Key Points
Question
What is the role for plant-based dietary patterns in the primary prevention of type 2 diabetes among adults?
Findings
In this systematic review and meta-analysis of prospective observational studies assessing the association between plant-based dietary patterns and risk of type 2 diabetes among adults, higher adherence to plant-based dietary patterns was associated with a lower risk of type 2 diabetes; this association was strengthened when healthy plant-based foods, such as fruits, vegetables, whole grains, legumes, and nuts, were included in the pattern. Findings were broadly consistent in several prespecified subgroups and in sensitivity analyses.
Meaning
Greater adherence to plant-based dietary patterns, especially those rich in healthful plant-based foods, is associated with lower risk of type 2 diabetes.
Abstract
Importance
Accumulating epidemiologic evidence has suggested favorable associations between plant-based dietary patterns and risk of type 2 diabetes, although there is a lack of a quantitative summary of evidence substantiating this important association.
Objective
To quantitatively synthesize available prospective observational evidence on the association between plant-based dietary patterns and risk of type 2 diabetes.
Data Sources
A systematic search of PubMed and MEDLINE, Embase, Web of Science, and reference lists through February 15, 2019, was conducted. Data analysis was conducted between December 2018 and February 2019.
Study Selection
All prospective observational studies that examined the association between adherence to plant-based dietary patterns and incidence of type 2 diabetes among adults 18 years or older were identified.
Data Extraction and Synthesis
Meta-analysis of Observational Studies in Epidemiology guidelines for data abstraction and reporting were followed, and a National Heart, Lung, and Blood Institute assessment tool was used to evaluate study quality. Two authors independently conducted full-text assessments and data abstraction. Meta-analysis was conducted using the random-effects method to calculate the overall relative risk (RR) and 95% CI.
Main Outcomes and Measures
Level of adherence to a plant-based diet and incidence of type 2 diabetes.
Results
A total of 9 studies were identified, totaling 307 099 participants with 23 544 cases of incident type 2 diabetes. A significant inverse association was observed between higher adherence to a plant-based dietary pattern and risk of type 2 diabetes (RR, 0.77; 95% CI, 0.71-0.84) in comparison with poorer adherence, with modest heterogeneity across studies (I2 = 44.5%; P = .07 for heterogeneity). Similar findings were obtained when using the fixed-effects model (RR, 0.80; 95% CI, 0.75-0.84). Consistent associations were observed across predefined subgroups. This association was strengthened when healthy plant-based foods, such as fruits, vegetables, whole grains, legumes, and nuts, were included in the definition of plant-based patterns (RR, 0.70; 95% CI, 0.62-0.79). Most studies were deemed to have good quality in terms of dietary assessment, disease outcomes, and statistical adjustment for confounding factors. Using restricted cubic splines, a significant inverse linear dose-response association was identified between plant-based dietary indices and risk of type 2 diabetes.
Conclusions and Relevance
Plant-based dietary patterns, especially when they are enriched with healthful plant-based foods, may be beneficial for the primary prevention of type 2 diabetes.
This systematic review and meta-analysis of prospective observational studies quantitatively synthesizes available evidence on the association between plant-based dietary patterns and risk of type 2 diabetes.
Introduction
Plant-based dietary patterns, which emphasize foods derived from plant sources and include lower consumption of or exclusion of animal products, have gained significant attention in recent years for their potential to prevent or manage several major chronic diseases, including type 2 diabetes, cardiovascular disease, and cancer.1,2,3,4 Suggested mechanisms for their protective association against the development of type 2 diabetes include higher consumptions of plant foods that are rich in vitamins, minerals, and antioxidants and lower consumption of red and processed meats.5,6,7 Meanwhile, depending on the definition of plant-based dietary patterns (eg, lacto-ovo-vegetarian and vegan), the patterns may also exclude certain animal products that are potentially beneficial for the prevention of cardiometabolic diseases, such as yogurt and fish.8,9,10 Moreover, refined grains, starches, and sugars can also be characterized as plant-based, although they are independently associated with a higher risk of type 2 diabetes.10,11 Hence, the overall effect of plant-based diets on the incidence of type 2 diabetes remains to be well characterized, particularly given the potentially opposing health effects of certain plant-based foods on the risk of type 2 diabetes.12 A recent meta-analysis13 of observational studies suggested that vegetarian dietary patterns are associated with a lower risk of type 2 diabetes, although this study was limited by the inclusion of mostly cross-sectional studies and only 2 prospective studies, both of which were performed among US Seventh Day Adventists.14,15 Hence, it is difficult to generalize the potential beneficial effects of plant-based dietary patterns to populations of other racial/ethnic groups or countries. In addition, while meta-analyses of clinical trials demonstrated beneficial effects of plant-based or vegan dietary patterns on body weight, glycemic control, and cardiovascular risk factors in patients with type 2 diabetes,16,17 whether plant-based diets have a role in the primary prevention of type 2 diabetes remains unclear.
To fill this knowledge gap, we conducted a systematic review and meta-analysis of prospective studies to assess the association between plant-based dietary patterns and the risk of type 2 diabetes. We hypothesized that plant-based dietary patterns are inversely associated with risk of type 2 diabetes.
Methods
Registration of Review Protocol
This review was registered at PROSPERO International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/PROSPERO/) with the registration No. CRD42018111250.
Data Sources and Searches
Three databases, including PubMed and MEDLINE, Embase, and Web of Science, were systematically searched through February 15, 2019. Reference lists of prior systematic reviews or relevant original research articles were also searched to identify any studies that were not found in the initial database search. Search terms included “plant-based diet,” “vegetarian,” “vegan,” and “type 2 diabetes.” The specific search terms used for each database along with the number of records retrieved can be found in eTable 1 in the Supplement. We did not identify any unpublished abstracts or studies that satisfied our inclusion criteria. We followed all of the recommended standards listed in the Meta-analysis of Observational Studies in Epidemiology checklist.18
Study Selection
The current analysis included prospective studies that examined associations between adherence to plant-based dietary patterns and the incidence of type 2 diabetes among adults (aged ≥18 years). Only studies published in English were included. Studies that exclusively reported cross-sectional associations or reported outcomes of type 1 diabetes or gestational diabetes were excluded. Detailed inclusion and exclusion criteria can be found in eTable 2 in the Supplement.
