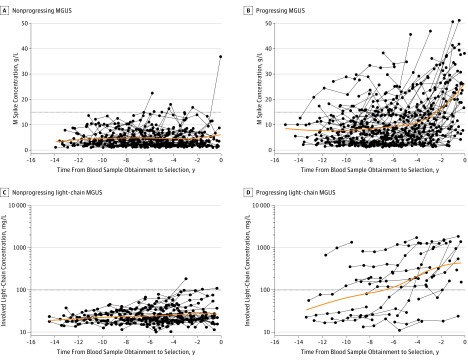
We used information for all available serum samples from multiple myeloma cases (n = 159) and matched controls (n = 156). Specifically, we plotted the M spike concentration and the involved serum FLC concentration from individual samples against the years before the multiple myeloma diagnosis (for cases) and selection (for controls) (Figure 1 and eFigure 2 in the Supplement). Increasing M spike concentration (P = .06 for linear term and P = .02 for quadratic term), increasing involved serum FLC levels (P = .01), and an increasing number of suppressed uninvolved immunoglobulins (P < .001) were associated with higher risk of progression to multiple myeloma.
Figure 1. Progression From Monoclonal Gammopathy of Undetermined Significance (MGUS) to Multiple Myeloma and Progression From Light-Chain MGUS to Light-Chain Multiple Myeloma.
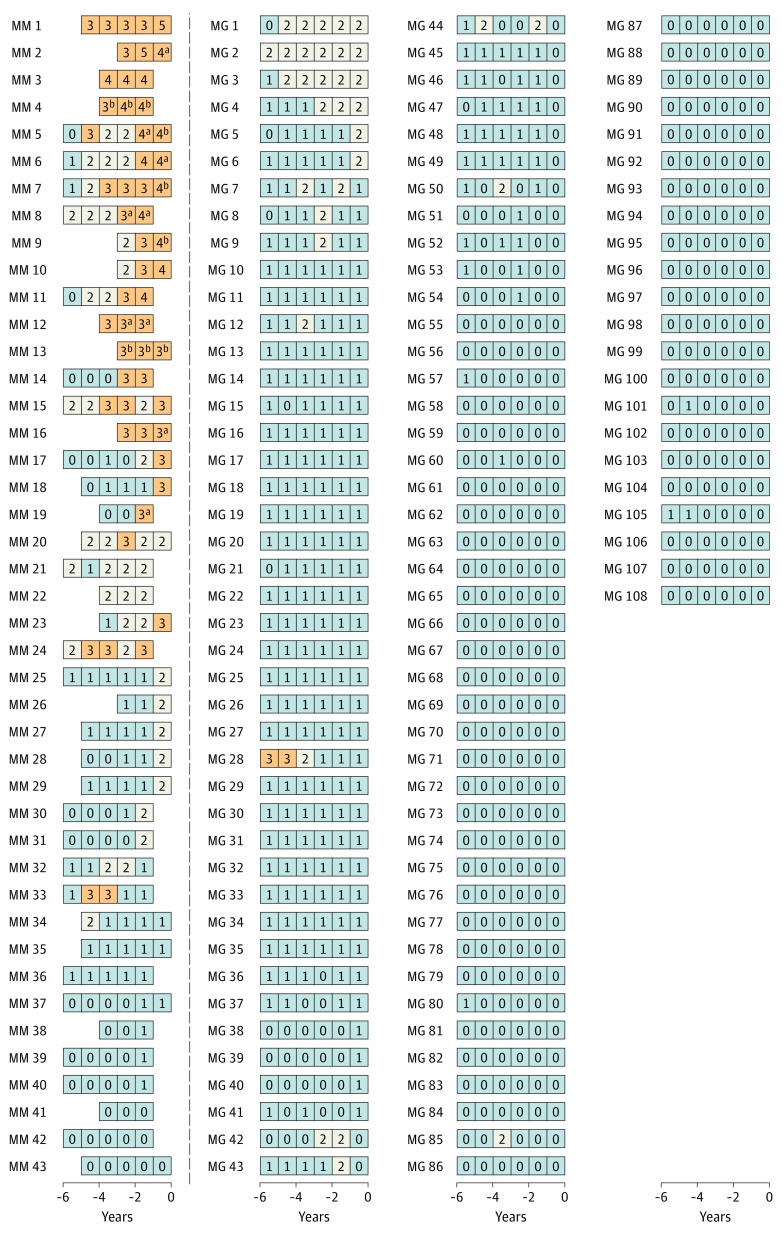
To investigate patterns of serum markers, we conducted another analysis of longitudinal data (eMethods in the Supplement). These analyses were limited to individuals with at least 1 prediagnostic serum sample within 2 years of diagnosis and at least 3 serial samples. For patients with MGUS without progression to multiple myeloma, we required at least 8 years of follow-up. Our final selection for these analyses included 43 multiple myeloma cases and 108 controls with MGUS without progression. Twenty-three of the 43 selected case patients with MGUS that progressed to multiple myeloma (53%) had a high risk score before multiple myeloma diagnosis, and only 1 of 108 controls with MGUS that did not progress to multiple myeloma had a high risk score (P < .001) (Figure 2). Furthermore, among case patients with MGUS that progressed and had a high risk score before multiple myeloma diagnosis, 16 (70%) had preceding blood samples with low-risk and/or intermediate-risk scores. Among those with disease progression, a high risk score was detected up to 5 years before multiple myeloma diagnosis (Figure 2). Among those with disease progression without a high risk score, 9 had available stored blood samples 1 year before multiple myeloma diagnosis; 5 had intermediate-risk MGUS, and 4 had low-risk MGUS (Figure 2 and eTable 2 in the Supplement). Of individuals with disease progression, 9 (21%) fulfilled the diagnostic blood-based criteria for smoldering multiple myeloma (ie, M spike concentration ≥30 g/L),19 and 6 (14%) fulfilled the diagnostic blood-based criteria for multiple