This cohort study examines factors associated with lymphedema after neoadjuvant chemotherapy and axillary lymph node dissection in women with node-positive breast cancer.
Key Points
Question
What factors are associated with breast cancer–related lymphedema after neoadjuvant chemotherapy and axillary dissection?
Findings
In this cohort study of 486 patients with breast cancer, increasing body mass index and neoadjuvant chemotherapy duration of 144 days or longer were associated with lymphedema symptoms, neoadjuvant chemotherapy duration of 144 days or longer was associated with a 20% limb volume increase, and removal of 30 nodes or more and higher number of positive nodes were associated with a 10% limb volume increase.
Meaning
The findings suggest that patients with longer duration of neoadjuvant chemotherapy or obesity have higher lymphedema risk and may benefit from enhanced prospective lymphedema surveillance.
Abstract
Importance
Most lymphedema studies include a heterogeneous population and focus on patients treated with adjuvant chemotherapy.
Objective
To examine factors associated with lymphedema after neoadjuvant chemotherapy (NAC) and axillary lymph node dissection in women with node-positive breast cancer.
Design, Setting, and Participants
This cohort study included data from 701 women 18 years or older with cT0-T4N1-2M0 breast cancer with documented axillary nodal metastasis at diagnosis who were enrolled in the American College of Surgeons Oncology Group Z1071 (Alliance for Clinical Trials in Oncology) trial, which took place from January 1, 2009, to December 31, 2012. Data analysis was performed from January 11, 2018, to November 9, 2018.
Interventions
All participants received NAC, breast operation, and axillary lymph node dissection. Participants underwent prospective arm measurements and symptom assessment after NAC completion and at 6-month intervals to 36 months postoperatively.
Main Outcomes and Measures
Factors associated with lymphedema were defined as self-reported arm heaviness or swelling (lymphedema symptoms) or an arm volume increase of 10% or more (V10) or 20% or more (V20).
Results
A total of 486 patients (mean [SD] age, 50.1 [10.8] years) were included in this study. Median follow-up for the 3 measures was 2.2 to 3.0 years. Cumulative lymphedema incidence at 3 years was 37.8% (95% CI, 33.1%-43.2%) for lymphedema symptoms, 58.4% (95% CI, 53.2%-64.1%) for V10, and 36.9% (95% CI, 31.9%-42.6%) for V20. Increasing body mass index (hazard ratio [HR], 1.04; 95% CI, 1.01-1.06) and NAC for 144 days or longer (HR, 1.48; 95% CI, 1.01-2.17) were associated with lymphedema symptoms. The V20 incidence was higher among patients who received NAC for 144 days or longer (HR, 1.79; 95% CI, 1.19-2.68). The V10 incidence was highest in patients with 30 nodes or more removed (HR, 1.70; 95% CI, 1.15-2.52) and increased with number of positive nodes (HR, 1.03; 95% CI, 1.00-1.06). On multivariable analysis, obesity was significantly associated with lymphedema symptoms (HR, 1.03; 95% CI, 1.01-1.06), and NAC length was significantly associated with V20 (HR, 1.74; 95% CI, 1.15-2.62).
Conclusions and Relevance
In this study, longer NAC duration and obesity were associated with increased lymphedema incidence, suggesting that patients in these groups may benefit from enhanced prospective lymphedema surveillance.
Introduction
The American Cancer Society estimates 268 600 new female breast cancer cases will be diagnosed in 2019,1 with an estimated 3 million women in the United States having had breast cancer in 2018.2 Lymphedema is a well-known complication of breast cancer treatment, resulting in an accumulation of protein-rich fluid in the interstitial spaces in the ipsilateral upper extremity.3 Despite the introduction of sentinel lymph node (SLN) surgery, which is associated with a lower risk of lymphedema compared with axillary lymph node dissection (ALND), recent data demonstrate that 10% to 30% of breast cancer survivors will develop lymphedema.4
The primary lymphedema symptoms of swelling and progressive fibrotic skin changes can be debilitating and result in decreased quality of life. In addition, lymphedema is also associated with recurrent soft-tissue infections, often resulting in hospitalization for antibiotic therapy, thus contributing to health care costs that might be lessened with prospective surveillance and early intervention.5,6,7 Treatment-related factors associated with the development of lymphedema include breast cancer–related surgery, including both ALND and, to a lesser extent, SLN surgery,4 radiotherapy to the regional lymphatics,8 chemotherapy, and endocrine therapy.9,10,11 Modifiable lifestyle factors include a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 25 or higher and increased sedentariness.9,12
Chemotherapy is often recommended as part of the treatment regimen for patients diagnosed with breast cancer, and there are conflicting reports regarding the association of chemotherapy with development of lymphedema.11,13 Anthracyclines and taxanes, some of the most commonly used agents in breast cancer, are associated with fluid retention and potentially associated with lymphedema.10 Increasingly, chemotherapy is administered before breast and axillary operation in the neoadjuvant setting, especially in patients with high-risk tumor biology and those with positive nodes identified at diagnosis. Neoadjuvant chemotherapy (NAC) can downstage disease in the breast and axillary nodes, often permitting less extensive breast operation and potentially less invasive axillary operation.14 Most studies13,15,16,17 on lymphedema have focused on patients treated with adjuvant chemotherapy, and lymphedema risk in patients treated with NAC is not well delineated.10
The American College of Surgeons Oncology Group (ACOSOG) Z1071 trial enrolled patients with node-positive breast cancer who were treated with NAC followed by SLN surgery and ALND. Therefore, this homogenous cohort at high risk for lymphedema development would be appropriate for evaluation of factors associated with lymphedema. ACOSOG is now part of the Alliance for Clinical Trials in Oncology. The substudy evaluation concerning rates of lymphedema in the trial was published previously.18 The rate of lymphedema varied by the criterion for definition of lymphedema. We report factors associated with the development of lymphedema in patients with node-positive breast cancer after NAC and ALND in the Z1071 lymphedema substudy.
Methods
Eligibility Criteria
This cohort study included women 18 years or older with cT0-T4N1-2M0 breast cancer who underwent fine-needle aspiration or core needle biopsy of an axillary node that revealed nodal metastasis at diagnosis, were treated with NAC, and were eligible for enrollment in the ACOSOG Z1071 trial.18 All patients received NAC followed by breast operation and ALND. The lymphedema substudy included arm measurements and prospective lymphedema questionnaires. The protocol was approved by each institutional review board of the participating sites. Written informed consent was obtained from all study participants, and data were deidentified. Data collection took place at 136 Alliance for Clinical Trials in Oncology and Cancer Therapy Evaluation Program at the National Cancer Institute participating sites across the United States from January 1, 2009, to December 31, 2012. Statistical analyses were conducted by the Alliance Statistics and Data Center. Only enrollees in the lymphedema substudy with measurements at baseline (after NAC, before operation) and at least 1 follow-up time point after the postoperative period were included in this analysis. Data analysis was performed January 11, 2018, to November 9, 2019.
