Abstract
Staphylococcus aureus is an important and common Gram-positive bacteria which causes clinical infections and food-poisoning cases. Therapeutic schedules for treatment of S. aureus infections are facing a challenge because of the emergence of multidrug resistance strains. It is urgent to find new antiinfective drugs to control S. aureus infection. S. aureus strains are capable of producing the golden carotenoid pigment: staphyloxanthin, which acts as an important virulence factor and a potential target for antivirulence drug design. This review is aimed at presenting an updated overview of this golden carotenoid pigment of S. aureus from the biosynthesis of staphyloxanthin, its function, and the genes involved in pigment production to staphyloxanthin: a novel target for antivirulence therapy.
Keywords: staphyloxanthin, target, antiinfective drug, antivirulence therapy
Introduction
Staphylococcus aureus is an important and common Gram-positive bacteria which can cause clinical infections and food-poisoning cases through producing a large number of extracellular virulence factors such as hemolysin, coagulase, enterotoxins, TSST-1, and protein A.1 Diseases caused by this bacterium are minor skin infections, pneumonia, sepsis, and endocarditis.2,3 S. aureus is widely distributed in nature. In the past few decades, numerous antibiotics that mostly aim to inhibit bacterial growth have been developed and used for S. aureus infections.4 However, the emergence of methicillin-resistant S. aureus (MRSA) limits the usage of antibiotics.5,6 The linezolid-resistant MRSA (LRSA) and vancomycin-intermediate and -resistant MRSA (VISA and VRSA),7 have almost destroyed the entire “antibio-defense.”8 The World Health Organization (WHO) first published its “priority list” of antibiotic-resistance in 2016, MRSA, VISA and VRSA were classified as high priority.9 MRSA infections led to more deaths than HIV/AIDS in the United States.10 Hence, it is urgent to search for an alternative strategy in this post-antibiotic era,11 and antivirulence drugs seem to be a viable option. Virulence factors are products of the pathogen, which could damage the host immune system.12,13 Antivirulence drugs aim to reduce the production of virulence factors without affecting bacterial growth to impede the possible emergence of drug resistance.14 Bacterial virulence factor is a novel target that has attracted strong research interest in recent years. Sortase A is a transpeptidase that assembles surface proteins into an envelope around S. aureus to form abscess lesions or cause lethal bacteremia. Jie Zhang et al15 reported that small molecule inhibitors of Sortase A could protect from S. aureus bacteremia in a mouse model.15 Small molecule inhibitors of type II and type III secretion systems associated with virulence expression could act as potential antivirulence agents for Gram-negative bacteria.16 Flagellin proteins giycosylated with pseudaminic acid (Pse) is critical in the pathogenicity of Helicobacter pylori, small molecule compounds targeting the Pse biosynthetic pathway could inhibit the formation of flagella as a antivirulence drug.17 In conclusion, earlier works revealed that antivirulence drugs exhibited significant advantages over antibiotics. Researches have confirmed that the carotenoid pigment staphyloxanthin is an important virulence factor in S. aureus which can protect S. aureus from innate immune defense systems.18,19 Studying of antivirulence drug based on the pigment biosynthetic pathway in S. aureus has a great significance to developing new antimicrobials. Therefore, the overall aim of this review is to illuminate the biosynthetic pathway, function and regulation approach of staphyloxanthin in S. aureus for promoting the development of antivirulence drugs.
Biosynthesis of staphyloxanthin
The yellow-to-orange pigment is an eponymous feature for identification of S. aureus. Ogston20 isolated a bacteria from surgical abscesses and named the genus Staphylococcus (staphylo means grape in Greek) to describe the grape-like clusters of bacteria observed under a microscope. In 1884, Rosenbach21 categorized different Staphylococci isolates as S. aureus (golden, in Latin) and S. albus (white, in Latin) based on pigmentation. Later, it was confirmed this classification was not very accurate. For example, pigmentation was unstable and partly dependent on growth media.22 Most strains isolated from natural sources produced orange colonies,21 those bovines isolates or multidrug resistance strains were usually yellow.23 Non-pigmented (white) S. aureus could appear in pigmented colonies.24 Then a more advanced classification method based on other characteristics of S. aureus replaced this simple classification.25 Although more than 90% S. aureus isolated from human infections were golden pigmented,26 studies had verified that some S. aureus isolates were non-pigmented.
