Abstract
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), a monogenic disorder caused by AIRE mutations, presents with several autoimmune diseases. Among these, endocrine organ failure is widely recognized, but the prevalence, immunopathogenesis, and treatment of non-endocrine manifestations such as pneumonitis remain poorly characterized. We enrolled 50 patients with APECED in a prospective observational study and comprehensively examined their clinical and radiographic findings, performed pulmonary function tests, and analyzed immunological characteristics in blood, bronchoalveolar lavage fluid, and endobronchial and lung biopsies. Pneumonitis was found in >40% of our patients, presented early in life, was misdiagnosed despite chronic respiratory symptoms and accompanying radiographic and pulmonary function abnormalities, and caused hypoxemic respiratory failure and death. Autoantibodies against BPIFB1 and KCNRG and the homozygous c.967_979del13 AIRE mutation are associated with pneumonitis development. APECED pneumonitis features compartmentalized immunopathology, with accumulation of activated neutrophils in the airways and lymphocytic infiltration in intraepithelial, submucosal, peribronchiolar, and interstitial areas. Beyond APECED, we extend these observations to lung disease seen in other conditions with secondary AIRE deficiency (thymoma and RAG deficiency). Aire-deficient mice had similar compartmentalized cellular immune responses in the airways and lung tissue, which was ameliorated by deficiency of T and B lymphocytes. Accordingly, T and B lymphocyte-directed immunomodulation controlled symptoms and radiographic abnormalities and improved pulmonary function in patients with APECED pneumonitis. Collectively, our findings unveil lung autoimmunity as a common, early, and unrecognized manifestation of APECED and provide insights into the immunopathogenesis and treatment of pulmonary autoimmunity associated with impaired central immune tolerance.
INTRODUCTION
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) or autoimmune polyglandular syndrome type 1 is a monogenic disorder most often caused by biallelic mutations in the thymus-enriched autoimmune regulator (AIRE) gene (1,2). Aire deficiency results in impaired central immune tolerance and the peripheral escape of self-reactive CD4+ T lymphocytes, which are sufficient and necessary to promote end-organ damage (1). Aire deficiency also features breakdown in B lymphocyte tolerance (3); Aire−/− B lymphocytes have been implicated in contributing to organ-specific autoimmune damage via direct priming of T lymphocytes (4, 5). APECED manifests with chronic mucocutaneous candidiasis (CMC) and autoimmunity that targets endocrine and non-endocrine organs (2, 6, 7). Among the non-endocrine manifestations, pneumonitis has been described in only a small subset of all published patient cohorts (~2%, 15 of 698 reported patients with APECED) (7–26), albeit with reported fatal outcomes (7,21,26). Although autoantibodies against the lung-specific bactericidal/permeability-increasing fold-containing B1 (BPIFB1) and the potassium channel regulator KCNRG (22,27,28) have been associated with the development of APECED pneumonitis, the immunopathogenesis of APECED pneumonitis in humans remains elusive, and no effective treatment is known.
To fill these important knowledge gaps, we comprehensively examined clinical, radiographic, pulmonary, microbiological, genetic, autoantibody, laboratory, and immunological features of 50 patients with APECED enrolled consecutively in a prospective observational natural history study. These findings were recapitulated in a mouse model of Aire deficiency and led us to test an intervention in patients with APECED.
RESULTS
Pneumonitis is an early, common, and life-threatening APECED manifestation
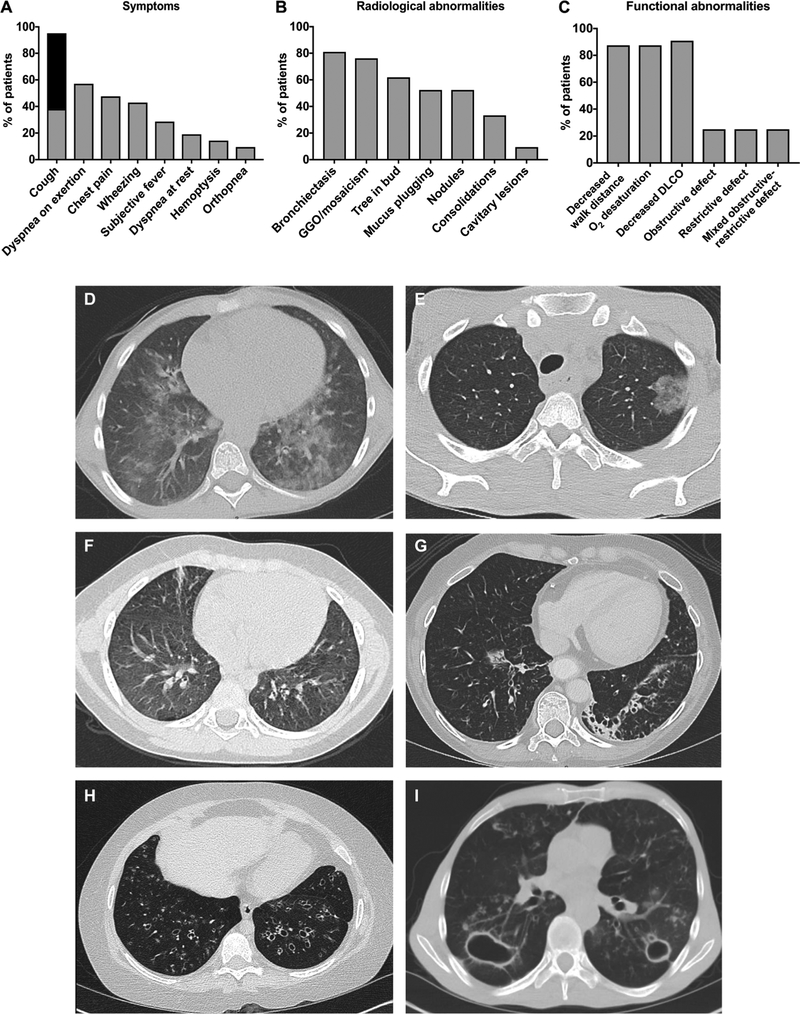
Previous studies had indicated that pneumonitis is an uncommon, poorly characterized manifestation of APECED (7–26). In the course of the comprehensive evaluation of 50 consecutive patients with APECED at the National Institutes of Health (NIH) Clinical Center, we found that 42% (n = 21) had pneumonitis. Females (16 of 30, 53.3%) were more commonly affected than males (5 of 20, 25%). Six (28.6%) were children with a mean age of 11.8 years (range, 9 to 16 years) (table S1). Eighteen were from the United States, and one each was from Canada, Argentina, and Australia. Seventeen were Caucasian, and four were Hispanic. Given the high prevalence of pneumonitis in our cohort, we sought to define the clinical, radiographic, and pulmonary characteristics of affected patients. Chronic cough was seen in all but one patient (n = 20; 95.2%) who was asymptomatic at the time of evaluation with radiographic abnormalities [ground-glass opacities (GGO)] and biopsy-proven pneumonitis (table S2, patient 5). Among the 20 patients who presented with chronic cough, sputum production was seen in only 12 (60%). Nocturnal bouts of cough, frequently awakening patients from sleep, occurred in 12 (60%). Dyspnea on exertion, pleuritic chest pain, wheezing, and subjective fevers were seen less frequently (Fig. 1A).
Fig. 1. Clinical, radiographic, and pulmonary function abnormalities of APECED pneumonitis.
(A) Clinical symptoms associated with APECED pneumonitis assessed by a standardized questionnaire. Chronic cough is classified as dry (gray shaded area) and with sputum production (black shaded area) (n = 21). (B) Radiographic features of APECED pneumonitis assessed by noncontrast chest computed tomography (CT) (n = 21). (C) Abnormalities in pulmonary function testing (n = 12) and 6-min walk test (n = 7) in patients with active APECED pneumonitis. DLCO, diffusing capacity of the lungs for carbon monoxide. (D to I) Representative radiographic abnormalities of APECED pneumonitis on chest CT imaging. GGO predominate early on (D to F). As disease progresses, bronchiectasis is prominent (G and H) and can lead to recurrent infections, including cavitary pulmonary nontuberculous mycobacteria (NTM) infection (I).
Cough onset occurred at a mean of 13.5 years (median, 4 years; range, 0.17 to 50 years) and persisted for a mean of 12 years (median, 9 years; range, 0 to 47 years) before the diagnosis of pneumonitis was made. Pneumonitis developed in eight patients (38%) before meeting a classic diagnostic dyad for APECED (i.e., any two manifestations among CMC, hypoparathyroidism, and adrenal insufficiency); the mean interval between onset of pneumonitis and development of a classic diagnostic dyad in seven of these patients was 3 years (median, 3 years; range, 0.17 to 5 years). The remaining patient developed pneumonitis at 18 months (currently 15 years old) and has a genetic diagnosis of APECED but has not yet developed a classic diagnostic dyad. The respiratory symptoms of these eight patients were attributed to asthma/bronchitis (n = 5; 62.5%) or pneumonia (n = 1; 12.5%); in two patients (25%), no diagnosis was established. In the 13 patients who developed pneumonitis after the classic diagnostic criteria for APECED were met, no diagnosis (n = 9; 69.2%) or misdiagnoses of bronchitis/asthma (n = 2; 15.3%) and recurrent pneumonia (n = 2; 15.3%) were made. Only one patient (4.8%) carried the diagnosis of pneumonitis before enrollment in our study.