Measurement and Assessment of Lymphedema
Limb measurements were taken on both arms at 5 anatomical locations: (1) the axilla, (2) halfway from the antecubital fossa to the axilla, (3) antecubital fossa, (4) halfway from the antecubital fossa to the wrist, and (5) the wrist, with volume calculated using the truncated cone formula.19,20 The circumferential measurement protocol was drawn from established methods,19,20 as applied in earlier cooperative group clinical trials, including Cancer and Leukemia Group B 70305.21,22
Symptoms were assessed using the Lymphedema Breast Cancer Questionnaire (LBCQ),19,20,23 a validated and reliable questionnaire to assess lymphedema indicators.23 The LBCQ is a self-report tool composed of 19 lymphedema-related symptoms drawn from the published literature, patient interview, and clinical observation. Bilateral circumferential limb measurements were obtained and the LBCQ was administered at the following time points: before the operation (after completion of NAC), 1 to 2 weeks after operation, and 6, 12, 18, 24, and 36 months after the operation.
Definitions of lymphedema were a volume increase of 10% or more (V10) or a volume increase of 20% or more (V20) compared with baseline and/or the contralateral limb19,20,23 and self-report of symptoms of heaviness and swelling (lymphedema symptoms).19,20,21,23 The volume measure was corrected for any change in the contralateral arm from baseline at the same time point. For V10, the increase was calculated as (ipsilateral time point measurement minus ipsilateral baseline measurement) minus (contralateral time point measurement minus contralateral baseline measurement). This number had to be 10% or more to count as a 10% or greater volume increase. A similar calculation was performed for V20. In addition, reports of LBCQ symptoms were assessed, with particular focus on lymphedema symptoms as associated with limb volume increase.19,21,22,23 Overall incidence of lymphedema by each measure was reported previously.18
Statistical Analysis
The database used for these analyses was locked on May 22, 2018. Baseline patient and disease characteristics were compared between groups with a 2-tailed t test for continuous variables and a χ2 test for categorical variables. The cumulative incidence was generated with Kaplan-Meier estimators. Patients who were unavailable for follow-up for a lymphedema event were censored at the date of their last arm measurement or symptom assessment. Comparisons of lymphedema incidence among different patient groups were made with a log-rank test. Univariable and multivariable Cox proportional hazards regression models were used to evaluate the association between baseline patient and disease characteristics and time to a lymphedema event. Duration of NAC was analyzed by tertiles (<105, 105-143, and ≥144 days). Point estimates (eg, number [percentage] of patients, hazard ratios [HRs]) and corresponding 95% CIs were used to summarize variables and associations. Statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc).
Results
Of the 701 eligible and evaluable patients registered to the Z1071 trial, 486 patients (mean [SD] age, 50.1 [10.8] years) consented to the lymphedema substudy. Median length of follow-up for the 3 measures of lymphedema ranged from 2.2 to 3.0 years. The lymphedema substudy patient group was not different from those patients who did not participate (Table 1).
Table 1. Baseline Characteristics by Statusa.
| Characteristic | Participated in Lymphedema Substudy (n = 486) | Not Included in This Analysis (n = 215) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 50.1 (10.8) | 51.1 (11.4) | .25 |
| Race | |||
| White | 391 (80.4) | 174 (80.9) | .15 |
| Black | 71 (14.6) | 28 (13.0) | |
| Asian | 15 (3.1) | 4 (1.9) | |
| Native American | 0 | 2 (0.9) | |
| >1 Race | 0 | 1 (0.5) | |
| Unknown or not reported | 9 (1.8) | 6 (2.8) | |
| Ethnicity | |||
| Hispanic or Latino | 42 (8.6) | 24 (11.2) | .40 |
| Not Hispanic or Latino | 418 (86.0) | 183 (85.1) | |
| Unknown or not reported | 26 (5.4) | 8 (3.7) | |
| Baseline performance status | |||
| 0 | 385 (79.2) | 177 (82.3) | .59 |
| 1 | 100 (20.6) | 38 (17.7) | |
| 2 | 1 (0.2) | 0 | |
| Smoking status | |||
| Current | 56 (11.5) | 29 (13.5) | .16 |
| Never | 342 (70.4) | 139 (64.6) | |
| Past | 74 (15.2) | 34 (15.8) | |
| Unknown | 14 (2.9) | 13 (6.0) | |
| Diabetes | |||
| No | 443 (91.2) | 201 (93.5) | .30 |
| Yes | 43 (8.8) | 14 (6.5) | |
| Peripheral vascular disease | |||
| No | 484 (99.6) | 213 (99.1) | .59 |
| Yes | 2 (0.4) | 2 (0.9) | |
| Arthritis | |||
| No | 447 (92.0) | 206 (95.8) | .06 |
| Yes | 39 (8.0) | 9 (4.2) | |
| Cardiac disease | |||
| No | 356 (73.2) | 166 (77.2) | .27 |
| Yes | 130 (26.8) | 49 (22.8) | |
| Neoadjuvant chemotherapy regimen | |||
| Anthracycline-containing regimen | 23 (4.7) | 21 (9.8) | .08 |
| Taxane-containing regimen | 84 (17.3) | 38 (17.7) | |
| Anthracycline- and taxane-containing regimen | 371 (76.3) | 152 (70.7) | |
| Other | 8 (1.6) | 4 (1.9) | |
| BMI categoryb | |||
| <25 | 137 (28.3) | 59 (27.4) | .06 |
| 25-29.99 | 127 (26.2) | 78 (36.3) | |
| 30-34.99 | 118 (24.4) | 40 (18.6) | |
| 35-39.99 | 62 (12.8) | 26 (12.1) | |
| ≥40 | 40 (8.3) | 12 (5.6) | |
| Estrogen receptor status | |||
| Positive | 299 (61.5) | 128 (59.5) | .73 |
| Negative | 186 (38.3) | 87 (40.5) | |
| Not done | 1 (0.2) | 0 | |
| Progesterone receptor status | |||
| Positive | 253 (52.1) | 110 (51.2) | .76 |
| Negative | 232 (47.7) | 104 (48.4) | |
| Not done | 1 (0.2) | 1 (0.5) | |
| Tumor biologyc | |||
| HR positive/ERBB2 negative | 225 (46.6) | 92 (42.8) | .55 |
| ERBB2 positive | 141 (29.2) | 71 (33.0) | |
| Triple negative | 117 (24.2) | 52 (24.2) | |
| T stage | |||
| T0 | 6 (1.2) | 0 | .55 |
| Tis | 1 (0.2) | 1 (0.5) | |
| T1 | 61 (12.6) | 29 (13.5) | |
| T2 | 270 (55.6) | 115 (53.5) | |
| T3 | 127 (26.1) | 58 (27.0) | |
| T4 | 21 (4.3) | 12 (5.6) | |
| N stage | |||
| N1 | 460 (94.6) | 203 (94.4) | .90 |
| N2 | 26 (5.4) | 12 (5.6) | |
| Radiotherapy receivedd | |||
| Yes | 416 (87.0) | 175 (81.4) | .053 |
| No | 62 (13.0) | 40 (18.6) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Data are presented as number (percentage) of patients unless otherwise indicated.