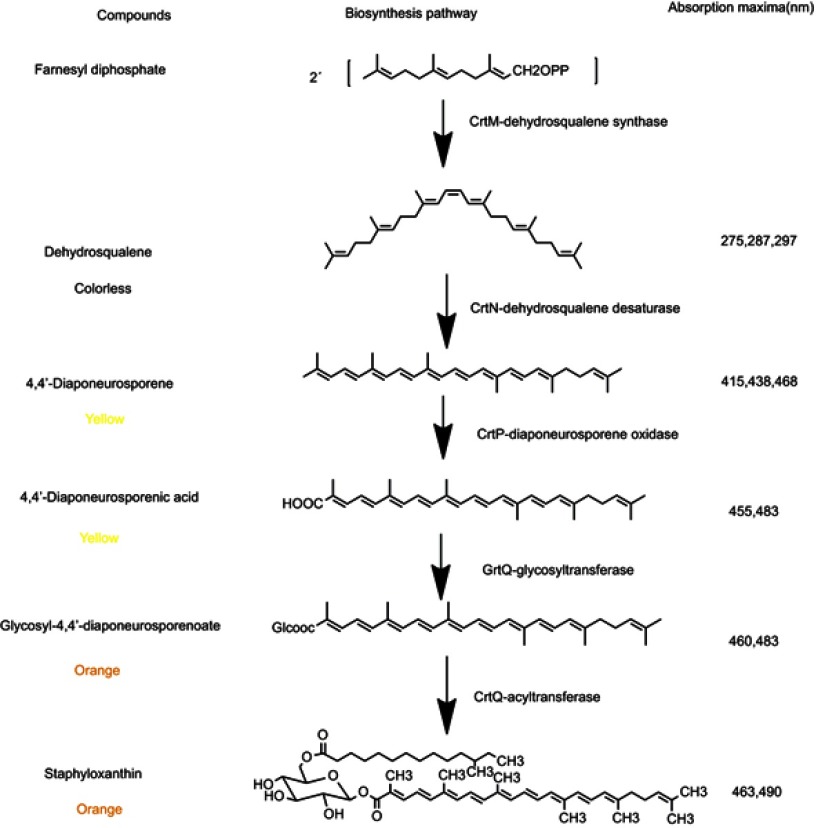
There was general agreement that these orange or yellow pigments are carotenoids, while there was considerable disagreement about their precise chemical structure. Carotenoids are structurally unique natural products that generally contain a long carbon chain skeleton in the central chain with two terminal rings. They generally have 40 carbons. Besides, carotenoids can also have 30 or 50 carbons when produced by certain bacteria via different intermediates.27 This carbon chain presents a series of conjugated double bonds that give it antioxidant capacity.28 The most important structural feature of carotenoids is the abundant delocalization of the polyene π-electrons which enables them to absorb visible light, conferring them intense colours that vary from yellow to red.29–31 Until 1972, the major orange pigment of S. aureus was named staphyloxanthin by Marshall and Rodwell.21 Marshall and Wilmoth21 extracted the pigments from S. aureus S41 with methanol and identified the chemical structures of 17 intermediary compounds. They were all triterpenoid carotenoids possessing a C30 structure rather than the common C40 carotenoid structure which usually existed in most organisms. The major pigment was staphyloxanthin, an α-D-glucopyranosyl-1-O-(4,4ʹ-diaponeurosporene-4-oate)6-O-(12-methyltetradecanoate). While in 2005, Staphyloxanthin was identified as β-D-glucopyranosyl-1-O-(4,4ʹ-diaponeurosporen-4-oate)-6-O-(12-methyltetradecanoate) by NMR spectroscopy, in which glucose was esterified with a triterpenoid carotenoid carboxylic acid at the C1”position and a C15 fatty acid at C6” position (Figure 1).32
Figure 1.
The pathways of staphyloxanthin biosynthesis. The pigments were dissolved in ethyl acetate and the absorption spectra was measured.
In the beginning, the postulated biosynthetic pathway of the triterpenoid carotenoids of S. aureus was reported in 1981.33 This pathway has been verified by Wieland et al34 who cloned the genes and analyzed the functions of CrtM and CrtN enzymes involved in the staphyloxanthin biosynthesis. The results also indicated that yellow pigments were observed only in those clones containing intact crtM and crtN. In 2005, the complete staphyloxanthin biosynthesis operon crtOPQMN was analyzed according to products analysis of crt mutants and protein sequences similarity comparisons.18 The five genes involved in staphyloxanthin biosynthesis were located in an operon crtOPQMN and a σB-dependent promoter lied in upstream of crtO. SigB plays a essential role in regulating staphyloxanthin biosynthesis, biofilm formation and virulence expression of S. aureus.35–37 The genes and enzymatic reactions were shown in Figure 1.
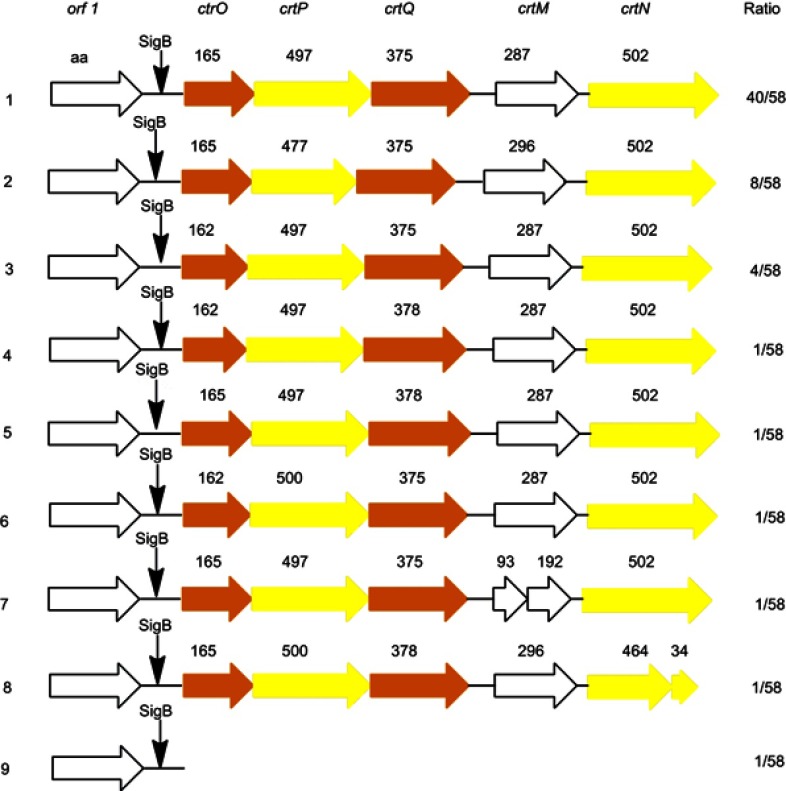
To investigate whether the crtOPQMN was ubiquitous in S. aureus, we analyzed the crt genes cluster of 58 S. aureus completed genomes published in NCBI database. The protein sequences of crtOPQMN from 58 S. aureus were aligned in this study. The crt genes cluster was ubiquitous and had similar gene organization in 58 S. aureus. The organization of the crt genes cluster could be mainly divided into 9 types (Figure 2). Most standard S. aureus belonged to type 1 such as S. aureus N315, S. aureus Newman, S. aureus ATCC25923. Type 7 (S. aureus MRSA 252) and type 8 (S. aureus CN1) had unconsecutive crtM and crtN genes respectively which has never been reported. Type 9 (S. aureus MSHR1132, a clonal complex 75 clinical isolate) lacked the crt genes and was non-pigmented. Besides, it reported that 126 CC75 clinical isolates could not produce pigment in 2011.37 The relationship between the other eight organizations of the crt genes cluster and colony color is still unknown.
