Abstract
“Probiotics: From Bench to Market” was a one-day conference convened by the New York Academy of Sciences on June 11, 2010, with the goal of stimulating discussion of the physiological effects of probiotics on the gastrointestinal, nervous, and immune systems. The program included speakers from academia, industry, and government to give conference participants a full understanding of the state of the field of probiotics. The overall goal of the program was to increase communication and collaboration among these groups to advance probiotic research and probiotic contributions to public health. The conference was divided into three sessions and included both oral and visual presentations as well as panel discussions.
Keywords: probiotics, prebiotics, microbiota, investigational new drug
Session I: Basic mechanisms of action
The first session was moderated by Tri Duong and Howard Young and functioned as an overview of the state-of-the-field of probiotics by describing the mechanisms of probiotics in the gastrointestinal, nervous, and immune systems. The first presentation was the keynote lecture given by Mary Ellen Sanders (Dairy and Food Culture Technologies, Centennial, CO). Her lecture “Probiotics: myth vs. facts” set the tone for the session and provided a broad context for the subsequent presentations. She identified and discussed common myths and misconceptions regarding probiotics and identified challenges faced by researchers and producers/suppliers in this area.
Common misconceptions include the understanding of the benefits of probiotics and the associated mechanisms; the proper dosage required to achieve a beneficial effect; and even what constitutes a probiotic. Indeed, commensal and fermentation microorganisms belonging to several genera, including Lactobacillus, have been labeled as probiotics.1 However, by definition, probiotics must confer proven health effects that must be considered to be strain specific, unless otherwise demonstrated. Some have suggested that the term probiotic has out lived its usefulness and have instead proposed the term pharmabiotics.2 This term refers to live, dead, or components of organisms that are part of the natural microbiota. But the term probiotic encompasses only live microbes from any source (human, animal, or environmental) so long as they are safe for their intended use and have documented health effects. Probiotics are commonly marketed to promote gastrointestinal (GI) health by “balancing” the GI microbiota. The concept of microbial balancing is undefined, but some evidence exists suggesting that certain probiotic strains can stabilize the gut microbiota by reducing the change in fecal bacteria communities in response to specific stress or health conditions3 or by promoting the return to a baseline microbial community following a perturbing event such as antibiotic therapy.4 Dosages required to achieve beneficial effects are commonly reported to be on the order of 1–10 billion CFU/day. However, effective doses are sometimes greater or less than this recommended amount. For example, administration of 100 million CFU/day of certain probiotics can reduce abdominal discomfort in irritable bowel syndrome (IBS) patients5 and improve colicky symptoms in infants.6 Thus, controlled studies must be performed to determine the appropriate dose, not a general dose, before probiotic effectiveness can be advanced. These and other misconceptions are attributable to inadequate understanding within the scientific, industrial, and regulatory communities. Minimizing these misconceptions will occur through a more complete understanding of the effects of probiotics in health and disease as well as increased communication among these communities.
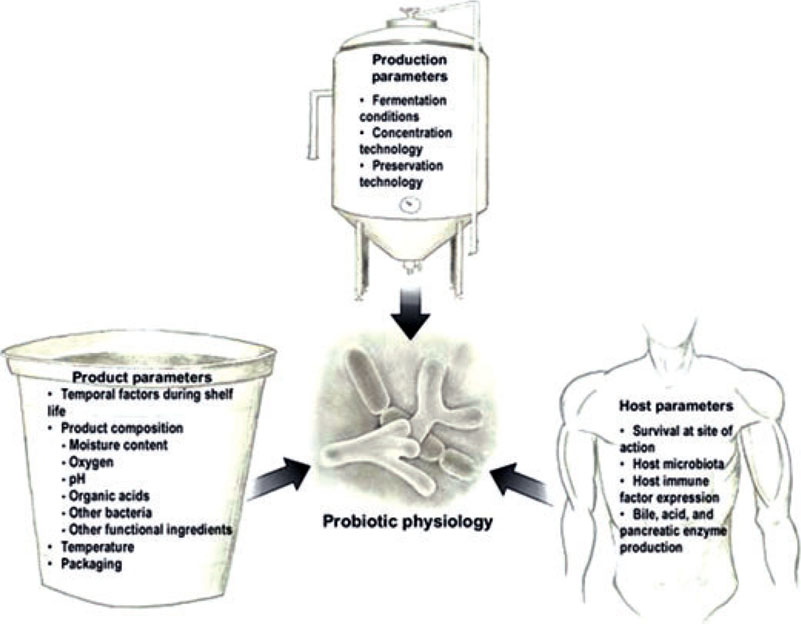
Within the probiotic industry, there are several notable challenges. The effects of manufacturing, product matrix, and host on the physiology and functionality of probiotic organisms is not well understood. Controlled research in humans is required to determine the bioequivalency of specific probiotics under varied conditions (Fig. 1). Furthermore, probiotics and their benefits are not well understood by consumers and physicians, causing confusion regarding which products can achieve certain health benefits. Several resources for physicians have been prepared by ISAPP,7 the World Gastroenterology Organisation,8 and others.9 Perhaps the most important challenge faced by this industry is the validation of health benefit claims for probiotics. Mechanisms are in place to seek approval for health benefit claims with domestic and international regulatory bodies; however, no probiotics have currently been approved for health benefit claims. While human trials studying the health benefits of probiotics rely upon human disease endpoints, validated probiotic-specific biomarkers for specific benefits will be required to gain regulatory approval. This is a major problem for the industry, as these markers have not yet been identified or clearly defined.
Figure 1.
An important challenge facing the probiotic industry today is to understand the extent to which different factors influence probiotic physiology. Probiotic physiology will, in turn, influence in vivo functionality and stability. The extent to which these factors influence probiotic physiology should be expected to be strain-specific unless demonstrated otherwise. Demonstration of bioequivalency among different delivery formats and production methods is an important step in developing evidence-substantiating efficacy.
