Abstract
Background:
Clinical genome and exome sequencing (CGES) are being used in an expanding range of clinical settings. Most approaches to offering patients choices about learning CGES results classify results according to expert definitions of clinical actionability. Little is known about how patients conceptualize different categories of CGES results.
Methods:
The MedSeq Project is a randomized controlled trial studying the use of whole-genome sequencing (WGS) in primary care and cardiology. We surveyed 202 patient-participants about different kinds of WGS results and conducted qualitative interviews with 49 of these participants. Interview data was analyzed both inductively and deductively using thematic content analysis.
Results:
Participants demonstrated high levels of study understanding and genetic literacy. A small majority of participants wanted to learn all of their WGS results (n=123, 61%). Qualitative data provided a deeper understanding of participants’ perspectives about different types of WGS results. Participants did not have the same views about which WGS results would be actionable or upsetting to learn. They conceptualized variants of uncertain significance (VUS) in a variety of different ways. Many participants expressed optimism that the uncertainty associated with VUS results could be reduced over time.
Conclusions:
Proposals to determine which WGS /CGES results to disclose by soliciting patient preferences may fail to appreciate the complex ways patients think about disease and the information WGS/CGES can produce. Our findings challenge prevailing methods of facilitating patient choice and assessing the benefits and harms related to the return of WGS/CGES results, which mostly rely on expert definitions of clinical utility to categorize the kinds of results patients can learn.
Introduction
Clinical genome and exome sequencing (“CGES”) are being used in a growing range of clinical settings (Biesecker and Green 2014). The increasing use of CGES raises questions about optimal strategies for managing the return of CGES results to patients, including those that are unrelated to the original indication for genetic testing. Many discussions about the return of CGES results focus on the scope of a clinician’s duty to return incidental findings and the role of patient preferences in decisions about the return of these results (Wolf, Annas, and Elias 2013; McGuire et al. 2013; Green, Lupski, and Biesecker 2013). Although guidelines from the American College of Medical Genetics and Genomics (ACMG) and the President’s Commission for the Study of Bioethical Issues suggest that patients should play some role in decisions about the return of results, clinician judgment about the benefits and harms of disclosing certain findings is also deemed important (President’s Commission for the Study of Bioethical Isues 2016; Green et al. 2013).
One challenge related to returning CGES results and determining which results may cause patients psychological harm is that little is known about how patients conceptualize the different types of results CGES can produce. Exacerbating this confusion, CGES research protocols use varied approaches to categorizing and describing CGES results. While prevailing approaches to informed consent and results management categorize results according to their degree of clinical utility or actionability, the criteria used to construct the relevant categories of results differ (Lindor et al. 2013). At least one approach incorporates additional disease information into its classification scheme, including information about disease severity, age of onset, and whether a disease is psychiatric or somatic (Bunnik, Schermer, and Janssens 2012). Another excludes the reproductive implications of CGES results as a consideration in determining the clinical actionability of a result (Hunter et al. 2016).
The lack of consistency in the definition of results categories is problematic given the emphasis bioethicists place on a patient’s right to play a role in decisions about the return of CGES results (Graves et al. 2015). Moreover, some guidance about clinical CGES disclosure is based on empirically unsupported assumptions about the types of results patients consider sensitive or upsetting (Berg, Khoury, and Evans 2011).
In this paper, we begin with the premise that understanding how patients conceptualize the different categories of CGES results is essential in order to obtain meaningful informed consent from patients undergoing CGES and to achieve shared decision-making about the return of CGES results. To gather empirical data on this topic, we surveyed patient-participants (“participants”) who were recruited into a clinical whole genome sequencing (WGS) research study. We then conducted semi-structured interviews with 49 of these individuals to better understand how they viewed different categories of possible WGS results prior to undergoing sequencing. Although our study employed WGS only, we infer that our findings have relevance in settings where a range of other genome-wide sequencing tests are being used. While the baseline interview component of our study was designed to examine how participants in our study viewed the WGS process, it did not examine broader questions about which kinds of WGS results can or should be returned to patients in a clinical setting.
Methods
Study Design
This study was carried out as part of a randomized controlled trial comparing WGS to standard of care in primary care and cardiology clinical practices. We recruited patients of cardiologists and primary care physicians at a single large urban network of academic hospitals and outpatient practices in Boston, Massachusetts between December 2012 and November 2014. Eligible primary care patients were ostensibly healthy adults aged 40–65. Eligible cardiology patients were adults of any age with hypertrophic or dilated cardiomyopathy diagnoses. Details of the overall study design have been described elsewhere (Vassy et al. 2014). All study procedures were approved by the Partners Healthcare Human Research Committee and the Baylor College of Medicine Institutional Review Board.