Figure 2.
The organization of the crt genes in 58 published S. aureus genomes. The color of the arrows represents the color of the product encoded by corresponding gene.
Function of staphyloxanthin
Pigmented (orange or yellow) S. aureus can produce non-pigmented derivatives which show enhanced susceptibility to linoleic acid and desiccation.38 MRSA strains which produce golden carotenoid pigments and have a wide distribution in the hospital can survive for a longer time than those that produce a small amount of pigment and have a restricted distribution.39 These phenomena strongly indicate pigmented strains have some advantages over non-pigmented strains. Pathogens are eliminated by host phagocytes mainly through releasing reactive oxygen species (ROS) such as O2–, H2O2 and HOCl which are produced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.40 Staphyloxanthin is a typical carotenoid pigment with numerous conjugated double bonds which are capable of absorbing excess energy from ROS.41 Reports have confirmed that some bacterial carotenoids such as those produced by S. aureus could protect against these defense molecules.42–44 Likewise, Liu et al45 reported that the carotenoid pigments could increase S. aureus virulence and neutrophil resistance because of its antioxidative activity, and significantly promote the development of subcutaneous abscess in a mouse model. Interestingly, heterologous expression of S. aureus carotenoids in the Streptococcus pyogenes (non-pigmented) exhibited increased antioxidative activity and neutrophil resistance. Compared with the wild-type strain, the non-pigmented S. aureus mutant was seen to be more easily killed by superoxide radical, hydrogen peroxide, hypochloride, hydroxyl radical and singlet oxygen.45,46 It’s worth noting that the survival advantages conferred by the S. aureus pigments were out of action in the blood of mice and patients lacking oxidative burst function.45 However, the role of staphyloxanthin in oxidative defense is complicated.47 Research has shown that the antioxidant of staphyloxanthin that protects bacteria from neutrophil oxidants depends on the dose of reactive chlorine species that bacteria encounter in the phagosome and other antioxidants.
In addition to its antioxidant property, staphyloxanthin ccan reduce cell membrane (CM) fluidity and stabilize the CM structure, changing the chemical composition and functional activity of the CM.48 Most cationic antimicrobial peptides (CAPs) in mammals initially reacted with microbial electronegative CM, so it is important to examine the correlations between pigment production and susceptibility to CAPs. Mishra et al49 compared the effect of a set of host defense peptides with different structures, sources and charges on a isogenic MSSA strain set which had different ability of pigmentation. The results showed that there was no significant difference in asymmetry, fatty acid profiles, phospholipid composition, cell wall thickness, and surface charge among the strain set, while the CM of the carotenoid-overproducing strain was more rigid than the wild-type and carotenoid-deficient strains, which led to higher resistance to host antimicrobial peptides. These indicated that carotenoids in S. aureus could enhance resistance to non-oxidative host defense mediated by cationic antimicrobial peptides through increasing CM rigidity.
In contrast, Liu et al45 reported there was no difference in the susceptibility of wild-type and ΔcrtM S. aureus to cathepsin G and human neutrophil elastase. Carotenoid-deficient and wild-type S. aureus were susceptible to murine CRAMP (an 18-amino-acid CAP which carries a charge of +5). It seems that the potential function of carotenoids in non-oxidative host defense need further studies.
Genes involved in pigment production
The yellow-to-orange (golden) pigments have been recognized as a virulence factor in S. aureus due to its antioxidant activity.45,46 The genes involved in pigments synthesis belong to an operon crtOPQMN regulated by SigB.50 The activity of SigB is controlled by a series of Rsb proteins encoded by rsb genes (rsbUVWsigB).51 Researches have proved that the rsbUVWsigB system and crtOPQMN operon are essential for the pigmentation in S. aureus.52,53 Besides these genes, other genes can also regulate the pigments production (Table 1). Katzif et al54 found that the susceptibility of S. aureus COL to human lysosomal cathepsin G (CG117-136) was associated with the expression of cold shock protein CspA, CspA could regulate genes expression at the level of transcription when acted as a single-strand DNA-binding protein and it was overproduced during cold shock. Later, they found that a highly pigmented strain COL became non-pigmented when the cspA gene was deleted,55 which meant the CspA was required for pigmentation in S. aureus. Loss of cspA resulted in decreasing expression of crtMN and sigB. It was unclear whether CspA regulated the expression of crtMN directly or indirectly because ΔcspA mutant exhibited decreased expression of sigB and asp23, a SigB-regulated gene. Taken together, CspA could control pigment production of S. aureus which was dependent on SigB.
Table 1.
The genes involved in pigmentation
| Mutation site | Pigmentationa | Expression of crtM or sigBb | |
|---|---|---|---|
| CspA | − | CrtM ↑ sigB ↑ | |
| hfq | + | CrtM ↑ | |
| dank | − | Unknown | |
| TCA cycle | CitZ | + | CrtM → sigB → |
| CitG | + | CrtM → sigB → | |
| SAV2365 | + | CrtM → sigB → | |
| Oxidative phosphorylation | CtaA | + | CrtM ↑ |
| QoxB | + | CrtM → sigB → | |
| Purine biosynthesis | PurN | + | CrtM ↑ sigB ↑ |
| PurH | + | CrtM ↑ sigB ↑ | |
| PurD | + | CrtM ↑ sigB ↑ | |
| PurA | + | CrtM ↑ sigB ↑ | |
Notes: a −, Reduced pigmentation compared to the corresponding wild-type strain, +, enhanced pigmentation compared to the corresponding wild-type strain.
b ↑, Increased expression level observed in mutants. →, No significant change in expression level.