Evidence suggests that probiotics have significant potential in the treatment of GI disorders, including certain types of diarrhea and functional and inflammatory bowel disorders. Yehuda Ringel (University of North Carolina, Chapel Hill) reviewed a range of studies in his talk “The effect of probiotics on intestinal function and symptoms” that suggest a potential role of probiotics in promoting GI health, with particular focus on functional bowel disorders. Research conducted by Ringel and others has established significant qualitative and quantitative differences in GI microbiota in disease states. These differences suggest the importance of the fecal and mucosal niches of the microorganisms in patients with bowel function disorders as compared to healthy controls.10 Additionally, epidemiological studies suggest that disturbances in GI microbiota, including acute gastroenteritis, small-bowel bacterial overgrowth, and antibiotic treatment, contribute to the development of IBS or irritable bowel symptoms. Much of our understanding of the relationship between GI microbiota and physiological bowel function comes from studies using germ-free animals. These animals, which lack any GI microbiota, exhibit markers of reduced bowel function, including delayed gastric emptying, slowed intestinal transit, and increased cecal size.11,12 Re-introduction of microbiota could reverse these changes. In addition to their effect on physiological function, gut microbiota can affect visceral sensation.13 Human studies have shown that treatment with probiotics may be beneficial to IBS patients,14,15 potentially by improving physiological function16,17 and reducing visceral hypersensitivity.18,19 Thus, microbiota play an important role in intestinal function and pathophysiology of functional bowel disorders (FBD) and manipulation of the GI microbiota; pre- and probiotics may be beneficial for patients with functional bowel symptoms. However, Ringel cautioned that targeting physiological function does not always correlate with clinical benefit.
Neurogastroenterology is an area of research concerned with the interactions between the nervous system and the gastrointestinal tract. In his presentation entitled “Impact of probiotics on the central nervous system?” Emeran Mayer (University of California at Los Angeles) provided a review of evidence suggesting that gut microbiota may play a role in the communication between the gut and the brain. It is understood that mucosal immune responses in the gut are modulated by the nervous system.20 More recently, interactions of lumenal factors, including intestinal microorganisms, with sensory nervous, endocrine, and immune cells at the mucosa and the brain’s responses to these signals, have been recognized.21 Stress-induced changes to gastric acid production, GI motility, mucus secretion, and gut pH can influence the gut microbial community. Postnatal stress reduced total microbiota22 in infant rhesus monkeys, potentially predisposing them to opportunistic infection and increased systemic inflammatory responses. Postnatal stress also caused altered fecal microbiota in rats.23 Stress-related changes in neurotransmitter levels can directly influence bacteria present in the GI tract. For example, norepinephrine has been shown to promote virulence in pathogens, including enterohemorrhagic Escherichia coli24 and Campylobacter jejuni.25 Probiotic therapy may be able to reduce the effect of stress on the GI microbiota and stress hormone levels26,27 and attenuate visceral hypersensitivity.18,28 This preclinical evidence suggests the involvement of microbiota in gut–brain immune modulation and stress and pain responses. There are even preliminary indications that differences in gut microbe populations exist in autistic children, although a causal relationship has not yet been established. These data indicate that much more research is needed to determine a clinical relevance with respect to microbiome and nervous system interactions.
The GI tract is the major site of interaction between environmental microorganisms and host tissues. Dendritic cells (DC) located at the gut mucosa are a critical component of the immune system as they are able to stimulate local mucosal immunity as well as systemic immunity. Mansour Mohamadzadeh (Northwestern University, Evanston, IL) described the efforts of his group in the development of probiotic Lactobacillus as an oral delivery vector for vaccines29 in his lecture “Pro-biotic impact on the immune system.” DCs are antigen-presenting cells able to sample microbial antigens either directly in the intestinal lumen or indirectly through those antigens that cross the mucosal epithelium through M cells. DCs then present captured antigens to B and T cells to initiate antibody and cell-mediated immune responses.30 Additionally, DC-targeting peptides have been identified that, when fused to microbial antigens, increase DC-antigen binding and subsequent stimulation of T cell and B cell immune responses.31 Specific probiotic Lactobacillus species have adjuvant-like effects on mucosal and systemic immunity and are able to induce regulated inflammatory responses against infection and activate DCs.32 Thus, engineering probiotic Lactobacillus species producing DC-targeted antigens represents a novel and powerful approach to oral vaccine delivery. Using this approach, a Lactobacillus-based DC-targeted vaccine for Bacillus anthracis conferred protective immunity and survival at levels comparable to, or better than, a traditional vaccine strategy.33 Furthermore, the Mohamadzadeh laboratory has found that different strains of Lactobacillus can elicit different anti-inflammatory cytokine responses (e.g., IL-10 expression) from macrophages. Such responses could contribute to a localized dampening of inflammation. Mohamadzadeh and his collaborators are actively pursuing the use of this technology as a new approach for vaccination against influenza and as a potential treatment for breast cancer and inflammatory bowel disease.
While considerable information exists about the composition of GI microbiota, a functional and mechanistic understanding is needed to understand how this ecosystem can be manipulated for our benefit. Justin Sonnenburg (Stanford University, Palo Alto, CA) addressed this topic in his talk “Understanding and altering the intestinal micro-biota.” Using a gnotobiotic mouse model system, his group investigates carbohydrate metabolism by the gut microbiota. Bacteriodes thetaiotaomicron is a major component of the human GI micro-biota, with greater than 5% of its genome encoding carbohydrate-digesting enzymes.34 Transcriptional profiling of B. thetaiotaomicron monoassociated with mice fed a polysaccharide-rich diet identified over 100 glycoside hydrolases related to plant polysaccharide utilization. Eliminating plant polyscaccharides from the mouse diet downregulated expression of these genes while upregulating expression of genes encoding enzymes responsible for degradation of host factors such as mucus polysaccharides.35 When co-colonized in mice with the probiotic Bifidobacterium longum, B. thetaiotaomicron upregulated expression of additional glycoside hydrolases, thus expanding its niche.36 However, when co-colonized with the methanogen Methanobrevibacter smithii, this organism down-regulated glycoside hydrolases but upregulated expression of a single locus involved in fructan utilization,37 resulting in higher-density growth. Additionally, in mice co-colonized with B. thetaiotaomicron and Bifidobacterium species, administration of the prebiotic inunlin induced predictable shifts in the gut microbial community.38 These studies demonstrate the ability of GI tract microorganisms to adapt to changing conditions of nutrient availability and competition with other organisms. These data further suggest that microbiota can be manipulated by introducing other microorganisms or by altering nutrients. These ideas are central to the use of pre- and probiotics, but it will remain to be seen if similar changes can be achieved in the context of the total microbial communities present in the gut.