myeloma (ie, serum FLC ratio ≥100 and involved serum FLC concentration ≥10 mg/dL)20 before clinical multiple myeloma diagnosis; none of those without disease progression fulfilled these criteria (P < .001) (eTable 2 in the Supplement). Blood-based criteria for multiple myeloma were detected up to 5 years before diagnosis (Figure 2). Severe immunoparesis (2 uninvolved immunoglobulins below the lower level of normal) was found in prediagnostic blood samples from 18 of 43 individuals with disease progression (42%) and 4 of 108 without disease progression (4%) (P < .001) (eTable 2 in the Supplement).
Figure 2. Longitudinal Analysis of Risk Scores Among Selected Individuals With and Without Progression From Monoclonal Gammopathy of Undetermined Significance (MGUS) to Multiple Myeloma.
A risk score was used to assess and label individual blood samples as follows: low-risk MGUS, 0 to 1 (gray); intermediate-risk MGUS, 2 (white); high-risk MGUS, 3 or higher (orange). Each series of boxes represents a unique patient, each box represents a unique blood sample, and the x-axis represents number of years before multiple myeloma diagnosis (for case patients) and number of years before selection (for controls). Each box includes a number that represents the risk score. MG indicates nonprogressing MGUS; and MM, progressing MGUS.
aFulfilled the blood-based criteria for smoldering multiple myeloma (ie, M spike concentration ≥30 g/L).
bFulfilled the blood-based criteria for multiple myeloma (ie, serum free light chain ratio ≥100 and involved serum free light chain concentration ≥10 mg/dL).
Risk Factors and Patterns of Progression Among Individuals With Light-Chain MGUS
Cross-sectional Modeling
Among individuals with light-chain MGUS, those with a highly skewed serum FLC ratio (defined as <0.1 or >10) at the time point most proximal to diagnosis or selection were more likely to experience progression to light-chain multiple myeloma compared with those for whom the serum FLC ratio was less skewed (adjusted OR, 44.0; 95% CI, 14.2-136.3; 75% vs 6%; P < .001) (Table 2). Similar to our findings among case patients with MGUS, we found more severe immunoparesis in those with light-chain MGUS that progressed to light-chain multiple myeloma compared with controls with stable MGUS (adjusted OR, 48.6; 95% CI, 9.5-248.2; 36% vs 2%; P < .001). Based on our clinical risk score (Table 1), we plotted longitudinal patterns of progression to light-chain multiple myeloma (eFigure 1B in the Supplement).