Recruitment and Informed Consent Process
Participants were recruited via a multi-step process that provided prospective participants with opportunities to learn about the study, review the study’s informed consent document with study staff, and ask questions before deciding to enroll (Vassy et al. 2014).
The informed consent document (see supplementary materials) was developed by a multi-disciplinary team informed by a review of relevant literature (Biesecker et al. 2009; Lautenbach et al. 2013; McGuire and Beskow 2010). Participants in this study were not able to make choices about which types of genetic test results to receive. The results the study planned to return fell into categories commonly used in clinical testing: carrier status, pharmacogenomic results, polygenic risk estimates, and known pathogenic or likely pathogenic Mendelian variants, including some variants of uncertain significance deemed resolvable by clinical evaluation. The results were described as information that may indicate one’s risk of developing certain diseases, including diseases that are preventable, treatable but not preventable, or neither preventable nor treatable. Participants were also told that they could receive information about genetic disorders that could affect family members, or genetic changes that influence the onset of or treatment of heart disease, certain medication reactions, and blood type. Variants of uncertain significance (VUS) were described as changes in one’s genome whose meaning for health are unknown.
On average, the informed consent sessions lasted 16 minutes, with a maximum duration of 41 minutes. Of the 514 patients in contact with the study staff via phone, 173 (33%) actively declined study participation, 93 (18%) were unresponsive after expressing initial interest, and the remainder were either ineligible (41, 8%) or were waitlisted (2, 0.4%). The most frequently cited reasons for declining were: time constraints and logistics (59% of active decliners), fear of insurance discrimination (28% of active decliners), concerns about the psychosocial impact of results (13% of decliners), and general privacy concerns (8%) of decliners. These findings have been reported in detail elsewhere (Robinson et al. 2016).
Survey Methods
At the baseline visit and prior to learning whether they would be randomized to receive WGS, participants completed surveys and an online family history assessment, and had blood drawn for the potential WGS analysis. Surveys measured broad domains including beliefs and attitudes toward genetics, health behaviors and healthcare utilization, understanding of the study and knowledge of genomics, socio-demographics, and preferences for receiving different categories of genetic test results.
In the baseline survey, we assessed participants’ hypothetical preferences for receiving the following 9 different categories of genetic test results: 1) not preventable, 2) not treatable, 3) serious but not life-threatening, 4) life-threatening, indicating a 5) slightly, 6) moderately, or 7) substantially increased risk, 8) decreased risk, and 9) results that even doctors do not know what they mean (i.e., VUS). These terms were not defined further for the respondents, and examples were not given. Response options were on a 5-point Likert-type scale anchored by strongly disagree to strongly agree. Statistical tests (chi square or ANOVA as appropriate) were conducted to determine whether socio-demographics or study cohort (primary care vs. cardiology) were associated with participants’ preferences for different categories of results. Other factors included in analyses were participants’ understanding of the study and knowledge of genomics.
Informed consent understanding was assessed with 22 true or false items based on the informed consent document, and a summary score was created by summing the number of correct items per participant. Genomic knowledge was assessed using a modified version of an existing 11-item scale, where a summary score was created by summing the number of correct items per participant (Kaphingst et al. 2012).
Qualitative Interview Methods
We conducted in-depth, semi-structured interviews with a subset of study participants (n=49) prior to learning their randomization status or receiving study results. The objective of these interviews was to describe participants’ perceptions of and anticipated responses to WGS results in their own words (Sandelowski 2000). Interview participants were sampled purposively to include roughly equal numbers from the two study cohorts (primary care participants (n=25); cardiology participants (n=24)) and to maximize variability with respect to the cardiologists and primary care physicians these participants receive care from. The goal of this sampling strategy was to achieve maximum variation and informational redundancy (Sandelowski 1995).
Interviews were conducted using an interview guide that was developed based on both a review of the empirical bioethics literature related to genomic sequencing and the clinical experiences of the two genetic counselors on the study team. The interview guide was subsequently refined as themes emerged from the first eight transcripts. Interview domains included motivations for participation, attitudes and expectations, and how participants believed they and their physicians would respond to different kinds of results were they to be randomized to receive WGS.