Improved gut barrier function, enhanced immunity, competitive exclusion, and direct antagonism are proposed mechanisms for the anti-infective activity of probiotics. Colin Hill (University College Cork, Ireland), in his presentation entitled “Bacteriocin production as a probiotic trait to combat infection,” reported on his investigations of the direct antagonism of pathogenic bacteria by bacteriocins produced by probiotic bacteria. Bacteriocins are antimicrobial peptides produced by microorganisms that have a very specific range of bactericidal activity. Listeria monocytogenes is a food-borne intracellular pathogen whose pathogenesis is well understood. While the mortality rate can be high, the incidence of listeriosis is insufficient to necessitate vaccination strategies to prevent infection, and thus it represents an opportunity for the use of probiotic-based interventions. Administration of the probiotic Lactobacillus salivarius UCC118 conferred significant protection and improved survival when mice were challenged with L. monocytogenes.39 Analysis of the genome of L. salivarius UCC118 revealed the presence of a gene encoding the bacteriocin, Abp118. Deletion of the bacteriocin gene resulted in loss of the antilisterial activity, proving that this bacteriocin is responsible for Listeria inhibition in vivo. After decades of supposition of the importance of bacteriocins in anti-infective activity, this research was the first to provide conclusive evidence. Interestingly, L.salivarius UCC118 also protects mice from Salmonella infection. However, bacteriocin-negative mutants of this probiotic protected equally well against Salmonella, demonstrating that this same bacteriocin did not play a role in the anti-Salmonella effect, and confirming that multiple mechanisms can play a role in anti-infective activity of probiotics. Clostridium difficile is a common cause of antibiotic-associated diarrhea and is another clinical condition in which there is evidence to suggest the efficacy of treatment with probiotics. Thuricin CD, a bacteriocin produced by Bacillus thuringiensis, has very specific activity against C.difficile.40 Additionally, in a human distal colon model, Thuricin CD was equally effective against C. difficile as vancomycin and metronidazole, with little impact on the remaining microbiota as compared to the antibiotics.41 Thuricin is an example of another bacteriocin that could be useful for anti-infective applications. These studies demonstrate the potential prophylactic and therapeutic use of probiotics in the prevention and treatment of infection. Additionally, understanding these mechanisms of pro-biotic action may lead to the development of pharmaceuticals for the treatment of bacterial infections without the negative impact on beneficial gut microbial populations currently seen following antibiotic therapy.
Appropriate model systems for studying the efficacy of probiotics are important in the development of probiotics for use in humans. Glenn Gibson (University of Reading, UK) provided an overview of in vitro and in vivo models currently in use in his talk, “Models for studying efficacy in probiotics.” Batch and continuous fermentation systems are currently used to investigate the physiology and fermentation properties of probiotic cultures under various conditions. Probiotics can also be co-cultured with other microbes, including pathogens, to investigate microbe–microbe interactions. Furthermore, single- and multi-stage fermentation systems can be used to model specific portions or the entire GI tract. Gibson and colleagues have developed a continuous multistage system replicating conditions (e.g., volumes and pH) present in the large intestine. Validated with gut microbiome specimens taken from sudden death victims, this system accurately models the intestinal lumen. The utility of these in vitro systems is limited, and these systems are perhaps most useful as screening tools to evaluate a variety of interventions or conditions to determine what does not look promising to pursue with further research. Human studies are essential in order to make a claim of pre- or probiotic benefit. However, in vitro models serve as useful screening tools in the development of probiotics for human use. Using prebiotic galactooligosaccahrides (GOS) as an example, Gibson described research on the selectivity and persistence of bifidogenic effects of the microbial populations in the gut, using an in vitro system and progressing to human studies. A double-blind, placebo-controlled study involving 30 adults determined that the prebiotic effect of GOS was dose-dependent and attributed solely to bifidogenicity.42 Additionally, GOS concomitantly improved symptoms and increased numbers of Bifidobacterium in IBS patients,43 significantly increased bifidobacteria, and positively influenced pro- and anti-inflammatory immune markers in elderly adults.44 GOS also reduced the incidence and duration of traveler’s diarrhea.45 The development of such model systems and sophisticated molecular tools has made possible the analysis of microbial diversity and changes in microbiota in response to specific interventions. Combining these in vitro and in vivo models with metabalomic analysis will allow researchers to study diet-mediated alterations of the gut micro-biota and determine if they affect metabolism of the host.
The seven presentations in the first session highlighted the progress and research required to better understand gaps in our knowledge of probiotics. Such preclinical research is important to set the stage for well-designed, hypothesis-driven human studies needed to fully understand the benefits of probiotics to human health, from both an industry and academic perspective. These talks underscored the need for further understanding of the role of probiotics in health and disease. Progress in the microbiology, physiology, and epidemiology of probiotics, paired with strong clinical trials, could result in novel treatment options for a variety of diseases.
Session II: Data blitz
The second session, moderated by Mary Ellen Sanders, consisted of a series of short presentations of new data generated by industry and academic researchers. The aim of the session was to highlight innovative approaches to substantiate the health effects of probiotic strains. Presenters were selected from submitted abstracts.
Duane Charbonneau (Procter & Gamble) began the session with the presentation “Development of Bifidobacterium longum infantis 35624 for a probiotic supplement.” Charbonneau described a progression of studies that, taken together, provides support for the role of B. infantis 35624 in helping to reduce symptoms of IBS. Work in a mouse infection model system indicated that strain 35624 was effective in reducing pathogen load, even in the context of the mixed microbiota environment of the gut microbiome. In a mouse model, strain 35624 demonstrated antimicrobial activity, which is the ability to positively modify microbial communities and influence anti-inflammatory immune biomarkers.46,47 Following the mouse model studies, two human clinical trials were conducted to determine if benefits could be documented in human subjects. One study tested B. infantis 35624 versus L.salivarius UCC4331 delivered in fermented milk.48 The B. infantis strain reduced symptoms, whereas the L.salivarius strain did not. This evidence of strain-specific effects provides support for pursuing strain 35624 as a probiotic therapy to manage symptoms of IBS. A second study, this time in women, confirmed this efficacy, and documented that 108 CFU/day was an effective dose.5 The lack of response associated with a 1010 dose was attributed to incomplete release of the bacteria from the encapsulated delivery device.