The Hfq (a host factor for RNA phage Qβ) protein was considered to be an RNA chaperone, which was a multifunctional regulator in bacteria.56 Deletion of hfq gene in S. aureus 8325-4 (a SigB deficiency, non-pigmented) could increase the yellow carotenoid pigmentation by increasing mRNA level of crtM as well as the resistance to oxidant and neutrophils, which meant hfq regulated crtM expression negatively in sigB-deficient mutant. It is strange that the survival of Δhfq mutants in the whole blood was lower than that of wild-type and the toxicity of mutants was significantly decreased in cells and murine peritonitis model, which suggested the pathogenicity of the mutant was reduced despite pigment production being increased.57 It is controversial about the function of Hfq in S. aureus. Liu et al thought Hfq was crucial in the infection of S. aureus as a multifunctional regulator.57 However, this option was opposed by Bohn et al58 who reported Hfq did not play an important role in spa mRNA expression, stress response and exoprotein expression in S. aureus RN6390, COL and Newman. The different expression patterns of the Hfq protein in different S. aureus strains may cause the opposite conclusion.
DnaK proteins are molecular chaperones playing a critical role in bacterial stress tolerance,59–61 which belong to the Heat-shock proteins (Hsps) family in all organisms.62 Staphylococcal dnaK was important in protecting S. aureus from oxidation, heat, and antibiotic stress. It also has an important influence on autolysis, pigmentation and survival in a mouse model.60 The ΔdnaK mutants of S. aureus were shown to be still capable of producing pigments, but only producing fewer carotenoids (pale yellow orange colonies) compared with wild-type strain SH1000 (bright golden-yellow colonies). The mutants demonstrated an increased susceptibility to oxidative stress conditions and a decreased survival in a mouse model.60 But the mechanism of action of dnaK deletion in pigmentation is not yet known.
Lan et al63 screened a group of S. aureus pigmentation mutants by transposon insertion, finding that 15 unidentified genes could affect pigmentation. It is worth noting that disrupted metabolic pathways such as the tricarboxylic acid cycle (TCA), purine biosynthesis, and oxidative phosphorylation result in enhanced pigmentation, while the purine biosynthesis mutants and oxidative phosphorylation mutants exhibit significantly attenuated virulence in a murine abscess model. Inactivation of TCA cycle genes (citZ, citG, and SAV2365) could enhance pigmentation but the expression level of sigB or crtM was not elevated. Farnesyl diphosphate, a substrate of pigmentation, was produced through the mevalonate pathway in S. aureus. Inactivation of the TCA cycle genes may lead acetyl-CoA flux to the mevalonate pathway to produce farnesyl diphosphate, which results in increased pigmentation. Disrupting oxidative phosphorylation genes (ctaA and qoxB) gives increased pigmentation, crtM mRNA level was elevated in the ctaA mutant while crtM, sigB mRNA levels showed no significant change in a qoxB mutant. The enhanced pigmentation in purine biosynthetic (purN, purH, purD, or purA) mutants is likely caused by increased expression of sigB.
The biosynthetic pathway of staphyloxanthin: a novel target for antivirulence therapy
In the past several decades, numerous antibiotics have been developed and used for bacterial infections. However, the rate of discovering new antibiotics has significantly decreased.64 Furthermore, current usage of bactericidal compounds is often unsuccessful because of the emergence of MRSA.5,6 Hence, the antivirulence approach aimed to reduce the production of virulence factors without affecting bacterial growth to impede the possible emergence of drug resistance has attracted strong research interest. Staphyloxanthin, a yellow-to-orange pigment, has been recognized as an important virulence factor of S. aureus.45 To a certain extent, if we can find a method to control or inhibit the production of staphyloxanthin, S. aureus may not be able to infect and survive in the host. Therefore, the staphyloxanthin biosynthesis pathway is a potential target to treat S. aureus infections.
Researches have found some natural compounds could inhibit staphyloxanthin biosynthesis. Sakai et al65 established a convenient assay-paper disks to observe staphyloxanthin production of MRSA. They found four inhibitors of lipid metabolism (cerulenin, dihydrobisvertinol, xanthohumol, and zaragozic acid) and two anthraquinones (6-deoxy-8-O-methylrabelomycin and Tetrangomycin) could inhibit staphyloxanthin production without affecting S. aureus growth. Staphyloxanthin consisted of a polyprenyl moiety, a sugar moiety and an acyl moiety. Therefore, it seemed reasonable that these inhibitors of lipid metabolism could inhibit staphyloxanthin production because they inhibit the production of polyprenyl and acyl residue in the staphyloxanthin structure. But the accurate mechanism of action of the two anthraquinones were unclear.
Flavonoids are ubiquitous in plants and are commonly found in fruit, vegetables, seeds, stems , and flowers. They are biologically active in combating diseases in humans because of their diverse biological functions, such as antioxidative, antifungal, antiviral, antibacterial, and anticarcinogenic activities.66 Flavone, a backbone compound of flavonoids, markedly reduces the production of staphyloxanthin and α-hemolysin without inhibiting the planktonic growth of S. aureus at subinhibitory concentration (50 µg/mL). This staphyloxanthin reduction renders the S. aureus cells 100 times more vulnerable to hydrogen peroxide in the presence of flavone.67 Although the exact action mechanisms of flavone’s antivirulence activity remains to be determined, the results suggests that the screening of a larger library of flavonoids will generate more potent therapeutic options for S. aureus. Rhodomyrtone [6,8-dihydroxy-2,2,4,4ʹ-tetramethyl-7-(3-methyl-1-oxobutyl)-9-(2-methylpropyl)-4,9-dihydro-1Hxanthene-1,3(2H)-di-one] was isolated from Rhodomyrtus tomentosa (Aiton) Hassk and exhibited prominent antibacterial activity against many Gram-positive bacteria.69,70 The MIC and MBC values of rhodomyrtone against MRSA was close to those of vancomycin.71 Rhodomyrtone-treated S. aureus exhibited reduced pigmentation and increased susceptibility to H2O2 and singlet oxygen killing probably by inducing the activity of the CrtM enzyme and inhibiting the CrtN enzyme. Consequently, the survival ability of the treated S. aureus was decreased in human whole blood.72
The biosynthesis of staphyloxanthin started with condensation of two molecules of farnesyl diphosphate to form dehydrosqualene, catalyzed by CrtM.33 The crystal structure of S. aureus CrtM at 1.58 Å (PDB accession number 2ZCO) was very similar to that of human squalene synthase (SQS:PDB accession number 1EZF) involved in cholesterol biosynthesis in humans. The superposition of crtM and SQS showed a 5.5 Å root-mean-square (RMS) deviation between the Ca atoms. Liu et al64 reported three SQS inhibitors (phosphonosulfonates BPH-652, BPH-698 and BPH-700) could inhibit CrtM activity, resulting in non-pigmented S. aureus with increased susceptibility to human blood and innate immune system in a mouse model. Furthermore, these three phosphonosulfonates did not affect the growth of three human cell lines (MCF-7, NCI-H460 and SF-268). Among three phosphonosulfonates, BPH-652 had a good IC50(110nM) in pigment inhibition.
