Key Points
Question
What is the scope of pregnancy-related adverse events associated with isotretinoin reported to the US Food and Drug Administration?
Findings
In this analysis of pregnancy-related adverse events associated with isotretinoin reported to the US Food and Drug Administration, there were 6740 total pregnancies among women exposed to isotretinoin reported from 1997 to 2017, peaking with 768 pregnancies at the 2006 initiation of the iPLEDGE program, for a rate of 0.65% per female of childbearing potential (768 of 117 784). Although reports of pregnancies, abortions, and fetal defects have decreased since 2006, several hundred pregnancies among women taking isotretinoin have been reported annually in the last decade.
Meaning
Although the number of reports of pregnancies among women taking isotretinoin has decreased from peaks in the mid-2000s, these pregnancies persist in the iPLEDGE era.
Abstract
Importance
iPLEDGE is a rigorous program initiated in 2006 to reduce fetal exposure to isotretinoin, a disease-modifying medication for acne that carries a risk of teratogenesis. Despite the imposition of iPLEDGE requirements on patients and clinicians, the scope of isotretinoin-related adverse events is unknown.
Objective
To determine the frequency and rate of pregnancy and pregnancy-related adverse events among women taking isotretinoin reported to the US Food and Drug Administration (FDA).
Design, Setting, and Participants
Pregnancy reports from the FDA Adverse Event Reporting System, a public database of medication adverse event reports filed by prescribers, consumers, and manufacturers, were used to perform a retrospective analysis of pregnancy-related adverse events associated with isotretinoin from January 1, 1997, to December 31, 2017. Each individual reporting any pregnancy-related adverse event signified 1 pregnancy. Abortions, pregnancies that occurred while contraception was used, and fetal defects were counted as subgroups of total pregnancy events.
Main Outcomes and Measures
The frequency of pregnancy and of pregnancy-related events (abortions, pregnancies that occurred while using contraception, and fetal defects) were stratified by year that the FDA was notified of the event and by age. The rates of adverse events were calculated using isotretinoin prescribing data.
Results
There was a total of 6740 pregnancies among women taking isotretinoin reported to the FDA from 1997 to 2017, peaking in 2006 (768 pregnancies) before settling into a range of 218 to 310 annual reports of pregnancy after 2011. The mean (SD) age of the women was 24.6 (7.1) years. The rate of pregnancy for females of childbearing potential was between 0.33% (388 of 115 925) and 0.65% (768 of 117 784), with a peak in 2006. Although pregnancies, abortions, and fetal defects among women taking isotretinoin have decreased since the initiation of iPLEDGE in 2006, all 3 persist.
Conclusions and Relevance
The number of reports of pregnancies, abortions, and fetal defects among women taking isotretinoin has decreased since peaking around the initiation of iPLEDGE in 2006. Explanations for this trend include a broader national decrease in teenage pregnancies and abortion rates, improvements in access to effective long-term and emergency contraception, stringent iPLEDGE requirements, and reporting fatigue over time. Despite the decrease, persistent reporting of pregnancy-related events in the last decade warrants investigation into the efficacy of iPLEDGE and exploration of new approaches for lowering fetal exposure to isotretinoin.
This analysis uses the FDA Adverse Event Reporting System to determine the frequency and rate of pregnancy among women taking isotretinoin and of pregnancy-related adverse events associated with isotretinoin.
Introduction
Isotretinoin is a disease-modifying treatment for acne in which a primary concern is teratogenesis in females of childbearing potential.1 Fetal exposure to isotretinoin has a risk of spontaneous abortion of approximately 20% and a risk of embryopathy of 18% to 28% in live births.2
Since the approval by the US Food and Drug Administration (FDA) of isotretinoin in 1982, there have been 4 Risk Evaluation and Mitigation Strategy systems to verify the proper use of drugs in which risks may otherwise exceed benefits.3 The iPLEDGE program, the most recent iteration, was implemented in 2006 to reduce fetal exposure to isotretinoin.4
The iPLEDGE program mandates registration of wholesalers, clinicians, and pharmacies who provide isotretinoin, as well as patients with a prescription for isotretinoin, and requires females of childbearing potential to have a pregnancy test with negative results and to verify 2 forms of contraception monthly.4 Despite these efforts, which place both time and financial strain on patients,4,5 it is unclear whether iPLEDGE has decreased fetal exposure to isotretinoin compared with prior Risk Evaluation and Mitigation Strategy programs.6
The scope of isotretinoin-related adverse events in the age of iPLEDGE is unknown. In this study, we evaluate the trends in pregnancies and pregnancy-related adverse events among patients taking isotretinoin in the United States between 1997 and 2017.
