Key Points
Question
Is direct oral anticoagulant a better option than warfarin for secondary prevention in older survivors of ischemic stroke who have atrial fibrillation?
Findings
In this observational study of 11 662 patients with atrial fibrillation who had had an ischemic stroke and were anticoagulation naive, patients discharged while receiving direct oral anticoagulants had more days at home postdischarge and were less likely to experience major adverse cardiovascular events, all-cause mortality, all-cause readmissions, cardiovascular readmissions, or hemorrhagic strokes, despite a small but significant increase in gastrointestinal bleeding.
Meaning
Direct oral anticoagulants appear to be an effective and safe treatment option compared with warfarin for patients with atrial fibrillation who have ischemic stroke.
This cohort assesses the clinical effectiveness of direct oral anticoagulants vs warfarin for stroke prevention in patients with atrial fibrillation.
Abstract
Importance
Current guidelines recommend direct oral anticoagulants (DOACs) over warfarin for stroke prevention in patients with atrial fibrillation (AF) who are at high risk. Despite demonstrated efficacy in clinical trials, real-world data of DOACs vs warfarin for secondary prevention in patients with ischemic stroke are largely based on administrative claims or have not focused on patient-centered outcomes.
Objective
To examine the clinical effectiveness of DOACs (dabigatran, rivaroxaban, or apixaban) vs warfarin after ischemic stroke in patients with AF.
Design, Setting, and Participants
This cohort study included patients who were 65 years or older, had AF, were anticoagulation naive, and were discharged from 1041 Get With The Guidelines–Stroke–associated hospitals for acute ischemic stroke between October 2011 and December 2014. Data were linked to Medicare claims for long-term outcomes (up to December 2015). Analyses were completed in July 2018.
Exposures
DOACs vs warfarin prescription at discharge.
Main Outcomes and Measures
The primary outcomes were home time, a patient-centered measure defined as the total number of days free from death and institutional care after discharge, and major adverse cardiovascular events. A propensity score–overlap weighting method was used to account for differences in observed characteristics between groups.
Results
Of 11 662 survivors of acute ischemic stroke (median [interquartile range] age, 80 [74-86] years), 4041 (34.7%) were discharged with DOACs and 7621 with warfarin. Except for National Institutes of Health Stroke Scale scores (median [interquartile range], 4 [1-9] vs 5 [2-11]), baseline characteristics were similar between groups. Patients discharged with DOACs (vs warfarin) had more days at home (mean [SD], 287.2 [114.7] vs 263.0 [127.3] days; adjusted difference, 15.6 [99% CI, 9.0-22.1] days) during the first year postdischarge and were less likely to experience major adverse cardiovascular events (adjusted hazard ratio [aHR], 0.89 [99% CI, 0.83-0.96]). Also, in patients receiving DOACs, there were fewer deaths (aHR, 0.88 [95% CI, 0.82-0.95]; P < .001), all-cause readmissions (aHR, 0.93 [95% CI, 0.88-0.97]; P = .003), cardiovascular readmissions (aHR, 0.92 [95% CI, 0.86-0.99]; P = .02), hemorrhagic strokes (aHR, 0.69 [95% CI, 0.50-0.95]; P = .02), and hospitalizations with bleeding (aHR, 0.89 [95% CI, 0.81-0.97]; P = .009) but a higher risk of gastrointestinal bleeding (aHR, 1.14 [95% CI, 1.01-1.30]; P = .03).
Conclusions and Relevance
In patients with acute ischemic stroke and AF, DOAC use at discharge was associated with better long-term outcomes relative to warfarin.
Introduction
Oral anticoagulant treatment with warfarin or direct oral anticoagulants (DOACs) plays a pivotal role in stroke prevention for patients with nonvalvular atrial fibrillation (AF) who are at high risk.1,2,3,4 Meta-analysis5 from clinical trials suggests that DOACs can reduce the risks of stroke, intracranial hemorrhage, and mortality but have similar risk of major bleeding and increased gastrointestinal bleeding compared with warfarin. Consequently, DOACs have increasingly been used as alternatives to warfarin for stroke prevention and are recommended over warfarin in the 2019 American College of Cardiology/American Heart Association/Heart Rhythm Society guideline for the management of patients with AF.6,7,8 Despite demonstrated efficacy in pivotal clinical trials, real-world evidence to date is largely based on administrative claims without clinical details and does not address a high-risk population, such as older adults with acute ischemic stroke who are at increased risk for both recurrent ischemic events and bleeding complications and therefore need secondary prevention; other studies are from European or Asian countries with different treatment patterns and guideline recommendations.9,10,11,12,13,14,15,16,17,18,19,20,21,22 From a patient’s perspective, an important measure of the benefit of anticoagulant treatment beyond survival is the prevention of recurrent events or prolonged hospital stays. Survivors of stroke have identified “being alive at home, without recurrent stroke, or being hospitalized for complications”23(p37) as the most desirable outcome.24,25 Such patient-centered outcomes have not been well studied as an end point in research on DOACs.
Using data from the American Heart Association/American Stroke Association (AHA/ASA) Get With The Guidelines–Stroke (GWTG-Stroke) clinical registry, linked with Centers for Medicare & Medicaid Services (CMS) claims, we examined the clinical effectiveness of DOACs vs warfarin in older survivors of acute ischemic stroke with AF and who were prescribed oral anticoagulants at discharge. Based on input from patients and stakeholders, we evaluated home time, a patient-centered outcome as a measure of functional status and independence after hospital discharge, as well as freedom from major adverse cardiovascular events (MACE) after anticoagulation treatment.23,25,26
Methods
Study Design and Data Sources
Details of the design and conduct of the Patient-Centered Research into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) study (ClinicalTrials.gov identifier: NCT02146274) have been previously described.23,27,28,29 The PROSPER study builds on the GWTG-Stroke program, a national stroke registry and quality-improvement initiative sponsored by the AHA/ASA.30,31 Standardized data collection includes patient demographics, medical history, medications prior to admission, diagnostic testing, brain imaging, treatment, in-hospital outcomes, and medication prescribed at discharge. Dabigatran and rivaroxaban prescribed at discharge was added to the registry in October 2011, followed by apixaban in October 2013 and edoxaban in September 2015. The validity and reliability of data collection have been reported previously.32 Each participating hospital received either human research approval to enroll patients without individual consent under the Common Rule or a waiver of authorization and exemption from subsequent review by their institutional review board.33 This study was approved by the institutional review board of Duke University.