Data for 2 participants in the lymphedema substudy were unknown or not reported; thus, n = 484.
Data for 3 participants in the lymphedema substudy were unknown or not reported; thus, n = 483.
Data for 8 participants in the lymphedema substudy were unknown or not reported; thus, n = 478.
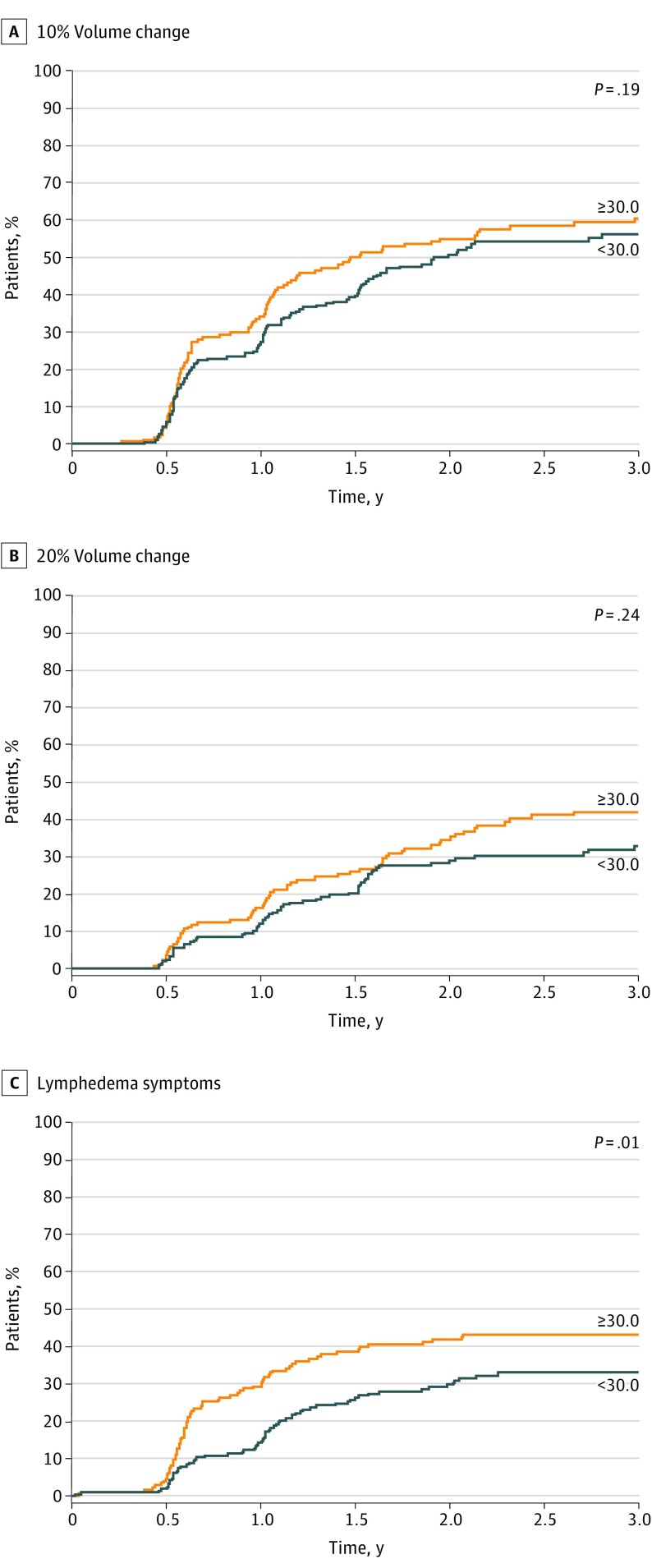
Cumulative incidence of lymphedema as defined by lymphedema symptoms was 37.8% (95% CI, 33.1%-43.2%) at 3 years and was associated with increased BMI, with lymphedema risk increasing with each point of increase in BMI. Figure 1A-C shows the incidence of lymphedema by BMI category (<30 or ≥30). Duration of NAC was also associated with lymphedema symptoms (Figure 2) for 144 days or more of NAC compared with shorter intervals.
Figure 1. Lymphedema Incidence by Body Mass Index (BMI) Category .
BMI is calculated as weight in kilograms divided by height in meters squared.