Methods
We performed a retrospective analysis of FDA reports of pregnancy-related adverse events associated with isotretinoin from January 1, 1997, to December 31, 2017, using the FDA Adverse Event Reporting System (FAERS), a database of medication adverse event reports filed by prescribers, consumers, and manufacturers. The data were accessed using FDAble, a search engine for FAERS.7 The study was deemed exempt by the Partners Institutional Review Board, as it used publicly available national data. These data were all submitted to the FDA by patients, clinicians, and drug manufacturers. These data were deidentified by the FDA.
In FAERS, adverse events are classified by reaction terms. We identified 145 unique reaction terms coded within the category “pregnancy, puerperium and perinatal conditions.” Of these, 8 pertaining to breastfeeding and male patients were omitted, and the remaining 137 were sorted by pregnancy-related outcome: spontaneous abortion, therapeutic abortion, pregnancy while using contraception, and fetal defect (eTable in the Supplement).
Because multiple reaction terms were sometimes reported for a unique pregnancy, each individual patient was counted only once as a pregnancy regardless of how many different reaction terms were reported for that individual. Counts were sorted into year of occurrence by the year that the FDA was notified of the event and stratified by age group.
Using these counts of adverse events and prescribing data from the Dermatologic and Ophthalmic Drugs Advisory Committee,8,9 we calculated the rates of adverse events for the years in which data were publicly available (2006 and 2008-2010). The number of patients enrolled in iPLEDGE in 2008-2010 is reported cumulatively since the start of the program; thus, we could not calculate an event rate for 2008. Data for all other years, including 2007, were not available. Because data for year 1 of iPLEDGE include December 31, 2006, through February 28, 2007, monthly prescriptions were calculated and multiplied by 12 to estimate prescriptions in 2006.
Results
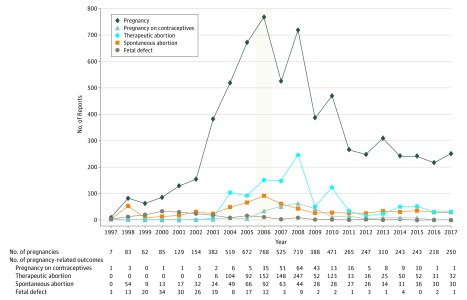
A total of 6740 pregnancies were reported to the FDA for patients taking isotretinoin from 1997 to 2017, peaking in 2006 with 768 pregnancies. A total of 4647 of 6740 pregnancies (68.9%) were reported after the introduction of iPLEDGE in 2006. Abortions (n = 291), of which 247 (84.9%) were therapeutic, and pregnancies that occurred while taking contraception (n = 64) peaked in 2008. All pregnancies and abortions decreased after this peak. Abortions comprised 28.1% of all pregnancy reports (1896 of 6740); however, only 10.9% of pregnancy reports (733 of 6740) were spontaneous abortions. Fetal defect reports peaked in 2000 (n = 34) and decreased to single digits (0-4) annually after 2008 (Figure), representing a small percentage of pregnancy-related adverse events annually after 2008.
Figure. Data on Pregnancies and Pregnancy-Related Adverse Outcomes Reported to the US Food and Drug Administration Among Patients Taking Isotretinoin by Year, 1997-2017.
Pregnancy-related outcomes represent subgroups of total pregnancies. The year 2006 is emphasized with shading and represents the implementation year of iPLEDGE.
Of the 41.3% of reports (2784 of 6740) that included the patient’s age, the mean (SD) age was 24.6 (7.1) years. The highest numbers of reported pregnancies (1510), therapeutic abortions (317), and spontaneous abortions (227) occurred among patients 20 to 29 years of age (Table 1).
Table 1. Reported Pregnancies and Pregnancy-Related Outcomes by Agea.
| Characteristic | Patients Age | Total No. | |||
|---|---|---|---|---|---|
| 15-19 | 20-29 | 30-39 | ≥40 | ||
| Pregnancy | 565 (20.3) | 1510 (54.2) | 536 (19.3) | 173 (6.2) | 2784 |
| Pregnancy-related outcomesb | |||||
| Pregnancy while taking contraception | 42 (17.1) | 147 (60.0) | 44 (18.0) | 12 (4.9) | 245 |
| Abortion | |||||
| Therapeutic | 120 (21.2) | 317 (55.9) | 117 (20.6) | 13 (2.3) | 567 |
| Spontaneous | 65 (17.1) | 227 (59.7) | 75 (19.7) | 13 (3.4) | 380 |
| Fetal defect | 12 (30.8) | 13 (33.3) | 8 (20.5) | 6 (15.4) | 39 |
Unless otherwise indicated, data are reported as number (percentage) of women.
Pregnancy-related outcomes represent subgroups of total pregnancies.