Until now, only one compound BPH-652 has been confirmed can decrease S. aureus survival in a mouse model. The crystal structure of CrtM and SQS have published in PDB date base. Molecular docking is an effective approach to predict the binding mode of substrate in its receptor, which has been successfully used in drug design.73 It suggests that it is a simple and potent way to develop antivirrulence drugs by screening CrtM inhibitors that have no effect on SQS through molecular docking. CrtN enzyme can also be an important target for antivirulence therapy except CrtM. CrtN enzyme catalyzed dehydrosqualene to form 4,4ʹ-diaponeurosporene, a first yellow carotenoid intermediate which means CrtN inhibitors may also block staphyloxanthin biosynthesis. Chen’s research group revealed that a antifungal agent, Naftifine hydrochloride (NTF) which is FDA-approved, could block the STX biosynthesis pathway by targeting CrtN to treat MRSA infections.73 Subsequently, on the basis of the results above, his group designed a novel compound 47 which displays superior pigment inhibitory activity and excellent efficacy against MRSA in vivo.
The protein sequences of crtOPQMN in 58 S. aureus were downloaded from NCBI date base and aligned by using MEGA6.0 software in this study. The percentage of CrtN protein sequence variable sites (8.17%) was lower than that of the other four protein sequences (CrtM: 15.20%, CrtO: 9.7%, CrtP: 8.20%, CrtQ: 12.96%). It indicated crtN is more conserved than crtOPQM and may be an optimal target for antivirulence drugs. Our hypothesis was confirmed by Cueno and Imai’s77 study that an effective antivirulence drug against S. aureus STX biosynthesis would involve targeting crtN activity, 4,4ʹ-diaponeurosporene levels, or both components by network analytics.
Conclusions
The complete staphyloxanthin biosynthesis was illuminated in 2005,32 there were five genes organized in an operon crtOPQMN invoved in staphyloxanthin biosynthesis. Dehydrosqualene catalyzed by the CrtM was not a carotenoid, which was colorless. The yellow carotenoids 4,4ʹ-diaponeurosporene and 4,4ʹ-diaponeurosporenic acid catalyzed by CrtN, CrtP could convert to an orange end product, staphyloxanthin.32 There were yellow, orange, and white colonies of S. aureus isolated from the environment, it’s tempting to propose that the major pigments of yellow colonies were 4,4ʹ-diaponeurosporene or 4,4ʹ-diaponeurosporenic acid. The yellow carotenoids were also capable of absorbing excess energy from ROS.41 It seemed that researchers had paid little attention to the difference in antioxidant capacity between the yellow and orange isolates which is worth further studying. There were nine types of organization of the crt genes in the published 58 S. aureus genomes, it’s remains unknown what the difference is on the biosynthesis of pigment between the white, yellow, and orange isolates. The relationship between the organization of the crt genes and colony color needs further study.
Carotenoid pigments of S. aureus could serve as a protective function against neutrophil killing of the host due to their antioxidant ability.45–56 There has been considerable disagreement about the relationship between pigments and CAPs45,49 The potential function of pigments in non-oxidative innate host defenses needs further study.
The rsbUVWsigB system and crtOPQMN operon were necessary for the pigmentation in S. aureus.52,53 Besides these genes, other genes could regulate the pigment production (Table 1). CspA and dnaK positively regulated pigment production in S. aureus. While hfq and genes involved in the TCA cycle, purine biosynthetic and oxidative phosphorylation regulated pigmentation negatively.
The expansion in bacterial resistance to antibiotics has created an urgent need for effective antimicrobial agents as well as antivirulence compounds. Researches have shown that some compounds could inhibit the pigmentation of S. aureus, which increased susceptibility to innate immune clearance. Three phosphonosulfonates blocked staphyloxanthin biosynthesis in vitro by inhibiting CrtM activity.64 Four inhibitors of lipid metabolism could inhibit staphyloxanthin production because they inhibited the production of polyprenyl and acyl residue in the staphyloxanthin structure. But the accurate mechanism of action of two anthraquinones need to be studied.65 Furthermore, Sakai et al65 did not mention whether the pathogenicity of S. aureus was decreased. Flavone, usually seen as an antioxidant and antibacterial showed good inhibition on pigmentation, while the mechanism of action was unknown.67,68 Rhodomyrtone-treated S. aureus exhibited reduced pigmentation, that probably induced the activity of the CrtM and CrtN enzyme.71
In 2008, Eric’s group first reported that cholesterol biosynthesis inhibitor BPH-652 could result in colorless S. aureus with increased susceptibility to innate immune system in a mouse model and nontoxic to three human cell lines. In 2016, an FDA-approved antifungal agent, Naftifine hydrochloride (NTF) and his derivative 47 could block the STX biosynthesis pathway by targeting CrtN to treat MRSA infections.73 In conclusion, it is hard to clarify the relationship between staphyloxanthin and S. aureus virulence. Some researches indicated that lacking of staphyloxanthin could impair S. aureus virulence compared with wild type.64,74 Whereas, Liu’s study showed the SigB mutation which could not produce staphyloxanthin still retained bacterial virulence in animal killing and skin abscess formation in mouse models.75 Disrupting metabolic pathways of purine biosynthesis and oxidative phosphorylation resulting in enhanced pigmentation along with attenuated virulence in a murine abscess model. There is no doubt staphyloxanthin is a virulence factor of S. aureus, but S. aureus pathogenicity is multifactorial, the pigments are just one factor. It is not possible to evaluate the toxicity of S.aureus only by the amount of pigment production. The relationship between colony pigment, virulence and drug resistance is still unknown.