The primary exposure of interest was adherence to plant-based dietary patterns, defined as higher consumption of plant-based foods and lower consumption or exclusion of animal-based foods. By this definition, vegetarian dietary patterns or vegan dietary patterns were also considered plant-based dietary patterns. In studies that classified adherence to plant-based dietary patterns using overall or healthful plant-based dietary indices, the association for overall plant-based dietary index was included in the pooled risk estimate, and the associations for healthful plant-based dietary index were included in a sensitivity analysis. The overall plant-based dietary index may also include less healthful plant foods, such as refined grains, starches (eg, white potatoes), or sugars (eg, sweets, desserts, or sugar-sweetened beverages). For studies that used dietary indices, we used the risk estimate that compared the highest with lowest quantiles, which represent the best (highest quantile) and poorest (lowest quantile) adherence to the plant-based dietary pattern. For studies that compared a priori–defined plant-based dietary patterns, including the vegan diet and vegetarian diet, we considered the study estimates comparing diets that are most restrictive of animal-based foods (vegan or semivegetarian) with the least restrictive, such as omnivorous diets.
Data Extraction and Assessment of Quality
Data from included studies were independently extracted by 2 of us (F.Q. and G.L.). Any discrepancies were evaluated by another of us (Q.S.) and discussed among all 3 of us. We extracted the following information from each study: number of participants and cases of diabetes, mean age, mean body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), sex composition (percentage of men), follow-up duration, dietary assessment method, method of scoring adherence to plant-based diet, diabetes ascertainment, and covariates that were adjusted for in the statistical models. We contacted study authors when we needed to obtain additional information that was not available in the online publications or supplementary materials. We assessed the quality of the studies using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies available on the National Heart, Lung, and Blood Institute website (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools), which assigns a score out of 14 to each study (where 0 indicates that the study does not meet any of the criteria for quality assessments and 14 indicates that the study meets all 14 criteria) based on potential areas of bias such as duration of follow-up, assessment of exposure and outcomes, and rates of loss to follow-up. Studies identified to be at high risk of bias (quality score <10) were excluded in a sensitivity analysis.
Data Synthesis and Analysis
Data analysis was performed between December 2018 and February 2019. We calculated a pooled relative risk (RR) using random-effects meta-analysis of hazards ratio, RR, or odds ratio from each study. The risk estimate with the greatest degree of statistical adjustment was included in the meta-analysis. Statistical heterogeneity was assessed with the Cochran Q-test (P < .10) and I2 statistic. To characterize potential sources of heterogeneity, subgroup analyses were performed, including analyses stratified by age, sex, global region, and definitions of plant-based diets (eg, vegetarian or vegan pattern vs plant-based dietary scores). In addition, we performed meta-regressions using age, BMI, sex, and duration of follow-up (as a continuous variable or using the median value to define short vs long follow-up duration), as well as the study quality score, to explore the potential sources of heterogeneity. Sensitivity analyses included those using inverse-variance fixed-effects meta-analysis as well as sequentially excluding individual studies to examine the effect on the overall risk estimate. Publication bias was assessed visually with funnel plots and with the Egger or Begg-Mazumdar regression tests.19
In a subset of studies that defined adherence to plant-based dietary patterns using plant-based diet indices, we performed a dose-response meta-analysis to assess potential nonlinearity. Restricted cubic splines with 3 knots (set at the 10th, 50th, and 90th percentiles for scores on the plant-based diet indices) were used to model the association of interest (rc_spline command in Stata). We used a generalized least-squares regression approach (glst package from Stata) to estimate the log-linear dose-response slope within each individual study and then pooled these slopes to derive the overall RR and 95% CI.20 All analyses were conducted with Stata, version 14.0 (Stata Corp). All P values were from 2-sided tests, and the results were deemed statistically significant at P < .05 unless stated otherwise.