The rate of pregnancy per isotretinoin prescription in 2006 and 2008-2010 ranged from 0.07% (388 of 519 641) to 0.31% (768 of 245 404) (Table 2), with a high of 0.31% in 2006. The rate of pregnancy per female of childbearing potential registered in iPLEDGE in 2006 and 2009-2010 ranged from 0.33% (388 of 115 925) to 0.65% (768 of 117 784), also peaking in 2006 at 0.65%.
Table 2. Rates of Pregnancy-Related Adverse Events.
| Event | Year 1 (2006), %a | Year 3 (2008), %b | Year 4 (2009), % | Year 5 (2010), % |
|---|---|---|---|---|
| Rate of Events per Total No. of Isotretinoin Prescriptions Among Females of Childbearing Potential | ||||
| Pregnancy (total) | 0.3113 | 0.1490 | 0.0747 | 0.1050 |
| Pregnancy while taking contraception | 0.0143 | 0.0133 | 0.0083 | 0.0029 |
| Abortion (total) | 0.0994 | 0.0602 | 0.0154 | 0.0337 |
| Therapeutic abortion | 0.0619 | 0.0511 | 0.0100 | 0.0274 |
| Fetal defects (total) | 0.0049 | 0.0019 | 0.0004 | 0.0004 |
| Rate of Events per Total No. of Females of Childbearing Potential Registered in iPLEDGE | ||||
| Pregnancy (total) | 0.6486 | NA | 0.3347 | 0.5144 |
| Pregnancy while taking contraception | 0.0042 | NA | 0.0371 | 0.0142 |
| Abortion (total) | 0.2072 | NA | 0.0690 | 0.1649 |
| Therapeutic abortion | 0.1290 | NA | 0.0449 | 0.1343 |
| Fetal defects (total) | 0.0102 | NA | 0.0017 | 0.0022 |
Abbreviation: NA, not available.
Calculated based on monthly prescriptions from December 31, 2006, to February 28, 2007.
The number of patients registered is only available cumulatively since 2006; thus, the number of females of childbearing potential enrolled in 2008 is unknown. Total number of prescriptions to females of childbearing potential: 2006, 245 404; 2008, 481 364; 2009, 519 641; and 2010, 448 625. Total number of females of childbearing potential enrolled in iPLEDGE: 2006, 117 784 (approximated from 14-month period); 2009, 115 925; and 2010, 91 556.
Discussion
The iPLEDGE program was established in 2006 with the aim of reducing fetal exposure to isotretinoin. Our findings demonstrate that reports of pregnancy among women taking isotretinoin are concentrated among those aged 20 to 29 years, peaked in 2006, and have been consistent since 2011. For the years in which data were available, the calculated annual rates of pregnancy while using isotretinoin per prescription for females of childbearing potential (<0.5%) and per female of childbearing potential enrolled in iPLEDGE (<0.65%) were low (Table 2).
Although a spike in reporting coincided with the implementation of iPLEDGE, the subsequent downward trend may be due to several factors. Decreasing isotretinoin-related pregnancies must be considered in the context of national pregnancy and contraception practices. Since 2000, the increased use of long-term birth control, intrauterine devices, and emergency contraception has been associated with reductions in teenage pregnancy.10,11,12
Reports of both spontaneous and therapeutic abortions have also decreased since the start of iPLEDGE. Abortions comprised 28.1% of all pregnancy reports; however, only 10.9% of pregnancy reports were spontaneous abortions, which is less than the previously published rate of 20%.2 Fetal defects were also reported less frequently than the estimated 18% to 28% risk of embryopathy,2 a very small proportion of annual pregnancy-related events after 2008. Therapeutic abortions of affected fetuses and incomplete reporting may explain the trends in these outcomes.
It is also possible that iPLEDGE’s stringent contraceptive requirements are independently associated with the decrease in pregnancies. The number of annual isotretinoin prescriptions is similar before and after the implementation of iPLEDGE,13,14 which suggests that there is a true decrease in reporting of pregnancies relative to prescriptions. Although there is no control group for comparison, the number of pregnancy reports remained persistently high for several years after the implementation of iPLEDGE in 2006 and plateaued at lower numbers beginning in 2011. If iPLEDGE were the primary reason for the decreasing number of pregnancies among women taking isotretinoin, then a decrease in the pregnancy rate would have been identified sooner after the implementation of iPLEDGE.
Although the number of reports of pregnancy among women taking isotretinoin is decreasing, a substantial number of pregnancies and abortions persist. Despite being more stringent than its predecessor, SMART (the System to Manage Accutane Related Teratogenicity), iPLEDGE did not lower the rate of fetal exposure to isotretinoin compared with SMART, which resulted in a rate of 0.29% exposed pregnancies per isotretinoin treatment course.6 Randomized clinical trials comparing iPLEDGE with alternative Risk Evaluation and Mitigation Strategy plans are warranted to determine the optimum approach to modifying risk for patients taking isotretinoin.