We prospectively performed chest CT imaging in all 50 patients to characterize all radiographic abnormalities, regardless of symptoms. Patients had a mean of four radiographic abnormalities (range, 1 to 7), with bilateral disease being the most common (n = 18; 85%). Bronchiectasis was the most common radiographic finding (81%) followed by GGO or mosaicism (76.2%), a tree-in-bud pattern, nodular opacities, or mucus plugging, each of which was seen in more than half of the patients (Fig. 1B). Bronchiectasis and/or GGO were seen in all patients with radiographic evidence of pneumonitis. Although evaluation of mediastinal lymphadenopathy was limited by the absence of intravenous contrast use in our chest CT imaging, 7 of 21 (33%) patients with pneumonitis still had evident mediastinal lymphadenopathy compared to 0 of 29 (0%) patients without pneumonitis (fig. S1).
Pulmonary function tests (PFTs) and 6-min walk were performed at the time of active disease in 12 and 7 patients, respectively. PFT results were notable for decreased DLCO in 90.9% of patients (mean, 52.5%; range, 25 to 80%), whereas obstructive, restrictive, or mixed obstructive-restrictive ventilatory defects were each seen in 25% of patients. Decreased (i.e., <500 m) walk distance (mean, 371.6 m; range, 30 to 480 m) and oxygen desaturation (mean, 6.9%; range, 2 to 15%) were seen during the 6-min walk test, both in 85.7% of patients (Fig. 1C).
In the course of the study evaluations, we encountered patients across the spectrum of pneumonitis severity, which provided an opportunity to characterize clinical and radiographic features of APECED pneumonitis at various stages in its progression. Dry cough associated with GGO and/or tree-in-bud radiographic pattern without bronchiectasis occurred in early-stage disease (Fig. 1, D to F). When untreated, pneumonitis appeared to progress to bronchiectasis-associated structural lung disease (Fig. 1, G and H) with associated productive cough and bacterial airway colonization. Progressively worsening bronchiectasis-associated lung disease occurred later in untreated pneumonitis, associated with Gram-negative or Gram-positive bacteria (n = 4) or NTM (n = 2). Hypoxemia requiring home oxygen therapy developed in four patients (19%), one of whom died at 14 years from recurrent infections and hypoxemic respiratory failure (mortality, 4.8%; Fig. 1H).
The progression from disease onset to end-stage lung disease was highly variable. A 54-year-old man (table S2, patient 1) developed chronic cough at 5 years old and over the course of 40 years developed cavitary pulmonary NTM infection (Fig. 1I) complicated by bronchopulmonary fistula. In contrast, the aforementioned 14-year-old progressed from cough to home oxygen therapy and death in 5 and 7 years, respectively. Together, pneumonitis is a common, overlooked, and misdiagnosed manifestation of APECED that presents early as chronic cough with GGO and/or bronchiectatic radiographic abnormalities and can impair pulmonary function, leading to hypoxemic respiratory failure and death.
BPIFB1 and KCNRG autoantibodies and AIRE c.967_979del13 homozygosity correlate with APECED pneumonitis
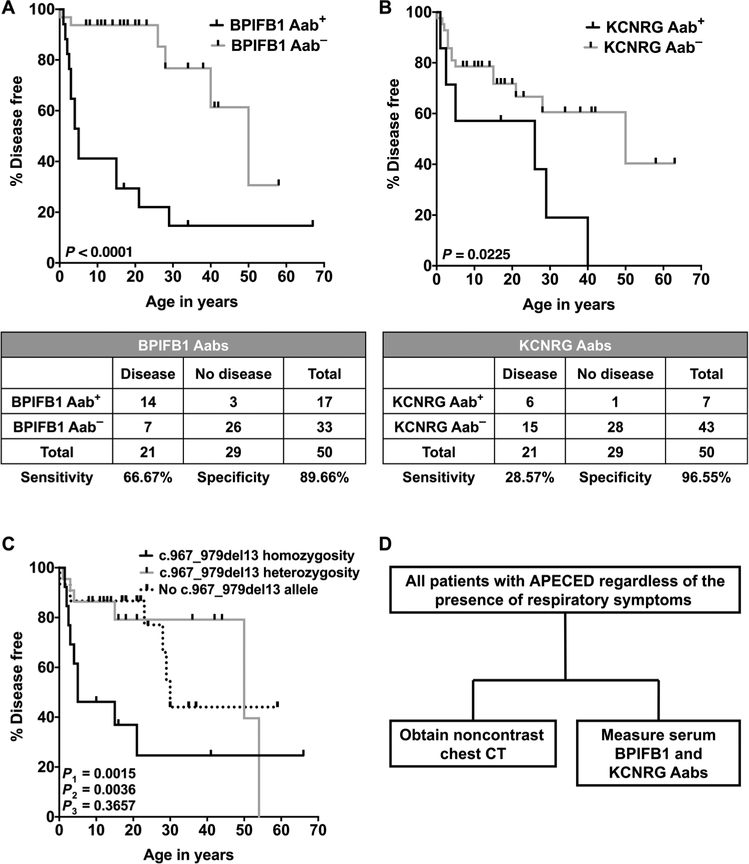
We sought autoantibody, genetic, laboratory, and/or peripheral blood immunological characteristics that might distinguish patients with APECED with and without pneumonitis. Autoantibodies against BPIFB1 and KCNRG have been associated with APECED pneumonitis in previous studies (6,22,27,28). In agreement, we found both autoantibodies to be highly specific for pneumonitis (~90 to 95%). Autoantibodies against BPIFB1 exhibited greater sensitivity (66.7%) compared to KCNRG (28.6%). Autoantibodies against both BPIFB1 and KCNRG were significantly (P < 0.0001 and P = 0.0225, respectively) associated with the time to development of pneumonitis (Fig. 2, A and B). Instead, no correlation was found with autoantibody positivity against 13 other tissue-specific antigens or cytokines and the time to development of pneumonitis (fig. S2). Collectively, BPIFB1 and/or KCNRG autoantibodies were detected in 16 (76.2%) patients with pneumonitis, whereas the remaining 5 cases were negative for both autoantibodies, including the asymptomatic patient with GGO at the time of diagnosis. We tested our patients with APECED pneumonitis for autoantibodies against interferon-induced helicase C domain-containing protein 1 (IFIH1)/melanoma differentiation-associated protein 5 (MDA5), which were reported in patients with polymyositis- and dermatomyositis-associated interstitial lung disease (29), but did not detect these autoantibodies (table S3).
Fig. 2. BPIFB1 and KCNRG autoantibodies and the homozygous c.967_979del13 AIRE mutation associate with the development of APECED pneumonitis.
(A and B) Kaplan-Meier curves illustrating the relationship between the presence of autoantibodies against BPIFB1 (A) or KCNRG (B) and the time to development of pneumonitis. P values are based on the log-rank test. The tables show the sensitivity and specificity of the corresponding autoantibodies (n = 50). (C) Kaplan-Meier curve illustrating the relationship between carrying homozygous c.967_979del13 AIRE mutations (group A), carrying a heterozygous c.967_979del13 AIRE mutation (group B), and carrying no c.967_979del13 AIRE mutations (group C) with the time to development of pneumonitis (n = 50). P1 represents the P value between groups A and B; P2 represents the P value between groups A and C, and P3 represents the P value between groups B and C. P values are based on the log-rank test. (D) Proposed diagnostic algorithm aimed at promoting earlier diagnosis of APECED pneumonitis. Aab, autoantibody.
We found an association between carrying the AIRE c.967_979del13 mutation in homozygosity and the time to development of pneumonitis (Fig. 2C); no other genotype-phenotype correlations were observed, nor did we identify specific human leukocyte antigen (HLA) haplotype associations with the time to development of pneumonitis. Moreover, there was no significant enrichment in specific peripheral blood T or B lymphocyte subsets in the patients with pneumonitis (tables S4 and S5). In a subset, erythrocyte sedimentation rate, C-reactive protein, and total immunoglobulin concentrations were increased (figs. S3 and S4). Together, chest CT was the most sensitive diagnostic test and identified all patients with APECED pneumonitis, including an early asymptomatic case. BPIFB1 and KCNRG autoantibodies, when combined, captured ~75% of affected patients with high specificity (Fig. 2D).
Compartmentalized neutrophil and lymphocyte accumulation in the airways and lung tissue underlies APECED pneumonitis in humans
The immunopathogenesis and treatment of APECED pneumonitis in humans are poorly defined. Therefore, we investigated the cellular immunophenotype in the airways and submucosal and deeper lung tissues of affected patients to gain insight into the immunopathology. We performed bronchoscopies with bronchoalveolar lavage fluid (BAL) harvesting and endobronchial biopsies in five patients with biopsy-proven APECED pneumonitis. Transbronchial and/or open lung tissue biopsies of patients with APECED pneumonitis were also examined when available.