Results
Included Studies and Baseline Characteristics
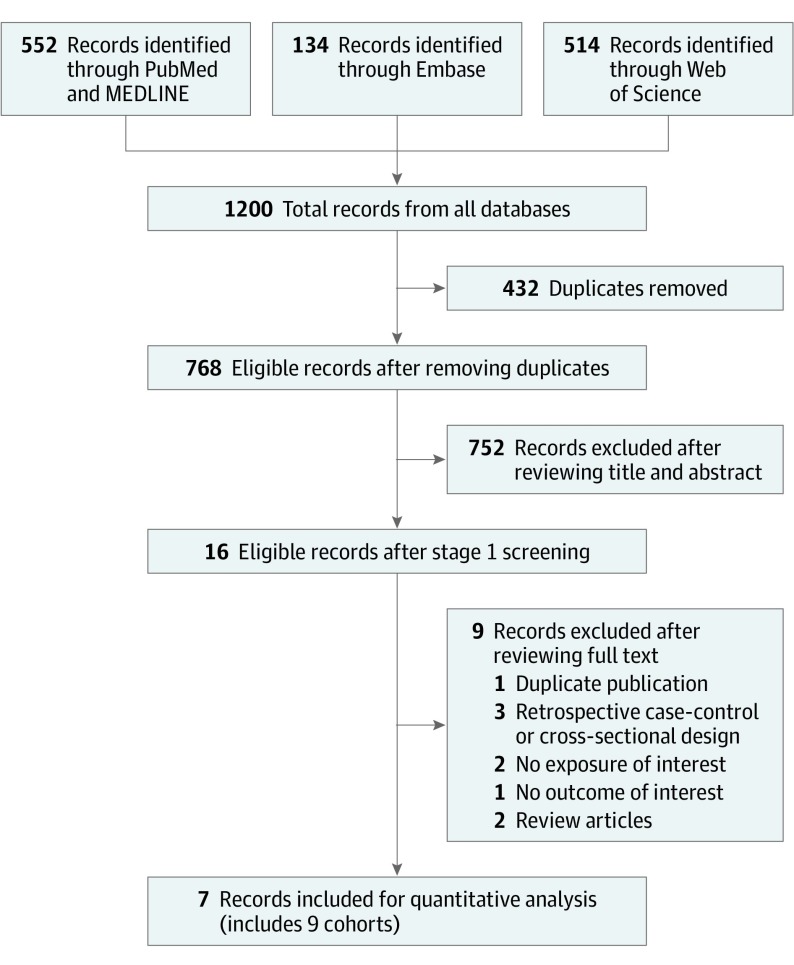
Figure 1 presents the search and screening results from all 3 databases. For the final quantitative synthesis, we included 9 study estimates from 7 publications.14,15,21,22,23,24,25 Table 1 shows the characteristics of the studies included in the meta-analysis. The meta-analysis includes 307 099 participants, 23 544 cases of incident type 2 diabetes, and studies with a duration of 2 to 28 years of follow-up. All of the studies used a prospective cohort design. Mean age ranged from 36.0 to 64.6 years, and mean BMI ranged from 23.0 to 26.7 at baseline. All studies used food frequency questionnaires to assess diet. Five studies (Nurses’ Health Study [NHS],22 Nurses’ Health Study II [NHSII],22 Health Professionals Follow-up Study [HPFS],22 Singapore Chinese Health Study [SCHS],23 and the Rotterdam Study24) characterized adherence to plant-based dietary patterns using plant-based dietary indices, 3 studies (Adventist Health Studies [AHS],14 AHS-2,15 and Tzu Chi Health Study [TCHS]25) compared individuals following a vegetarian or vegan dietary pattern with those who were not following a vegetarian dietary pattern, and 1 study (ATTICA Study21) derived a vegetarian dietary pattern using a factor analysis approach. Specific food compositions of the individual plant-based diets in each study are provided in eTable 3 in the Supplement. In most studies, incident cases of diabetes were identified through self-report and confirmed with validated questionnaires inquiring about symptoms or medication use. Statistical adjustments varied across the studies, with most to all studies adjusting for well-established diabetes risk factors, including age, BMI, smoking status, and family history of diabetes.
Figure 1. Flow Diagram of Prospective Observational Studies of Plant-Based Dietary Patterns and Incident Type 2 Diabetes.
Table 1. Baseline Characteristics of Published Studies Examining Plant-Based Dietary Patterns and Incident Type 2 Diabetes.
| Source | Study Name | Country | Type 2 Diabetes Cases, No./Total No. | Age, Mean, y | BMI, Mean | Men, % | Follow-up, y | Follow-up for Dose-Response Analysis, Person-Years | Dietary Assessment | Exposures | Diabetes Ascertainment | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vang et al,14 2008 | Adventist Health Study and Adventist Mortality Studies | United States | 543/8401 | 64.6 | 24.5 | 61.1 | Median, 17.0 | NA | FFQ | Long-term vegetarian vs long-term occasional meat intake vs long-term nonvegetarian | Self-reported | Age, sex, and BMI |
| Tonstad et al,15 2013 | Adventist Health Study-2 | United States | 616/41 387 | 58.0 | 26.7 | 36.7 | Median, 2.0 | NA | FFQ | Vegan, lacto-ovo-vegetarian, pesco-ovo-vegetarian, semi-vegetarian vs nonvegetarian | Self-reported with validated questionnaire | Age, BMI, race/ethnicity, sex, educational level, income, television watching, sleep, alcohol intake, physical activity, and smoking |
| Koloverou et al,21 2016 | ATTICA Cohort Study | Greece | 191/1485 | 45.2 | 26.4 | 49.3 | Median, 10.0 | NA | FFQ | Vegetarian-like dietary pattern characterized by fruits, vegetables, legumes, bread, Greek rusk, and pasta | Clinical examination with measurement of fasting blood glucose, diagnosis was based on the ADA criteria, namely, fasting glucose >125 mg/dL or the use of antidiabetic agents or insulin | Age, sex, family history of diabetes, waist circumference, and smoking |
| Satija et al,22 2016 | Nurses’ Health Study | United States | 7711/69 949 | 50.0 | 25.0 | 0 | Maximum, 28.0 | 1 662 705 | FFQ | PDI, hPDI, and uPDI, comparing extreme deciles | Self-reported with confirmation by a validated supplementary questionnaire, diagnosis criteria per the National Diabetes Data Group | Age, smoking, physical activity, alcohol intake, multivitamin use, family history of diabetes, total energy intake, hypertension, hypercholesterolemia, menopausal status or hormone replacement use, and BMI |
| Satija et al,22 2016 | Nurses’ Health Study II | United States | 5200/90 239 | 36.0 | 25.0 | 0 | Maximum, 20.0 | 1 656 996 | FFQ | PDI, hPDI, and uPDI, comparing extreme deciles | Self-reported with confirmation by a validated supplementary questionnaire, diagnosis criteria per the National Diabetes Data Group | Age, smoking, physical activity, alcohol intake, multivitamin use, family history of diabetes, total energy intake, hypertension, hypercholesterolemia, menopausal status or hormone replacement use, oral contraceptive, and BMI |