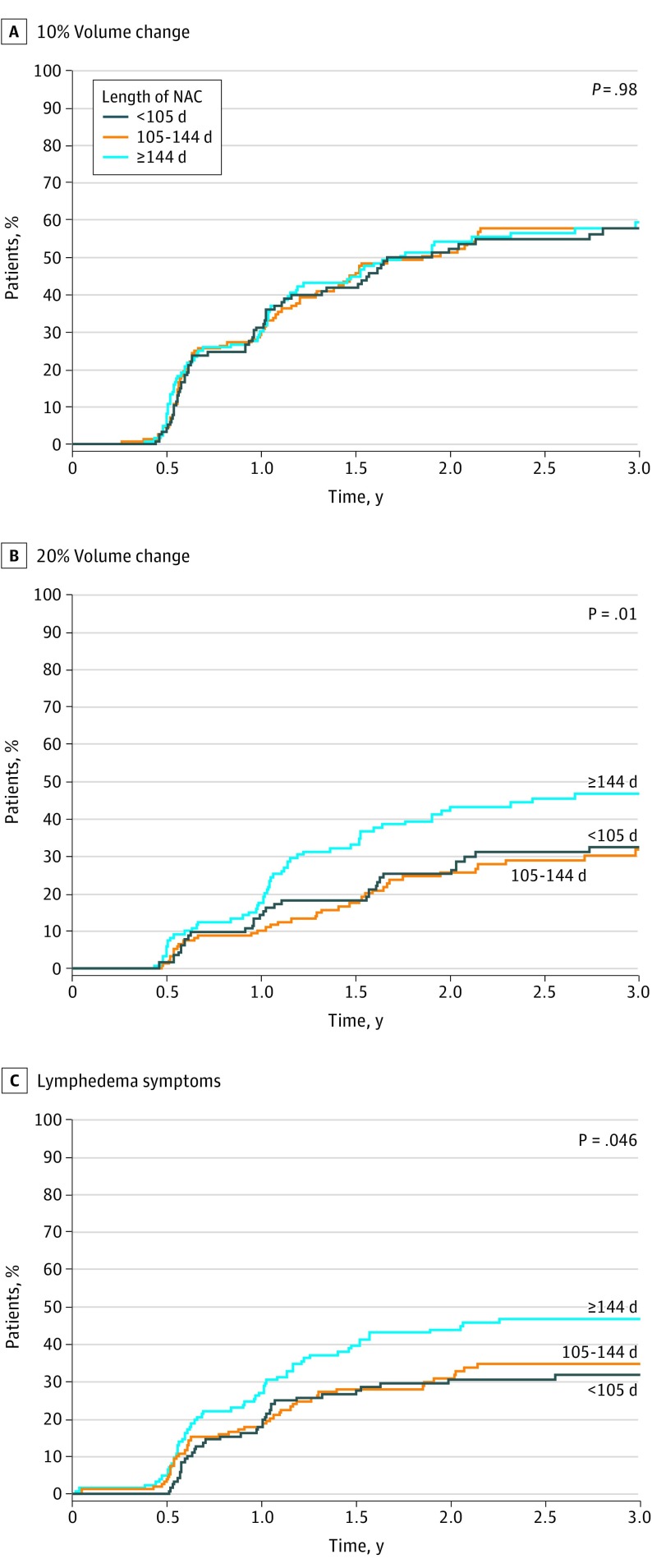
Figure 2. Lymphedema Incidence by Length of Neoadjuvant Chemotherapy (NAC) Category .
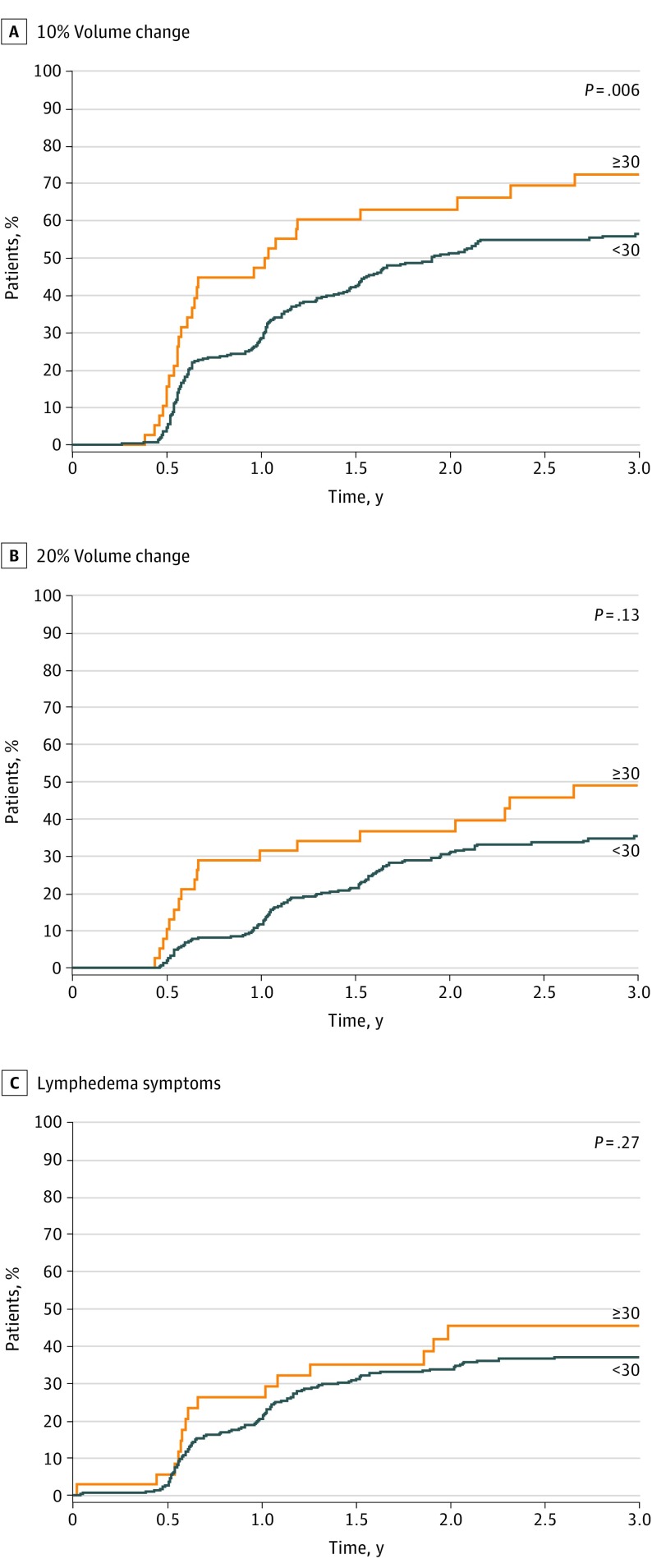
Cumulative LE incidence as defined by V10 was 58.4% at 3 years (95% CI, 53.2%-64.1%). Lymphedema rates were higher as the number of lymph nodes removed increased (Figure 3). The highest rates were seen in patients with 30 lymph nodes or more removed (V10: HR, 1.70 [95% CI, 1.15-2.52]; V20: HR, 1.56 [95% CI, 0.95-2.56]; symptoms: HR, 1.31 [95% CI, 0.78-2.21]). In addition, lymphedema rates by arm measurements increased with increasing number of positive lymph nodes from 1-3 to 4-9 (V10: HR, 1.03 [95% CI, 0.74-1.41] vs 1.73 [95% CI, 1.02-2.91]; V20: HR, 0.97 [95% CI, 0.64-1.46] vs 1.64 [95% CI, 0.84-3.23]) (Table 2).