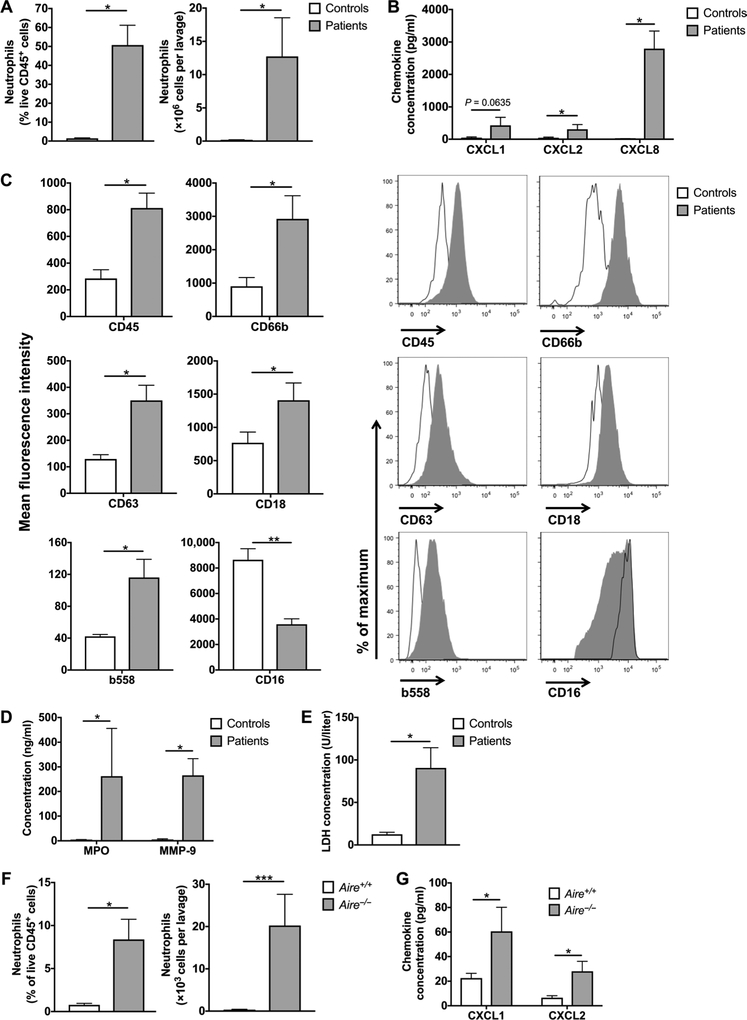
We found a significant number of neutrophils in the absence of bacterial infection in the airways of patients with APECED pneumonitis (Fig. 3A and fig. S5) along with neutrophil-targeted CXC chemokines in patient BAL (Fig. 3B). Neutrophils of patients with APECED pneumonitis displayed an activated phenotype. This was determined by increased surface expression of the extracellular epitope of the reduced form of nicotinamide adenine dinucleotide phosphate oxidase b558; primary, secondary, and tertiary granule contents (CD18, CD63, and CD66b); and CD45 and by decreased surface expression of CD 16 (Fig. 3C). Consonant with the increased number of activated neutrophils in patient airways, we found markedly elevated BAL concentrations of the neutrophil products myeloperoxidase (MPO) and matrix metallopeptidase-9 (MMP-9) (Fig. 3D), which have been implicated in the pathogenesis of bronchiectasis in patients with and without cystic fibrosis (30, 31), and significantly increased lactate dehydrogenase (LDH), indicative of airway tissue injury (Fig. 3E).
Fig. 3. Accumulation of activated neutrophils in the airways of patients with APECED pneumonitis.
(A) Neutrophils in the BAL of patients with APECED with pneumonitis (n = 5) and healthy controls (n = 4). Percentage of neutrophils within total CD45+ leukocytes (left) and total number of neutrophils per lavage (right). (B) Concentration of CXC neutrophil-targeted chemokines in the BAL of patients with APECED with pneumonitis (n = 5) and healthy controls (n = 4). (C) Neutrophil activation phenotype in BAL of patients with APECED with pneumonitis (n = 4 to 5) and healthy controls (n = 4). Shown are summary data (left) on mean fluorescence intensity and representative fluorescence-activated cell sorting histograms (right) for CD45, CD66b, CD63, CD18, b558, and CD16. (D) Concentration of the neutrophil products MPO and MMP-9 in the BAL of patients with APECED with pneumonitis (n = 5) and healthy controls (n = 4). (E) LDH concentration in the BAL of patients with APECED with pneumonitis (n = 5) and healthy controls (n=4). (F) Neutrophils in the BAL of Aire+/+ and Aire−/− mice (n = 8 to 10 per group; three independent experiments). Percentage of neutrophils within total CD45+ leukocytes (left) and total number of neutrophils per lavage (right). (G) Concentration of the CXC neutrophil-targeted chemokines CXCL1 and CXCL2 in the BAL of Aire+/+ and Aire−/− mice (n = 7 to 10 per group; three independent experiments). Differences between groups in all panels were determined using Mann-Whitney test with the exception of the differences between groups in the left panel of (F) (% of neutrophils within total CD45+ leukocytes) and on the right side of the graph in (G) (CXCL2 concentration), which were determined using unpaired t test with Welch’s correction. *P < 0.05, **P < 0.01, and ***p< 0.001. All quantitative data represent means ± SEM.
Because airway neutrophil accumulation is not a consistent feature of all types of interstitial lung diseases, we corroborated these findings using Aire−/− and wild-type (WT) littermate mice. As seen in the human airways, we observed a marked accumulation of neutrophils and induction of neutrophil-targeted CXC chemokines in the BAL of Aire−/− mice in the absence of any infectious challenge (Fig. 3, F and G). Hence, APECED pneumonitis appears to be associated with an increase of activated neutrophils, neutrophil-related products, and tissue injury markers in the airways. These products may collectively contribute to the development of bronchiectasis, a hallmark abnormality in APECED.
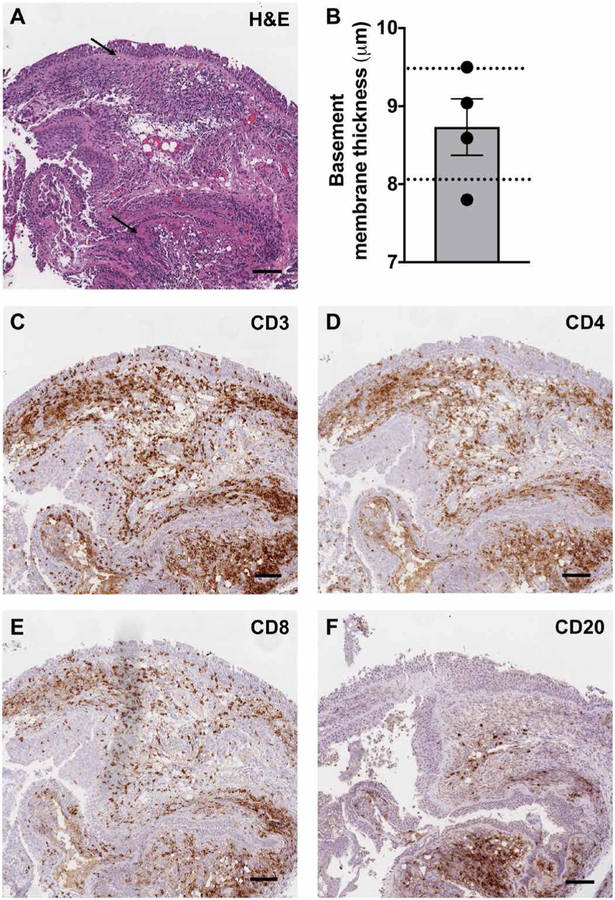
Histological and immunohistochemical examination of patient endobronchial biopsies revealed two consistent findings: (i) thickened basement membrane (mean, 8.7 jam; range, 7.8 to 9.5 jam), comparable to that reported in asthma (32) but without eosinophils (Fig. 4, A and B), and (ii) submucosal and intraepithelial lymphocytic inflammation, composed predominately of T lymphocytes with less infiltration by B lymphocytes. CD4+ T lymphocytes were more abundant than CD8+ T lymphocytes in most cases (80%) in the submucosa, whereas CD8+ T lymphocytes predominated in intraepithelial areas (Fig. 4, A and C to F). Consistent with the airway neutrophilia, neutrophils were occasionally seen as a purulent exudate on the bronchial epithelial surface but not invading the epithelium or the submucosa. Less often, squamous metaplasia was seen (40%).