Semi-structured interviews took place between March 2013 and September 2014 and lasted approximately 45 minutes. All interviews were audio recorded and transcribed. Interview data was analyzed using a combined deductive and inductive approach. Questions from the interview guide were used to develop an initial coding scheme, and inductive codes were added as transcripts were reviewed and additional themes were noted.
Eight transcripts were initially independently coded by members of the research team to develop a consensus-coding scheme. Once the coding scheme was being applied consistently, one member of the research team coded the remaining 41 transcripts. These data were independently reviewed by two other research team members and stored and managed using Atlas.ti software (version 7, ©2003–2012). Interviews were halted when the entire research team agreed that informational redundancy had been reached.
Results
A total of 202 participants completed baseline surveys. Participants were primarily white (87%), well educated (81% reported being college graduates or higher), and high-income earners (64% reported annual household incomes greater than $100,000). They also scored well on a scale measuring genomic knowledge (on average participants correctly answered 10 out of 11 items) and demonstrated a strong understanding of the study and what their participation entailed (on average participants answered 20 out of 22 items correctly). The characteristics of the 49 individuals who were interviewed did not differ from those in the overall study (see Table 1).
Table 1.
Participant socio-demographic and understanding characteristics
| Characteristic - no. (%) | All Participants n=202 | Interview Participants n=49 | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 55 (11.2) | 56 (11.9) | 0.885 |
| Age (years), range | 19 – 85 | 26 – 85 | |
| Female gender | 103 (51) | 22 (45) | 0.410 |
| Race/Ethnicity | |||
| Non-Hispanic white | 176 (87) | 41 (84) | 0.819 |
| Other | 26 (13) | 8 (16) | |
| Annual household income | |||
| <$100,000 | 73 (36) | 15 (31) | 0.353 |
| ≥$100,000 | 129 (64) | 34 (69) | |
| Highest education level | |||
| Did not graduate from college | 38 (19) | 8 (16) | 0.600 |
| College graduate or higher | 164 (81) | 41 (84) | |
| Genetic knowledge score, 11-items | |||
| Number of correct items, mean (SD) | 10 (1.2) | 10 (1.2) | 0.505 |
| Number of correct items, range | 4 – 11 | 7 – 11 | |
| Informed consent understanding score, 22-items | |||
| Number of correct items, mean (SD) | 20 (2.1) | 20 (1.6) | 0.203 |
| Number of correct items, range | 13 – 22 | 15 – 22 | |
Results from the baseline survey asking about hypothetical preferences showed that the majority of participants (n=123, 61%) agreed or strongly agreed that they wanted to receive every type of WGS result asked about (Figures 1 and 2). A substantial minority (39%), however, did not want all types of results, meaning they disagreed with at least one category of result type asked about. Results that a clinical laboratory could not interpret (i.e., VUS) were the least desired type of result as 25% neither agreed nor disagreed to strongly disagreed they would want to receive a VUS. Of those participants who completed the qualitative interview, 21 of 49 expressed some discomfort about learning all of their WGS results. We found no significant associations between participants’ characteristics and their preferences for receiving different types of WGS results (data not shown).
Figure 1.
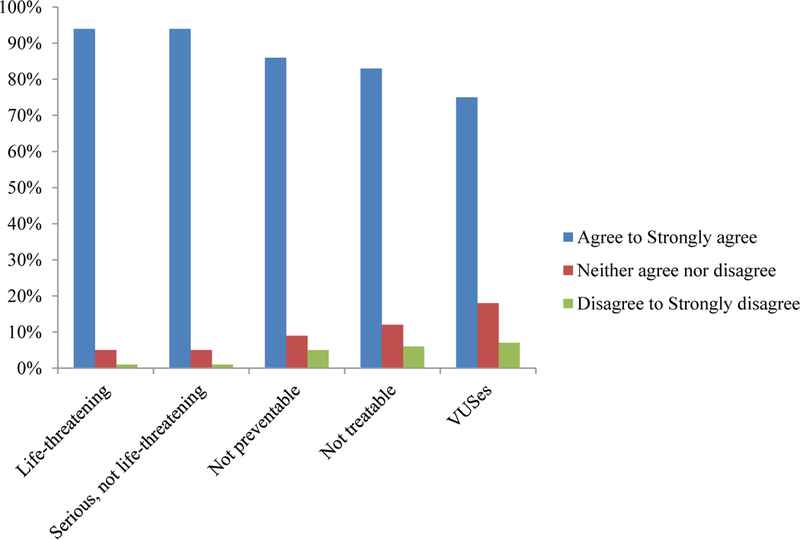
Patients’ preferences for receiving different types of genetic results
Figure 2.