Figure 3. Lymphedema Incidence by Total Number of Lymph Nodes Removed .
Table 2. Univariable and Multivariable Association of Baseline Variables With Lymphedema Using 3 Definitions for Lymphedema.
| Variable | Objective Measurement | Patient Report | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | 10% Volume Increase | 20% Volume Increase | No. | Lymphedema Symptoms (Heaviness or Swelling) | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||||
| Univariable Analysis | |||||||||
| Age, y | |||||||||
| <50 | 201 | 1 [Reference] | .32 | 1 [Reference] | .79 | 225 | 1 [Reference] | .99 | |
| 50-59 | 117 | 1.31 (0.96-1.76) | 1.12 (0.76-1.66) | 122 | 1.02 (0.70-1.49) | ||||
| 60-69 | 59 | 1.10 (0.74-1.64) | 1.27 (0.79-2.07) | 67 | 0.94 (0.59-1.52) | ||||
| ≥70 | 11 | 0.79 (0.32-1.94) | 1.12 (0.41-3.08) | 15 | 0.93 (0.34-2.55) | ||||
| Age, continuous, y | 388 | 1.00 (0.99-1.02) | .78 | 1.01 (0.99-1.03) | .27 | 429 | 1.00 (0.98-1.02) | .99 | |
| BMI category | |||||||||
| <30 | 216 | 1 [Reference] | .15 | 1 [Reference] | .17 | 235 | 1 [Reference] | .008 | |
| ≥30 | 170 | 1.22 (0.93-1.60) | 1.27 (0.90-1.79) | 192 | 1.56 (1.12-2.17) | ||||
| BMI, continuous | 386 | 1.01 (0.99-1.03) | .45 | 1.01 (0.99-1.04) | .34 | 427 | 1.04 (1.01-1.06) | .004 | |
| Tumor biology | |||||||||
| ERBB2 positive/ERBB2 negative | 185 | 1 [Reference] | .26 | 1 [Reference] | .38 | 202 | 1 [Reference] | .30 | |
| ERBB2 positive | 108 | 0.85 (0.61-1.19) | 0.96 (0.63-1.46) | 124 | 1.29 (0.88-1.90) | ||||
| Triple negative | 95 | 1.18 (0.85-1.63) | 1.29 (0.86-1.95) | 103 | 1.30 (0.86-1.96) | ||||
| Length of NAC, d | .009 | ||||||||
| <105 | 116 | 0.99 (0.71-1.37) | .86 | 1.10 (0.70-1.72) | 127 | 0.90 (0.59-1.38) | .03 | ||
| 105-143 | 151 | 1 [Reference] | 1 [Reference] | 165 | 1 [Reference] | ||||
| ≥144 | 121 | 1.08 (0.78-1.49) | 1.79 (1.19-2.68) | 137 | 1.48 (1.01-2.17) | ||||
| Length of NAC, continuous, d | 1.00 (1.00-1.01) | .28 | 1.01 (1.00-1.01) | .004 | 1.00 (1.00-1.01) | .11 | |||
| Chemotherapy regimen | |||||||||
| Anthracycline | 12 | 1 [Reference] | .32 | 1 [Reference] | .30 | 21 | 1 [Reference] | .45 | |
| Taxane | 71 | 1.20 (0.47-3.06) | 0.74 (0.25-2.20) | 76 | 1.28 (0.44-3.70) | ||||
| Both | 301 | 1.52 (0.62-3.69) | 1.11 (0.41-3.01) | 327 | 1.58 (0.58-4.29) | ||||
| Radiotherapy | |||||||||
| Received radiotherapy, including nodal radiotherapy | 252 | 1 [Reference] | .68 | 1 [Reference] | .15 | 276 | 1 [Reference] | .42 | |
| Received radiotherapy without nodal radiotherapy | 93 | 1.12 (0.82-1.53) | 1.40 (0.95-2.07) | 103 | 0.76 (0.50-1.15) | ||||
| Did not receive radiotherapy | 43 | 0.92 (0.58-1.45) | 1.43 (0.84-2.45) | 50 | 0.92 (0.53-1.58) | ||||
| Surgery type | |||||||||
| Mastectomy | 234 | 1 [Reference] | .19 | 1 [Reference] | .64 | 261 | 1 [Reference] | .90 | |
| Partial mastectomy | 152 | 1.20 (0.91-1.58) | 1.09 (0.77-1.54) | 166 | 0.98 (0.70-1.37) | ||||
| Total nodes removed | |||||||||
| <30 | 350 | 1 [Reference] | .008 | 1 [Reference] | .08 | 392 | 1 [Reference] | .30 | |
| ≥30 | 38 | 1.70 (1.15-2.52) | 1.56 (0.95-2.56) | 37 | 1.31 (0.78-2.21) | ||||
| Total nodes removed, continuous | 388 | 1.02 (1.00-1.03) | .048 | 1.01 (0.99-1.03) | .30 | 429 | 1.00 (0.98-1.03) | .62 | |
| Total positive nodes | |||||||||
| 0 | 164 | 1 [Reference] | .22 | 1 [Reference] | .33 | 183 | 1 [Reference] | .46 | |
| 1-3 | 126 | 1.03 (0.74-1.41) | 0.97 (0.64-1.46) | 135 | 1.28 (0.87-1.89) | ||||
| 4-9 | 74 | 1.73 (1.02-2.91) | 1.64 (0.84-3.23) | 85 | 1.23 (0.63-2.42) | ||||
| ≥10 | 24 | 1.11 (0.76-1.62) | 1.29 (0.82-2.04) | 26 | 1.35 (0.87-2.09) | ||||
| Total positive nodes, continuous | 388 | 1.03 (1.00-1.06) | .03 | 1.03 (0.99-1.07) | .15 | 429 | 1.02 (0.98-1.06) | .32 | |
| Multivariable Analysis | |||||||||
| Age, continuous, y | NA | 1.00 (0.99-1.01) | .95 | 1.01 (0.99-1.03) | .30 | NA | 1.00 (0.98-1.01) | .75 | |
| BMI, continuous | NA | 1.00 (0.98-1.02) | .95 | 1.01 (0.98-1.03) | .55 | NA | 1.03 (1.01-1.06) | .011 | |
| Length of NAC, d | |||||||||
| <105 | NA | 0.93 (0.66-1.30) | .85 | 1.05 (0.67-1.66) | .01 | NA | 0.88 (0.57-1.36) | .06 | |
| 105-143 | NA | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] | ||||
| ≥144 | NA | 1.02 (0.73-1.42) | 1.74 (1.15-2.62) | NA | 1.42 (0.96-2.09) | ||||
| Radiotherapy | |||||||||
| Received radiotherapy | NA | 1 [Reference] | .82 | 1 [Reference] | .35 | NA | 1 [Reference] | .91 | |
| Did not receive radiotherapy | NA | 0.95 (0.60-1.50) | 1.29 (0.76-2.19) | NA | 0.97 (0.56-1.67) | ||||
| Total nodes removed, continuous | NA | 1.02 (1.00-1.03) | .08 | 1.00 (0.98-1.03) | .68 | NA | 1.00 (0.98-1.02) | .82 | |
| Total positive nodes, continuous | NA | 1.03 (1.00-1.06) | .06 | 1.02 (0.98-1.06) | .40 | NA | 1.01 (0.97-1.05) | .61 | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; NA, not applicable; NAC, neoadjuvant chemotherapy.