To assess longitudinal outcomes, we linked GWTG-Stroke data to Medicare claims by matching the data on a series of indirect identifiers, including admission date, discharge date, the patient’s age or date of birth, and sex. This linkage method has been successfully completed and validated using Medicare inpatient claims.34 Previous work has shown that patients in the linked GWTG-Stroke and Medicare database are representative of the national Medicare population receiving care for ischemic stroke.35 The corporation IQVIA serves as the GWTG data collection and coordination center. The Duke Clinical Research Institute serves as the GWTG data analysis center and has an agreement to analyze the aggregate deidentified data for research purposes.
Study Population and Variable of Interest
This is a retrospective analysis of patients 65 years or older who were admitted to GWTG-Stroke hospitals and discharged alive between October 2011 and December 2014 for acute ischemic stroke and who had a medical history of AF or flutter or documented persistent or paroxysmal AF or flutter during their hospitalization. Unless the patient died during follow-up, each individual had at least 1 year of follow-up data after the index hospital discharge through Medicare (up to December 2015). Based on discharge medications abstracted from the medical record, we dichotomized patient groups as those prescribed DOACs (either dabigatran, rivaroxaban, or apixaban) vs warfarin at discharge. Edoxaban was approved by the US Food and Drug Administration in January 2015; therefore, edoxaban was not available during the index hospitalization.
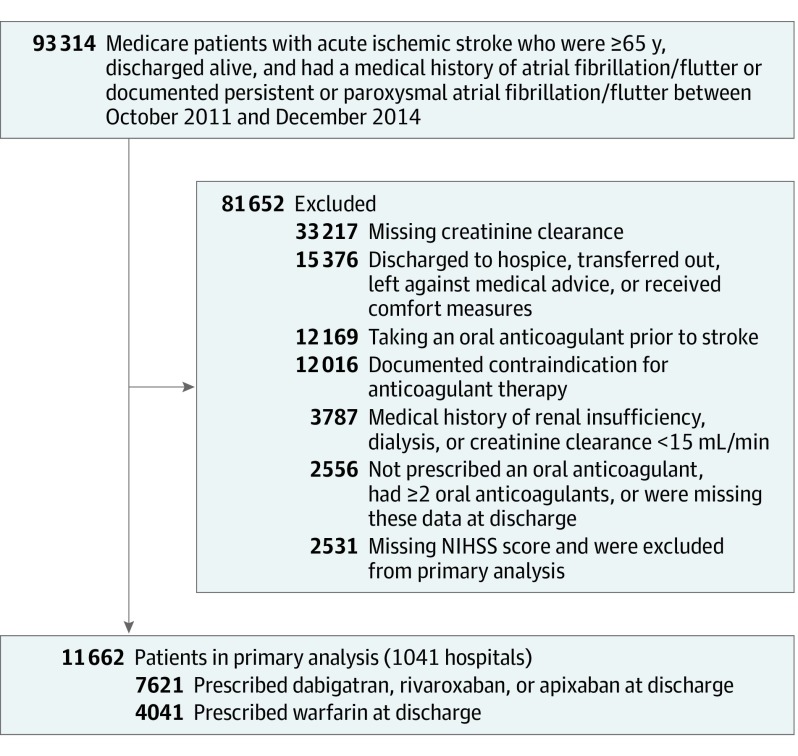
Figure 1 shows details of inclusion and exclusion criteria. Briefly, we excluded patients who were discharged to hospice; transferred to another hospital; received comfort measures only; had documented contraindications for anticoagulation treatment; had a medical history of renal insufficiency, dialysis, or creatinine clearance less than 15 mL/min; or with data missing, since these patients might not be eligible for DOACs. To avoid prevalent user bias, we further excluded those receiving chronic anticoagulation treatment before the index stroke admission. The National Institutes of Health Stroke Scale (NIHSS) score is a critical factor associated with outcomes in acute ischemic stroke, so we further excluded individuals with missing NIHSS scores (n = 2531) for the primary analysis. After these exclusions, the primary study population consisted of 11 662 survivors of ischemic stroke with complete NIHSS score data from 1041 GWTG-Stroke hospitals in the United States. Sensitivity analyses included individuals without NIHSS scores recorded (n = 14 193). The eTable in Supplement 1 displays characteristics of the patient cohorts of those with and without NIHSS scores recorded.
Figure 1. Study Population.
This figure displays the final study population from the initial cohort through exclusions. NIHSS indicates the National Institutes of Health Stroke Scale.
Outcome Measures
The primary outcomes were home time and MACE. Home time is defined as the total number of days alive and out of the hospital or a skilled nursing facility during the first year after the index hospital discharge, reflecting a patient’s desire of “being alive at home, without recurrent stroke, or being hospitalized for complications.”23(p37) Home time represents a patient-centered outcome measure for an episode of stroke care and is highly correlated with modified Rankin scale score,26 a gold standard measure of functional status commonly used in stroke clinical trials.36,37 The MACE end point is a composite measure of all-cause mortality, cardiovascular, or cerebrovascular readmission. Secondary outcomes included all-cause mortality, fatal bleeding (readmission for bleeding with in-hospital mortality), all-cause readmission, cardiovascular readmission, ischemic stroke readmission, systemic embolism readmission, hemorrhagic stroke readmission, gastrointestinal bleeding, and any bleeding requiring hospitalization. We determined the date of death through the Medicare denominator files and ascertained the date and cause of readmission from the Medicare hospital claims data, as has been done previously.27,29
Statistical Analyses
Consistent with Patient-Centered Outcomes Research Institute methods, we prespecified a statistical analysis plan similar to those used in clinical trials (Statistical Analysis Plan in Supplement 2).23,38 Means, medians, and percentages were used to describe the distribution of continuous and categorical variables. Standardized differences were used to compare baseline characteristics between patient cohorts. An absolute standardized difference greater than 10% indicates significant imbalance of a covariate, whereas a smaller value supports the assumption of balance between treatment groups.39
We first described the distribution of home time and the incidence rates for longitudinal outcomes by DOACs at discharge. Incidence rates were calculated as the number of new events divided by person-time in years at risk. Follow-up for all events of interests was censored at the event, the patient’s death, or December 31, 2015, whichever came first. We then used negative binomial model for the continuous outcome of home time and Cox proportional hazards model for binary outcomes such as MACE. These analyses accounted for within-hospital clustering by using hospital-specific random intercepts for negative binomial models and robust sandwich estimators for Cox proportional hazards models.