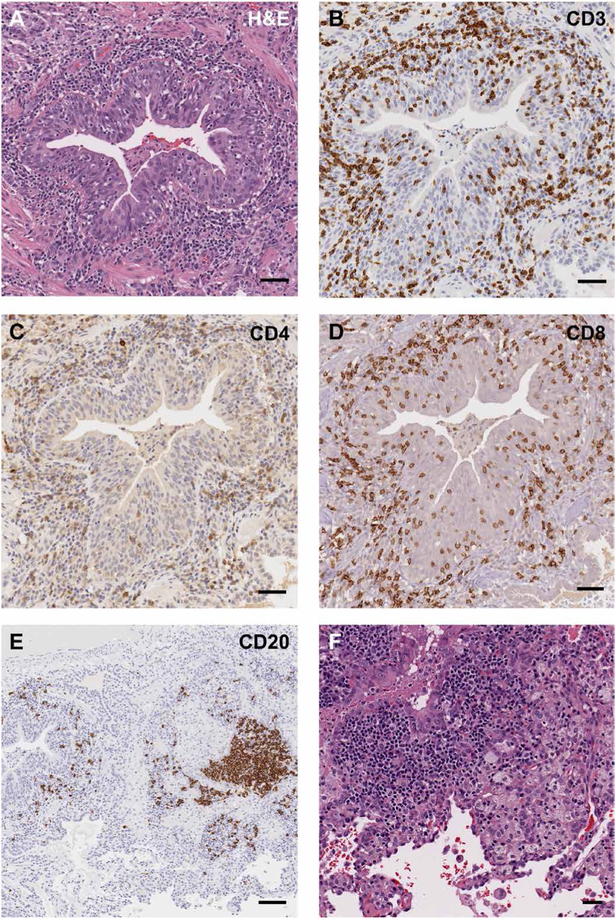
Fig. 4. Basement membrane thickening and lymphocyte infiltration in intraepithelial and submucosal tissue is seen in endobronchial biopsies of patients with APECED pneumonitis.
(A) Representative image of hematoxylin and eosin (H&E) staining of an endobronchial biopsy from a patient with APECED pneumonitis (patient 3; table S1). Black arrows indicate a thickened basement membrane. (B) Mean basement membrane thickness measured in micrometers (n = 4). The dotted lines outline the range of reported basement membrane thickness seen in patients with asthma (31). (C to F) Representative images of immunohistochemical staining with the lymphocyte markers CD3 (C), CD4 (D), CD8 (E), and CD20 (F) from the same endobronchial biopsy. Scale bars, 150 μm.
All six available deep lung tissue samples showed lymphocytic or lymphoplasmacytic bronchiolitis and/or peribronchiolar inflammation dominated by CD4+ and CD8+ T lymphocytes, with the latter prominent within intraepithelial areas. Mild-to-moderate peribronchiolar fibrosis was also observed (5 of 6; 83.3%) (Fig. 5, A to E). Less often (2 of 6; 33.3%), interstitial pneumonitis was seen consisting of lymphocytes, histiocytes with occasional poorly formed granulomas (Fig. 5F), and rare neutrophil accumulation without abscess formation; neither eosinophils nor vasculitis was observed. Deeper in peribronchial tissue, prominent B lymphocyte nodules were seen (4 of 6; 66.7%) (Fig. 5E), as were primary follicles (follicular bronchiolitis) (5 of 6; 83.3%) with occasional germinal center formation (1 of 6; 16.7%) (fig. S6).
Fig. 5. Lymphocyte infiltration in bronchiolar, peribronchiolar, and interstitial lung tissue underlies APECED pneumonitis.
Representative images of H&E staining (A and F) and immunohistochemical staining with the lymphocyte markers CD3 (B), CD4 (C), CD8 (D), and CD20 (E) obtained from resected lung tissue of a patient with APECED with pneumonitis (patient 1; table S1). Panel A is generated from the same data shown in (6). Scale bars, 50 μm.
Lung disease in secondary AIRE deficiencies displays common features with APECED pneumonitis
We next wondered whether the clinical, radiographic, histological, and autoantibody features of APECED pneumonitis are shared by other diseases that are associated with decreased AIRE expression and lung disease because shared characteristics might imply common pathogenetic mechanisms.
Patients with thymoma have impaired thymic AIRE expression (33), and a subset of them exhibit clinical manifestations similar to APECED (i.e., CMC and endocrine organ failure) and have autoantibodies against T helper 17 cytokines, 21-hydroxylase, NACHT leucinerich-repeat protein 5, and glutamic acid decarboxylase 65. However, less is known about the presentation and autoantibody profile of patients with thymoma with lung disease. We identified patients with thymoma with (13 of 62) or without (49 of 62) lung disease. Those with lung involvement had chronic cough and shortness of breath with radiographic evidence of GGO and bronchiectasis; 46.2% (6 of 13) had autoantibodies against KCNRG (3 of 13), BPIFB1 (1 of 13), or both (2 of 13) (Fig. 6, A and B). Patients with thymoma with lung involvement appeared to have a similar compartmentalized immune response to patients with APECED. In two such patients who underwent bronchoscopy, we found airway neutrophilia (i.e., 77% of leukocytes) and intraepithelial and submucosal lymphocytic inflammation on endobronchial and transbronchial biopsy (Fig. 6, C and D).
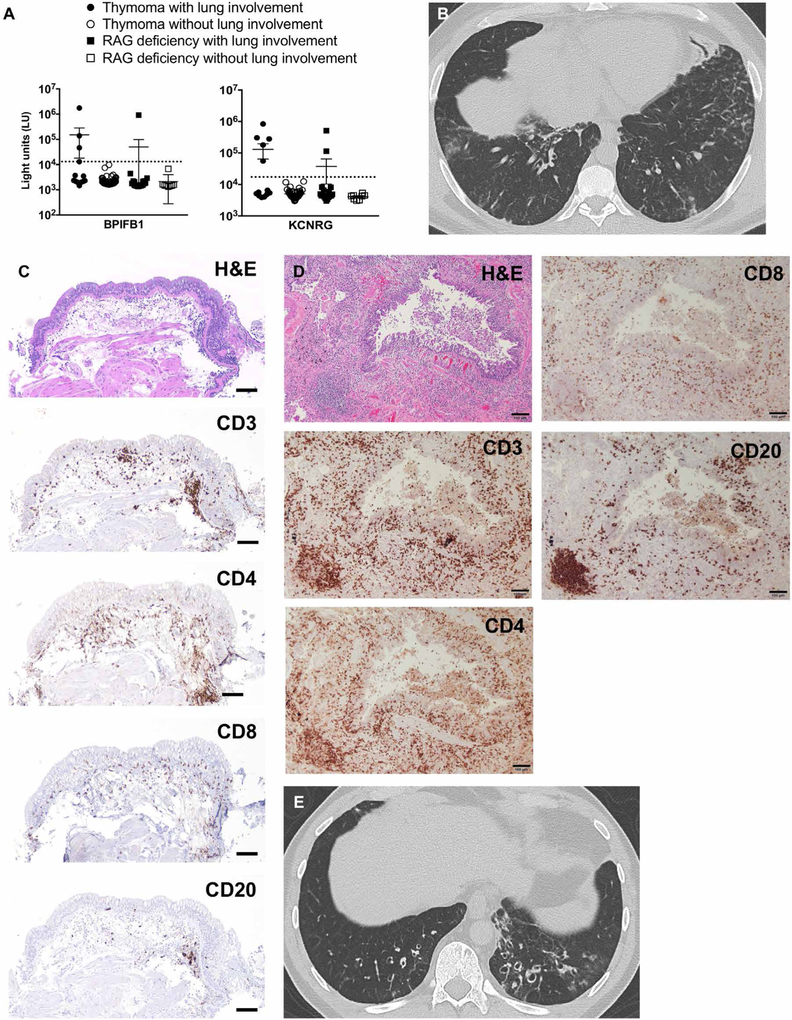
Fig. 6. Lung disease in patients with secondary AIRE deficiency exhibits shared features with APECED pneumonitis.
(A) Shown is the autoantibody immunoreactivity against BPIFB1 and KCNRG as light units (LU) using the luciferase immunoprecipitation systems (LIPS) immunoassay in 62 patients with thymoma with (n = 13) or without (n = 49) lung involvement, and in 27 recombination-activating gene (RAG)-deficient patients with (n = 19) or without (n = 8) lung involvement. Dotted lines represent the cutoff values for determining autoantibody seropositivity. (B) Representative chest CT image from a patient with thymoma with lung disease. (C and D) Representative images from an endobronchial (C) and transbronchial (D) biopsy of the same patient with thymoma with lung disease. Shown are H&E and immunohistochemical staining with the lymphocyte markers CD3, CD4, CD8, and CD20. Scale bars, 100 μm. (E) Representative chest CT image from a RAG-deficient patient with lung disease.
Besides thymoma, thymic AIRE is decreased in patients with biallelic hypomorphic RAG mutations who manifest immune deficiency, dysregulation, and autoimmunity (34). Patients with RAG deficiency display broad-spectrum autoantibodies against cytokines and tissue autoantigens (35), but no information exists regarding the presence of bronchial-targeted autoantibodies in RAG deficiency. We identified 27 RAG-deficient patients with (19 of 27) or without (8 of 27) lung involvement. Those with lung disease had chronic respiratory symptoms with GGO and bronchiectasis (Fig. 6E): 15.8% (3 of 19) of them had autoantibodies against KCNRG (2 of 19) or BPIFB1 (1 of 19) (Fig. 6A). Collectively, these data indicate that patients with thymic dysfunction, whether from APECED, thymoma, or RAG deficiency, share clinical and biochemical characteristics in common. These features unify the underlying mechanisms and pathophysiology of these conditions, at least concerning lung disease.