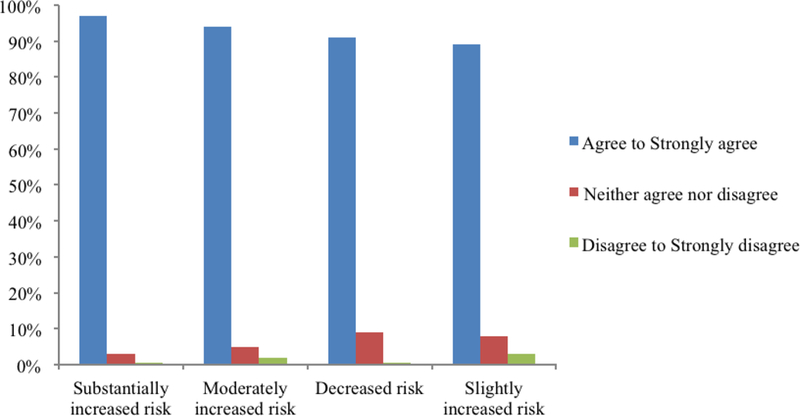
Patients’ preferences for receiving genetic results indicating varying degrees of risk
Our qualitative data provided a deeper understanding of how participants conceptualized WGS results before undergoing sequencing. In particular, we discovered themes in three areas related to how participants conceptualized WGS results: First, participants had nuanced ideas about what constitutes an actionable result. They could not readily distinguish or articulate examples of results associated with treatable/preventable conditions versus those untreatable/unpreventable. Second, they expressed differing beliefs about which kinds of results would be most upsetting for them to learn. Third, they conceptualized VUS in a variety of ways and were optimistic that uncertainty associated with a VUS could be reduced in the future.
1). Participants’ views about the distinction between different types of results categorized by actionability
Participants could not readily provide examples of results associated with preventable or treatable conditions as distinct from results associated with conditions that are not preventable or treatable. Participants gave several reasons why the distinction was unintuitive to them. Either they hadn’t given much thought to the distinction before, or they found the distinction difficult to make without clearer definitions of what the terms “preventable” and “treatable” meant.
In their own words, participants defined these concepts broadly to include things like lifestyle modifications they could implement at home, even for a disease like Alzheimer’s disease which is generally not considered to be treatable.
INTERVIEWER: And what kind of health conditions do you think fall in this [preventable or treatable] category?
PARTICIPANT: Potentially some stuff like the Alzheimer’s I mentioned earlier. I’ve done some reading about trying to keep the brain active by doing different types of puzzles and stuff, to hold it off a little bit.
Participants also found it hard to conceptualize different types of WGS results because they weren’t sure what conditions WGS could provide information about.
PARTICIPANT: I consider myself fairly well informed, but I really have very little idea what specific genes would be targeted and how comprehensive they are. So, you did mention the heart, so I can assume that is in there. I don’t know if there are other kinds of mental illnesses you can get a feeling for. Perhaps diseases involving seizures? I’m just guessing - or lack of muscle control. You know, I’m kind of guessing here…
When participants voiced their difficulty distinguishing results associated with preventable or treatable conditions from those with less objective clinical utility, it was often because they were thinking about other qualities of a WGS result besides the condition it could provide information about. To some participants, the degree of certainty associated with a result was as or more important to them than the nature of the condition it was associated with.
INTERVIEWER: Please tell me how you feel about receiving these results [associated with not preventable or treatable conditions]?
PARTICIPANT: I would have to ask again whether or not—it’s like a hundred percent sure, or this is an indication? I mean, how would it be framed? My assumption is that nobody is going to say something like, “You are definitely getting Parkinson’s,” or something like that, you know. “You are definitely getting pancreatic cancer.” So it probably would, it would depend…
Instead of referencing the preventability or treatability of disorders WGS could give them information about, participants used personal or familial illness histories to shape their perceptions of the results they might receive. They described their hypothetical WGS results by referencing disorders in their families and found it hard to think of conditions besides these.
INTERVIEWER: What diseases or conditions do you think might fall into this [preventable or treatable] category?
PARTICIPANT: Oh, well, the heart disease is one, obviously—one he’s mentioned. You know—gosh—what other things are out there other than cancer? I can’t think of too many other things that—I mean, I’ve never thought about ALS, although I have a friend whose brother died of it, but I don’t think about things like that. I hadn’t thought of those things. It’s never been in my family, so I just don’t think about it.