Charbonneau explained that while the mechanism for efficacy of B. infantis 35624 in treatment of IBS is not known, mouse models suggest that probiotic-stimulated release of anti-inflammatory cytokines, such as interleukin-10 (IL-10), may be involved.
Kevin A. Donato (Research Institute, Hospital for Sick Children, Toronto) presented a talk titled “Lactobacillus rhamnosus GG attenuates interferon γ and tumor necrosis factor-α–induced epithelial dys-function.” Donato first presented evidence that specific cytokines and microbes interfere with the integrity of epithelial tight junctions between cells and thus the barrier function of gut epithelial cells.49,50 He then posed the question, Do probiotics have beneficial effects on barrier function? To address this question, his group used a barrier function model system (specifically, a challenge of monolayers of gut epithelial cells grown in vitro) to assess the ability of a representative probiotic, L. rhamnosus GG, to prevent (or reduce) the proinflammatory effects of other microorganisms and proinflammatory cytokines on the gut epithelial barrier, known causes of barrier disruption. Donato and colleagues found that pretreatment of the epithelial monolayer with L. rhamnosus GG attenuated the disruption of barrier function caused by enterohemorrhagic Escherichia coli O157:H7 or proinflammatory cytokines. They also showed that this anti-inflammatory effect was species-specific, as two other lactobacilli (strains of L. farciminis and L. plantarum) were unable to protect the epithelial barrier function. For L. rhamnosus GG–associated positive effects, Donato and colleagues found that inflammatory TNF-α–induced chemokines (such as CXCL-8 and CCL-11) were reduced and that this was associated with reduced nuclear translocation of transcription factor nuclear factor κB.51
Patrick Veiga’s (Danone Research) talk, “Study of the interplay between gut microbiota and ingested beneficial bacteria in irritable bowel syndrome subjects with predominant constipation,” focused on the interplay between gut and ingested microbiota in IBS. Veiga described a study that showed that four-week consumption of a fermented milk product containing Bifidobacterium lactis DN-173 010 and strains of Lactococcus lactis, Steptococcus thermophilus, and Lactobacillus bulgaricus led to an improvement of IBS symptoms in women with constipation-predominant IBS.52
To identify changes in the gut microbiota that might be induced by probiotic consumption, Viega and colleagues collected fecal samples acquired before and after consumption of the fermented milk and analyzed them using phylogenetic microarrays (HitChips) and qPCR. These analyses indicated that the probiotics did not affect the global structure of the gut microbiome but rather had specific effects on phylogenetic groups. For example, there was a correlation between the presence of L. plantarum at baseline in the feces of subjects with the ability to recover B. lactis post-feeding. The presence of L. reuteri or L. plantarum correlated with recovery of L. lactis. The significance of these findings remain to be determined, but these data may suggest that the response to probiotic treatment may depend on a subject’s particular resident gut microbiota.
Howard Young (National Cancer Institute) presented his talk entitled “Exacerbation of DSS-induced colitis by localized delivery of IFN-β secreted by Lactobacillus acidophilus.” This presentation reported on an unexpected outcome observed in an animal model of gut inflammation and treatment with localized type I interferon. Young provided an overview on the conflicting reports of the role of type I interferons in gut inflammation and inflammatory bowel diseases,53–56 and then described research demonstrating that IFN receptor alpha (IFNRα)–knockout mice are more susceptible to dextran sulfate sodium (DSS)–induced colitis than are wild-type mice. One conclusion from such experiments is that type I interferon signaling would reduce DSS-induced colitis.
To test this, Young’s group designed an experiment with a localized delivery of type I interferon beta (IFN-β using probiotic bacteria genetically altered to expresses the cytokine. Specifically, recombinant L. acidophilus constitutively expressing murine IFN-βwas tested for its ability to reduce DSS-induced colitis in otherwise healthy mice. Surprisingly, mice treated with the transgenic lacto-bacilli secreting IFN-β prior to induction of acute DSS colitis showed greater weight loss and intestinal thickening/shortening, and an increase in the percentage of the specific type of CD4+ T cells in the small intestine that are known to play pathogenic roles in colitis. Young and colleagues also found increased levels of inflammatory cytokines (e.g., IL-6 and TNF) in the serum and in the supernatant from intestinal tissue culture. Further analysis indicated that lactobacilli secreting IFN-β resulted in down-regulation of IFNRα on intestinal DCs, thus phenocopying the results seen in the experiments with the IFNRα-knockout mice. Additional experiments using pretreatment with lactobacilli that were not transgenically altered to secrete IFN-β showed improvement in DSS-induced colitis symptoms. These experiments demonstrate that localized delivery of IFN-β by probiotic bacteria can exacerbate, rather than ameliorate, DSS-induced colitis.
Arthur Ouwehand’s (Health and Nutrition, Danisco) presentation, “Administration of probiotic Bifidobacterium lactis 420 reverses diabetic status in mice under high-fat diet,” described experiments intended to reverse or reduce the problematic effects of metabolic syndrome, one consequence of a high-fat diet. Metabolic syndrome occurs when an individual presents with at least three of the following five abnormal conditions: elevated blood pressure, high blood sugar levels, high levels of blood triglycerides, low levels of high-density lipids, and too much fat around the waist.57 Having metabolic syndrome increases the risk of heart disease, stroke, and diabetes. Concerning how the latter stems from metabolic syndrome, some work has indicated that the underlying causes of metabolic syndrome may be subacute inflammation in adipose tissues, which leads to impaired insulin metabolism and, ultimately, diabetes. Other work has indicated that bacterially derived lipopolysaccharide (LPS) may be involved in the development of metabolic syndrome. In one model, a high-fat diet leads to bacterial translocation across the gut endothelium (barrier breach), which ultimately causes increased LPS in the bloodstream.
Ouwehand described work by his group with probiotics to reduce intestinal inflammation and barrier breach, thus reducing the inflammatory effects of LPS in the bloodstream.58 Ouwehand and colleagues fed B. lactis 420 to mice on a high-fat diet and found improved intestinal epithelial barrier function and reduced bacterial translocation to adipose tissue. Furthermore, improved barrier function led to reduced plasma LPS, reduced inflammation, and improved glucose tolerance. Ouwehand concluded that probiotic treatment with B. lactis 420 may offer a new strategy for treatment of metabolic syndrome and comorbidities of obesity and chronic inflammation.