| Satija et al,22 2016 | Health Professionals Follow-up Study | United States | 3251/40 539 | 53.0 | 25.2 | 100 | Maximum, 24.0 | 782 670 | FFQ | PDI, hPDI, and uPDI, comparing extreme deciles | Self-reported with confirmation by a validated supplementary questionnaire, diagnosis criteria per the National Diabetes Data Group | Age, smoking, physical activity, alcohol intake, multivitamin use, family history of diabetes, total energy intake, hypertension, hypercholesterolemia, and BMI |
| Chen et al,23 2018 | Singapore Chinese Health Study | Singapore | 5207/45 411 | 55.2 | 23.0 | 42.7 | Median, 11.2 | 475 459 | FFQ | PDI, hPDI, comparing extreme quintiles | Self-reported through a validated questionnaire, validity was established through linkage with nationwide hospital discharge database, supplementary questionnaire inquiring about symptoms and treatment, or analysis of blood samples | Age, sex, dialect group, year of interview, energy intake, physical activity, BMI, education, smoking, hypertension, and alcohol use |
| Chen et al,24 2018 | Rotterdam Study I, II, and III | The Netherlands | 642/6770 | 62.0 | 26.6 | 41.3 | Median, 7.3 | 54 024 | FFQ | PDI, comparing extreme quintiles | Diagnosis information was collected from general practitioners' records, pharmacy databases, and follow-up examinations; diabetes diagnosis was defined as a fasting blood glucose concentration of 126 mg/dL, a nonfasting blood glucose concentration of 200 mg/dL (when fasting samples were unavailable), or the use of blood glucose-lowering drugs or dietary treatment and registration of the diabetes diagnosis; confirmation was judged by 2 independent study group physicians, discrepancies were settled by consulting an endocrinologist | Age, sex, energy intake, Rotterdam Study subcohort, education, smoking, family history of diabetes, physical activity, food supplement use, and BMI |
| Chiu et al,25 2018 | Tzu Chi Health Study | Taiwan | 183/2918 | 53.2 | 23.3 | 81.2 | Median, 5.0 | NA | FFQ | Vegetarian diet vs reverted vegetarian vs converted vegetarian vs nonvegetarian | Self-reported on questionnaires or HbA1c≥6.5%; in cases in which the diagnosis was uncertain, medical record review was performed | Age, sex, education, physical activity, family history of diabetes, follow-up methods (health examination or questionnaire only), use of lipid medication, and BMI |
Abbreviations: ADA, American Diabetes Association; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FFQ, food frequency questionnaire; HbA1c, hemoglobin A1c; hPDI, Healthful Plant-Based Diet Index; NA, not available; PDI, Plant-Based Diet Index; uPDI, Unhealthful Plant-Based Diet Index.
SI conversion factor: To convert glucose to millimoles per liter, multiply by 0.0555; HbA1c to proportion of total hemoglobin, multiply by 0.01.
Plant-Based Diet and Risk of Type 2 Diabetes
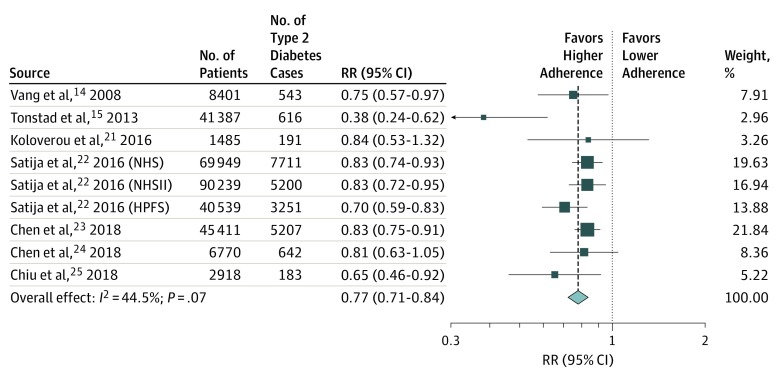
The forest plot in Figure 2 shows that greater adherence to plant-based dietary patterns is associated with a lower risk of type 2 diabetes, with a random-effects pooled RR of 0.77 (95% CI, 0.71-0.84).14,15,21,22,23,24,25 We observed modest heterogeneity among the studies with an I2 of 44.5% (P = .07 for heterogeneity). We found similar associations when using an inverse-variance fixed-effects model, with a pooled RR of 0.80 (95% CI, 0.75-0.84) (eFigure 1 in the Supplement). We did not observe any substantial changes to our main estimate with the exclusion of any single study (eFigure 2 in the Supplement). When we considered “healthful plant-based dietary index” instead of “overall plant-based dietary index” in 4 of the cohorts (ie, NHS, NHSII, HPFS, and SCHS), results were modestly strengthened. The random-effects RR associated with comparing extreme categories of intake was 0.70 (95% CI, 0.62-0.79), while the fixed-effects RR was 0.72 (95% CI, 0.69-0.76). In an exploratory analysis in 6 of the studies (AHS, NHS, NHSII, HPFS, the Rotterdam Study, and TCHS), we assessed the influence of BMI adjustment on the association between plant-based dietary patterns and risk of type 2 diabetes. As shown in eFigure 3 in the Supplement, the pooled RR was 0.53 (95% CI, 0.49-0.58) before BMI adjustment and 0.79 (95% CI, 0.74-0.85) after adjusting for BMI (P < .001 between groups).
Figure 2. Forest Plot of Studies Examining the Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes.
Pooled estimate calculated using random-effects meta-analysis. HPFS indicates Health Professionals Follow-up Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; and RR, relative risk.