2). Participants’ Beliefs About Which Results Would Be Upsetting to Learn
Participants understood that they might receive distressing results from WGS. However, they held different views about which results would be most upsetting to learn. Considerations such as a participant’s family history, personal illness history, and whether a participant had children mattered as much as the perceived actionability of a result that WGS could yield. Parents were especially apprehensive about learning they could transmit a genetic mutation to their children.
PARTICIPANT: Obviously the fear [associated with study enrollment] is to be told something bad is going to happen and there’s not a whole lot you can do about it. But again, that’s not the worst thing. The worst thing would be to find out that I pass something onto my kids that would impact them. That would be the worst thing.
Although there were no significant differences in preferences for types of results from the survey data by participants’ age, one common belief expressed in interviews was that results associated with an increased risk of developing a serious or untreatable disease would be more burdensome to learn about at younger ages, because of the impact such information could have on a young person’s life decisions.
PARTICIPANT: I’m not 20. It would be much more dangerous to find that [a genetic risk for Alzheimer’s or Lou Gehrig’s disease] out when you were 20 than to find it out when you’re in your late 50’s, because then you would be worrying about it for, like, 30 years…. Then you might say, ‘well, when I’m 70 I’m going to get Lou Gehrig’s disease. I probably shouldn’t get married and then I probably shouldn’t have children.’ I mean, it could manifest a lot of ethical decisions for a young person…
However, several participants with family histories of progressive illness expressed surprisingly little concern about receiving results related to those diseases, because their experiences with caring for affected family members had left them optimistic that they could deal with these diseases or hopeful for future treatments. These participants attached sentiments of hope and optimism about medical progress to diseases that had affected their families in the past.
PARTICIPANT: My mother, as I said, has Alzheimer’s… it’s like Groundhog Day, if you saw the movie, but she’s happy…so if I was like that, I wouldn’t mind it too much. It’s more of a hassle for the people around you than yourself…by the way, I kind of think that disease someday they’ll be able to control and maybe stop from happening…look at what they’ve done for cancer. So many cancers are now curable at least for a while if not totally.
Participants in the cardiology cohort referenced their personal experiences with a life-threatening illness when imagining the kinds of upsetting results they could learn from WGS. They often remarked that their experiences with cardiomyopathy had primed them to cope with difficult results better than the average person.
INTERVIEWER: How do you feel about receiving those results [associated with not preventable or treatable conditions]?
PARTICIPANT: I’ve faced death. I haven’t smiled about it… I’ve been dealing with it since I’m 29 years old. And I’ve died three times already, so one more. I’ve got nine, right? You get nine lives…. Is that the way it works? If you’re going to submit that result to any human being…I’d probably be the one to handle it the best.
3). How participants conceptualized variants of uncertain significance (VUS)
Participants responded to the possibility of receiving a VUS in different ways. Some participants normalized this possibility by comparing it to previous experiences of medical uncertainty or voicing acceptance that scientific knowledge has limits. Others seemed surprised that they could receive a result with uncertain significance from WGS, as though they hadn’t given the possibility much thought. While some felt that they would be more vigilant about their health if they received a VUS in a disease-related gene, others did not see any value in focusing on a VUS if their doctor didn’t know what it meant.
INTERVIEWER: The researchers may find sequence changes in your DNA they cannot interpret at this time. Tell me how you feel about receiving these results?
PARTICIPANT: Totally neutral because (laughing) I’d look at them like, “If you guys can’t figure it out then I guess that’s a good thing.” Like, there’s no way I’d be able to figure something like that out, so, pretty neutral about that…I just feel like if it’s not something that’s obvious or already studied, why worry about something if it’s not already known or studied?
When probed, participants described a variety of reasons why a result could have uncertain significance, including: the possibility their DNA was unusual, the volume and complexity of genome data, the novelty of tools and approaches used to analyze WGS, and insufficiently broad physician knowledge. Regarding their own physician’s knowledge, one participant asked:
PARTICIPANT: If he hasn’t seen it before…should I infer from that that it hasn’t been seen by anybody, or he’s just the wrong guy…you know, you’re talking specifically about my relationship with Dr. [P01 physician]--if he hasn’t seen it? Or are you implying that it just hasn’t been understood by current medicine? Or, he’s just not the specialist I should be seeing?