Lymphocyte ablation in mice ameliorates the compartmentalized immunopathology of APECED pneumonitis
We next examined histological features of lung disease in Aire−/− mice and found that Aire−/− mouse lung tissue exhibited histological abnormalities similar to those seen in patients with APECED pneumonitis. Specifically, we observed intraepithelial (fig. S7), submucosal, peribronchiolar, and interstitial infiltration composed of T and B lymphocytes in Aire−/− lung tissue, whereas B lymphocyte aggregates were more prominent deeper in the tissue (Fig. 7A). Given the importance of AIRE for central tolerance of lymphocytes, we asked whether Aire−/− T and/or B lymphocytes drive the compartmentalized neutrophilia in the airways and lymphocytic infiltration we observed in APECED pneumonitis. We generated Aire−/−Tcra−/− and Aire−/−Ighm−/− mice that lack αβ T or mature B lymphocytes, respectively, and compared their BAL neutrophil accumulation and lung histology with that of WT and Aire−/− littermate mice. The enhanced neutrophil accumulation seen in Aire−/− BAL was restored to WT levels in both Aire−/−Tcra−/− and Aire−/−Ighm−/− mice, indicating that Aire−/− αβ T and B lymphocytes promote airway neutrophilia (Fig. 7B).
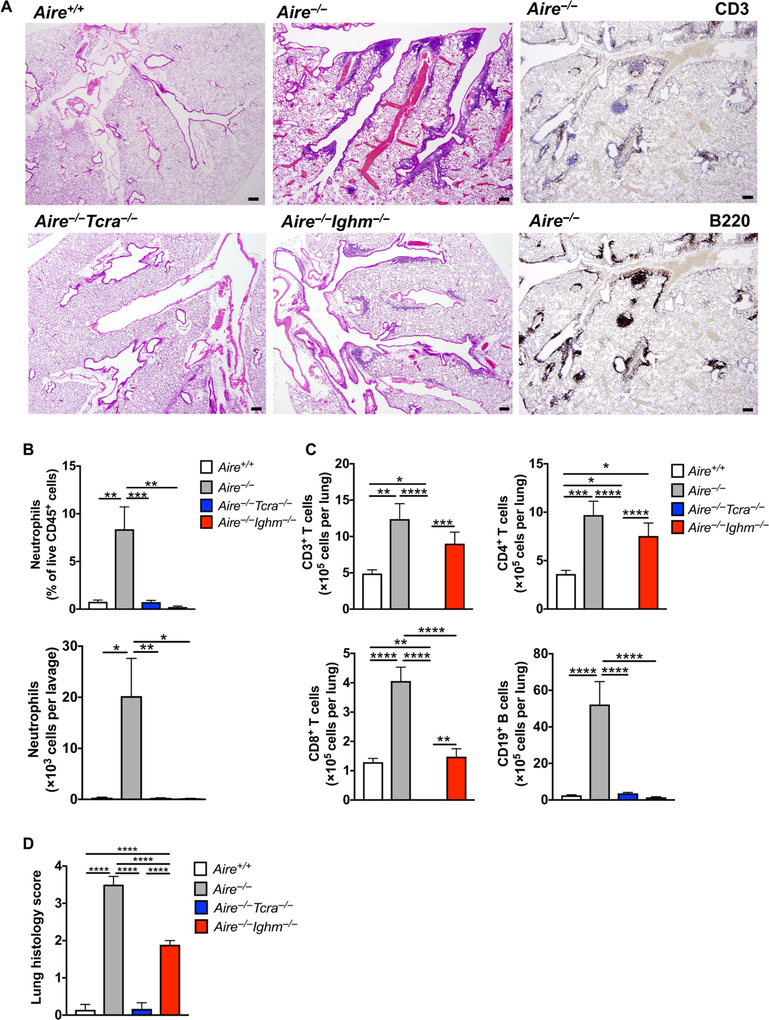
Fig. 7. Lymphocyte deficiency ameliorates airway neutrophil accumulation and lung tissue injury in Aire−/− mice.
(A) Representative images of H&E staining of Aire+/+,Aire−/−,Aire−/−Tcra−/−, and Aire−/−lghm−/− mouse lung and of immunohistochemical staining with the lymphocyte markers CD3 and B220 in Aire−/− mouse lung. Original magnification, 40×. Scale bars, 200 μm. n = 6 to 10 per group; one to three independent experiments. (B) Neutrophils in the BAL. Percentage of neutrophils within total CD45+ leukocytes (top; *P < 0.05) and total number of neutrophils per lavage (bottom; **P< 0.01 and ***P< 0.001). n = 7 to 10 per group; three to four independent experiments. (C) Lymphocyte populations in lung tissue. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. n = 7 to 10 per group; three independent experiments. (D) Lung histology scores. ****P < 0.0001. n = 6 to 10 per group; three independent experiments. Differences between groups were determined using one-way analysis of variance (ANOVA) with Tukey’s correction for multiple comparisons. Quantitative data represent means ±SEM.
In contrast, Aire−/− αβ T and B lymphocytes exhibited differential effects in mediating lymphocyte subset lung infiltration and resultant tissue injury. Specifically, Aire−/−Tcra−/− mouse lungs had absent CD4+ and CD8+ T lymphocytes, as expected, which was associated with decreased B lymphocyte accumulation to levels comparable to WT mice; Aire−/−Tcra−/− mouse lungs exhibited histological scores that were similar to those of WT mice (Fig. 7, A, C, and D). Aire−/−Ighm−/− mouse lungs had absent B lymphocytes, as expected, which was associated with CD8+ T lymphocyte accumulation at levels comparable to WT mice but with increased CD4+ T lymphocyte accumulation similar to Aire−/− lung tissue. CD4+ T lymphocytes are critical to promote pneumonitis (5), so as could be predicted, Aire−/−Ighn−/− mice manifested only partial amelioration of lung tissue injury relative to Aire−/− mice (Fig. 7, A, C, and D). Together, these data indicate that although both Aire−/− αβ T and B lymphocytes drive airway neutrophilia, Aire−/− αβ T lymphocytes predominate over Aire−/− B lymphocytes in promoting autoimmune lung tissue injury.
Combination lymphocyte-directed immunomodulation remits APECED pneumonitis in patients
Our mouse data suggested that T lymphocyte depletion with an agent such as CD52-targeting alemtuzumab would be promising, but it would also confer a high risk for opportunistic infections (36). Therefore, we elected to proceed with a T cell modulation strategy in combination with CD20-targeting rituximab to capitalize on the beneficial effects of B lymphocyte deficiency seen in mice. We chose to target T lymphocytes with the purine analog azathioprine because of its excellent safety profile in patients with APECED for autoimmune hepatitis. In patients with reduced thiopurine methyltrans-ferase (TPMT) activity, mycophenolate mofetil was used to target T lymphocytes to avoid azathioprine-induced toxicity (fig. S8). Thus, we treated five consecutive patients with APECED with biopsy-proven evidence of lymphocytic inflammation and radiographic evidence of pneumonitis and performed clinical, radiographic, pulmonary function and autoantibody evaluations at baseline and at 1 and 6 months after initiation of treatment [three females and two males; mean, 26.8 years (range, 7 to 53 years)]. Three patients were naïve to immunomodulation, whereas two were on azathioprine for a history of biopsy-proven autoimmune hepatitis, and rituximab was added (table S2, patients 3 and 4).
This lymphocyte-directed treatment resulted in obvious clinical improvement at both 1 and 6 months (Fig. 8A). Among the four patients who had chronic cough and shortness of breath at baseline, cough resolved in all four, and shortness of breath resolved in three and improved in one. In the two patients who had bronchiectasis-associated staphylococcal and pseudomonal infections before therapy (table S2, patients 1 and 2), no infection recurrences were observed after treatment. A marked improvement (mean, ~82 to 96%) was seen in pneumonitis-associated radiographic abnormalities at the 1- and 6-month evaluations using either a composite radiographic score or a three-dimensional (3D) volumetric assessment of radio-graphic abnormalities (Fig. 8, B and C; figs. S9 to S13; and table S2). The clinical and radiographic responses were accompanied by improved pulmonary functions, resolution of oxygen desaturation during the 6-min walk test, and increases in 6-min walk distance (Fig. 8, D to G). As expected, CD19+ B lymphocytes were undetectable at 1 month and were still decreased at 6 months compared to baseline, whereas CD3+, CD4+, and CD8+ T lymphocyte numbers did not significantly change (fig. S14). The clinical, radiographic, and functional responses after treatment occurred without a decline in the titers of BPIFB1 and KCNRG autoantibodies (Fig. 8H).