Dose-Response Meta-analysis
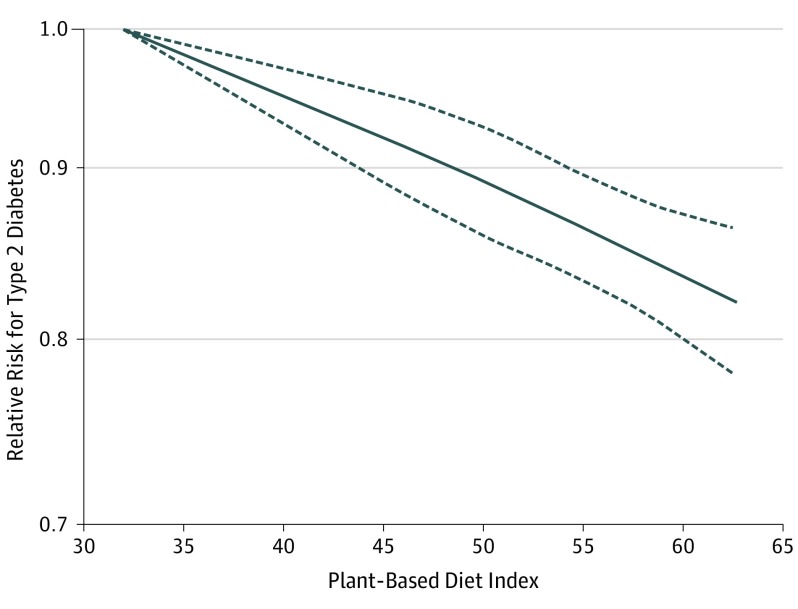
We performed a dose-response meta-analysis using data from 5 studies (NHS, NHSII, HPFS, SCHS, and the Rotterdam Study). These studies comprise most of the participants (82.4%) and cases of type 2 diabetes (93.5%). All 5 studies assessed dietary adherence using comparable plant-based diet indices, which ranged from 32.0 to 62.7, with higher numbers indicating greater adherence to a plant-based diet. Figure 3 shows a significant inverse association between plant-based indices and risk of type 2 diabetes, with no suggestion of potential nonlinearity.
Figure 3. Restricted Cubic Splines of the Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes Among 5 Studies That Used Plant-Based Dietary Indices to Classify Adherence.
P < .001 for linearity and P = .76 for nonlinearity, suggesting a largely linear association.
Subgroup Results and Meta-regression
Table 2 shows the results for analyses stratified by study characteristics.14,15,21,22,23,24,25 There was modest heterogeneity by dietary classification with nominally stronger associations observed for studies that used predefined dietary patterns (eg, vegetarian or vegan diet) compared with studies that used diet indices, with random-effects pooled RRs of 0.64 (95% CI, 0.49-0.86) for dietary pattern and 0.81 (95% CI, 0.77-0.86) for dietary indices (P = .04 for heterogeneity). We did not find any other sources of heterogeneity by age, sex, or global region. Likewise, we did not identify any potential sources of heterogeneity according to baseline values of age, BMI, sex composition, duration of follow-up, or study quality scores.
Table 2. Subgroup Analysis of Plant-Based Dietary Patterns and Risk of Type 2 Diabetes.
| Characteristic | Studies, No. | Relative Risk (95% CI) | I2, % | P Value for Heterogeneity Between Subgroups | |
|---|---|---|---|---|---|
| Inverse-Variance Fixed-Effects Meta-analysis | Random-Effects Meta-analysis | ||||
| Main estimate | 9 | 0.80 (0.75-0.84) | 0.77 (0.71-0.84) | 44.5 | .07 |
| Regiona | |||||
| North America | 5 | 0.78 (0.73-0.84) | 0.74 (0.64-0.86) | 66.9 | .77 |
| Europe | 2 | 0.82 (0.65-1.02) | 0.82 (0.65-1.02) | 0 | |
| Asia | 2 | 0.82 (0.74-0.89) | 0.78 (0.63-0.96) | 43.7 | |
| Dietary classificationb | |||||
| Dietary pattern | 4 | 0.67 (0.56-0.80) | 0.64 (0.49-0.86) | 56.3 | .04 |
| Diet index | 5 | 0.81 (0.77-0.86) | 0.81 (0.77-0.86) | 0 | |
| Age, yc | |||||
| <55 | 5 | 0.77 (0.70-0.84) | 0.77 (0.70-0.84) | 0 | .13 |
| ≥55 | 5 | 0.85 (0.78-0.92) | 0.84 (0.75-0.95) | 43.0 | |
| Sexd | |||||
| Male | 3 | 0.81 (0.76-0.87) | 0.81 (0.76-0.87) | 0 | .83 |
| Female | 4 | 0.80 (0.72-0.89) | 0.78 (0.63-0.96) | 6.7 | |
Studies from North America included Adventist Health Studies,14 Adventist Health Study-2,15 Nurses’ Health Study,22 Nurses’ Health Study II,22 and Health Professionals Follow-up Study.22 Studies from Europe included ATTICA21 and the Rotterdam Study.24 Studies from Asia included the Singapore Chinese Health Study23 and Tzu Chi Health Study.25
Studies that used prespecified dietary patterns included the Adventist Health Studies, Adventist Health Study-2, ATTICA, and Tzu Chi Health Study. Studies that used a plant-based diet index included the Nurses’ Health Study, Nurses’ Health Study II, Health Professionals Follow-up Study, Singapore Chinese Health Study, and the Rotterdam Study.
The included studies were the ATTICA, Nurses’ Health Study, Nurses’ Health Study II, Health Professionals Follow-up Study, and Singapore Chinese Health Study.
The included studies were the Nurses’ Health Study, Nurses’ Health Study II, Health Professionals Follow-up Study, Singapore Chinese Health Study, and Tzu Chi Health Study.
Assessment of Publication Bias and Risk of Bias in Individual Studies
Visual inspection of the funnel plot did not suggest obvious publication bias, although 1 study (AHS-2) rendered a large effect estimate that lay outside the pseudo 95% CI of the funnel plot (eFigure 4 in the Supplement). In addition, Egger regression tests (β = –1.79; 95% CI, –3.54 to –0.04; P = .046) and Begg-Mazumdar regression tests (continuity-corrected z score, 2.19; P = .03) indicated potential publication bias. We performed trim-and-fill analysis using the metatrim module in Stata (StataCorp LP) to assess the robustness of the association after accounting for potential publication bias (eFigure 5 in the Supplement). Our updated results remained essentially unchanged (random-effects RR, 0.81; 95% CI, 0.77-0.85; fixed-effects RR, 0.80; 95% CI, 0.72-0.89). Detailed assessment of study quality in each study using the National Heart, Lung, and Blood Institute tool can be found in eTable 4 in the Supplement. The most common weakness identified among the studies was the lack of a power or sample size calculation, with only 1 study performing this calculation.21 Another weakness was lack of repeated dietary measurements during follow-up, with only 4 studies (NHS, NHSII, HPFS, and TCHS) using repeated dietary assessments.22,25 We deemed 1 study14 to be of relatively poor quality (quality score of 9 of 14), which was owing to inadequate statistical adjustments for confounding factors, lack of additional measures to confirm self-reported diabetes, and high rates of loss to follow-up (>20%). However, exclusion of this study did not alter our risk estimate (RR, 0.77; 95% CI, 0.70-0.85; I2 = 50.6%; P = .05 for heterogeneity).