Up to now, antivirulence drugs against S. aureus infections targeting crtM and crtN have exhibited a promising prospect, while the similar structural features between S. aureus crtM and human squalene synthasean,64 the drugs targeting crtM may affect human cholesterol biosynthesis which indicate crtM may be an unfavorable target for antivirulence drugs. Researches should pay more attention to the antivirulence drugs against S.aureus targeting crtN activity, 4,4ʹ-diaponeurosporene levels, or both components. Also, to identify effective antivirulence drug targets by network analytics is a novel method which can be used in the drug development field in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vandendriessche S, Vanderhaeghen W, Soares FV, et al. Prevalence, risk factors and genetic diversity of methicillin-resistant Staphylococcus aureus carried by humans and animals across livestock production sectors. J Antimicrob Chemother. 2013;68(7):1510–1516. doi: 10.1093/jac/dkt047 [DOI] [PubMed] [Google Scholar]
- 2.Federspiel JJ, Stearns SC, Peppercorn AF, Chu VH, Fowler VG Jr. Increasing US rates of endocarditis with Staphylococcus aureus: 1999–2008. Arch Intern Med. 2012;172:363–365. doi: 10.1001/archinternmed.2011.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the international collaboration on endocarditis-prospective cohort study. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fry DE. The continued challenge of Staphylococcus aureus in the surgical patient. Am Surg. 2013;79(1):1–10. [PubMed] [Google Scholar]
- 5.Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: can we make evolution-proof drugs? Nat Rev Microbiol. 2014;12:300–308. doi: 10.1038/nrmicro3232 [DOI] [PubMed] [Google Scholar]
- 6.Shorr AF. Epidemiology of staphylococcal resistance. Clin Infect Dis. 2007;45:171–176. doi: 10.1086/519473 [DOI] [PubMed] [Google Scholar]
- 7.Russoa A, Campanileb F, Falconea M, et al. Staphylococcal bacteraemia: a multicentre case-case-control study in Italy. Int J Antimicrob Agents. 2015;45(3):255–261. doi: 10.1016/j.ijantimicag.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 8.Rossi F, Diaz L, Wollam A, et al. Transferable vancomycin resistance in a community-associated MRSA lineage. N Engl J Med. 2014;370(16):1524–1531. doi: 10.1056/NEJMoa1303359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics; World Health Organization,http://www.who.int/medicines/publications/global-priority-listantibiotic-resistant-bacteria/en/ (accessed February 27, 2016).
- 10.Bancroft EA. Antimicrobial resistance: it’s not just for hospitals. JAMA. 2007;298(15):1803–1804. doi: 10.1001/jama.298.15.1803 [DOI] [PubMed] [Google Scholar]
- 11.Dickey SW, Otto M. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discovery. 2017;16(7):457–471. doi: 10.1038/nrd.2017.23 [DOI] [PubMed] [Google Scholar]
- 12.Casadevall A, Pirofski LA. Host-pathogen interactions: redefining the basic concepts of virulenlence and pathogenicity. Infect Immun. 1999;67(8):3703–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clauditz A, Resch A, K P W, Peschel A, Götz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74(8):4950–4953. doi: 10.1128/IAI.00204-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho HS, Lee FH, Cho MH, Lee J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling. 2015;31:1–11. doi: 10.1080/08927014.2014.991319 [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Liu H, Zhu K, et al. Antiinfective therapy with a small molecule inhibitor of Staphylococcus aureus sortase. Proc Natl Acad Sci USA. 2014;111(37):13517–13522. doi: 10.1073/pnas.1408601111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felise HB, Nguyen HV, Pfuetzner RA, et al. An inhibitor of gram-negative bacterial virulence protein secretion. Cell Host Microb. 2008;4(4):325–336. doi: 10.1016/j.chom.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ménard R, Schoenhofen IC, Tao L, et al. Small-molecule inhibitors of the pseudaminic acid biosynthetic pathway: targeting motility as a key bacterial virulence factor. Antimicrob Agents Chemother. 2014;58(12):7430–7440. doi: 10.1128/AAC.03858-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khodade VS, Chandra MS, Banerjee A, et al. Bioreductively activated Reactive Oxygen Species (ROS) generators as MRSA inhibitors. ACS Med Chem Lett. 2014;5(7):777–781. doi: 10.1021/ml5001118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldfield E, Feng X. Resistance-resistant antibiotics. Trends Pharmacol Sci. 2014;35(12):664–674. doi: 10.1016/j.tips.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogston A. Report upon micro-organisms in surgical diseases. Br Med J. 1881;1(369):b2–375. doi: 10.1136/bmj.1.1054.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall JH, Wilmoth GJ. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol. 1981;147:900–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mashburn KL, Atkinson S. Evaluation of adrenal function in serum and feces of Steller sea lions (Eumetopias jubatus): influences of molt, gender, sample storage, and age on glucocorticoid metabolism. Gen Comp Endocrinol. 2004;136:371–381. doi: 10.1016/j.ygcen.2004.01.016 [DOI] [PubMed] [Google Scholar]
- 23.Willis AT, Jacobs SI, Goodburn GM. Pigment production, enzyme activity and antibiotic sensitivity of staphylococci. Subdivision of the Pathogenic Group. J Pathol Bacteriol. 1964;87:157–167. doi: 10.1002/path.1700870122 [DOI] [PubMed] [Google Scholar]