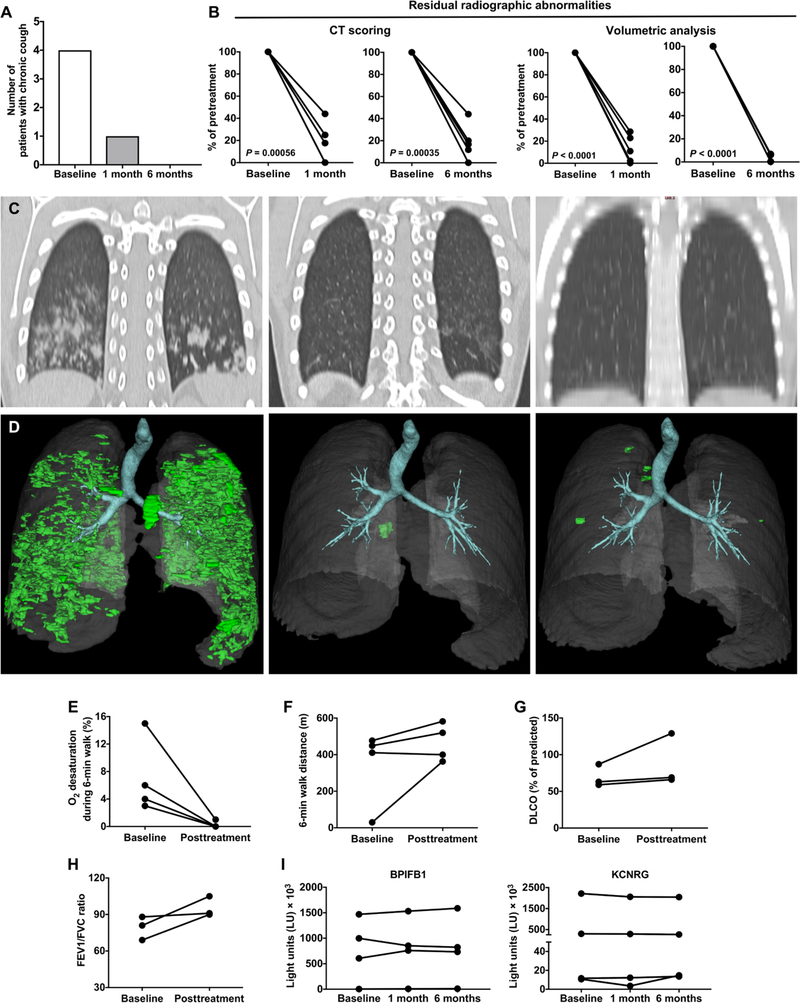
Fig. 8. Combination lymphocyte-directed immunomodulation remits pneumonitis in patients with APECED.
(A) Number of patients reporting cough at baseline and at 1 and 6 months after treatment initiation (n = 5). (B) Percent residual radiographic abnormalities calculated at 1 and 6 months after treatment initiation relative to the pretreatment radiographic abnormalities (n = 5). Statistical analysis of comparison data was performed by paired t test. (C) Representative coronal chest CT images at baseline and at 1 and 6 months after treatment initiation (patient 2; table S2). (D) 3D reconstructed CT images at baseline and at 1 and 6 months after treatment initiation (patient 5; table S2). (E to H) Pulmonary function assessed by measurements at baseline and after treatment initiation of % oxygen desaturation during the 6-min walk (n = 4) (E), the 6-min walk distance in meters (n = 4) (F), the ratio of forced expiratory volume at 1 s to forced vital capacity (FEV1/FVC) (n = 3) (G), and DLCO (n = 3) (H). (I) Shown is the autoantibody immunoreactivity against BPIFB1 and KCNRG as light units (LU) using the LIPS immunoassay at baseline and at 1 and 6 months after treatment initiation (n = 4).
During the 6-month posttreatment evaluations, we also assessed the efficacy of the lymphocyte-directed therapy in ameliorating other non-pneumonitis APECED manifestations. All four patients with Sjogren’s-like syndrome reported subjective improvement in sicca symptoms, and three patients in whom salivary flow rates were measured before and after therapy produced more saliva after treatment (fig. S15). All three patients with previous history of autoimmune hepatitis remained in remission during treatment. One of the three patients with nail dystrophy (table S2, patient 2) had complete resolution of her dystrophic nails (fig. S16). CMC did not resolve in any of the five affected patients, nor was there improvement in hypoparathyroidism (0 of 5), adrenal insufficiency (0 of 4), intestinal malabsorption (0 of 3), vitamin B12 deficiency (0 of 2), or alopecia (0 of 2). Together, these data indicate that beyond improving pneumonitis, lymphocyte-directed immunomodulation ameliorates APECED-associated autoimmunity in a manifestation-specific manner.
DISCUSSION
In this prospective observational natural history study of patients with APECED, we identified pneumonitis as an early and common clinical manifestation, and we defined lymphocyte-driven compartmentalized lung immunopathology that is ameliorated by lymphocyte-targeted immunomodulation. Our findings provide insight into the prevalence, pathogenesis, diagnosis, and treatment of autoimmune lung disease in the setting of impaired central immune tolerance.
We found that >40% of our patients with APECED had autoimmune lung disease, typically early in the course of the syndrome and often before the classic APECED diagnostic criteria were met. Despite the presence of chronic respiratory symptoms in ~95% of affected patients and of radiographic and pulmonary function abnormalities in 100% of affected patients, the overwhelming majority (~95%) of patients with pneumonitis were undiagnosed or misdiagnosed at the time of our evaluation. Because of the potential devastating sequelae of untreated lung disease, all patients with APECED warrant evaluation with chest CT imaging to assess for bronchiectasis and/or GGO. These radiographic features, when combined, were 100% sensitive for capturing autoimmune pneumonitis, even in asymptomatic patients who were still early in the course of their lung disease. Pediatricians and pulmonologists should have a high index of suspicion for APECED in children who have not yet met the classic diagnostic criteria but present with chronic respiratory symptoms and have a history of oral thrush, hypoparathyroidism, enamel hypoplasia, intestinal malabsorption, and/or urticarial eruption. Together, our findings reinforce the need for increased clinical awareness that APECED does not present solely with endocrinopathies but is also characterized by immune dysregulation that affects non-endocrine tissues with potential life-threatening complications (6).
Beyond chest CT, measurement of autoantibodies against BPIFB1 and KCNRG is a valuable, albeit less sensitive, screen for APECED pneumonitis (27,28). Both autoantibodies are highly specific, whereas autoantibodies against BPIFB1 have greater sensitivity. Some patients with pneumonitis are positive for either BPIFB1 or KCNRG auto-antibodies, indicating that both autoantibodies are useful screening tests. When combined, these autoantibodies captured the majority (~75%), but not all, of the affected patients. The identification of several patients with APECED with biopsy-proven autoimmune pneumonitis who were negative for both autoantibodies suggests that yet-unknown pulmonary autoantigens are the target of autoreactive AIRE-deficient lymphocytes in these patients.
An intriguing question arises with regard to the much greater prevalence of pneumonitis in our patients with APECED compared to other reported patient cohorts. We found an association between the homozygous c.967_979del13 AIRE mutation and development of pneumonitis. However, although this genotype may be partly contributing, the presence of homozygous c.967_979del13 AIRE mutations alone is unlikely to fully account for the greater prevalence of pneumonitis because in other APECED cohorts in which this genotype is enriched, such as the Norwegian and British, the reported frequency of autoimmune lung disease is low (16,21). It is possible that the uniform pulmonary evaluation of all our patients regardless of symptoms, including with CT chest imaging, by the same pulmonologists within our multidisciplinary team of specialists may have allowed us to diagnose pneumonitis with greater sensitivity in comparison to other APECED cohorts. Enrollment in our study and uniform pulmonary evaluation of European patients with APECED with c.967_979del13 homozygosity will help address this possibility. In addition, non-AIRE genetic modifiers maybe present in our patients and may act in synergy with AIRE deficiency, as recently demonstrated between AIRE and peripheral immune tolerance checkpoint molecules (37–39). Differential pulmonary microbiome and/or environmental factors in our cohort may also contribute to the enrichment of pneumonitis. Investigation of these genetic and microbiome factors via whole-exome and microbial metagenomic sequencing was not included in this study.
APECED pneumonitis features a characteristic compartmentalized lung immunopathology with large numbers of activated neutrophils in the airways and infiltration of T and B lymphocytes in intraepithelial, submucosal, peribronchiolar, and interstitial lung tissue. The abundance of activated airway neutrophils may contribute to the development of bronchiectasis, as previously suggested in patients with CF and non-CF bronchiectasis. This could be accomplished via secretion of toxic products such as MMP-9 and MPO and/or formation of neutrophil extracellular traps (30, 31, 40). We did not determine whether hematopoietic (i.e., alveolar macrophages) and/or nonhematopoietic (i.e., alveolar epithelial cells) cells produced neutrophil-targeted CXC chemokines in AIRE-deficient human and mouse airways. The abrogation of airway neutrophilia in Aire−/−Tcra−/− and Aire−/−Ighm−/− mice demonstrates a requirement for both Aire−/− T and B lymphocytes in promoting neutrophil accumulation in the Aire-deficient airways, via mechanisms that remain to be determined. The excess of neutrophils in the airways of patients with APECED pneumonitis is of considerable diagnostic value because all six patients with biopsy-proven pneumonitis who also had induced sputum examination had abundant neutrophils in the sputum sample during active disease. Therefore, induced sputum examination for neutrophil enrichment may be a noninvasive method to identify patients with APECED pneumonitis.