Guy Rousseau (Hôpital du Sacré-Coeur de Montreal) ended the data blitz session with the talk “Probiotics inhibit behavioral signs of depression after a myocardial infarction in a rat model.” He described a study designed to determine whether probiotics can attenuate depressive behavior that occurs in patents after myocardial infarction (MI), a problem seen, remarkably, in more than 20% of MI patients. The reasons for MI-associated depression are not clear. However, MI causes release of proinflammatory substances, and rat models of MI have demonstrated increased apoptosis in the limbic system after MI.59 Rousseau’s group previously demonstrated that pretreatment with probiotics reduces apoptosis observed in the limbic system after MI.60 They surmised that probiotics might reduce proinflammatory cytokine apoptosis in the limbic system that leads to depression after myocardial infarction.
In the current experiments, Rousseau evaluated the efficacy of Lactobacillus helveticus R0052 and B. longum R0175 administration (1 billion cells in 200 mL drinking water daily, starting seven days before MI and continuing between the seventh and fourteenth day post-MI) on behavior of mice 14 days post-MI using a number of tests. Behavioral tests included social interaction, forced swim and passive avoidance tests, and biological/biochemical-included measurements of MI infarct size, plasma concentration of IL-1β, and intestine integrity. While probiotics had no effect on MI infarct size, improvement was observed in socialization and forced swim tests, IL-1β was reduced, and integrity of the intestinal barrier was restored. Probiotic-treated MI rats showed the expected behavioral syndrome of depression, and probiotics had no impact on behavioral performance in sham rats.
Rousseau concluded that probiotics can interfere with the development of post-MI depressive behavior in the MI mouse model. The results suggest an important role of the gastrointestinal tract in mediating the post-MI response.
Session III: From clinical trials to market
Orally administered probiotics may be marketed as dietary supplements, conventional foods, medical foods, and drugs (biologics). At present, no pro-biotic product is licensed in the United States as a biological drug product for use in the treatment, prevention, cure, mitigation, or diagnosis of a specific human disease. Foods and dietary supplements cannot carry these types of claims. However, researchers often study probiotic products, which are marketed as supplements and foods, for the purpose of treating or preventing a disease. Because dietary supplements and foods differ from therapeutic agents in how they are regulated by the U.S. Food and Drug Administration (FDA), these study agents are considered therapeutic agents or investigational new drugs. Marguerite Klein, Office of Dietary Supplements, National Institutes of Health (NIH), explained in the introduction to the final session that the purpose of the session was to describe, distinguish, and discuss the U.S. regulatory experiences in the conduct of probiotic clinical research, the results of which could be used to substantiate health effects. Four speakers addressed their experiences from industry, regulatory, and academic perspectives. The discussion panel at the end of the session included legal and additional research perspectives.
The intended use of a product dictates how it will be regulated and the nature of the claims that can be made. Thus, a thorough understanding of the definitions of various products is critical to its proper regulation. A dietary supplement is an orally administered product intended to supplement the diet. By contrast, a drug is any article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease. One type of drug is a biological product, defined as containing any virus, therapeutic serum, toxin, antitoxin, or analogous product.61 Today, the definition of a virus in the regulatory context has been interpreted to encompass products containing microorganisms, such as bacteria and fungi.
Cary Frye, of the International Dairy Foods Association, presented “What are the major regulatory challenges to linking clinical studies to substantiation of structure/function or health claims on probiotic foods.” The Center for Food Safety and Applied Nutrition (CFSAN) at the FDA regulates probiotic products under the broad category of food, including dietary supplements. CFSAN is primarily responsible for post-marketing surveil-lance. The manufacturer is responsible for ensuring that the food or supplement is safe before it is marketed and is further responsible for substantiating labeling claims. Limited labeling claims may be made for products regulated as foods and dietary supplements. They may carry “structure/function” or “health” claims. These two types of claims are intended for the general population, which is interpreted to be the healthy or at-risk consumer. Structure/function claims focus on maintaining or supporting normal structures or functions of the body; for example, it helps maintain healthy intestinal microbiota, helps maintain regularity, relieves occasional constipation, or helps support immune function. Structure/function claims for foods do not need CFSAN approval or notification, but they must be truthful, not misleading, and substantiated by competent and reliable scientific evidence. However, dietary supplement companies must notify the FDA within 30 days of marketing a supplement that bears a structure/function claim. In addition, the structure/function claim must be accompanied on the label or labeling by the FDA disclosure statement that the agency has not evaluated the claim and that the product is not intended to be used in the diagnosis, mitigation, treatment, cure, or prevention of disease.
A regulatory challenge for industry is conveying the benefits of a food or dietary supplement containing probiotic organisms and also avoiding claims that would be viewed by the FDA as unauthorized health or drug claims.62 A health claim can be distinguished from a structure/function claim by its focus on risk reduction of disease or a health-related condition. An example of this type of claim that would be relevant to probiotic effects might be a statement claiming that the product reduces the risk of traveler’s diarrhea. However, under current interpretation of food law, this claim would likely not be considered appropriate because it addresses a short-term, acute condition, not a long-term, diet-related condition. All health claims require CFSAN authorization. The challenge is determining if there is sufficient scientific evidence in risk reduction to support petitioning the FDA to permit a health claim related to the relationship of a food or supplement containing a specific probiotic strain.63 There are currently no approved health claims in the United States for probiotic products.
Manufacturers or clinical researchers who wish to evaluate live biotherapeutic products (LBPs) to prevent, treat, or cure a human disease should submit an IND to the Center for Biologics Evaluation and Research (CBER) at the U.S. FDA. Cara Fiore spoke as a representative of the Office of Vaccines Research and Review (OVRR), the FDA office responsible for the regulation and oversight of LBPs (live microorganisms that are not vaccines and are intended to prevent, treat, or cure a human disease).
Individuals or companies who wish to study the use of probiotics for the treatment of a disease must follow a regulatory pathway similar to that followed for live vaccines to prevent infectious diseases. This pathway begins with an Investigational New Drug application, or IND, that must be submitted before studies in humans can begin. The IND must contain sufficient information for the FDA to evaluate how the product is made, to ensure that safe, high-quality manufacturing processes are used. In addition, data from preclinical animal toxicology studies may be required to demonstrate that it is safe to proceed with human clinical studies.