We used a propensity score–overlap weighting approach to control for potential selection bias.40 The overlap weighting is an extension of the propensity score method to balance covariates between 2 treatment groups. Each individual’s statistical weight is proportional to the probability of that individual being assigned to the opposite treatment group, derived from a generalized logistic regression model with treatment (DOACs vs warfarin) as the dependent variable, all observed patient-level and hospital-level characteristics as the independent variables, and generalized estimating equations to account for within-hospital correlations. The overlap weighting method minimizes the asymptotic variance of the nonparametric estimate of the weighted average treatment effect within the class of balancing weights and yields balance between treatment groups in the means of each covariate included in the model.40
Covariates included baseline patient sociodemographic and clinical factors, as well as hospital characteristics, that are expected to be associated with outcome and have been used in prior GWTG-Stroke analyses.27,41,42 These included patient age, sex, self-reported race/ethnicity (as recorded by admission staff, medical staff, or both, usually during the registration), and zip code–level socioeconomic status; medical history; emergency medical services transportation; on-hour arrival (defined as Monday through Friday from 7 am to 6 pm, except for holidays) or off-hour arrival (defined as all other times); NIHSS score at presentation; first measure of creatinine clearance, heart rate, and systolic blood pressure at admission; and body mass index (calculated as weight in kilograms divided by height in meters squared). Hospital characteristics included hospital bed size; annual ischemic stroke volume; AHA/ASA Joint Commission certification as a primary or comprehensive stroke center; academic status; rural or urban location; and hospital location (by US region).
To address heterogeneity of treatment effects, we performed these analyses in clinically relevant subgroups by age (65-80 and >80 years), sex, history of previous stroke or transient ischemic attack, myocardial infarction or coronary artery disease, diabetes mellitus, stroke severity (NIHSS score ≤4 and >4, which is the median NIHSS score in the cohort), and concomitant antiplatelet therapy at discharge. Because of the multiple outcomes assessed, we used P value adjustment with 99% CIs to be more conservative for the primary outcome of home time and MACE. We also reported hospital readmission with pneumonia and sepsis (either primary or secondary diagnosis) as negative outcome controls (falsification end points) to account for potential treatment selection bias.37,43 We selected these 2 conditions because they are expected to be unassociated with anticoagulant choice and common enough to provide sufficient power to detect false positives. The primary analyses focused on patients with complete NIHSS score data (n = 11 662). Sensitivity analyses included individuals without NIHSS scores recorded and excluded NIHSS scores as a covariate in the model (n = 14 193). Because home time is affected by institutional care, such as a transfer to skilled nursing facility or inpatient rehabilitation facility, a subgroup analysis of home time was performed in patients discharged to home (n = 5023). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). All P values were 2-sided, with P < .01 as the significance threshold for primary outcomes of home time and MACE and P < .05 as the threshold for secondary and negative outcome controls.
Results
Of 11 662 older survivors of ischemic stroke who had AF and complete NIHSS score data, 4041 patients (34.7%) were prescribed DOACs (1239 were prescribed dabigatran; 1842, rivaroxaban; and 960, apixaban), and 7621 patients (65.3%) were prescribed warfarin at discharge. Table 1 shows the baseline sociodemographic, clinical, and hospital characteristics according to the oral anticoagulant prescribed at discharge. The median age was 80 (interquartile range [IQR], 74-86) years, and 2277 individuals prescribed DOACs and 4292 individuals prescribed warfarin (56.3%) were female. The median (IQR) pre-stroke CHA2DS2-VASc score was 4 (3-5), with 11 452 patients (98.2%) scoring 2 or more, suggesting that most should have received anticoagulant therapy but were not treated before a stroke occurred. Except for NIHSS scores (median [IQR]: DOACs, 4 [1-9] vs warfarin, 5 [2-11]) and zip code–level unemployment rates (median [IQR]: DOACs, 6.9% [5.8%-8.1%]; warfarin, 7.3% [6.1%-8.7%]), all patient-level characteristics were similar between DOACs and warfarin cohorts. There was no systematic skew in those receiving DOACs or warfarin based on socioeconomic status. An inspection of propensity score distributions showed sufficient overlap between 2 treatment cohorts (eFigure 1 in Supplement 1). After propensity score–overlap weighting, patients were well balanced on all observed characteristics, with an absolute standardized difference less than 10% (eFigure 2 in Supplement 1).
Table 1. Baseline Characteristics of Study Population.