Cumulative incidence as defined by V20 was 36.9% (95% CI, 31.9%-42.6%) at 3 years and was associated with NAC duration, with each additional day associated with increased risk. With use of tertiles of duration of NAC (Figure 2), the highest incidence of lymphedema as measured by V20 and symptoms was seen in patients with NAC duration of 144 days or more.
In univariable analysis, increasing body mass index (HR, 1.04; 95% CI, 1.01-1.06) and NAC for 144 days or longer (HR, 1.48; 95% CI, 1.01-2.17) were associated with lymphedema symptoms. Obesity was associated with a higher rate of symptomatic lymphedema (HR, 1.56; 95% CI 1.12-2.17). The V20 incidence was also higher among patients who received NAC for 144 days or longer (HR, 1.79; 95% CI, 1.19-2.68). The V10 incidence was highest in patients with 30 nodes or more removed (HR, 1.70; 95% CI, 1.15-2.52) and increased with number of positive nodes (HR, 1.03; 95% CI, 1.00-1.06).
Adjuvant radiotherapy was not associated with lymphedema incidence for any of the 3 lymphedema definitions in this study, which may be because radiotherapy was administered to 416 participants (87.0%). Specifically, the cohort of patients who did not receive radiotherapy was small and may limit this analysis. Type of chemotherapy regimen (anthracycline-containing regimen vs taxane-containing regimen vs both), breast surgical procedure (lumpectomy vs mastectomy), and patient age were also not associated with lymphedema for any of the definitions.
On multivariable analysis (Table 2), severe lymphedema incidence was higher with longer duration of NAC (HR, 1.74; 95% CI, 1.15-2.62), and incidence of lymphedema by symptoms (HR, 1.03; 95% CI, 1.01-1.06) was higher in obese patients.
Discussion
The risk of lymphedema in patients with breast cancer is multifactorial, including patient factors, such as BMI, and treatment factors, including axillary operation, radiotherapy, and chemotherapy. Thus, patients with more advanced breast cancer, who often receive all these treatment modalities, are at a higher risk of lymphedema.24 In the ACOSOG Z1071 trial, patients with node-positive breast cancer treated with NAC and ALND (and in most cases with radiotherapy) comprised a cohort at high risk of lymphedema. This study applied rigorous anthropologic and symptom assessment protocols to collect data after NAC and before surgical intervention for patients with breast cancer and prospectively followed up patients for 2 to 3 years. The identified additional risk factors associated with higher risk of lymphedema development were length of NAC treatment and patient obesity.
Some studies10,25,26 have indicated a higher risk of lymphedema with administration of taxanes, whereas others15,27 have not. In a prospective cohort study27 of 1121 patients with unilateral breast cancer followed up postoperatively during a 2-year period, no significant association with the development of lymphedema and taxane-based therapy was seen; however, ALND, older age, and higher BMI were associated with lymphedema. Similarly, we did not see a significant difference in lymphedema rates by type of chemotherapy regimen used; however, the group of patients who received only anthracycline-based chemotherapy without a taxane was small, which limited our ability to evaluate the association between type of chemotherapy regimen and lymphedema risk.
Duration of chemotherapy regimen has not been evaluated in many studies. However, a previous study25 evaluating chemotherapy in the adjuvant setting demonstrated that arm edema rates were low after anthracycline administration but higher after completion of taxane-based therapy, concluding that lymphedema rates were higher after taxane-based chemotherapy. This finding may be attributable to the taxane therapy or could be associated with a cumulative effect from a longer duration of chemotherapy. The duration of NAC in our study was evaluated in tertiles, with one-third of patients treated with regimens longer than 144 days. The higher risk of lymphedema in patients with longer duration of NAC regimens could be associated with the total dose of chemotherapy received or could be associated with delays in chemotherapy. Delays in chemotherapy delivery caused by toxic effects of the chemotherapy are not known. It is possible that those patients with poor tolerance to the chemotherapy may have had delays in treatment and reduction in doses that could have affected the duration of chemotherapy. Although the longer duration of NAC may be associated with higher burden of disease, on multivariable analysis controlling for tumor size and number of positive nodes, the association remained significant. These findings require additional studies to understand the association between doses and types of chemotherapy in the NAC regimen and development of lymphedema.
Literature regarding lymphedema development in patients treated with NAC is limited. One retrospective review28 of 3136 patients treated with mastectomy reported a lymphedema rate of 10.4%, with a mean of 4.2 years of follow-up. Factors associated with lymphedema were postmastectomy radiotherapy (HR, 2.05; 95% CI, 1.58-2.66), ALND (HR, 1.79; 95% CI, 1.39-2.31), bilateral mastectomy (HR, 1.40; 95% CI, 1.10-1.78), and NAC (HR, 1.42; 95% CI, 1.10-1.84). The lymphedema rates seen in the current study were higher than that reported in other studies, likely because all patients had received NAC and ALND and 87% had received adjuvant radiotherapy. Another study10 investigated body weight changes of breast cancer survivors during NAC, which included 4 cycles of anthracycline plus cyclophosphamide followed by 4 cycles of taxane. Of 406 participants, 270 completed a telephone questionnaire assessment, which reported the development of lymphedema in 97 participants (35.9%). Multivariable analysis indicated that a BMI of 25 or greater (odds ratio, 1.72; 95% CI, 0.80-3.69) was an independent factor for the development of lymphedema. This finding is in keeping with the findings in the current study in which obesity (BMI ≥30) was associated with a higher rate of symptomatic lymphedema (HR, 1.56; 95% CI, 1.12-2.17). Our findings support those from studies29,30 in the adjuvant setting that suggest an association between higher BMI and risk for development of lymphedema. The risk of developing lymphedema was reported as 40% to 60% higher in breast cancer survivors with BMI categorized as overweight or obese compared with women with normal weight, according to findings from an earlier prospective study of longitudinal data.29 With further support of a direct association of lymphedema development with obesity, the findings suggest that patients with newly diagnosed breast cancer may be in a unique position for early intervention for lymphedema risk reduction by referral to an oncology dietician and involvement in programs designed to increase activity and exercise.30 Although the primary effect of early dietary intervention for weight loss would be beneficial for lymphedema risk reduction,29 potential secondary outcomes include a positive association with breast cancer recurrence, self-care, and overall health. Individuals with comorbidities, such as diabetes, cardiovascular diseases, or musculoskeletal diseases, may benefit from BMI reduction through dietary intervention or exercise, leading to improved quality of life.