In the lung parenchyma, instead of activated neutrophils, a characteristic pattern of lymphocytic infiltration was evident. In endobronchial biopsies, a consistent pattern of CD8 > CD4 intraepithelial lymphocytosis and CD4 > CD8 > B lymphocyte submucosal infiltration together with a thickened basement membrane was of diagnostic utility. Within the lung parenchyma, CD4 and CD8 lymphocyte peribronchial and bronchiolar infiltration with associated follicular bronchiolitis and large B lymphocyte aggregates deeper in the lung parenchyma was also consistently observed, whereas CD4 and CD8 interstitial tissue lymphocytosis was seen less often. The greater dependence on T over B lymphocytes for promoting deep lung injury in mice is consistent with previous studies (4,5). Our cellular immunophenotyping of unaffected enhanced CD4 T lymphocyte accumulation in Aire−/−Ighm−/− mouse lungs, which have residual autoimmune pneumonitis, further supports a substantial role for CD4 T lymphocytes in driving lung parenchymal autoimmune disease. The relative contributions of effector versus regulatory CD4 T lymphocytes in promoting lung autoimmunity in AIRE deficiency (41, 42) and the effector mechanisms and T lymphocyte receptor repertoires of AIRE-deficient CD4 T lymphocytes in the lung remain important questions.
Beyond being useful for diagnosis, the compartmentalized myeloid and lymphoid cell accumulation in the airways and bronchioles/deeper lung of APECED pneumonitis, respectively, provides a roadmap for the potential categorization of interstitial lung diseases that may share common pathogenetic mechanisms and thus may benefit from the lymphocyte-directed immunomodulation that is effective in APECED pneumonitis. We found similar clinical, radiographic, autoantibody, and histological features in patients with interstitial lung disease in the setting of secondary AIRE deficiency seen in thymoma and RAG deficiency. These data collectively provide evidence for a fundamental unification of underlying mechanisms and pathophysiology of conditions that affect thymic function as it relates to pulmonary immunopathology, whether due to APECED, thymoma, or RAG deficiency. These findings suggest that the lymphocyte-targeted immunomodulation that is effective in APECED pneumonitis may also be effective in autoimmune lung disease seen in other conditions of thymic dysfunction. The fact that most thymoma and RAG-deficient patients with pneumonitis did not carry BPIFB1 or KCNRG autoantibodies indicates that other yet-unknown pulmonary autoantigens may be involved in the pathogenesis of autoimmune pneumonitis in secondary AIRE deficiency.
Other autoimmune disorders that present with interstitial lung disease and share the myeloid-lymphoid compartmentalized immunopathologies in the airways and lung parenchyma of APECED pneumonitis include lung diseases associated with ulcerative colitis, Sjogren’s syndrome, and, occasionally, lupus and rheumatoid arthritis (43–46). It is possible that these conditions involve BPIFB1 and KCNRG autoantibodies, and associated pneumonitis may respond to lymphocyte-directed immunomodulation.
We provide proof of concept that lymphocyte-directed immunomodulation in patients with APECED results in remission of autoimmune pneumonitis with resolution of chronic respiratory symptoms, marked improvement in radiographic abnormalities, and improved pulmonary function. Our treatment data are based on a relatively small number of patients who received therapy in a nonrandomized, nonblinded study design; treatment of a larger number of patients with APECED pneumonitis will be required to validate our response data. In addition, longitudinal follow-up of our patients will be important to establish the durability of the observed pneumonitis remission and the proportion of treated patients who may require redosing with rituximab. Furthermore, because T lymphocytes predominate over B lymphocytes in driving the development of autoimmune pneumonitis, future comparison of T lymphocyte-targeted monotherapy with azathioprine versus combination therapy with azathioprine and rituximab will be important. The same lymphocyte-directed immunomodulation that we found effective in patients with APECED has been used successfully in granulomatous and lymphocytic interstitial lung disease seen in patients with common variable immunodeficiency (47), which suggests that this treatment approach may have broader applications in the treatment of autoimmune lung diseases in primary immunodeficiency disorders. We do not yet have several years of longitudinal follow-up in all patients with APECED to establish the precise natural history and the factors that influence patient-to-patient variation in the progression of autoimmune lung disease. Nonetheless, our findings represent an effective treatment regimen for autoimmunity in patients with APECED and form the foundation for future clinical trials that will determine the best therapy for APECED pneumonitis.
In summary, we show that lung autoimmunity is a common and early manifestation of AIRE deficiency that causes significant morbidity and mortality. Our work identifies a characteristic cellular immune response in the airways and lung parenchyma that has important diagnostic implications. This immunopathology is driven by T and B lymphocytes and is responsive to lymphocyte-directed immunomodulation in humans. Collectively, our shared findings in APECED and in conditions of secondary AIRE deficiency provide insights into the pathogenesis and treatment of lung autoimmunity in the setting of impaired central immune tolerance.
MATERIALS AND METHODS
Study design
In this observational natural history study, 50 consecutive patients with APECED prospectively underwent a comprehensive and uniform evaluation in a National Institute of Allergy and Infectious Diseases (NIAID) Institutional Review Board (IRB)-approved protocol to characterize the clinical, radiographic, pulmonary function, microbiologic, genetic, autoantibody, and laboratory features of APECED. Patients with APECED pneumonitis were offered bronchoscopy with BAL harvesting and endobronchial and lung tissue biopsies, which underwent flow cytometric, immunohistochemical, and Luminex analyses to characterize the cellular immunopheno-type underlying the autoimmune lung disease. Aire-deficient mouse lungs were evaluated in a similar manner as outlined in the Supplementary Materials and Methods. We generated Aire−/−Tcra−/− and Aire−/−Ighm−/− mice to gain insight into the T and B lymphocyte requirement for promoting airway and lung tissue immunopathology.
We treated patients with biopsy-proven APECED pneumonitis in a nonrandomized, nonblinded fashion with combination lymphocyte-directed immunomodulation and evaluated clinical, radiographic, and pulmonary function and autoantibody responses at baseline and at 1 and 6 months after initiation of treatment. To extend our observations in lung disease beyond APECED, we evaluated patients with thymoma and RAG deficiency who have secondary AIRE deficiency and found shared features with APECED pneumonitis.
All mice were maintained at an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility under specific pathogen-free conditions. All mouse studies were performed with adherence to the NIH Guide for the Care and Use of Laboratory Animals under a protocol approved by the NIAID Animal Care and Use Committee. The analysis of mouse histological and Luminex data was blinded with regard to the mouse genotype. Primary data are reported in data file S1.
Study participants
Fifty consecutive patients with APECED enrolled from 2013 to 2016 in a NIAID IRB-approved protocol (11-I-0187). Inclusion criteria consisted of either a clinical APECED diagnosis based on developing any two manifestations within the classic triad of CMC, hypoparathyroidism, and adrenal insufficiency (n = 46) or a genetic diagnosis (i.e., biallelic AIRE mutations and/or deletions) without a classic diagnostic dyad (n = 4).
Four healthy volunteers enrolled in a National Heart, Lung, and Blood Institute (NHLBI) IRB-approved protocol (07-H-0142) during the same time period to undergo bronchoscopy with BAL. Healthy volunteers were 18 to 75 years who did not meet the following exclusion criteria: (i) smoking history of ≥10 pack-years, a current smoker, or tobacco free <1 year; (ii) positive HIV status by HIV polymerase chain reaction; (iii) acute or chronic hepatitis based on viral hepatitis serologies; (iv) pregnant or breastfeeding; (v) history of pulmonary disorders; (vi) history of bleeding disorders or significant bruising or bleeding with intramuscular injections or blood draws; (vii) anticoagulant use; and (viii) taking immunosuppressive medications, cytotoxic medications, inhaled corticosteroids, or long-acting β-agonists within the last 3 months.
We obtained serum from and reviewed medical records of 62 patients with thymoma and 27 patients with RAG deficiency who were enrolled in National Cancer Institute (NCI) (12-CN-029) and NIAID (05-I-0213,16-I-N139,18-I-N128, and 18-I-0041) IRB-approved protocols, respectively. All study participants provided written informed consent in accordance with the Declaration of Helsinki.
Clinical evaluations
Irrespective of disease manifestations, all patients with APECED underwent comprehensive, uniform, prospective evaluation consisting of (i) a standardized pulmonary clinical history questionnaire (table S6); (ii) noncontrast chest CT; (iii) serum measurement of autoantibodies, inflammatory markers, and immunoglobulin concentrations; (iv) lymphocyte immunophenotyping from peripheral blood; (v) DNA extraction for AIRE and HLA sequencing; and (vi) consultations by the same multidisciplinary team of infectious disease, immunology, and pulmonology specialists. Pneumonitis was defined as the presence of chronic (≥4 weeks) cough and radio-graphic evidence of interstitial, nodular and/or GGO, and/or bronchiectasis. The diagnosis was biopsy-confirmed by lymphocyte infiltration in 12 of 21 pneumonitis patients, whereas the remaining 9 patients did not have biopsies at the time of active symptoms (table S7).
Patients with active respiratory symptoms and pulmonary radiographic abnormalities during the NIH visit underwent further pulmonary investigation including (i) PFT and 6-min walk test according to American Thoracic Society/European Respiratory Society guidelines, (ii) sputum induction, and/or (iii) bronchoscopy with BAL. Endobronchial biopsies were performed in some patients. BAL underwent microbiological, flow cytometric, autoantibody, enzyme-linked immunosorbent assay, and Luminex analyses, as outlined in the Supplementary Materials and Methods. Endobronchial, transbronchial, and/or open lung biopsies, performed at the NIH or outside hospitals, were subjected to histological and immunohistochemical analyses.