Changes of Marker Values
We used all available serum samples from light-chain multiple myeloma cases (n = 28) and matched light-chain MGUS controls (n = 56) and plotted the involved serum FLC concentration from individual samples by number of years before multiple myeloma diagnosis (for cases) and selection (for controls) (Figure 1, C and D). Increased involved serum FLC levels (P < .001) and increased number of suppressed uninvolved immunoglobulins (P < .001) were associated with higher risk of progression to multiple myeloma.
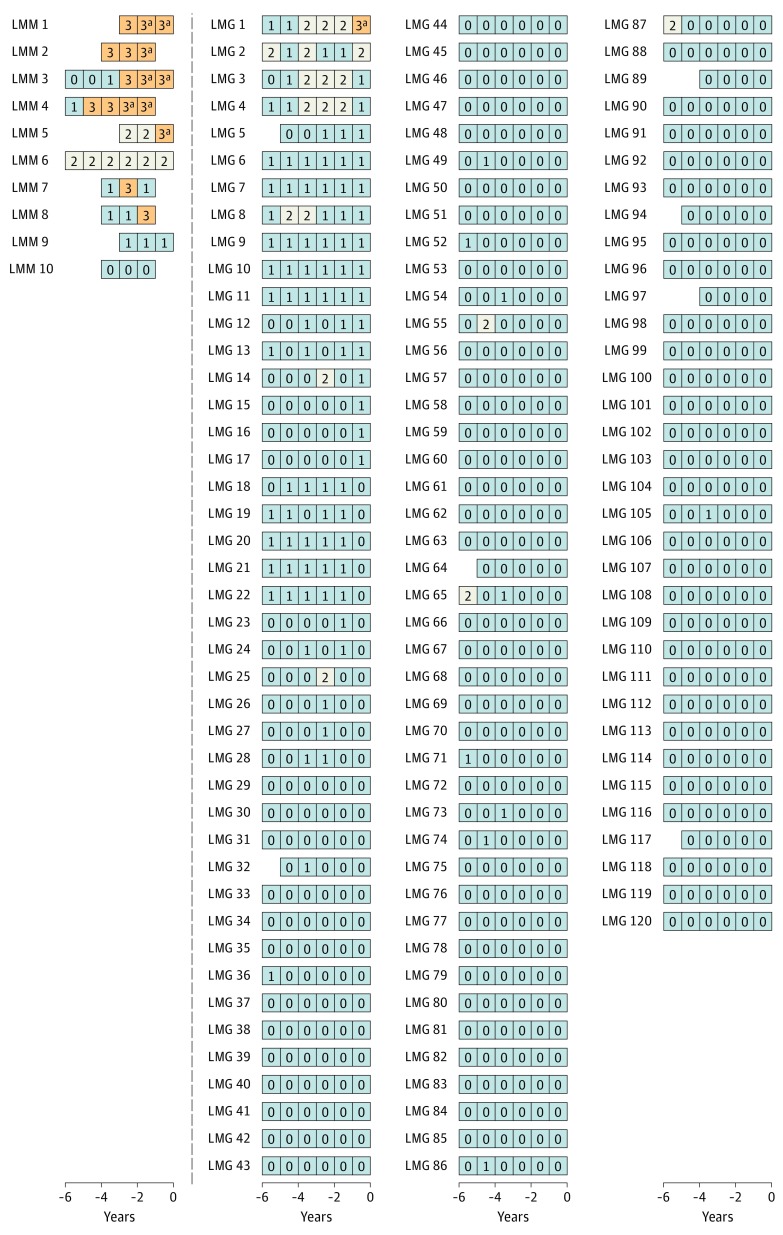
The second analysis of longitudinal data was limited to individuals with at least 1 prediagnostic serum sample within 2 years of diagnosis and at least 3 serial samples. For patients with MGUS without progression to light-chain multiple myeloma, we required at least 8 years of follow-up. Our final selection for these analyses included 10 light-chain multiple myeloma cases and 120 light-chain MGUS cases. Seven of the 10 patients with light-chain MGUS (70%) that progressed to light-chain multiple myeloma had a high risk score before the light-chain multiple myeloma diagnosis; only 1 of 120 without progression (0.8%) had a high risk score (P < .001) (Figure 3). Among case patients with light-chain MGUS that progressed who had a high-risk status before light-chain multiple myeloma diagnosis, 5 (71%) had preceding blood samples with low-risk and/or intermediate-risk scores. Among patients with disease progression, high-risk status was detected up to 5 years before multiple myeloma diagnosis (Figure 3). Blood samples were available for 2 individuals who were not at high risk before disease progression; they had intermediate-risk and low-risk MGUS 1 year before multiple myeloma diagnosis (Figure 3 and eTable 2 in the Supplement). Six cases patients with light-chain MGUS (60%) that progressed met the diagnostic blood-based criteria for light-chain multiple myeloma before their clinical multiple myeloma diagnosis, as did 1 of the individuals without progression (0.8%) (P < .001) (eTable 2 in the Supplement). Blood-based criteria for multiple myeloma were detected up to 5 years before multiple myeloma diagnosis. Severe immunoparesis was found in prediagnostic blood samples from 7 of 10 individuals with disease progression (70%) and 3 of 120 without progression (2%) (P < .001) (eTable 2 in the Supplement).