Fiore said that many of the INDs received in OVRR are placed on “clinical hold” because they do not include sufficient data about the product to assess the risks to subjects in the proposed study. If the institution or individual performing the research is not the company that makes the product, researchers may have difficulty obtaining proprietary manufacturing information. However, there are ways to overcome this difficulty, such as cross-referencing a master file (MF) provided by the product manufacturer. The information in this MF can be reviewed by the FDA but is not available to the IND holder. However, this requires that the manufacturer establish a MF. Dr. Fiore advised investigators to request a pre-IND meeting with the FDA to discuss the information required for an IND submission in order to avoid a clinical hold.
Once an IND is in effect, probiotics that are intended for use as LBPs must follow the same regulatory pathway as other biological products or drugs, including Phase 1, 2, and 3 clinical studies. After safety and efficacy of the product have been demonstrated, the manufacturer files a Biologics License Application (BLA), which permits them to market the product for the specific indication in the specific population. When the BLA is approved, the FDA may require post-marketing safety studies. In addition, the manufacturer may conduct additional studies to support post-approval labeling changes such as new indications or changes to the dose or dosing regimen. This process is intended to ensure that all biological products licensed for use in the United States are safe and effective (see Table 1).
Table 1.
Regulatory information for probiotics studied as investigational new drugs
| Ask CBER/FDA if an IND application is needed |
| Center for Biologies Evaluation and Research |
| Office of Communication, Training and Manufacturers Assistance |
| Manufacturers Assistance and Technical Training Branch |
| 800-835-4709 or 301-827-1800 |
| matt@cber.fda.gov |
| Comply with current good manufacturing practices |
| “Current Good Manufacturing Practice Regulation and Investigational New Drugs” |
| Draft guidance, “INDs—Approaches to Complying with CGMP During Phase 1” |
| http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064971.htm |
| Request a pre-IND meeting |
| “Guidance for Industry: Formal Meetings with Sponsors and Applicants for PDUFA Products” |
| http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM079744.pdf |
| Describe probiotic test product |
| “Content and Format of Chemistry, Manufacturing and Controls Information and Establishment Description for Vaccine or Related Product” |
| http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucmO76612.htm |
| “Guidelines for Drug Master Files” |
| http://www.fda.gov/cder/guidance/dmf.htm |
| Learn from other’s related experience |
| Shapiro, S. Z. 2002. The HIV/AIDS vaccine researchers’ orientation to the process of preparing a USFDA application for an investigational new drug (IND): what it is all about and how you start by preparing for your pre-IND meeting. Vaccine 20: 1261–1280. |
Dan Merenstein, Georgetown University, and Patricia Hibberd, Massachusetts General Hospital, addressed issues related to designing and implementing quality clinical trials of therapeutic probiotics in their respective talks “Probiotic foods: developing and implementing quality clinical trials” and “Therapeutic probiotics: designing and implementing quality clinical trials.” Both speakers addressed the large number of ongoing clinical studies, the majority of which continue to be conducted outside the United States. They expressed concern that the United States might fall further behind the rest of the world in probiotic clinical research, especially given the challenges of submitting IND applications.
A variety of probiotics are being studied in a wide range of ages, medical conditions, and via different routes of administration. The Cochrane collaboration has raised questions about the quality of previous probiotic trials, the consequence of which may limit recommendations for their use. Most studies in ClinicalTrials.gov are approved by institutional review boards (IRBs); however, IRB approval does not mean that the trial is of high quality, even if the IRB has regulatory oversight.
Merenstein and Hibberd questioned whether IND approval improves the credibility of probiotic trials with professionals and/or consumers. Both investigators have conducted probiotic studies with and without INDs and shared their experiences and perspectives. Both followed the CONSORT guidelines, received IRB approvals, convened data and safety monitoring boards (DSMBs), and registered their studies in ClinicalTrials.gov. In addition, on one occasion Merenstein received a letter exempting his study of acute sinusitis from an IND because the study was “investigator initiated.” This exemption was issued by the FDA’s Center for Drug Evaluation and Research (CDER), which had been responsible for probiotic INDs in the past. He questioned why the CBER would not do the same for investigator-initiated studies that were not intended to lead to a BLA. Hibberd also expressed concerns about the burden of preparing and submitting an investigator-initiated IND to the CBER vs. the CDER, but noted that there were benefits from the advice provided by the CBER. More recently, Merenstein conducted studies of a probiotic yogurt containing a strain available in marketed products, including infant formula. Because he proposed to study prevention of antibiotic-associated diarrhea in otherwise healthy children, the FDA required that the study be conducted under an IND. Hibberd has been required to obtain INDs regulated by the CBER for Phase I studies of infection prevention and enhancement of immunogenicity of influenza vaccine. Both investigators experienced significant delays in obtaining their INDs. CBER placed their studies on clinical hold. The delays related mostly to difficulties providing adequate chemistry, manufacturing, and control (CMC) information and CBER’s requirement to conduct Phase 1 studies in healthy individuals before proceeding to their NIH peer-reviewed and funded studies of sick, compromised individuals, or children. However, these studies have been expanded to evaluate the effects of probiotics on the microbiome. These studies are now proceeding with multiple layers of oversight from the FDA, NIH, DSMBs, and IRBs, and a contract research organization is also being used to assure data quality. Both investigators are still not able to conduct their originally proposed and funded NIH studies but are making progress toward achieving that goal.
Following the presentations, a panel convened to further discuss the regulatory experiences and issues. In addition to the speakers, other panelists included Philippe Caradec, The Dannon Company Inc.; Martin Hahn, Hogan Lovells US LLP; and Linda Duffy, National Center for Complementary and Alternative Medicine, NIH. The discussion was wide ranging and provocative. It included the need for scientific rigor for clinical studies, the need for Phase 1 studies and INDs, the legality of conducting clinical studies without INDs, endpoints and the use of biomarkers, Generally Recognized as Safe (GRAS) status, and first-to-market challenges.
The panelists generally agreed that in order for probiotic science to advance, studies need to be credible. The field is entering a new age with the integration of the microbiome projects with probiotic studies and the development of pyrosequencing and new molecular libraries and technologies. The best science and the best standards will move the field forward.