Whatever feelings they had about VUSs, most participants were optimistic that their physicians could reduce uncertainty by making follow-up referrals or staying abreast of relevant scientific discoveries. Several participants mentioned that they would expect updates about the status of a VUS in the future, either in the form of notifications from doctors, updates from researchers, or the opportunity to be re-sequenced.
PARTICIPANT: I would think it [a VUS] would go in my file and potentially the doctor would have to be vigilant about new studies and new things that come up and keep it in mind, or put me in like some sort of network where it would raise flags down the line.
Discussion
Our survey results showed that while a majority of participants would be amenable to receiving all types of WGS results, 39% of those enrolled in the study expressed some level of discomfort about receiving certain types of WGS results. In-depth interviews revealed that the constructs used to elicit participant preferences in the survey, which mirror those used in many informed consent processes, may not be aligned with how individuals are conceptualizing the information they could potentially learn from WGS. We identified three areas where participants had highly subjective and varied perceptions about WGS results or results disclosure: their perceptions of results associated with preventable or treatable conditions vs. conditions that were not preventable or treatable, their beliefs about which results would be upsetting to learn, and their understandings of and expectations related to a VUS.
Our finding that participants could not readily distinguish results associated with preventable or treatable conditions from those with less objective clinical actionability was linked to their nuanced understanding that most diseases are not purely determined by genetics and that the notion of a “treatment” can be context-dependent. This finding is consistent with a large body of research showing that illness perceptions and beliefs about disease causation vary across diseases and according to patient characteristics such as age, health status, gender, and sociocultural background (Marteau 1997; Klonoff and Landrine 1994; French et al. 2001; Kelly et al. 2005). It raises questions about informed consent and results management approaches that classify WGS/CGES results based solely upon expert-defined notions of clinical actionability.
Our findings are morally relevant because of their implications for both informed consent for WGS/CGES and shared decision-making about the management of WGS/CGES results. Motivated by the principle of respect for persons, informed consent is a two-way communicative process in which a patient makes an informed and voluntary decision to undergo WGS/CGES based on a sound understanding of what kinds of results the technology may produce. Informed consent processes that rely heavily on expert-defined notions of clinical actionability may fail to impart patients with a meaningful understanding about the possible outcomes of WGS/CGES, thereby undermining the purpose of informed consent in the first place. Moreover, ideally decisions about the return of WGS/CGES results are not confined to the informed consent process but occur as part of an ongoing clinical relationship. In the context of such a relationship, shared decision-making about the management of WGS/CGES results is only possible if the language used to discuss results is comprehensible and relevant to both clinicians and patients.
Adding to this complexity is the fact that disease ontologies, screening regimens, and treatments vary across healthcare settings. The known relationship between genetic variants and diseases will shift over time, as will knowledge about diseases themselves and their treatment options (Aronson et al. 2012). As such, it is unlikely that static, context-neutral definitions of “clinical utility” and “actionability” will be established in the near term. Our results bolster the idea that WGS/CGES information is best conceptualized as a resource that patients and clinicians “manage” together over time, as new scientific discoveries come to light and as the circumstances of patients’ lives, including family composition and health, evolve (Yu et al. 2013).
We also found that participants held different beliefs about which types of results would be most upsetting to learn. When explaining their perceptions of disease risk and severity, patients referenced their ages, family contexts, and prior illness experiences. This observation is consistent with evidence that intentions to learn different kinds of genetic test results are shaped by dispositional, emotional, and experiential variables (Vernon et al. 1999; Wade et al. 2012; Lautenbach et al. 2013; Taber et al. 2014) and that individuals incorporate heuristics into appraisals they make under conditions of uncertainty (Tversky and Kahneman 1974).
In the context of clinical WGS/CGES, these findings matter because the return of clinical WGS/CGES is not purely a function of patient preferences. Clinicians and clinician-researchers play more of a shared role in decisions about the return of WGS/CGES results than is typically expected of researchers in a research-only setting. This is because clinicians have ethical and fiduciary duties to maximize benefits and minimize harms to patients, using their professional judgment. Our results show that clinicians who wish to achieve shared decision-making about the return of WGS/CGES result should, to the best of their abilities, maximize their understanding of how an individual patient conceptualizes his or her WGS/CGES results, including which ones might be upsetting to learn. This will help clinicians to explain their decisions to return upsetting or unwanted results to patients when their clinical judgment compels them to do so in the best interest of a patient. Failure to justify such decisions in terms that are clear and sensitive to a patient could risk undermining the physician-patient relationship.