Figure 3. Longitudinal Analysis of Risk Among Selected Individuals With and Without Progression From Light-Chain Monoclonal Gammopathy of Undetermined Significance (MGUS) to Light-Chain Multiple Myeloma.
A risk score was used to assess and label individual blood samples as follows: low-risk light-chain MGUS, 0 to 1 (gray); intermediate-risk light-chain MGUS, 2 (white); high-risk light-chain MGUS, 3 or higher (orange). Each series of boxes represents a unique patient, each box represents a unique blood sample, and the x-axis represents number of years before multiple myeloma diagnosis (for case patients) and number of years before selection (for controls). Each box includes a number that represents the risk score. LMG, indicates nonprogressing light-chain MGUS; and LMM, progressing light-chain MGUS.
aFulfilled the blood-based criteria for multiple myeloma (ie, serum free light chain ratio ≥100 and involved serum free light chain concentration ≥10 mg/dL).
Discussion
With use of data from the PLCO Cancer Screening Trial, to our knowledge, the first prospective screening study was performed in 2009 that showed that multiple myeloma was consistently preceded by MGUS.21 We designed a study of longitudinal data to investigate dynamics of serum immune markers in individuals with MGUS. We provided novel insights by showing that an individual’s risk of progression from MGUS to multiple myeloma was not constant, and the same was true for individuals with light-chain MGUS. This study based on prospectively collected samples may provide better understanding of the findings of previous retrospective follow-up studies.7,8,9 Previously reported annual risk of progression from MGUS to multiple myeloma of 0.5% to 1.0%7,8,9 reflected the mean risk among all MGUS cases but were not applicable to individual patients. In the present study, we found that individual patients’ clinical risk categories could convert. Our data suggest that low-risk or intermediate-risk MGUS can convert to high-risk MGUS and progress to multiple myeloma in individuals within 5 years. The same conversion patterns were true for individuals with light-chain MGUS. This finding is clinically relevant and supports annual blood testing for all individuals diagnosed with MGUS or light-chain MGUS, as well as yearly assessment of a patient’s clinical risk status.