| Characteristics | Patients, No. (%) | Standardized Differencea | |
|---|---|---|---|
| Direct Oral Anticoagulants (n = 4041) | Warfarin (n = 7621) | ||
| Demographic | |||
| Age, median (IQR), y | 80 (74-86) | 80 (74-86) | 2.7 |
| Female | 2277 (56.3) | 4292 (56.3) | 0.1 |
| Race/ethnicity | |||
| Non-Hispanic white | 3504 (86.7) | 6592 (86.5) | 4.5 |
| Non-Hispanic black | 251 (6.2) | 502 (6.6) | |
| Hispanic | 115 (2.8) | 212 (2.8) | |
| Asian | 70 (1.7) | 97 (1.3) | |
| Other | 101 (2.5) | 215 (2.8) | |
| Zip code–level socioeconomic status, median (IQR) | |||
| Household income, $1000s | 53 (46-64) | 53 (45-64) | 1.7 |
| Home value, $1000s | 173 (132-263) | 179 (132-266) | 1.8 |
| High school degree, % | 88.3 (84.5-90.5) | 88.8 (85.3-90.9) | 11.6 |
| College degree, % | 30.2 (22.8-36.6) | 30.2 (22.4-36.3) | 2.9 |
| Unemployment, % | 6.9 (5.8-8.1) | 7.3 (6.1-8.7) | 23.3 |
| Medical history | |||
| Atrial fibrillation/flutter | 2597 (64.3) | 4580 (60.1) | 8.6 |
| Persistent or paroxysmal atrial fibrillation/flutter documented during the hospitalization | 3872 (95.8) | 7339 (96.3) | 2.4 |
| Coronary artery disease or prior myocardial infarction | 1217 (30.1) | 2412 (31.6) | 3.3 |
| Carotid stenosis | 145 (3.6) | 255 (3.3) | 1.3 |
| Diabetes mellitus | 1012 (25.0) | 1994 (26.2) | 2.6 |
| Peripheral vascular disease | 183 (4.5) | 364 (4.8) | 1.2 |
| Hypertension | 3284 (81.3) | 6166 (80.9) | 0.9 |
| Smoker | 287 (7.1) | 480 (6.3) | 3.2 |
| Dyslipidemia | 1986 (49.1) | 3780 (49.6) | 0.9 |
| Heart failure | 476 (11.8) | 1008 (13.2) | 4.4 |
| Previous stroke | 704 (17.4) | 1326 (17.4) | 0.1 |
| Previous transient ischemic attack | 348 (8.6) | 595 (7.8) | 2.9 |
| Anemia | 886 (21.9) | 1969 (25.8) | 9.2 |
| Chronic obstructive pulmonary disease | 838 (20.7) | 1759 (23.1) | 5.7 |
| Liver disease | 77 (1.9) | 166 (2.2) | 1.9 |
| Drug or alcohol abuse | 315 (7.8) | 629 (8.3) | 1.7 |
| Sleep apnea | 217 (5.4) | 388 (5.1) | 1.3 |
| Patient information | |||
| Arrival by emergency medical services | 2198 (55.6) | 4498 (60.9) | 10.7 |
| On-hour arrivalb | 1935 (48.0) | 3576 (47.1) | 2.0 |
| NIHSS score, median (IQR) | 4 (1-9) | 5 (2-11) | 20.0 |
| Prestroke CHA2DS2-VASc score, median (IQR)c | 4 (3-5) | 4 (3-5) | 2.6 |
| Prestroke CHA2DS2-VASc score ≥2c | 3959 (98.0) | 7493 (98.3) | 2.6 |
| Creatinine clearance, median (IQR), mL/min | 48.4 (37.5-61.4) | 46.2 (35.3-59.9) | 8.4 |
| Heart rate, median (IQR), beats per minute | 78 (67-93) | 80 (68-95) | 6.6 |
| Systolic blood pressure, median (IQR), mmHg | 155 (138-176) | 153 (136-173) | 7.0 |
| BMI, median (IQR) | 26.4 (23.3-30.3) | 26.5 (23.3-30.7) | 2.6 |
| Hospital characteristics | |||
| Rural hospital | 139 (3.4) | 329 (4.3) | 4.5 |
| Region | |||
| West | 606 (15.0) | 1130 (14.8) | 23.5 |
| South | 1733 (42.9) | 2462 (32.3) | |
| Midwest | 749 (18.5) | 1822 (23.9) | |
| Northeast | 953 (23.6) | 2207 (29.0) | |
| Primary/comprehensive stroke center | 1032 (25.5) | 2154 (28.3) | 6.1 |
| Academic hospital | 3050 (76.0) | 5729 (75.8) | 0.5 |
| No. of beds, median (IQR) | 394 (251-608) | 376 (248-583) | 3.7 |
| Annual ischemic stroke volume, median (IQR) | 256 (170-403) | 244 (165-385) | 10.2 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHA2DS2-VASc, congestive heart failure, hypertension, age 75 years or older (doubled), diabetes, stroke/transient ischemic attack or thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65 to 75 years, sex category (female); IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale.
Standardized differences were calculated as the differences in means or proportions divided by a pooled estimate of the standard deviation. A standardized difference greater than 10 is typically considered a meaningful difference between 2 groups.
On-hour arrival is defined as Monday through Friday from 7 am to 6 pm, except for holidays.
The CHA2DS2-VASc is a clinical tool for estimating the risk of stroke in patients with atrial fibrillation with scores ranging from 0 to 9; a score of 0 or 1 corresponds to low to moderate risk of stroke; 2 or higher, to high stroke risk.
Home Time and MACE
Primary Analyses
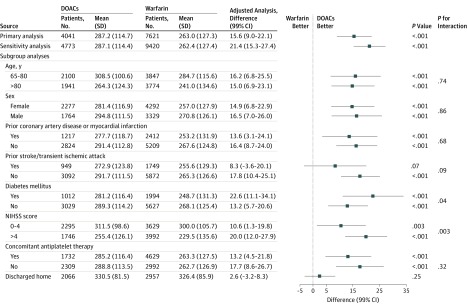
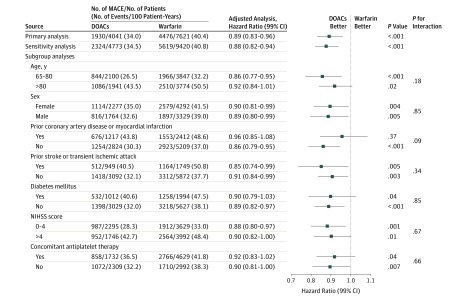
The median duration of follow-up for patients receiving DOACs and patients receiving warfarin were 1470 (IQR, 885-1507) and 1462 (IQR, 464-1509) days, respectively, for those discharged in 2011; 1163 (IQR, 608-1317) and 1137 (IQR, 429-1293) days, respectively, for those discharged in 2012; 813 (IQR, 599-953) and 812 (IQR, 444-963) days, respectively, for those discharged in 2013; and 488 (IQR, 393-593) and 484 (IQR, 369-615) days, respectively, for those discharged in 2014. The unadjusted mean (SD) home time was 287.2 (114.7) days, and the median (IQR) was 348 (259-365) days during the first year postdischarge for patients discharged receiving DOACs; the same values were 263.0 (127.3) days and 331 (201-360) days for those discharged receiving warfarin (Table 2). A MACE occurred in 1930 patients (34.0% per year) who were discharged receiving DOACs, compared with 4476 patients (40.4% per year) who were discharged receiving warfarin. After propensity score–overlap weighting, patients treated with DOACs at discharge were more likely to spend more days alive and out of the hospital or a skilled nursing facility (adjusted home time difference, 15.6 [99% CI, 9.0-22.1] days) and had a significantly lower risk of MACEs (adjusted hazard ratio [HR], 0.89 [99% CI, 0.83-0.96]) than those receiving warfarin at discharge. In sensitivity analyses that included patients with missing NIHSS data, the association of DOACs with primary outcomes of home time (21.4 [99% CI, 15.3-27.4] days) and MACE (adjusted HR, 0.88 [99% CI, 0.82-0.94]) remained essentially unchanged (Figures 2 and 3).