Patients with more advanced disease, including larger tumors and positive nodes, require more intensive treatment, often including chemotherapy, operation, and radiotherapy, and these patients are at the greatest risk for lymphedema. Enhanced surveillance for lymphedema is likely warranted for all these patients. The findings from our study suggest that patients with obesity and those with longer duration of NAC may be at greatest risk of lymphedema.
Multivariable analysis of data from the Iowa Women’s Health Study13 found that the following cancer treatment factors were positively associated with lymphedema: tumor stage, number of excised nodes, positive nodes, and adjuvant chemotherapy, as well as personal health characteristics of baseline BMI, waist and hip circumference, and general health. In a recent prospective study31 from 4 Chinese hospitals, independent risk factors for lymphedema included axillary dissection, number of positive nodes, BMI, and radiotherapy. This finding was similar in the current study with NAC; on univariable analysis, the number of positive nodes and the number of nodes removed were associated with higher risk of lymphedema, with the highest risk of lymphedema in patients with 30 or more nodes removed. In a study32 that compared lymphedema risk in 68 patients treated with NAC with 161 patients who received adjuvant chemotherapy, risk of lymphedema was not significantly different in those who received NAC (15%) than in those who received adjuvant chemotherapy only (23%); however, in patients treated with NAC, the presence of residual positive axillary nodes was associated with higher risk of lymphedema.
With improvements in targeted therapy and use of chemotherapy in the neoadjuvant setting, the number of positive nodes may decrease. In addition, the results from the Z1071 trial, along with other prospective clinical trials, have led to changes in axillary management; thus, more patients are undergoing SLN surgery, and routine ALND is used less frequently in this setting. The successor trial to Z1071, the Alliance A011202 trial, is evaluating the need for ALND in patients with a positive SLN after NAC and comparing ALND with axillary radiotherapy.33 The lymphedema substudy from this ongoing trial is expected to provide important information on lymphedema rates in this setting.
The results of the present study validate a previous systematic review9 emphasizing the importance of BMI and suggest that the prevention of lymphedema is not only the responsibility of the surgeon and the radiation therapist but also the patient and the medical oncologist.
Limitations
In addition to chemotherapy and endocrine therapy used to treat breast cancer, medications prescribed to treat comorbidities may also produce peripheral edema. Cardiovascular disease accounts for most noncommunicable disease–associated deaths (17.7 million people annually), followed by cancer (8.8 million), respiratory diseases (3.9 million), and diabetes (1.6 million).34 As patients live longer, they are likely to develop chronic conditions that require concomitant medical therapy. Drug classifications commonly identified that may influence the development and severity of lymphedema include (1) calcium channel blockers, (2) nonsteroidal anti-inflammatory drugs, (3) anticonvulsants and antineuralgics, (4) thiazolidinediones, (5) tricyclic antidepressants, and (6) corticosteroids.35,36 Patients receiving taxanes are more likely to receive corticosteroids during their chemotherapy. These drugs may disrupt capillary filtration; increase renal tubular sodium, resulting in fluid retention; lead to possible weight gain; or otherwise cause peripheral edema. We did not have records of all medications for patients enrolled in the ACOSOG Z1071 trial. Evaluation of the pharmacologic profiles of patients undergoing breast cancer treatment and concurrent medical therapy for chronic disease is recommended as a component of prospective surveillance for lymphedema and for incorporation of data in future clinical trials that involve associations of pharmaceuticals with lymphedema development and presentation.
Another limitation of this study is the lack of pre-NAC limb measurement and symptom assessment to further clarify possible preexisting symptoms attributable to comorbidities and limb asymmetry at baseline. These limitations can be rectified in future prospective longitudinal studies. In addition, across the study time frame of enrollment, the chemotherapy protocols for different types of breast cancer were changing. Over time, chemotherapy agents and protocols change with advancements in cancer care, adjunct radiotherapy protocols evolve, and individualization of treatment becomes more frequent. These factors may prove to be associated with variance in lymphedema incidence.
Conclusions
In the prospective assessment of lymphedema in patients treated with NAC and ALND, lymphedema rates were high, and factors associated with lymphedema were obesity and duration of NAC. The findings suggest that patients receiving multimodality therapy that includes longer NAC regimens and patients with higher BMIs should be considered for enhanced lymphedema surveillance to allow early detection and intervention.
References
- 1.American Cancer Society 2019 Cancer facts and figures. 2019. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019. Accessed June 2, 2019.
- 2.American Cancer Society 2018 Cancer facts and figures. 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed June 20, 2018.