Discussion
In this meta-analysis of prospective observational studies, we found that greater adherence to a plant-based dietary patterns was inversely associated with the risk of type 2 diabetes. These findings were broadly consistent across subgroups defined by various population characteristics and robust in sensitivity analyses. This association was strengthened when only healthy plant-based foods, such as fruits, vegetables, whole grains, legumes, and nuts, were included in the definition of plant-based patterns. To our knowledge, the present study provides the most comprehensive evidence on the association between plant-based dietary patterns and incidence of type 2 diabetes.
Several potential mechanisms may explain the observed favorable associations. Plant-based diets typically emphasize fruits, vegetables, nuts, legumes, and whole grains, which contain fiber, vitamins and minerals, antioxidants, phenolic compounds, and unsaturated fatty acids. Clinical trials and observational studies have shown that these foods individually and jointly improve insulin sensitivity and blood pressure,26,27 reduce long-term weight gain, and ameliorate systemic inflammation,28,29 pathways involved in the cause of type 2 diabetes. On the other hand, these diets also deemphasize or avoid red and processed meats, which have been shown to adversely affect risk of type 2 diabetes, possibly owing to their high heme iron or dietary cholesterol contents.30 In addition, the interaction of animal-derived compounds, such as choline and l-carnitine, with the gut microbiome and the production of trimethylamine-N-oxide have been implicated in the risk of developing gestational diabetes and type 2 diabetes, although the current evidence remains inconclusive.31,32 By analyzing dietary patterns, we can quantify the synergistic effects of dietary compositions on overall disease risk, including the substitution and replacement of major macronutrient types (ie, plant sources of fat and protein for the respective animal sources).33,34
More importantly, plant-based diets may also reduce the risk of type 2 diabetes through lowering the risk of excess weight gain.17 As such, our observed associations may underestimate the true associations because all studies adjusted for BMI in statistical analyses. Our exploratory analysis in a subset of the studies demonstrated that the association with plant-based dietary patterns is substantially altered with adjustment for BMI, which may serve as both a confounder and a mediator. Multiple interventional and observational studies have indicated that increased consumption of plant-based foods can lead to short-term weight loss or prevention of long-term weight gain.35,36,37 In turn, it is likely that a considerable proportion of the protective association between plant-based diets and risk of type 2 diabetes can be attributable to weight control. At the same time, small-scale interventional studies demonstrated that plant-based dietary patterns improved measures of glycemic control independently of body weight for individuals with and without type 2 diabetes, suggesting that the health benefits extend beyond weight control.16,26,38 Moreover, plant-based diets may also improve the profile of adiposity-related risk markers, including leptin, adiponectin, high-sensitivity C-reactive protein, and interleukin-6.39,40 Nevertheless, additional experimental studies are warranted to further shed light on other pathways, such as interactions with the gut microbiota, that may account for the beneficial associations observed for plant-based diets and risk of type 2 diabetes. In addition, metabolomic studies can identify specific signatures of plant-based dietary patterns and provide insights into how specific constituents within these dietary patterns can influence disease risk.
Several studies in our meta-analysis demonstrated benefits of strict vegetarianism or veganism on the development of type 2 diabetes.14,15,25 In many populations across the world, self-reported strict vegetarianism or veganism comprises a minority of the total population, ranging from 1% to 3% in Australia and New Zealand, 3% to 9% in North America and Europe, and 8.5% in Israel, although there has been growing popularity for these dietary patterns across the world.41 Concerns have been raised that strict vegan diets that exclude dairy and fish may lead to inadequate intakes of certain nutrients in the general populations, including vitamins B12 and D and calcium, consumption of which is associated with lower risk of type 2 diabetes.42,43 However, consuming animal products is not the only way to prevent nutritional deficiencies for these specific nutrients. The consumption of a balanced plant-based dietary pattern with the inclusion of fortified foods and the use of dietary supplements can help individuals who practice a vegan or vegetarian diet meet their needs for these nutrients. The dose-response association observed in our analysis suggests that, in general populations that do not practice strict vegetarian or vegan diets, replacing animal products with healthful plant-based foods is likely to exert a significant reduction in the risk of diabetes. Meanwhile, the Dietary Guidelines for Americans 2015-2020 and the recent EAT-Lancet commission report noted that modest consumption of poultry, fish, and dairy in the context of a predominantly plant-based diet is unlikely to result in adverse health consequences.44,45 In all studies included in our dose-response meta-analysis, the highest category of adherence to plant-based dietary patterns still included a significant amount of animal foods (NHS, 3.2 servings per day; NHSII, 2.8 servings per day; HPFS, 3.9 servings per day; SCHS, 1.66 servings per day; and the Rotterdam Study, 219.3 g per day) (eTable 3 in the Supplement). Whether further reduction in animal foods in a plant-based dietary pattern exerts additional health benefits warrants additional studies.
Moreover, our findings for plant-based dietary patterns are broadly consistent with the protective associations against type 2 diabetes found for several other dietary patterns that also emphasize plant-based foods but do not completely exclude animal foods, including the Mediterranean dietary pattern, the Dietary Approaches to Stop Hypertension (DASH) pattern, and the Alternative Healthy Eating Index.46 A subgroup analysis from the Prevencion con Dieta Mediterranea randomized trial observed a significantly lower risk of type 2 diabetes among individuals in the combined Mediterranean diet groups compared with those following a low-fat control diet, although this beneficial effect was mostly driven by the group that received extra-virgin olive oil.47 Moreover, our finding of broadly consistent associations of the plant-based diets with risk of type 2 diabetes across different subgroups further supports a likely causal role of this dietary pattern in the prevention of type 2 diabetes. Overall, the totality of current evidence supports health benefits for increasing plant-based food consumption in lowering the risk of type 2 diabetes and, potentially, other cardiometabolic diseases.