Table 2. Association Between Discharge Oral Anticoagulants and Clinical Outcomes.
| Outcomes | Events, No. (Events per 100 Patient-Years, No.) | P Value | |||
|---|---|---|---|---|---|
| Direct Oral Anticoagulants (n = 4041) | Warfarin (n = 7621) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | ||
| Primary Outcomes | |||||
| Home time during the first year postdischarge, d | |||||
| Median (IQR) | 348 (259-365) | 331 (201-360) | 27.5 (20.5-34.6)a | 15.6 (9.0-22.1)a | <.001 |
| Mean (SD) | 287.2 (114.7) | 263.0 (127.3) | |||
| Major adverse cardiovascular events | 1930 (34.0) | 4476 (40.4) | 0.82 (0.76-0.87)b | 0.89 (0.83-0.96)b | <.001 |
| Secondary Outcomesc | |||||
| Mortality | |||||
| All-cause | 1183 (15.8) | 3028 (19.6) | 0.78 (0.73-0.84) | 0.88 (0.82-0.95) | <.001 |
| Fatal bleedingd | 59 (0.8) | 164 (1.1) | 0.71 (0.53-0.93) | 0.84 (0.63-1.12) | .23 |
| Readmission | |||||
| All-cause | 2353 (53.1) | 5052 (62.4) | 0.84 (0.80-0.88) | 0.93 (0.88-0.97) | .003 |
| Cardiovascular | 1267 (22.3) | 2770 (25.0) | 0.86 (0.81-0.92) | 0.92 (0.86-0.99) | .02 |
| Ischemic stroke | 380 (5.6) | 770 (5.6) | 0.97 (0.86-1.09) | 1.01 (0.89-1.14) | .91 |
| Systemic embolism | 42 (0.6) | 100 (0.7) | 0.84 (0.58-1.21) | 0.95 (0.66-1.38) | .80 |
| Hemorrhagic stroke | 54 (0.8) | 151 (1.0) | 0.69 (0.51-0.93) | 0.69 (0.50-0.95) | .02 |
| Gastrointestinal bleeding | 367 (5.4) | 714 (5.1) | 1.01 (0.90-1.15) | 1.14 (1.01-1.30) | .03 |
| Any bleedinge | 728 (11.4) | 1717 (13.4) | 0.82 (0.75-0.89) | 0.89 (0.81-0.97) | .009 |
| Negative outcome control | |||||
| Pneumoniaf | 546 (8.1) | 1256 (9.2) | 0.85 (0.77-0.93) | 0.96 (0.87-1.06) | .40 |
| Sepsisf | 481 (7.0) | 1151 (8.3) | 0.81 (0.73-0.90) | 0.95 (0.85-1.06) | .37 |
Abbreviation: IQR, interquartile range.
Differences with 99% CIs; all other values are reported as hazard ratios.
Hazard ratios with 99% CIs (all secondary outcomes are reported with 95% CIs).
All secondary outcomes are reported as hazard ratios with 95% CIs.
Rehospitalization for bleeding with in-hospital mortality.
Rehospitalization for bleeding.
Either primary or secondary diagnoses.
Figure 2. Association Between Direct Oral Anticoagulants (DOACs) at Discharge and Home Time During the First Year Postdischarge.
MACE indicates major adverse cardiovascular event; NIHSS, National Institutes of Health Stroke Scale.
Figure 3. Association Between Direct Oral Anticoagulants (DOACs) at Discharge and Major Adverse Cardiovascular Events (MACEs).
NIHSS indicates National Institutes of Health Stroke Scale.
Subgroup Analyses
Of 11 662 survivors of ischemic stroke, 5715 (49.0%) were 80 years or older, 6569 (56.3%) were female, 5738 (49.2%) had an NIHSS score greater than 4, 3629 (31.1%) had a history of prior myocardial infarction or coronary artery disease, 2698 (23.1%) had had a previous stroke or transient ischemic attack, 3006 (25.8%) had diabetes, 6361 (54.5%) received concomitant antiplatelet therapy at discharge, and 5023 were discharged to home directly (DOACs, 2066 [51.1%]; warfarin, 2957 [38.8%]). Except for patients with prior stroke or transient ischemic attack (home time: adjusted differences, 8.30 [99% CI, −3.6 to 20.1] days), prior myocardial infarction or coronary artery disease (MACE: adjusted HR, 0.96 [99% CI, 0.87-1.05]), and those who were discharged to home (home time: adjusted difference, 2.6 [99% CI, −3.2 to 8.3] days), the associated benefit of DOACs over warfarin in terms of longer home time and lower risk of MACE, were consistent across all other subgroups. However, diabetes and concomitant antiplatelet therapy subgroups for MACE were significant at 95% CI but not at 99% CI (Figures 2 and 3).
Secondary Outcomes
Except for ischemic stroke readmission (DOACs, 380 of 4041 patients [5.6% per year] vs warfarin, 770 of 7621 patients [5.6% per year]) and gastrointestinal bleeding (DOACs, 367 of 4041 [5.4% per year] vs warfarin, 714 of 7621 [5.1% per year]), the unadjusted incidences of mortality and readmissions were lower among patients treated with DOACs at discharge (Table 2). After propensity score–overlap weighting, discharge DOAC treatment was associated with a lower risk of all-cause mortality (DOACs, 1183 [15.8% per year] vs warfarin, 3028 [19.6% per year]; adjusted HR, 0.88 [95% CI, 0.82-0.95]; P < .001), all-cause readmissions (DOACs, 2353 [53.1% per year] vs warfarin, 5052 [62.4% per year]; adjusted HR, 0.93 [95% CI, 0.88-0.97]; P = .003), cardiovascular readmissions (DOACs, 1267 [22.3% per year] vs warfarin, 2770 [25.0% per year]; adjusted HR, 0.92 [95% CI, 0.86-0.99]; P = .02), hemorrhagic strokes (DOACs, 54 [0.8% per year] vs warfarin, 151 [1.0% per year]; adjusted HR, 0.69 [95% CI, 0.50-0.95]; P = .02), and hospitalizations with bleeding (DOACs, 728 [11.4% per year] vs warfarin, 1717 [13.4% per year]; adjusted HR, 0.89 [95% CI, 0.81-0.97]; P = .009) but a higher risk of gastrointestinal bleeding (DOACs, 367 [5.4% per year] vs warfarin, 714 [5.1% per year]; adjusted HR, 1.14 [95% CI, 1.01-1.30]; P = .03). However, there were no significant differences in fatal bleeding (DOACs, 59 [0.8% per year] vs warfarin, 164 [1.1% per year]; adjusted HR 0.84 [95% CI, 0.63-1.12]), ischemic stroke readmissions (DOACs, 380 [5.6% per year] vs warfarin, 770 [5.6% per year]; adjusted HR, 1.01 [95% CI, 0.89-1.14]), and systemic embolism (DOACs, 42 [0.6% per year] vs warfarin, 100 [0.7% per year]; adjusted HR, 0.95 [95% CI, 0.66-1.38]) between the cohorts.