It has been proposed that, based on clinical observations and retrospective data sets, evolving changes in monoclonal protein levels are associated with progression.11,12,13 In this prospective study, we found that significantly increasing M spike and involved serum FLC concentrations were detectable in patients with disease MGUS progression more than 5 years before multiple myeloma diagnosis; case patients with progressing light-chain MGUS similarly had significantly increasing involved serum FLC concentrations. When we combined individual markers into a risk score for progression, 53% of patients with progressing MGUS but only 1 of 108 patients with nonprogressing MGUS had a high risk score. Similarly, we found evidence of high-risk status in 70% of case patients with progressing light-chain MGUS but only 1 of 120 controls with nonprogressing light-chain MGUS. Most patients who developed multiple myeloma after a preceding state of high-risk MGUS had converted from low-risk or intermediate-risk stages within 5 years before multiple myeloma diagnosis; similar patterns were found in patients with light-chain MGUS progression. Four of 43 (9%) individuals who progressed from MGUS to multiple myeloma had low-risk score 1 year before the multiple myeloma diagnosis; 1 of 10 (10%) of progressing light-chain MGUS were low-risk 1 year before light-chain multiple myeloma diagnosis.
To our knowledge, there are no previous data on the rate of smoldering multiple myeloma development on the pathway to multiple myeloma. Using serum markers before formal diagnosis of multiple myeloma, we found that only 21% of patients with MGUS progression fulfilled the blood-based criteria for smoldering multiple myeloma,19 and 14% fulfilled the criteria for multiple myeloma.20 Of the patients with light-chain MGUS progression, 60% fulfilled the blood-based criteria for light-chain multiple myeloma before formal diagnosis.20 These blood-based criteria were detectable up to 5 years before formal diagnosis of both multiple myeloma and light-chain multiple myeloma. However, we did not have access to bone marrow biopsy results; therefore, we could not evaluate data on plasma cell percentage.
The biologic features of multiple myeloma are complex, and DNA sequencing studies have shown that the mutational landscape is already altered in earlier stages of the disease process.22 Although there are only limited data available, it has been proposed that the host’s biologic features play a role in the regulation of stable precursor disease vs progression.7,9,23 In our cross-sectional analysis, immunosuppression (≥1 suppressed uninvolved immunoglobulins) was found in 58% of patients with MGUS progression compared with 20% of those without MGUS progression. Among patients with light-chain MGUS, immunosuppression was found in 54% with disease progression and 12% without progression. Among patients with MGUS and light-chain MGUS, those with severe immunosuppression (≥2 suppressed uninvolved immunoglobulins) had a significantly elevated risk of progression (MGUS: 29% among those with progression vs 3% among those without progression; light-chain MGUS, 18% among those with progression vs 2% among those without progression). Future studies should investigate biologic interactions between nontumor and tumor cells in association with progression.
Limitations
Limitations include the lack of access to bone marrow biopsy or aspirate material for clinical and biologic characterization.
Conclusions
The findings suggest that individuals with low-risk or intermediate-risk MGUS, including those with light-chain MGUS, can experience progression to high-risk MGUS multiple myeloma within 5 years. Our results may be clinically relevant and support annual blood tests for all individuals diagnosed with MGUS or light-chain MGUS.