Several improvements to iPLEDGE have been suggested. Although iPLEDGE successfully informs women about teratogenicity, counseling regarding contraception options is limited.15 Facilitating access to contraception by automatically providing emergency contraception alongside isotretinoin prescriptions may also reduce the number of pregnancies.
Limitations
These data must be considered in the context of our study design. Data in FAERS are limited by reliance on proper reporting. Reporting fatigue among physicians and patients may have influenced our results. The emphasis on pregnancy prevention and mandated reporting by iPLEDGE suggests that most events reported to physicians should be recorded, and behaviors associated with reporting variability should be consistent over time and are unlikely to affect overall trends. Because the precise classification of pregnancy in FAERS is reporter-dependent, pregnancy-related outcomes are likely underreported, particularly in the category of pregnancy while using contraception, because information regarding the nature of contraception in these reports is lacking. In addition, the lack of a denominator in number of isotretinoin courses for every year investigated limits our ability to determine the true rate of pregnancy-related events.
Conclusions
Although the number of pregnancy-related adverse events for patients taking isotretinoin has decreased since 2006, pregnancies, abortions, and fetal defects associated with to isotretinoin exposure continue to be a problem. Further research is required to determine the most efficacious system to reduce complications for patients and administrative requirements for physicians while at the same time maintaining access to this important drug.
eTable. Reaction Term Grouping Strategy for Pregnancy and Pregnancy-Related Outcomes
References
- 1.Vallerand IA, Lewinson RT, Farris MS, et al. Efficacy and adverse events of oral isotretinoin for acne: a systematic review. Br J Dermatol. 2018;178(1):1175-1179. doi: 10.1111/bjd.15668 [DOI] [PubMed] [Google Scholar]
- 2.Sladden MJ, Harman KE. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin? Arch Dermatol. 2007;143(9):1187-1188. doi: 10.1001/archderm.143.9.1187 [DOI] [PubMed] [Google Scholar]
- 3.Slatko GH. Risk evaluation and mitigation strategy (REMS): FDA perspective on what physicians need to know. Am Fam Physician. 2015;92(9):771-772. [PubMed] [Google Scholar]
- 4.Charrow A, Xia FD, Lu J, Waul M, Joyce C, Mostaghimi A. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PLoS One. 2019;14(3):e0210445. doi: 10.1371/journal.pone.0210445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori WS, Houston N, Moreau JF, et al. Personal burden of isotretinoin therapy and willingness to pay for electronic follow-up visits. JAMA Dermatol. 2016;152(3):338-340. doi: 10.1001/jamadermatol.2015.4763 [DOI] [PubMed] [Google Scholar]
- 6.Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65(6):1117-1125. doi: 10.1016/j.jaad.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 7.Pratt LA, Danese PN. More eyeballs on AERS. Nat Biotechnol. 2009;27(7):601-602. doi: 10.1038/nbt0709-601 [DOI] [PubMed] [Google Scholar]
- 8.Dermatologic and Opthalmic Drugs Advisory Committee, Food and Drug Administration Briefing document for iPLEDGE year one update. 2007. https://wayback.archive-it.org/7993/20171102022007/https://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4311b1-02-ipledge.pdf. Accessed June 13, 2019.
- 9.Dermatologic and Opthalmic Drugs Advisory Committee, Food and Drug Administration Briefing document for iPLEDGE. https://wayback.archive-it.org/7993/20170405213524/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOphthalmicDrugsAdvisoryCommittee/UCM281376.pdf. Accessed June 13, 2019.
- 10.Hamilton BE, Mathews TJ. Continued declines in teen births in the United States, 2015. NCHS Data Brief. 2016;(259):1-8. [PubMed] [Google Scholar]
- 11.Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63(2):253-256. doi: 10.1016/j.jadohealth.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels K, Jones J, Abma J. Use of emergency contraception among women aged 15-44: United States, 2006-2010. NCHS Data Brief. 2013;(112):1-8. [PubMed] [Google Scholar]
- 13.Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77(3):456-463.e4. doi: 10.1016/j.jaad.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 14.Mendelsohn AB, Governale L, Trontell A, Seligman P. Changes in isotretinoin prescribing before and after implementation of the System to Manage Accutane Related Teratogenicity (SMART) risk management program. Pharmacoepidemiol Drug Saf. 2005;14(9):615-618. doi: 10.1002/pds.1111 [DOI] [PubMed] [Google Scholar]
- 15.Werner CA, Papic MJ, Ferris LK, Schwarz EB. Promoting safe use of isotretinoin by increasing contraceptive knowledge. JAMA Dermatol. 2015;151(4):389-393. doi: 10.1001/jamadermatol.2014.4171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Reaction Term Grouping Strategy for Pregnancy and Pregnancy-Related Outcomes