There was disagreement as to whether conducting studies with IND guidance improves their rigor, although studies not sponsored by NIH or other institutional oversight could benefit more if they were conducted under IND guidance when there is inadequate oversight to assure product characterization and safety assessment. Also, how studies are reported in the literature may jeopardize the perceived study quality. The quality of reporting probiotic studies, as with other natural products studies, needs to be enhanced.
Hahn disagreed with the views of the FDA and indicated that, apart from NIH-supported grants, there may be flexibility in conducting studies under an IND application. There may be a lawful basis to conduct a study with disease endpoints of a lawfully marketed over-the-counter product or dietary supplement, for example. The intended use will dictate regulation, but there are subcategories of foods, such as medical foods. For example, foods for special dietary use are designed for use by people with a disease that has specific nutritional requirements. Conducting a clinical study of the product to assess its affect on the disease state does not convert the product into a drug. There may be a legal basis for proceeding without IND oversight, especially if the industry is trying to determine the use of a food and is not shipping the product across interstate lines. An example is necrotizing enterocolitis (NEC), a very serious disease in babies. The infant formula industry has been studying how to design formulas or diets to minimize NEC without IND oversight. Hahn said that FDA has been dealing with this issue ever since the enactment of the Dietary Supplement Health Education Act of 1994 and suggests that the research field needs comprehensive guidance; the need for guidance was echoed by the panelists and meeting participants.
A concern that Hahn expressed about the IND requirement for clinical studies is that industry could be barred from marketing a probiotic product as a food or dietary supplement. If a probiotic product is studied under IND guidance as a drug or LBP and the study results are published, or if there is a substantial number of other published clinical investigations before the product has been marketed as a food or dietary supplement, then it cannot be marketed as a food or dietary supplement. Researchers developing new strains and conducting studies under IND guidance may be forced into the drug or LBP category and be precluded from marketing the product as a food or dietary supplement.
A number of panelists and conference participants challenged the need to conduct Phase 1 studies of probiotic microorganisms that have been in the marketplace for a long time and in instances where their investigations are not “first in humans.” One participant questioned the need for IND oversight if the probiotic microorganism was GRAS, as determined by notification of the FDA or self-affirmation. However, GRAS does not apply to “drug” use and is not recognized by the CBER as a safety measure. Participants and panelists also discussed how rigorous these Phase 1 studies need to be. For example, other dietary factors, such as prebiotics, influence gut microbiota and their metabolites or actions. The need to control or monitor dietary intake was discussed. Dietary assessment is a challenge in any setting. In addition, metabolic ward studies where food intake is controlled are expensive. The simplest solution may be to prohibit foods and supplements known to influence gut micro-biota. This discussion led to another concern about Phase 1 studies: for academicians, it is hard to acquire support for these early phase studies that are not scientifically interesting and often do not result in publication.
In conclusion, this session identified benefits of conducting studies under IND oversight but also recognized the challenges (e.g., delays to research, numerous hours of investigator time) of the IND application process and the possible limitations to new dietary ingredients researched under IND oversight. In addition, the participants questioned the rationale for requiring IND oversight for studies of products not intended to be marketed as drugs. Several recommendations that emerged throughout the session are presented in Table 2.
Table 2.
Recommendations related to probiotic research
U.S. Food and Drug Administration
|
National Institutes of Health
|
Probiotic food and supplement manufacturers
|
Researchers
|
Acknowledgments
Event sponsored by an unrestricted education grant from Dannon Company Inc. Funding for this conference was also made possible in part by 1R13AI088836–01 from the National Institute of Allergy and Infectious Diseases. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government. [Correction added after online publication November 24, 2010: acknowledgement wording amended.]
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Hill C 2010. Probiotics and pharmabiotics. Bioengineered Bugs 1: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanahan F, Stanton C, Ross P & Hill C. 2009. Pharmabiotics: Bioactives from mining host-microbe-dietary interactions. Functional Food Reviews 1: 20–25. [Google Scholar]
- 3.Kubota A et al. 2009. Lactobacillus strains stabilize intestinal microbiota in Japanese cedar pollinosis patients. Microbiol. Immunol 53: 198–205. [DOI] [PubMed] [Google Scholar]
- 4.Engelbrektson A et al. 2009. Probiotics to minimize the disruption of faecal microbiota in healthy subjects undergoing antibiotic therapy. J. Med. Microbiol 58: 663–670. [DOI] [PubMed] [Google Scholar]
- 5.Whorwell PJ et al. 2006. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol 101: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 6.Savino F, Pelle E, Palumeri E, et al. 2007. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 119: e124–e130. [DOI] [PubMed] [Google Scholar]
- 7.ISAPP. 2009. Probiotics: A Consumer Guide for Making Smart Choices (www.isapp.net).
- 8.WGO. 2008. WGO Practice Guidline—Probiotics and Prebiotics (http://www.worldgastroenterology.org/probioticsprebiotics.html).