eMethods. Study Population and Sample Selection; Laboratory Testing; and Statistical Analyses of Longitudinal Data
eTable 1. Demographic Characteristics of Study Participants
eTable 2. Patterns of Serum Protein Markers in Selected Progressing Versus Nonprogressing MGUS and Light-Chain MGUS: Clinical Risk Score Analysis
eFigure 1. Panel A: Progression to Multiple Myeloma, by MGUS Risk Category; Panel B: Progression to Light-chain Multiple Myeloma, by Light-chain MGUS Risk Category
eFigure 2. Progression From MGUS to Multiple Myeloma, and Average Involved sFLC Concentrations Over Time
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962-2972. doi: 10.1182/blood-2007-10-078022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes R, Leung N, Kyle R, Winearls CG. Myeloma kidney: improving clinical outcomes? Adv Chronic Kidney Dis. 2012;19(5):342-351. doi: 10.1053/j.ackd.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 4.Landgren O, Kyle RA, Rajkumar SV. From myeloma precursor disease to multiple myeloma: new diagnostic concepts and opportunities for early intervention. Clin Cancer Res. 2011;17(6):1243-1252. doi: 10.1158/1078-0432.CCR-10-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgren O, Graubard BI, Kumar S, et al. Prevalence of myeloma precursor state monoclonal gammopathy of undetermined significance in 12372 individuals 10-49 years old: a population-based study from the National Health and Nutrition Examination Survey. Blood Cancer J. 2017;7(10):e618. doi: 10.1038/bcj.2017.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgren O, Graubard BI, Katzmann JA, et al. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia. 2014;28(7):1537-1542. doi: 10.1038/leu.2014.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turesson I, Kovalchik SA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014;123(3):338-345. doi: 10.1182/blood-2013-05-505487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362-1369. doi: 10.1056/NEJMoa054494 [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Larson DR, Therneau TM, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378(3):241-249. doi: 10.1056/NEJMoa1709974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyle RA, Durie BG, Rajkumar SV, et al. ; International Myeloma Working Group . Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121-1127. doi: 10.1038/leu.2010.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosiñol L, Bladé J, Esteve J, et al. Smoldering multiple myeloma: natural history and recognition of an evolving type. Br J Haematol. 2003;123(4):631-636. doi: 10.1046/j.1365-2141.2003.04654.x [DOI] [PubMed] [Google Scholar]
- 12.Ravi P, Kumar S, Larsen JT, et al. Evolving changes in disease biomarkers and risk of early progression in smoldering multiple myeloma. Blood Cancer J. 2016;6(7):e454. doi: 10.1038/bcj.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández de Larrea C, Isola I, Pereira A, et al. Evolving M-protein pattern in patients with smoldering multiple myeloma: impact on early progression. Leukemia. 2018;32(6):1427-1434. doi: 10.1038/s41375-018-0013-4 [DOI] [PubMed] [Google Scholar]
- 14.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375(9727):1721-1728. doi: 10.1016/S0140-6736(10)60482-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prorok PC, Andriole GL, Bresalier RS, et al. ; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team . Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6)(suppl):273S-309S. doi: 10.1016/S0197-2456(00)00098-2 [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829-836. doi: 10.1080/01621459.1979.10481038 [DOI] [Google Scholar]
- 18.Killick R, Fearnhead P, Eckley IA. Optimal detection of changepoints with a linear computational cost. J Am Stat Assoc. 2012;107(500):1590-1598. doi: 10.1080/01621459.2012.737745 [DOI] [Google Scholar]
- 19.Landgren O. Shall we treat smoldering multiple myeloma in the near future? Hematology Am Soc Hematol Educ Program. 2017;2017(1):194-204. doi: 10.1182/asheducation-2017.1.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi: 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 21.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-5417. doi: 10.1182/blood-2008-12-194241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mailankody S, Kazandjian D, Korde N, et al. Baseline mutational patterns and sustained MRD negativity in patients with high-risk smoldering myeloma. Blood Adv. 2017;1(22):1911-1918. doi: 10.1182/bloodadvances.2017005934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosani T, Mailankody S, Korde N, et al. Host-related immunodeficiency in the development of multiple myeloma. Leuk Lymphoma. 2018;59(5):1127-1132. doi: 10.1080/10428194.2017.1361026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Population and Sample Selection; Laboratory Testing; and Statistical Analyses of Longitudinal Data
eTable 1. Demographic Characteristics of Study Participants
eTable 2. Patterns of Serum Protein Markers in Selected Progressing Versus Nonprogressing MGUS and Light-Chain MGUS: Clinical Risk Score Analysis
eFigure 1. Panel A: Progression to Multiple Myeloma, by MGUS Risk Category; Panel B: Progression to Light-chain Multiple Myeloma, by Light-chain MGUS Risk Category
eFigure 2. Progression From MGUS to Multiple Myeloma, and Average Involved sFLC Concentrations Over Time