Negative Outcome Control (Falsification Analyses)
The unadjusted incidences of hospitalization with pneumonia and sepsis were numerically lower in patients discharged with DOACs (pneumonia: DOACs, 546 of 4041 patients [8.1%] vs warfarin, 1256 of 7621 [9.2%]; sepsis: DOACs, 481 of 4041 [7.0%] vs warfarin, 1151 of 7621 [8.3%]; Table 2). After propensity score–overlap weighting, these differences were not significant (pneumonia: adjusted HR, 0.96 [95% CI, 0.87-1.06]; sepsis: adjusted HR, 0.95 [95% CI, 0.85-1.06]), which suggests that the significant differences between DOACs and warfarin with regard to primary and secondary outcomes were less likely attributable to treatment selection bias.
Discussion
Using a nationwide contemporary registry of patients with AF hospitalized for acute ischemic stroke, we found that a new prescription of DOACs at discharge compared with warfarin was associated with more institution-free home time and a lower risk of MACE, all-cause mortality, all-cause readmissions, cardiovascular readmissions, hemorrhagic strokes, and rehospitalizations with bleeding, albeit with a small but significantly higher risk of gastrointestinal bleeding. The clinical benefits associated with DOACs in terms of home time and MACE were consistent across a broad range of clinically relevant subgroups. In addition, DOACs were as effective as warfarin for the secondary prevention of ischemic stroke and systemic embolism. Importantly, these differences do not appear to be dependent on socioeconomic status, at least in Medicare beneficiaries. Collectively, these findings suggest that the efficacy of DOACs in clinical trials appears to translate into effectiveness in clinical practice and support the current guideline recommendations of DOACs over warfarin for secondary prevention in patients with AF at high risk.8,44,45,46,47,48
Anticoagulation therapy is central to the management of patients with AF at high risk. While warfarin has been the most widely used oral anticoagulants for more than 60 years, all 4 phase III clinical trials1,2,3,4 have shown comparable or even better efficacy and safety profiles of DOACs compared with warfarin. Unlike patients enrolled in pivotal clinical trials, this study included a higher proportion of vulnerable patients known to be at increased risk for both ischemic events and bleeding. Understanding the associated benefits and risks in this broader population may be important for stakeholders who make decisions about the extent or limits of DOACs in practice. The included ischemic stroke population was older than patients in other trials, with more women, comorbidities, and underlying risk factors for recurrent stroke (mean poststroke CHADS2 score: this study, 3.9 vs the pooled analysis of the Randomized Evaluation of Long-Term Anticoagulant Therapy [RE-LY], Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation [ROCKET AF], Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation [ARISTOTLE], and Global Study to Assess the Safety and Effectiveness of Edoxaban [DU-176b] vs Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation [ENGAGE AF-TIMI 48] trials, 2.6).5 These vulnerable risk profiles may have explained the higher number of MACE and recurrent ischemic strokes observed in this cohort. Nevertheless, the benefit of DOACs over warfarin in terms of longer home time and MACE reduction was observed across a broad range of prespecified subgroups. Additionally, DOACs were associated with a 12% relative risk reduction in all-cause mortality, which is similar to the results from a meta-analysis of trial data (10% reduction).5 For the secondary prevention of ischemic stroke, DOACs were as effective as warfarin, but they were associated with fewer hemorrhagic strokes, despite a small but significantly higher risk of gastrointestinal bleeding. Overall, these findings indicate that DOACs have a favorable risk-benefit profile compared with warfarin for secondary prevention in patients with ischemic stroke and AF.
While warfarin is highly effective in preventing stroke in patients with AF, proper levels of anticoagulation with warfarin are often difficult to maintain.49 The median time in therapeutic range has been reported in the range of 58% to 68% in the ARISTOTLE, RE-LY, and ROCKET AF trials.2,50,51 Adherence to warfarin remains poor outside a clinical trial setting, with less than 60% of patients actually taking warfarin in community practice.52 Therefore, poor warfarin adherence and time in therapeutic range control may explain some of the benefits seen with DOACs. Nonetheless, there has been controversy regarding DOAC adherence, given that the lesser monitoring requirement, high out-of-pocket cost, and twice-daily dosing schedules for dabigatran and apixaban may also lead to poor adherence.53,54
Our findings could potentially be biased by early discontinuation, switching to a different agent, interruption in therapy, and poor adherence or time in therapeutic range. While information on medication adherence and international normalized ratios during follow-up is not available, it could be argued that this study follows the intention-to-treat principle and better reflects clinical practice effectiveness rather than the efficacy of DOACs observed in clinical trials. The findings partially mitigate the concern that the efficacy of DOACs reported in clinical trials may not translate into usual clinical care, especially among older patients with multiple comorbidities. Future research is needed to evaluate long-term medication adherence between warfarin and DOACs and its association with outcomes.