Participants in our study pinpointed several reasons why a WGS/CGES result could have uncertain meaning. These findings highlight a need to develop better practices for communicating about the uncertainty associated with many WGS/CGES results, which may take multiple forms depending on its sources, focal issues, or the persons it implicates. Each of these forms may warrant different courses of follow-up action in a clinical context (Han, Klein, and Arora 2011). Overall, individuals enrolled in the MedSeq Project were optimistic that their physicians could resolve the uncertainty associated with some WGS/CGES results. Given the limited availability of tools for reclassifying variants and updating clinical test reports (Aronson et al. 2012), physicians should be mindful to encourage realistic expectations about their ability to resolve uncertain results. To do otherwise may prevent patients from making informed decisions about whether to undergo WGS/CGES and may risk patient disillusionment during this early phase of clinical WGS/CGES adoption.
It is important to note that the MedSeq Project participants who completed in-depth interviews discussed their preferences for WGS/CGES results and results disclosure even though they were not offered any choices about which results to receive and even though preference-setting about the return of results was not a central focus of the informed consent process for this study. As such, the views they expressed cannot tell us about how patients view the distinction between primary and incidental findings. While we acknowledge that questions about the scope of clinicians’ duties and practical abilities to return incidental findings without clinical relevance are important, our study was not designed to explore those issues directly. Although participants in our study were not asked to choose what types of results to learn, we believe that similar variability in perceptions are likely to exist in contexts where patients are setting preferences for the return of secondary findings.
Another limitation of our study is that participants were recruited into the MedSeq Project by their own physicians in whom they demonstrated high levels of trust. Thus their views may not reflect those of other populations undergoing WGS/CGES. Participants in our study had relatively high scores on measures of study understanding and genomic knowledge. It remains an open question whether other patient populations might demonstrate greater or less variation in the ways they conceptualize WGS/CGES results compared to this highly scientifically literate group.
Supplementary Material
ACKNOWLEDGMENTS:
The authors thank the members and participants in the MedSeq Project for their important contributions. The authors also thank 5AM Solutions, Inc. (Rockville, MD, USA), for their help in customizing the workflow of the “My Family Health Portrait” web tool for this study.
FUNDING: This work was supported by NIH grants U01-HG006500 (Green).
Footnotes
CONFLICTS OF INTEREST: None.
ETHICAL APPROVAL: This study was approved by the institutional review board(s) at Partners Healthcare and the Baylor College of Medicine.
References:
- Aronson SJ, Clark EH, Varugheese M, Baxter S, Babb LJ, and Rehm HL. 2012. Communicating new knowledge on previously reported genetic variants. Genetics in Medicine: Official Journal of the American College of Medical Genetics 14(8): 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JS, Foreman AKM, O’Daniel JM, et al. 2016. A semiquantitative metric for evaluating clinical actionability of incidental or secondary findings from genome-scale sequencing. Genetics in Medicine 18(5): 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JS, Khoury MJ, and Evans JP. 2011. Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time. Genetics in Medicine: Official Journal of the American College of Medical Genetics 13(6): 499–504. [DOI] [PubMed] [Google Scholar]
- Biesecker LG, and Green RC. 2014. Diagnostic clinical genome and exome sequencing. The New England Journal of Medicine 371(12): 1170. [DOI] [PubMed] [Google Scholar]
- Biesecker LG, Mullikin JC, Facio FM, et al. 2009. The ClinSeq project: Piloting large-scale genome sequencing for research in genomic medicine. Genome Research 19(9): 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnik EM, Schermer MH, and Janssens ACJW. 2012. The role of disease characteristics in the ethical debate on personal genome testing. BMC Medical Genomics 5(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French DP, Senior V, Weinman J, and Marteau TM. 2001. Causal attributions for heart disease: A systematic review. Psychology & Health 16(1): 77–98. [Google Scholar]
- Graves KD, Sinicrope PS, McCormick JB, Zhou Y, Vadaparampil ST, and Lindor NM. 2015. Public perceptions of disease severity but not actionability correlate with interest in receiving genomic results: Nonalignment with current trends in practice. Public Health Genomics 18(3): 173–183. [DOI] [PubMed] [Google Scholar]