We observed a modest strengthening in our overall RR when we included the risk estimates for “healthful plant-based dietary index” (random-effects RR, 0.70) rather than “overall plant-based dietary index” (RR, 0.77) in 4 studies (NHS, NHSII, HPFS, and SCHS) that examined both indices. In 3 of the studies, an unhealthful plant-based dietary pattern defined by increased consumption of refined grains, starches, and sugars was consistently associated with a higher risk of type 2 diabetes.22 This finding is consistent with prior observations that not all plant foods are equally beneficial and that the quality and food matrix (eg, whole grains vs refined grains) play an important role in determining their health effects.48 As such, current nutrition recommendations, such as the Dietary Guidelines for Americans 2015-2020,44 should continue to place specific emphasis on the quality of foods that comprise any dietary pattern.
Strengths and Limitations
Our study has several strengths, including the comprehensive synthesis of all available prospective studies with large numbers of participants and cases of type 2 diabetes, broadly consistent findings using several analytic methods and across different subgroups, and assessment of dose-response association.
Several limitations should be noted. First, all of the dietary exposures were assessed using self-reports through food frequency questionnaires, which have inherent measurement errors and misclassifications, particularly because most of the studies did not conduct repeated assessments of diet. In studies that prospectively assess diet and disease, misclassifications tend to be nondifferential and would be expected to bias true associations toward the null, potentially resulting in an underestimation of the true effect size. In addition, all of the studies were observational, which means that residual or unmeasured confounding cannot be ruled out. There was moderate heterogeneity across the studies, which may be owing to differences in the ways that plant-based dietary patterns were defined or dissimilar characteristics of individuals adhering to plant-based diets in various countries. However, because of the relatively small number of studies and limited reporting of subgroup results, we were not able to explore all potential sources of heterogeneity. Nevertheless, our findings using a random-effects model were not considerably different from the fixed-effects method. Future studies could elucidate whether other demographic or clinical characteristics, including history of metabolic disorders (glucose intolerance or hypertension), may modify the association between plant-based dietary patterns and risk of type 2 diabetes. Our meta-analysis included only studies from relatively high-income countries, and the findings may not be generalizable to populations following plant-based dietary patterns in low- or middle-income countries, where the food quality and composition (ie, greater availability of refined starches) may differ substantially.49,50
Conclusions
Our findings suggest that plant-based dietary patterns were associated with lower risk of type 2 diabetes, even after adjustment for BMI. Owing to the overall low feasibility of randomized clinical trials directly testing plant-based dietary patterns for the prevention of type 2 diabetes, our study provides important supporting evidence in conjunction with randomized clinical trials on intermediate end points to suggest a possible protective role of these dietary patterns against the development of type 2 diabetes. Further experimental evidence could help provide insights into other novel pathways that could mediate the beneficial association between plant-based dietary patterns and type 2 diabetes.
eTable 1. Search Terms and Number of Records
eTable 2. Inclusion/Exclusion Criteria for Literature Search
eTable 3. Food Composition of Plant-Based Diets
eTable 4. Assessment of Individual Study Bias
eFigure 1. Forest Plot of Prospective Studies Examining the Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes
eFigure 2. Changes to the Overall Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes When Removing One Study at a time, Calculated Using Random-Effects Meta-Analysis
eFigure 3. Forest Plot of Prospective Studies Examining the Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes, With and Without Adjustments for Body Mass Index (BMI)
eFigure 4. Funnel Plot of Prospective Studies Examining the Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes
eFigure 5. Fill and Trim Analysis to Account for Potential Publication Bias
References
- 1.Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640-3649. doi: 10.1080/10408398.2016.1138447 [DOI] [PubMed] [Google Scholar]
- 2.McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14(5):342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satija A, Bhupathiraju SN, Spiegelman D, et al. . Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411-422. doi: 10.1016/j.jacc.2017.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanou AJ, Svenson B. Reduced cancer risk in vegetarians: an analysis of recent reports. Cancer Manag Res. 2010;3:1-8. doi: 10.2147/CMAR.S6910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr. 2003;78(3)(suppl):544S-551S. doi: 10.1093/ajcn/78.3.544S [DOI] [PubMed] [Google Scholar]
- 6.Kahleova H, Levin S, Barnard N. Cardio-metabolic benefits of plant-based diets. Nutrients. 2017;9(8):E848. doi: 10.3390/nu9080848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song M, Fung TT, Hu FB, et al. . Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(10):1453-1463. doi: 10.1001/jamainternmed.2016.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr. 2016;103(4):1111-1124. doi: 10.3945/ajcn.115.123216 [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Picard-Deland E, Marette A. Fish and marine omega-3 polyunsatured fatty acid consumption and incidence of type 2 diabetes: a systematic review and meta-analysis. Int J Endocrinol. 2013;2013:501015. doi: 10.1155/2013/501015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187-225. doi: 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhupathiraju SN, Tobias DK, Malik VS, et al. . Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100(1):218-232. doi: 10.3945/ajcn.113.079533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemler EC, Hu FB. Plant-based diets for cardiovascular disease prevention: all plant foods are not created equal. Curr Atheroscler Rep. 2019;21(5):18. doi: 10.1007/s11883-019-0779-5 [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Park K. Adherence to a vegetarian diet and diabetes risk: a systematic review and meta-analysis of observational studies. Nutrients. 2017;9(6):E603. doi: 10.3390/nu9060603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann Nutr Metab. 2008;52(2):96-104. doi: 10.1159/000121365 [DOI] [PubMed] [Google Scholar]