Limitations
This study has some limitations. First, this was a retrospective observational analysis. While we used propensity score–overlap weighting to balance the differences between DOACs and warfarin, unmeasured and residual confounding may exist. Second, the GWTG-Stroke registry only includes patients who experienced stroke; therefore, these findings cannot be extrapolated to individuals with AF who take oral anticoagulation for primary stroke prevention. Third, the primary analysis focused on individuals with complete NIHSS scores. It is unlikely that physicians will report stroke severity differently according to the oral anticoagulation prescribed at hospital discharge. Importantly, both primary analyses excluding those with missing NIHSS scores and the sensitivity analysis including these individuals produced similar results. Fourth, this study analyzed patients in the GWTG-Stroke and Medicare linked database, with complete renal function information. Despite being, to our knowledge, the largest stroke registry in the United States, these results might not be extrapolated to younger patients, those with impaired renal function, those treated in non–GWTG-Stroke hospitals, or those in other countries. Importantly, the exclusion of 33 217 patients with missing creatinine clearance carries the risk of selection bias, given that renal function is a crucial factor for treatment decisions and dosages of the DOACs. Fifth, in patients with a severe stroke, physicians may wait 1 to 2 weeks and then start anticoagulation shortly after discharge. Exclusion of individuals who did not receive warfarin or DOACs at discharge may introduce selection bias, especially in sicker patients. Sixth, unlike DOACs, patients treated with warfarin need dosage adjustment. There may have been a bias against patients treated with warfarin in terms of home time, since these patients tended to have longer lengths of stay and are discharged to skilled nursing facilities or inpatient rehabilitation facilities more often while they received care to get their international normalized ratio in the therapeutic range. Nonetheless, the differential discharge pattern should not affect other outcomes, such as MACE and readmissions. Finally, the results of this analysis support the premise that, compared with warfarin, DOACs (as a class) are associated with improved long-term outcomes; however, this study is not designed as a comparative study of each individual agent or associated dosage strategies. Further studies should evaluate the clinical effectiveness of specific DOACs against each other and warfarin and develop algorithms to aid in treatment selection according to individual risk profiles, preferences, and values.
Conclusions
Among survivors of acute ischemic stroke who had AF, DOAC use at discharge was associated with improved long-term outcomes relative to warfarin. These findings provide empirical support for current guideline recommendations and confirm the improved risk-benefit ratio in DOAC vs warfarin for secondary prevention in patients with AF.
eTable. Baseline Characteristics between Patients with and without NIHSS
eFigure 1. Propensity Score Distribution Plots
eFigure 2. Balance Plots Before and After Propensity Score Overlap Weighting
Statistical Analysis Plan.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJ, et al. ; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, et al. ; ENGAGE AF-TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 5.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 6.Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69(20):2475-2484. doi: 10.1016/j.jacc.2017.03.540 [DOI] [PubMed] [Google Scholar]
- 7.Patel PA, Zhao X, Fonarow GC, et al. Novel oral anticoagulant use among patients with atrial fibrillation hospitalized with ischemic stroke or transient ischemic attack. Circ Cardiovasc Qual Outcomes. 2015;8(4):383-392. doi: 10.1161/CIRCOUTCOMES.114.000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019;0(0):CIR0000000000000665.30686041 [Google Scholar]
- 9.Yavuz B, Ayturk M, Ozkan S, et al. A real world data of dabigatran etexilate: multicenter registry of oral anticoagulants in nonvalvular atrial fibrillation. J Thromb Thrombolysis. 2016;42(3):399-404. doi: 10.1007/s11239-016-1361-4 [DOI] [PubMed] [Google Scholar]
- 10.Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GYH. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha M-J, Choi E-K, Han K-D, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2017;48(11):3040-3048. doi: 10.1161/STROKEAHA.117.018773 [DOI] [PubMed] [Google Scholar]
- 12.Lip GYH, Skjøth F, Nielsen PB, Kjældgaard JN, Larsen TB. Effectiveness and safety of standard-dose nonvitamin k antagonist oral anticoagulants and warfarin among patients with atrial fibrillation with a single stroke risk factor: a nationwide cohort study. JAMA Cardiol. 2017;2(8):872-881. doi: 10.1001/jamacardio.2017.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933-2944. doi: 10.1161/STROKEAHA.118.020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lip GYH, Larsen TB, Skjøth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60(8):738-746. doi: 10.1016/j.jacc.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 15.Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GYH, Larsen TB. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2017;356:j510. doi: 10.1136/bmj.j510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saji N, Kimura K, Tateishi Y, et al. ; daVinci Study Group . Safety and efficacy of non-vitamin K oral anticoagulant treatment compared with warfarin in patients with non-valvular atrial fibrillation who develop acute ischemic stroke or transient ischemic attack: a multicenter prospective cohort study (daVinci study). J Thromb Thrombolysis. 2016;42(4):453-462. doi: 10.1007/s11239-016-1376-x [DOI] [PubMed] [Google Scholar]
- 17.Coleman CI, Peacock WF, Bunz TJ, Alberts MJ. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke. 2017;48(8):2142-2149. doi: 10.1161/STROKEAHA.117.017474 [DOI] [PubMed] [Google Scholar]
- 18.Zapata-Wainberg G, Masjuan J, Quintas S, et al. The neurologist’s approach to cerebral infarct and transient ischaemic attack in patients receiving anticoagulant treatment for non-valvular atrial fibrillation: ANITA-FA study. Eur J Neurol. 2019;26(2):230-237. doi: 10.1111/ene.13792 [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura S, Koga M, Sato S, et al. ; SAMURAI Study Investigators . Two-year outcomes of anticoagulation for acute ischemic stroke with nonvalvular atrial fibrillation -SAMURAI-NVAF study. Circ J. 2018;82(7):1935-1942. doi: 10.1253/circj.CJ-18-0067 [DOI] [PubMed] [Google Scholar]
- 20.Okumura Y, Yokoyama K, Matsumoto N, et al. ; SAKURA AF Registry Investigators . Three-year clinical outcomes associated with warfarin vs. direct oral anticoagulant use among Japanese patients with atrial fibrillation -findings from the SAKURA AF registry. Circ J. 2018;82(10):2500-2509. doi: 10.1253/circj.CJ-18-0535 [DOI] [PubMed] [Google Scholar]