- Green RC, Berg JS, Grody WW, et al. 2013. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in Medicine: Official Journal of the American College of Medical Genetics 15(7): 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Lupski JR, and Biesecker LG. 2013. Reporting Genomic Sequencing Results to Ordering Clinicians: Incidental, but Not Exceptional. JAMA 310, no. 4 (July 24): 365–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han PKJ, Klein WMP, and Arora NK. 2011. Varieties of uncertainty in health care: A conceptual taxonomy. Medical Decision Making: An International Journal of the Society for Medical Decision Making 31(6): 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Irving SA, Biesecker LG, et al. 2016. A standardized, evidence-based protocol to assess clinical actionability of genetic disorders associated with genomic variation. Genetics in Medicine: Official Journal of the American College of Medical Genetics 18: 1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst KA, Facio FM, Cheng M-R, et al. 2012. Effects of informed consent for individual genome sequencing on relevant knowledge. Clinical Genetics 82(5): 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K, Leventhal H, Andrykowski M, et al. 2005. Using the common sense model to understand perceived cancer risk in individuals testing for BRCA1/2 mutations. Psycho-Oncology 14(1): 34–48. [DOI] [PubMed] [Google Scholar]
- Klonoff EA, and Landrine H. 1994. Culture and gender diversity in commonsense beliefs about the causes of six illnesses. Journal of Behavioral Medicine 17(4): 407–418. [DOI] [PubMed] [Google Scholar]
- Lautenbach DM, Christensen KD, Sparks JA, and Green RC. 2013. Communicating genetic risk information for common disorders in the era of genomic medicine. Annual Review of Genomics and Human Genetics 14: 491–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor NM, Johnson KJ, McCormick JB, Klee EW, Ferber MJ, and Farrugia G. 2013. Preserving personal autonomy in a genomic testing era. Genetics in Medicine: Official Journal of the American College of Medical Genetics 15(5): 408–409. [DOI] [PubMed] [Google Scholar]
- Marteau TM 1997. Illness representations after The Human Genome Project: The perceived role of genes in causing illness. In Perceptions of Health and Illness, ed. Petrie KJ and Weinman JA, 241–266. Harwood Academic Publishers. [Google Scholar]
- McGuire AL, and Beskow LM. 2010. Informed consent in genomics and genetic research. Annual Review of Genomics and Human Genetics 11: 361–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Joffe S, Koenig BA, et al. 2013. Point-counterpoint: Ethics and genomic incidental findings. Science 340(6136): 1047–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- President’s Commission for the Study of Bioethical Issues. Anticipate and Communicate: Ethical Management of Incidental and Secondary Findings in the Clinical, Research, and Direct-to-Consumer Contexts Available at: http://bioethics.gov/sites/default/files/FINALAnticipateCommunicate_PCSBI_0.pdf (accessed June 6, 2016) [DOI] [PubMed]
- Robinson JO, Carroll TM, Feuerman LZ, et al. 2016. Participants and study decliners’ perspectives about the risks of participating in a clinical trial of whole genome sequencing. Journal of Empirical Research on Human Research Ethics: JERHRE 11(1): 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelowski M 1995. Sample size in qualitative research. Research in Nursing & Health 18(2): 179–183. [DOI] [PubMed] [Google Scholar]
- Sandelowski M 2000. Whatever happened to qualitative description? Research in Nursing & Health 23(4): 334–340. [DOI] [PubMed] [Google Scholar]
- Taber JM, Klein WMP, Ferrer RA, Lewis KL, Biesecker LG, and Biesecker BB. 2014. Dispositional optimism and perceived risk interact to predict intentions to learn genome sequencing results. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association 34(7): 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, and Kahneman D. 1974. Judgment under uncertainty: Heuristics and biases. Science 185(4157): 1124–1131. [DOI] [PubMed] [Google Scholar]
- Vassy JL, Lautenbach DM, McLaughlin HM, et al. 2014. The MedSeq Project: A randomized trial of integrating whole genome sequencing into clinical medicine. Trials 15: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon SW, Gritz ER, Peterson SK, Perz CA, Marani S, Amos CI, and Baile WF. 1999. Intention to learn results of genetic testing for hereditary colon cancer. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 8(4): 353–360. [PubMed] [Google Scholar]
- Wade CH, Shiloh S, Roberts JS, Hensley Alford S, Marteau TM, and Biesecker BB. 2012. Preferences among diseases on a genetic susceptibility test for common health conditions: An ancillary study of the multiplex initiative. Public Health Genomics 15(6): 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Annas GJ, and Elias S. 2013. Patient autonomy and incidental findings in clinical genomics. Science 340(6136): 1049–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JH, Jamal SM, Tabor HK, and Bamshad MJ. 2013. Self-guided management of exome and whole-genome sequencing results: Changing the results return model. Genetic Medicine 15(9): 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