- 24.Servin-Massieu M. Spontaneous appearance of sectored colonies in Staphylococcus aureus cultures. J. Bacteriol. 1961;82:316–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenfeld M, Ramsey BW, Gibson RL. Pseudomonas acquisition in young patients with cystic fibrosis: pathophysiology, diagnosis, and management. Curr Opin Pulm Med. 2003;9:492–497. [DOI] [PubMed] [Google Scholar]
- 26.Lennette EH, Balows A, Hausler WJ Jr, Shadomy JH. Manual of Clinical Microbiology. 4th ed. Washington (DC): American Society for Microbiology; 1985. [Google Scholar]
- 27.Ribeiro D, Freitas M, Silva AMS, Fé C, Fernandes E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem Toxicol. 2018. doi: 10.1016/j.fct.2018.07.060 [DOI] [PubMed] [Google Scholar]
- 28.Siems W, Wiswedel I, Salerno C, et al. b-carotene breakdown products may impair mitochondrial functions - potential side effects of high-dose b-carotene supplementation. J Nutr Biochem. 2005;16:385–397. doi: 10.1016/j.jnutbio.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 29.Siems W, Sommerburg O, Schild L, Augustin W, Langhans CD, Wiswedel I. b-Carotene cleavage products induce oxidative stress in vitro by impairing mitochondrial respiration. FASEB J. 2002;16:1289. doi: 10.1096/fj.01-0765fje [DOI] [PubMed] [Google Scholar]
- 30.Walter MH, Strack D. Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep. 2011;28:663–692. doi: 10.1039/c0np00036a [DOI] [PubMed] [Google Scholar]
- 31.Nagao A. Bioavailability of dietary carotenoids: intestinal absorption and metabolism. JARQ. 2014;48:385–391. doi: 10.6090/jarq.48.385 [DOI] [Google Scholar]
- 32.Pelz A, Wieland KP, Putzbach K, Hentschel P, Albert K, Gotz F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem. 2005;280:32493–32498. doi: 10.1074/jbc.M505070200 [DOI] [PubMed] [Google Scholar]
- 33.Marshall JH, Wilmoth GJ. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: evidence from a study of mutants. J Bacteriol. 1981;147:914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wieland B, Feil C, Gloria-Maercker E, et al. Genetic and biochemical analyses of the biosynthesis of the yellow carotenoid 4,4ʹ-diaponeurosporene of Staphylococcus aureus. J Bacteriol. 1994;176:7719–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell G, Fugère A, Pépin Gaudreau K, et al. SigB is a dominant regulator of virulence in Staphylococcus aureus small-colony variants. PLoS One. 2013;8:e65018. doi: 10.1371/journal.pone.0065018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuchscherr L, Löffler B. Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection. Curr Genet. 2016;62:15–17. doi: 10.1007/s00294-015-0503-0 [DOI] [PubMed] [Google Scholar]
- 37.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33:5967–5982. [DOI] [PubMed] [Google Scholar]
- 38.Grinsted J, Lacey RW. Ecological and genetic implications of pigmentation in Staphylococcus aureus. J Gen Microbiol. 1973;75:259–267. doi: 10.1099/00221287-75-2-259 [DOI] [PubMed] [Google Scholar]
- 39.Beard-Pegler MA, Stubbs E, Vickery AM. Observations on the resistance to drying of staphylococcal strains. J Med Microbiol. 1988;26:251–255. doi: 10.1099/00222615-26-4-251 [DOI] [PubMed] [Google Scholar]
- 40.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004 [DOI] [PubMed] [Google Scholar]
- 41.El-Agamey A, Lowe GM, McGarvey DJ, et al. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430:37–48. doi: 10.1016/j.abb.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 42.Rosen H, Klebanoff SJ. Bactericidal activity of a superoxide anion-generating system. A Model for the Polymorphonuclear Leukocyte. J Exp Med. 1979;149:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahl TA, Midden WR, Hartman PE. Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen. J Bacteriol. 1989;171:2188–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu GY, Doran KS, Lawrence T, et al. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci USA. 2004;101:14491–14496. doi: 10.1073/pnas.0406143101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu GY, Essex A, Buchanan JT, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–215. doi: 10.1084/jem.20050846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clauditz A, Resch A, Wieland K-P, Peschel A, Götz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74:4950–4953. doi: 10.1128/IAI.00204-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beavers WN, Skaar EP. Neutrophil-generated oxidative stress and protein damage in Staphylococcus aureus. Pathog Dis. 2016;74. doi: 10.1093/femspd/ftw060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.IuA P, Kaprel’iants AS, Ostrovskii DN, Ignatov VV. Study of the membranes of pigment-free mutant of Staphylococcus aureus. Biokhimiia. 1976;41:1116–1120. [PubMed] [Google Scholar]