- 21.Le Heuzey J-Y, Bassand J-P, Berneau J-B, et al. ; GARFIELD-AF Investigators . Stroke prevention, 1-year clinical outcomes and healthcare resource utilization in patients with atrial fibrillation in France: data from the GARFIELD-AF registry. Arch Cardiovasc Dis. 2018;111(12):749-757. doi: 10.1016/j.acvd.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 22.Staerk L, Fosbøl EL, Lip GYH, et al. Ischaemic and haemorrhagic stroke associated with non-vitamin K antagonist oral anticoagulants and warfarin use in patients with atrial fibrillation: a nationwide cohort study. Eur Heart J. 2017;38(12):907-915. [DOI] [PubMed] [Google Scholar]
- 23.Xian Y, O’Brien EC, Fonarow GC, et al. Patient-centered research into outcomes stroke patients prefer and effectiveness research: implementing the patient-driven research paradigm to aid decision making in stroke care. Am Heart J. 2015;170(1):36-45, 45.e1-45.e11. doi: 10.1016/j.ahj.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 24.O’Brien EC, Xian Y, Fonarow GC, Olson DM, Schwamm LH, Hernandez AF. Clinical commentary on “certain uncertainty: life after stroke from the patient’s perspective”. Circ Cardiovasc Qual Outcomes. 2014;7(6):970. doi: 10.1161/CIRCOUTCOMES.114.001484 [DOI] [PubMed] [Google Scholar]
- 25.Hannah D, Lindholm B, Maisch L. Certain uncertainty: life after stroke from the patient’s perspective. Circ Cardiovasc Qual Outcomes. 2014;7(6):968-969. doi: 10.1161/CIRCOUTCOMES.114.001315 [DOI] [PubMed] [Google Scholar]
- 26.Fonarow GC, Liang L, Thomas L, et al. Assessment of home-time after acute ischemic stroke in Medicare beneficiaries. Stroke. 2016;47(3):836-842. doi: 10.1161/STROKEAHA.115.011599 [DOI] [PubMed] [Google Scholar]
- 27.Xian Y, Wu J, O’Brien EC, et al. Real world effectiveness of warfarin among ischemic stroke patients with atrial fibrillation: observational analysis from Patient-Centered Research into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) study. BMJ. 2015;351:h3786. doi: 10.1136/bmj.h3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xian Y, O’Brien EC, Liang L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA. 2017;317(10):1057-1067. doi: 10.1001/jama.2017.1371 [DOI] [PubMed] [Google Scholar]
- 29.O’Brien EC, Greiner MA, Xian Y, et al. Clinical effectiveness of statin therapy after ischemic stroke: primary results from the statin therapeutic area of the Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) study. Circulation. 2015;132(15):1404-1413. doi: 10.1161/CIRCULATIONAHA.115.016183 [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Reeves MJ, Smith EE, et al. ; GWTG-Stroke Steering Committee and Investigators . Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes. 2010;3(3):291-302. doi: 10.1161/CIRCOUTCOMES.109.921858 [DOI] [PubMed] [Google Scholar]
- 31.Schwamm LH, Fonarow GC, Reeves MJ, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119(1):107-115. doi: 10.1161/CIRCULATIONAHA.108.783688 [DOI] [PubMed] [Google Scholar]
- 32.Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J. 2012;163(3):392-398, 398.e1. doi: 10.1016/j.ahj.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 33.Office for Human Research Protections Federal policy for the protection of human subjects ('common rule'). https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html. Published March 18, 2016. Accessed June 18, 2019.
- 34.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157(6):995-1000. doi: 10.1016/j.ahj.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves MJ, Fonarow GC, Smith EE, et al. Representativeness of the Get With The Guidelines-Stroke Registry: comparison of patient and hospital characteristics among Medicare beneficiaries hospitalized with ischemic stroke. Stroke. 2012;43(1):44-49. doi: 10.1161/STROKEAHA.111.626978 [DOI] [PubMed] [Google Scholar]
- 36.Wilson JTL, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243-2246. doi: 10.1161/01.STR.0000027437.22450.BD [DOI] [PubMed] [Google Scholar]
- 37.Saver JL, Filip B, Hamilton S, et al. ; FAST-MAG Investigators and Coordinators . Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin focused assessment (RFA). Stroke. 2010;41(5):992-995. RFA. doi: 10.1161/STROKEAHA.109.571364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patient-Centered Outcomes Research Institute (PCORI) Methodology Committee The PCORI methodology report. https://www.pcori.org/sites/default/files/PCORI-Methodology-Report.pdf. Published 2017. Accessed April 27, 2018.
- 39.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113(521):390-400.doi: 10.1080/01621459.2016.1260466 29930437 [DOI] [Google Scholar]
- 41.Smith EE, Shobha N, Dai D, et al. Risk score for in-hospital ischemic stroke mortality derived and validated within the Get With the Guidelines–Stroke Program. Circulation. 2010;122(15):1496-1504. doi: 10.1161/CIRCULATIONAHA.109.932822 [DOI] [PubMed] [Google Scholar]
- 42.Fonarow GC, Pan W, Saver JL, et al. Comparison of 30-day mortality models for profiling hospital performance in acute ischemic stroke with vs without adjustment for stroke severity. JAMA. 2012;308(3):257-264. doi: 10.1001/jama.2012.7870 [DOI] [PubMed] [Google Scholar]
- 43.Lipsitch M, Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383-388. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 45.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 46.Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 47.January CT, Wann LS, Alpert JS, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary, a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280. doi: 10.1016/j.jacc.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 48.Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121-1201. doi: 10.1016/j.chest.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 49.Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) study. Arch Intern Med. 2007;167(3):229-235. doi: 10.1001/archinte.167.3.229 [DOI] [PubMed] [Google Scholar]
- 50.Wallentin L, Lopes RD, Hanna M, et al. ; Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Investigators . Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127(22):2166-2176. doi: 10.1161/CIRCULATIONAHA.112.142158 [DOI] [PubMed] [Google Scholar]
- 51.Wallentin L, Yusuf S, Ezekowitz MD, et al. ; RE-LY investigators . Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376(9745):975-983. doi: 10.1016/S0140-6736(10)61194-4 [DOI] [PubMed] [Google Scholar]
- 52.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131(12):927-934. doi: 10.7326/0003-4819-131-12-199912210-00004 [DOI] [PubMed] [Google Scholar]
- 53.Brown JD, Shewale AR, Talbert JC. Adherence to rivaroxaban, dabigatran, and apixaban for stroke prevention in incident, treatment-naïve nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2016;22(11):1319-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borne RT, O’Donnell C, Turakhia MP, et al. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the Veterans Health Administration. BMC Cardiovasc Disord. 2017;17(1):236. doi: 10.1186/s12872-017-0671-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Baseline Characteristics between Patients with and without NIHSS
eFigure 1. Propensity Score Distribution Plots
eFigure 2. Balance Plots Before and After Propensity Score Overlap Weighting
Statistical Analysis Plan.