- 15.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23(4):292-299. doi: 10.1016/j.numecd.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4(5):373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet. 2015;115(6):954-969. doi: 10.1016/j.jand.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 19.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676-680. doi: 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 20.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66-73. doi: 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koloverou E, Panagiotakos DB, Georgousopoulou EN, et al. ; ATTICA Study Group . Dietary patterns and 10-year (2002-2012) incidence of type 2 diabetes: results from the ATTICA Cohort Study. Rev Diabet Stud. 2016;13(4):246-256. doi: 10.1900/RDS.2016.13.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satija A, Bhupathiraju SN, Rimm EB, et al. . Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. doi: 10.1371/journal.pmed.1002039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen GC, Koh WP, Neelakantan N, Yuan JM, Qin LQ, van Dam RM. Diet quality indices and risk of type 2 diabetes mellitus: the Singapore Chinese Health Study. Am J Epidemiol. 2018;187(12):2651-2661. doi: 10.1093/aje/kwy183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Zuurmond MG, van der Schaft N, et al. . Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: the Rotterdam Study. Eur J Epidemiol. 2018;33(9):883-893. doi: 10.1007/s10654-018-0414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu THT, Pan WH, Lin MN, Lin CL. Vegetarian diet, change in dietary patterns, and diabetes risk: a prospective study. Nutr Diabetes. 2018;8(1):12. doi: 10.1038/s41387-018-0022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahleova H, Tura A, Hill M, Holubkov R, Barnard ND. A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: a 16-week randomized clinical trial. Nutrients. 2018;10(2):E189. doi: 10.3390/nu10020189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama Y, Nishimura K, Barnard ND, et al. . Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014;174(4):577-587. doi: 10.1001/jamainternmed.2013.14547 [DOI] [PubMed] [Google Scholar]
- 28.Kahleova H, Matoulek M, Malinska H, et al. . Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med. 2011;28(5):549-559. doi: 10.1111/j.1464-5491.2010.03209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haghighatdoost F, Bellissimo N, Totosy de Zepetnek JO, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2017;20(15):2713-2721. doi: 10.1017/S1368980017001768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515-524. doi: 10.1007/s11883-012-0282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Zhong C, Li S, et al. . Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am J Clin Nutr. 2018;108(3):603-610. doi: 10.1093/ajcn/nqy116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svingen GF, Schartum-Hansen H, Pedersen ER, et al. . Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin Chem. 2016;62(5):755-765. doi: 10.1373/clinchem.2015.250761 [DOI] [PubMed] [Google Scholar]
- 33.Malik VS, Li Y, Tobias DK, Pan A, Hu FB. Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol. 2016;183(8):715-728. doi: 10.1093/aje/kwv268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice Bradley BH. Dietary fat and risk for type 2 diabetes: a review of recent research. Curr Nutr Rep. 2018;7(4):214-226. doi: 10.1007/s13668-018-0244-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang RY, Huang CC, Hu FB, Chavarro JE. Vegetarian diets and weight reduction: a meta-analysis of randomized controlled trials. J Gen Intern Med. 2016;31(1):109-116. doi: 10.1007/s11606-015-3390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392-2404. doi: 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith JD, Hou T, Ludwig DS, et al. . Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am J Clin Nutr. 2015;101(6):1216-1224. doi: 10.3945/ajcn.114.100867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnard ND, Cohen J, Jenkins DJ, et al. . A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89(5):1588S-1596S. doi: 10.3945/ajcn.2009.26736H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baden MY, Satija A, Hu FB, Huang T. Change in plant-based diet quality is associated with changes in plasma adiposity-associated biomarker concentrations in women. J Nutr. 2019;149(4):676-686. doi: 10.1093/jn/nxy301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eichelmann F, Schwingshackl L, Fedirko V, Aleksandrova K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes Rev. 2016;17(11):1067-1079. doi: 10.1111/obr.12439 [DOI] [PubMed] [Google Scholar]
- 41.Ruby MB. Vegetarianism: a blossoming field of study. Appetite. 2012;58(1):141-150. doi: 10.1016/j.appet.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 42.Craig WJ. Health effects of vegan diets. Am J Clin Nutr. 2009;89(5):1627S-1633S. doi: 10.3945/ajcn.2009.26736N [DOI] [PubMed] [Google Scholar]
- 43.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017-2029. doi: 10.1210/jc.2007-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietary Guidelines for Americans 2015-2020. 8th ed. December 2015. US Department of Health and Human Services and US Department of Agriculture website. https://health.gov/dietaryguidelines/2015/guidelines/. Accessed June 21, 2019.
- 45.Willett W, Rockström J, Loken B, et al. . Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447-492. doi: 10.1016/S0140-6736(18)31788-4 [DOI] [PubMed] [Google Scholar]
- 46.Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147(6):1174-1182. doi: 10.3945/jn.116.242552 [DOI] [PubMed] [Google Scholar]
- 47.Salas-Salvadó J, Bulló M, Estruch R, et al. . Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. 2014;160(1):1-10. doi: 10.7326/M13-1725 [DOI] [PubMed] [Google Scholar]
- 48.AlEssa HB, Bhupathiraju SN, Malik VS, et al. . Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am J Clin Nutr. 2015;102(6):1543-1553. doi: 10.3945/ajcn.115.116558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pawlak R. Vegetarian diets in the prevention and management of diabetes and its complications. Diabetes Spectr. 2017;30(2):82-88. doi: 10.2337/ds16-0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dehghan M, Mente A, Zhang X, et al. ; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390(10107):2050-2062. doi: 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Terms and Number of Records
eTable 2. Inclusion/Exclusion Criteria for Literature Search
eTable 3. Food Composition of Plant-Based Diets
eTable 4. Assessment of Individual Study Bias
eFigure 1. Forest Plot of Prospective Studies Examining the Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes
eFigure 2. Changes to the Overall Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes When Removing One Study at a time, Calculated Using Random-Effects Meta-Analysis
eFigure 3. Forest Plot of Prospective Studies Examining the Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes, With and Without Adjustments for Body Mass Index (BMI)
eFigure 4. Funnel Plot of Prospective Studies Examining the Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes
eFigure 5. Fill and Trim Analysis to Account for Potential Publication Bias