- 9.Floch MH et al. 2008. Recommendations for Probiotic Use—2008. J. Clin. Gastroenterol 42(Suppl. 2): S104–S108. [DOI] [PubMed] [Google Scholar]
- 10.Ringel Y & Carroll IM. 2009. Alterations in the intestinal microbiota and functional bowel symptoms. Gastrointest. Endosc. Clin. N. Am 19: 141–150. [DOI] [PubMed] [Google Scholar]
- 11.Barbara G et al. 2005. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am. J. Gastroenterol 100: 2560–2568. [DOI] [PubMed] [Google Scholar]
- 12.Husebye E, Hellstrom PM, Sundler F, et al. 2001. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am. J. Physiol. Gastrointest. Liver Physiol 280: G368–G380. [DOI] [PubMed] [Google Scholar]
- 13.Bercik P et al. 2004. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology 127: 179–187. [DOI] [PubMed] [Google Scholar]
- 14.McFarland LV & Dublin S. 2008. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J. Gastroenterol 14: 2650–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moayyedi P et al. 2010. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 59: 325–332. [DOI] [PubMed] [Google Scholar]
- 16.Bar F et al. 2009. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol. Motil 21: 559–566, e516–e557. [DOI] [PubMed] [Google Scholar]
- 17.Marteau P et al. 2002. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment. Pharmacol. Ther 16: 587–593. [DOI] [PubMed] [Google Scholar]
- 18.Verdu EF et al. 2006. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 55: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ait-Belgnaoui A et al. 2006. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut 55: 1090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tracey KJ 2002. The inflammatory reflex. Nature 420: 853–859. [DOI] [PubMed] [Google Scholar]
- 21.Mayer EA & Bushnell MC. 2009. Synthesis In Functional Pain Syndromes: Presentation and Pathophysiology. Mayer EA & Bushnell MC, Eds. International Association for the Study of Pain. [Google Scholar]
- 22.Bailey MT & Coe CL. 1999. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol 35: 146–155. [PubMed] [Google Scholar]
- 23.O’Mahony SM et al. 2009. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 65: 263–267. [DOI] [PubMed] [Google Scholar]
- 24.Waldor Matthew K. & Sperandio V. 2007. Adrenergic Regulation of Bacterial Virulence. J. Infect. Dis 195: 1248–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cogan TA et al. 2007. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut 56: 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gareau MG, Jury J, MacQueen G, et al. 2007. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56: 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudo N et al. 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol 558: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousseaux C et al. 2007. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med 13: 35–37. [DOI] [PubMed] [Google Scholar]
- 29.Mohamadzadeh M, Duong T, Hoover T & Klaenhammer TR. 2008. Targeting mucosal dendritic cells with microbial antigens from probiotic lactic acid bacteria. Expert Rev. Vaccines 7: 163–174. [DOI] [PubMed] [Google Scholar]
- 30.Mohamadzadeh M & Luftig R. 2004. Dendritic cells: In the forefront of immunopathogenesis and vaccine development-A review. J. Immune Based Ther. Vaccines 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curiel TJ et al. 2004. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J. Immunol 172: 7425–7431. [DOI] [PubMed] [Google Scholar]
- 32.Mohamadzadeh M et al. 2005. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 102: 2880–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohamadzadeh M, Duong T, Sandwick SJ, et al. 2009. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc. Natl. Acad. Sci. USA 106: 4331–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J et al. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299: 2074–2076. [DOI] [PubMed] [Google Scholar]
- 35.Sonnenburg JL et al. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307: 1955–1959. [DOI] [PubMed] [Google Scholar]
- 36.Sonnenburg JL, Chen CT & Gordon JI. 2006. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 4: e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuel BS & Gordon JI. 2006. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. USA 103: 10011–10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonnenburg ED et al. 2010. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 141: 1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corr SC et al. 2007. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 104: 7617–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rea MC et al. 2010. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. USA 107: 9352–9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rea MC et al. 2010. Microbes and Health Sackler Colloquium: Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc. Natl. Acad. Sci. USA [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Depeint F, Tzortzis G, Vulevic J, et al. 2008. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr 87: 785–791. [DOI] [PubMed] [Google Scholar]
- 43.Silk DB, Davis A, Vulevic J, et al. 2009. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther 29: 508–518. [DOI] [PubMed] [Google Scholar]
- 44.Vulevic J, Drakoularakou A, Yaqoob P, et al. 2008. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr 88: 1438–1446. [DOI] [PubMed] [Google Scholar]
- 45.Drakoularakou A, Tzortzis G, Rastall RA & Gibson GR. 2010. A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galactooligosaccharide mixture in reducing travellers’ diarrhoea. Eur. J. Clin. Nutr 64: 146–152. [DOI] [PubMed] [Google Scholar]
- 46.O’Mahony C et al. 2008. Commensal-Induced Regulatory T Cells Mediate Protection against Pathogen-Stimulated NF-κ B Activation. PLoS Pathog 4: e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheil B et al. 2006. Role of interleukin (IL-10) in probiotic-mediated immune modulation: an assessment in wild-type and IL-10 knock-out mice. Clin. Exp. Immunol 144: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Mahony L et al. 2005. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 128: 541–551. [DOI] [PubMed] [Google Scholar]
- 49.Donato KA et al. 2008. Escherichia albertii and Hafnia alvei are candidate enteric pathogens with divergent effects on intercellular tight junctions. Microbial Pathogenesis 45: 377–385. [DOI] [PubMed] [Google Scholar]
- 50.Turner JR 2009. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol 9: 799–809. [DOI] [PubMed] [Google Scholar]
- 51.Donato KA, Gareau MG, Wang YJ, et al. 2010. Lacto-bacillus rhamnosus GG attenuates interferon- {gamma} and tumor necrosis factor-{alpha -induced barrier }{ dysfunction} Microbiology. [Epub ahead and pro-inflammatory signaling of print] [DOI] [PubMed] [Google Scholar]
- 52.Agrawal A et al. 2009. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173–010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther 29: 104–114. [DOI] [PubMed] [Google Scholar]
- 53.Katakura K et al. 2005. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest 115: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musch E, Andus T & Malek M. 2002. Induction and maintenance of clinical remission by interferon-beta in patients with steroid-refractory active ulcerative colitis-an open long-term pilot trial. Aliment. Pharmacol. Ther 16: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 55.Pena-Rossi C et al. 2008. Clinical trial: a multicentre, randomized, double-blind, placebo-controlled, dose-finding, phase II study of subcutaneous interferon-beta-la in moderately active ulcerative colitis. Aliment. Pharmacol. Ther 28: 758–767. [DOI] [PubMed] [Google Scholar]
- 56.Steidler L et al. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289: 1352–1355. [DOI] [PubMed] [Google Scholar]
- 57.Alberti KG et al. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 58.Putaala H et al. 2008. Effect of four probiotic strains and Escherichia coli O157:H7 on tight junction integrity and cyclo-oxygenase expression. Res. Microbiol 159: 692–698. [DOI] [PubMed] [Google Scholar]
- 59.Kaloustian S et al. 2009. Tumor necrosis factor-alpha participates in apoptosis in the limbic system after myocardial infarction. Apoptosis 14: 1308–1316. [DOI] [PubMed] [Google Scholar]
- 60.Girard S-A et al. 2009. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br. J. Nutr 102: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 61.Public Health Service Act of 1944, 42, U.S.C. 262.
- 62.FDA. 2008. Guidance for Industry: Substantiation for Dietary Supplement Claims Made Under Section 403(r) (6) of the Federal Food, Drug, and Cosmetic Act. [Google Scholar]
- 63.FDA. 2009. Guidance for Industry: Evidence-Based Review System for the Scientific Evaluation of Health Claims. [Google Scholar]