- 3.Executive Committee The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Lymphology. 2016;49(4):170-184. [PubMed] [Google Scholar]
- 4.Miller CL, Specht MC, Skolny MN, et al. Risk of lymphedema after mastectomy: potential benefit of applying ACOSOG Z0011 protocol to mastectomy patients. Breast Cancer Res Treat. 2014;144(1):71-77. doi: 10.1007/s10549-014-2856-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García Nores GD, Ly CL, Savetsky IL, et al. Regulatory T cells mediate local immunosuppression in lymphedema. J Invest Dermatol. 2018;138(2):325-335. doi: 10.1016/j.jid.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostby PL, Armer JM, Dale PS, Van Loo MJ, Wilbanks CL, Stewart BR. Surveillance recommendations in reducing risk of and optimally managing breast cancer-related lymphedema. J Pers Med. 2014;4(3):424-447. doi: 10.3390/jpm4030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stout NL, Pfalzer LA, Springer B, et al. Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92(1):152-163. doi: 10.2522/ptj.20100167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avraham T, Yan A, Zampell JC, et al. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-β1-mediated tissue fibrosis. Am J Physiol Cell Physiol. 2010;299(3):C589-C605. doi: 10.1152/ajpcell.00535.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500-515. doi: 10.1016/S1470-2045(13)70076-7 [DOI] [PubMed] [Google Scholar]
- 10.Park S, Lee JE, Yu J, et al. Risk factors affecting breast cancer-related lymphedema: serial body weight change during neoadjuvant anthracycline plus cyclophosphamide followed by taxane. Clin Breast Cancer. 2018;18(1):e49-e54. doi: 10.1016/j.clbc.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 11.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959-1972. [DOI] [PubMed] [Google Scholar]
- 12.Wanchai A, Armer JM, Stewart BR, Lasinski BB. Breast cancer-related lymphedema: a literature review for clinical practice. Int J Nurs Sci. 2016;3(2):202-207. doi: 10.1016/j.ijnss.2016.04.006 [DOI] [Google Scholar]
- 13.Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat. 2011;130(3):981-991. doi: 10.1007/s10549-011-1667-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le-Petross HT, McCall LM, Hunt KK, et al. Axillary ultrasound identifies residual nodal disease after chemotherapy: results from the American College of Surgeons Oncology Group Z1071 Trial (Alliance). AJR Am J Roentgenol. 2018;210(3):669-676. doi: 10.2214/AJR.17.18295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, He X, Tang B, et al. Risk factors of lymphedema on affected side of upper limb after breast cancer surgery–report from a single center of China. Int J Clin Exp Med. 2017;10(1):1592-1601. [Google Scholar]
- 16.Kim M, Park IH, Lee KS, et al. Breast Cancer-Related Lymphedema after Neoadjuvant Chemotherapy. Cancer Res Treat. 2015;47(3):416-423. doi: 10.4143/crt.2014.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):498-503. doi: 10.1016/j.ijrobp.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 18.Armer JM, Ballman KV, McCall L, et al. Lymphedema symptoms and limb measurement changes in breast cancer survivors treated with neoadjuvant chemotherapy and axillary dissection: results of American College of Surgeons Oncology Group (ACOSOG) Z1071 (Alliance) substudy. Support Care Cancer. 2019;27(2):495-503. doi: 10.1007/s00520-018-4334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3(4):208-217. doi: 10.1089/lrb.2005.3.208 [DOI] [PubMed] [Google Scholar]
- 20.Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest. 2005;23(1):76-83. doi: 10.1081/CNV-48707 [DOI] [PubMed] [Google Scholar]
- 21.Paskett ED, Liu H, Oliveri J, et al. Effects of a lymphedema prevention intervention on range of motion among women receiving lymph node dissection for breast cancer treatment (Alliance) CALGB 70305. J Clin Oncol. 2018;36(7)(suppl):123-123. doi: 10.1200/JCO.2018.36.7_suppl.123 [DOI] [Google Scholar]
- 22.Paskett ED, Le-Rademacher J, Oliveri J, et al. Prevention of lymphedema in women with breast cancer (BC): results of CALGB (Alliance) 70305. J Clin Oncol. 2017;35(5)(suppl):104-104. doi: 10.1200/JCO.2017.35.5_suppl.104 [DOI] [Google Scholar]
- 23.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52(6):370-379. doi: 10.1097/00006199-200311000-00004 [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TT, Hoskin TL, Habermann EB, Cheville AL, Boughey JC. Breast cancer–related lymphedema risk is related to multidisciplinary treatment and not surgery alone: results from a large cohort study. Ann Surg Oncol. 2017;24(10):2972-2980. doi: 10.1245/s10434-017-5960-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M-J, Beith J, Ward L, Kilbreath S. Lymphedema following taxane-based chemotherapy in women with early breast cancer. Lymphat Res Biol. 2014;12(4):282-288. doi: 10.1089/lrb.2014.0030 [DOI] [PubMed] [Google Scholar]
- 26.Jung S-Y, Shin KH, Kim M, et al. Treatment factors affecting breast cancer-related lymphedema after systemic chemotherapy and radiotherapy in stage II/III breast cancer patients. Breast Cancer Res Treat. 2014;148(1):91-98. doi: 10.1007/s10549-014-3137-x [DOI] [PubMed] [Google Scholar]
- 27.Swaroop MN, Ferguson CM, Horick NK, et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: results from a large prospective cohort. Breast Cancer Res Treat. 2015;151(2):393-403. doi: 10.1007/s10549-015-3408-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basta MN, Wu LC, Kanchwala SK, et al. Reliable prediction of postmastectomy lymphedema: the risk assessment tool evaluating lymphedema. Am J Surg. 2017;213(6):1125-1133.e1. doi: 10.1016/j.amjsurg.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 29.Mahamaneerat WK, Shyu C-R, Stewart BR, Armer JM. Breast cancer treatment, BMI, post-op swelling/lymphoedema. J Lymphoedema. 2008;3(2):38-44. [PMC free article] [PubMed] [Google Scholar]
- 30.ClinicalTrials.gov Breast Cancer WEight Loss Study (BWEL Study). Alliance for Clinical Trials in Oncology A011401; 2016. NCT02750826; NCI-2015-01918. https://clinicaltrials.gov/ct2/show/NCT02750826. Accessed November 6, 2018.
- 31.Zou L, Liu FH, Shen PP, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer. 2018;25(3):309-314. doi: 10.1007/s12282-018-0830-3 [DOI] [PubMed] [Google Scholar]
- 32.Specht MC, Miller CL, Skolny MN, et al. Residual lymph node disease after neoadjuvant chemotherapy predicts an increased risk of lymphedema in node-positive breast cancer patients. Ann Surg Oncol. 2013;20(9):2835-2841. doi: 10.1245/s10434-012-2828-y [DOI] [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov Comparison of Axillary Lymph Node Dissection With Axillary Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy. Alliance for Clinical Trials in Oncology; 2018. NCT01901094. https://clinicaltrials.gov/ct2/show/study/NCT01901094. Accessed June 20, 2018.
- 34.World Health Organization Noncommunicable Diseases. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases. Accessed June 20, 2018.
- 35.Tesar E, Armer JM. Effect of common medications on breast cancer-related lymphedema. Rehabil Oncol. 2018;36(1):7-12. doi: 10.1097/01.REO.0000000000000105 [DOI] [Google Scholar]
- 36.Keeley V. Drugs and lymphoedema: those which may cause oedema or make lymphoedema worse. LymphLink. 2012;24(4):1-3. [Google Scholar]