- 49.Mishra NN, Liu GY, Yeaman MR, et al. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob Agents Chemother. 2011;55:526–531. doi: 10.1128/AAC.00680-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bischoff M, Dunman P, Kormanec J, et al. Microarray-based analysis of the Staphylococcus aureus sigma B regulons. J Bacteriol. 2004;186:4085–4099. doi: 10.1128/JB.186.13.4085-4099.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Schaik W, Abee T. The role of σB in the stress response of Gram-positive bacteria-targets for food preservation and safety. Curr Opin Biotechnol. 2005;16(2):218–224. doi: 10.1016/j.copbio.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 52.Giachino P, Engelmann S, Bischoff M. Sigma B activity depends on RsbU in Staphylococcus aureus. J Bacteriol. 2001;183:1843–1852. doi: 10.1128/JB.183.6.1843-1852.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palma M, Cheung AL. Sigma B activity in Staphylococcus aureus is controlled by RsbU and an additional factor(s) during bacterial growth. Infect Immun. 2001;69:7858–7865. doi: 10.1128/IAI.69.12.7858-7865.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katzif S, Danavall D, Bowers S, Balthazar JT, Shafer WM. The major cold shock gene, cspA, is involved in the susceptibility of Staphylococcus aureus to an antimicrobial peptide of human cathepsin G. Infect Immun. 2003;71:4304–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katzif S, Lee EH, Law AB, Tzeng YL, Shafer WM. CspA regulates pigment production in Staphylococcus aureus through a SigB-dependent mechanism. J Bacteriol. 2005;187:8181–8184. doi: 10.1128/JB.187.23.8181-8184.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vassilieva IM, Rouzanov MV, Zelinskaya NV, Moll I, Blasi U, Garber MB. Cloning, purification, and crystallization of a bacterial gene expression regulator-Hfq protein from Escherichia coli. Biochemistry (Mosc). 2002;67:1293–1297. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Wu N, Dong J, et al. Hfq is a global regulator that controls the pathogenicity of Staphylococcus aureus. PLoS One. 2010;5(9):e13069. doi: 10.1371/journal.pone.0013069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bohn C, Rigoulay C, Bouloc P. No detectable effect of RNA-binding protein Hfq absence in Staphylococcus aureus. BMC Microbiol. 2007;7:10. doi: 10.1186/1471-2180-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Craig EA. The heat shock response. CRC Crit Rev Biochem. 1985;18:239–280. [DOI] [PubMed] [Google Scholar]
- 60.Hu B, Mayer MP, Tomita M. Modeling Hsp70-mediated protein folding. Biophys J. 2006;91:496–507. doi: 10.1529/biophysj.106.083394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh VK, Utaida S, Jackson LS, Jayaswal RK, Wilkinson BJ, Chamberlain NR. Role for dnaK locus in tolerance of multiple stresses in Staphylococcus aureus. Microbiology. 2007;153:3162–3173. doi: 10.1099/mic.0.2007/009506-0 [DOI] [PubMed] [Google Scholar]
- 62.Al Refaii A, Alix JH. Ribosome biogenesis is temperature-dependent and delayed in Escherichia coli lacking the chaperones DnaK or DnaJ. Mol Microbiol. 2009;71:748–762. doi: 10.1111/j.1365-2958.2008.06561.x [DOI] [PubMed] [Google Scholar]
- 63.Lan L, Cheng A, Dunman PM, Missiakas D, He C. Golden pigment production and virulence gene expression are affected by metabolisms in Staphylococcus aureus. J Bacteriol. 2010;192:3068–3077. doi: 10.1128/JB.00928-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu CI, Liu GY, Song Y, et al. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science. 2008;319:1391–1394. doi: 10.1126/science.1153018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakai K, Koyama N, Fukuda T, Mori Y, Onaka H, Tomoda H. Search method for inhibitors of staphyloxanthin production by methicillin-resistant Staphylococcus aureus. Biol Pharm Bull. 2012;35:48–53. [DOI] [PubMed] [Google Scholar]
- 66.Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee JH, Park JH, Cho MH, Lee J. Flavone reduces the production of virulence factors, staphyloxanthin and alpha-Hemolysin, in Staphylococcus aureus. Curr Microbiol. 2012;65:726–732. doi: 10.1007/s00284-012-0229-x [DOI] [PubMed] [Google Scholar]
- 68.J W H, Yang J, Guo H, et al. The\r, staphylococcus aureus\r, airSR two-component system mediates reactive oxygen species resistance via transcriptional regulation of staphyloxanthin production[J]. Infect Immun. 2017,85(2):IAI.00838-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Limsuwan S, Voravuthikunchai SP. Boesenbergia pandurata (Roxb.) Schltr., Eleutherine americana Merr. and Rhodomyrtus tomentosa (Aiton) Hassk. as antibiofilm producing and antiquorum sensing in Streptococcus pyogenes. FEMS Immunol Med Microbiol. 2008;53:429–436. doi: 10.1111/j.1574-695X.2008.00445.x [DOI] [PubMed] [Google Scholar]
- 70.Saising J, Hiranrat A, Mahabusarakam W, Ongsakul M, Voravuthikunchai SP. Rhodomyrtone from Rhodomyrtus tomentosa (Aiton) hassk. as a natural antibiotic for staphylococcal cutaneous infections. J Health Sci. 2008;54:589–595. doi: 10.1248/jhs.54.589 [DOI] [Google Scholar]
- 71.Limsuwan S, Trip EN, Kouwen TRHM, et al. A new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine. 2009;16:645. doi: 10.1016/j.phymed.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 72.Leejae S, Hasap L, Voravuthikunchai SP. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J Med Microbiol. 2013;62:421–428. doi: 10.1099/jmm.0.047316-0 [DOI] [PubMed] [Google Scholar]
- 73.Vijayalakshmi P, Nisha J, Rajalakshmi M. Virtual screening of potential inhibitor against FtsZ protein from Staphylococcus aureus. Interdiscip Sci. 2014;6(4):331–339. doi: 10.1007/s12539-012-0229-3 [DOI] [PubMed] [Google Scholar]
- 74.Chen F, Di H, Wang Y, et al. Small molecule targeting of a diapophytoene desaturase inhibits S. aureus virulence. Nat Chem Biol. 2016;12(3):174–179. doi: 10.1038/nchembio.2003 [DOI] [PubMed] [Google Scholar]
- 75.Liu, H, Shang, W, Hu, Z et al.. A novel SigB(Q225P) mutation in Staphylococcus aureus retains virulence but promotes biofilm formation. Emerg Microbes Infect. 2018;7:72. doi: 10.1038/s41426-018-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]