Lymphocyte-directed treatment and follow-up
Five patients with biopsy-proven pneumonitis were treated with the combination of rituximab and azathioprine (1 mg/kg per day) (n = 3) or 500 mg twice daily of mycophenolate mofetil in those with reduced TPMT activity (n = 2) (fig. S8). Four adults received 1-g rituximab every 2 weeks for a total of two doses, and one child received 187.5 mg/m2 on week 1 followed by 375 mg/m2 weekly for three additional weeks. All patients were premedicated 1 hour before each rituximab infusion with acetaminophen, diphenhydramine, and methylprednisolone (1 mg/kg). Another 0.5 mg/kg dose of methylprednisolone was given 24 hours later to prevent delayed rituximab infusion reactions. We performed prospective clinical evaluations, noncontrast chest CT with blinded radiographic scoring, PFTs, peripheral blood immunophenotyping, and serum autoantibody measurements before treatment and at 1 and 6 months after treatment. PFT and 6-min walk testing were performed in three and four of five patients, respectively, because one patient was wheelchair-bound and suffered from intractable hiccups (table S2, patient 5) and was unable to perform both tests, whereas another patient with tracheostomy (table S2, patient 1) was unable to perform PFTs.
Statistical analyses
Comparison of the frequency and/or absolute numbers of different peripheral blood and BAL immune cell subsets between patients with APECED and healthy donors and between patients with APECED with pneumonitis and those without pneumonitis and comparison of chemoattractant and cell activation molecules in human and mouse experiments were performed using an unpaired t test (with Welch’s correction, where necessary), Mann-Whitney test, or one-way ANO VA (with Tukey’s correction, as appropriate), where appropriate, using GraphPad Prism 7.0 and were presented as means ± SEM. Radiographic responses of patients with APECED at 1 and 6 months after lymphocyte-directed immunomodulation were compared to baseline using paired t tests. Kaplan-Meier curves and log-rank tests were used to analyze time to development to pneumonitis. A chi-square test (with or without Yates’ correction, where appropriate) was used in the analysis of (i) pneumonitis frequency between genders and (ii) frequency of associated mediastinal lymphadenopathy in patients with or without pneumonitis. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments:
We thank our patients and their families for participation in our study and the NIAID clinical fellows, residents, and nursing staff for dedication to patient care. We thank S. A. Kim for providing histopathology pictures from thymoma lung tissue.
Funding: This research was supported by the Division of Intramural Research of the NIH, USA: NIAID (E.M.N.F., T.J.B., M.S., W.G., S.H., A.B., K.D., A.P.H., L.N.S., D.N.D., S.M.H., L.D.N., and M.S.L.), NIH Clinical Center (D.J., L.R.F., D.J.M., and T.A.F.), NHLBI (K.P.F. and K.N.O.), NCI (M.A., D.E.K., C.-C.R.L., and A.R.), NIDCR (P.D.B.), NIDDK (T.H.), and NICHD (K.W.H.). This project was also funded, in part, with federal funds from the NCI, NIH, under contract no. HHSN261200800001E (D.B.K. and W.G.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human and Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
SUPPLEMENTARY MATERIALS
stm.sciencemag.org/cgi/content/full/11/495/eaav5597/DC1
Supplementary Materials and Methods
Fig. S1. Enlarged mediastinal lymph nodes in patients with APECED pneumonitis.
Fig. S2. Association of autoantibody reactivity against cytokines and tissue-specific autoantigens and the time to development of pneumonitis in patients with APECED.
Fig. S3. Serum inflammatory markers in patients with APECED pneumonitis.
Fig. S4. Immunoglobulin concentrations in patients with APECED pneumonitis.
Fig. S5. Leukocyte subsets other than neutrophils do not exhibit increased accumulation in the airways of patients with APECED pneumonitis.
Fig. S6. Primary lymphoid follicle and germinal center formation in lung tissue of patients with APECED pneumonitis.
Fig. S7. Intraepithelial lymphocyte infiltration in Aire−/− mouse lung tissue.
Fig. S8. Schematic illustration of the lymphocyte-directed immunomodulation used in this study.
Fig. S9. Treatment response assessed by radiography in patient 1.
Fig. S10. Treatment response assessed by radiography in patient 2.
Fig. S11. Treatment response assessed by radiography in patient 3.
Fig. S12. Treatment response assessed by radiography in patient 4.
Fig. S13. Treatment response assessed by radiography in patient 5.
Fig. S14. Lymphocyte-directed immunomodulation causes rapid depletion of B, but not T, lymphocytes in peripheral blood of patients with APECED with pneumonitis.
Fig. S15. Lymphocyte-directed immunomodulation improves salivary production in patients with APECED with Sjogren’s-like syndrome.
Fig. S16. Lymphocyte-directed immunomodulation caused resolution of nail dystrophy in one of the three patients with APECED with this condition.
Table S1. Demographic and geographic origin characteristics of our patients with APECED.
Table S2. Demographic, clinical, and radiographic response characteristics of the five patients with APECED pneumonitis who received lymphocyte-directed immunomodulation.
Table S3. Patients with APECED pneumonitis do not carry serum autoantibodies against MDA5.
Table S4. Percent of lymphocyte subsets within corresponding lymphocytes in the peripheral blood of patients with APECED with or without pneumonitis.
Table S5. Absolute numbers of lymphocyte subsets in the peripheral blood of patients with APECED with or without pneumonitis.
Table S6. Standardized pulmonary clinical history questionnaire used for the evaluation of clinical features of APECED pneumonitis in our study.
Table S7. Clinical, radiographic, lung biopsy, and autoantibody features of pneumonitis in the 21 affected patients with APECED included in this study.
Data file S1. Primary data.
Competing interests: L.R.F. is an inventor on patent no. 8,406,493, which is held by the Henry M Jackson Foundation for Advancement of Military Medicine Inc. on the use of “Multi-grayscale overlay window: Universal CT trauma window”, and on patent no. 9,541,822, which is held by the U.S. Department of Health and Humans Services (HHS) on “Radiographic inclination device for portable chest imaging.” L.R.F. has a research agreement with Carestream Health and is on their advisory board.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Mathis D, Benoist C, Aire. Annu. Rev. Immunol 27,287–312 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Constantine GM, Lionakis MS, Lessons from primary immunodeficiencies: Autoimmune regulator and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Immunol. Rev 287,103–120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sng J, Ayoglu B, Chen JW, Schickel J-N, Ferre EMN, Glauzy S, Romberg N, Hoenig M, Cunningham-Rundles C, Utz PJ, Lionakis MS, Meffre E, AIRE expression controls the peripheral selection of autoreactive B cells. Sci. Immunol 4, eaav6778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavanescu I, Benoist C, Mathis D, B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: A therapeutic approach for APECED patients. Proc. Natl. Acad. Sci. U.S.A 105,13009–13014 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devoss JJ, Shum AK, Johannes KP, Lu W, Krawisz AK, Wang P, Yang T, Leclair NP, Austin C, Strauss EC, Anderson MS, Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J. Immunol 181,4072–4079 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferre EM, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, Rosen LB, Break TJ, Gu W, Hunsberger S, Browne SK, Hsu AP, Rampertaap S, Swamydas M, Collar AL, Kong HH, Lee C-CR, Chascsa D, Simcox T, Pham A, Bondici A, Natarajan M, Monsale J, Kleiner DE, Quezado M, Alevizos I, Moutsopoulos NM, Yockey L, Frein C, Soldatos A, Calvo KR, Adjemian J, Similuk MN, Lang DM, Stone KD, Uzel G, Kopp JB, Bishop RJ, Holland SM, Olivier KN, Fleisher TA, Heller T, Winer KK, Lionakis MS, Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight 1, e88782 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahonen P, Myllärniemi S, Sipila I, Perheentupä J, Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N. Engl. J. Med 322,1829–1836 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Cetani F, Barbesino G, Borsari S, Pardi E, Cianferotti L, Pinchera A, Marcocci C, A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J. Clin. Endocrinol. Metab 86,4747–4752 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Perheentupa J, Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J. Clin. Endocrinol. Metab 91,2843–2850 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Wolff AS, Erichsen MM, Meager A, Magitta NF, Myhre AG, Bollerslev J, Fougner KJ, Lima K, Knappskog PM, Husebye ES, Autoimmune polyendocrine syndrome type 1 in Norway: Phenotypic variation, autoantibodies, and novel mutations in the autoimmune regulator gene. J. Clin. Endocrinol. Metab 92,595–603 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Dominguez M, Crushell E, Ilmarinen T, McGovern E, Collins S, Chang B, Fleming P, Irvine AD, Brosnahan D, Ulmanen I, Murphy N, Costigan C, Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in the Irish population. J. Pediatr. Endocrinol. Metab 19,1343–1352 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Meloni A, Willcox N, Meager A, Atzeni M, Wolff ASB, Husebye ES, Furcas M, Rosatelli MC, Cao A, Congia M, Autoimmune polyendocrine syndrome type 1 : An extensive longitudinal study in Sardinian patients. J. Clin. Endocrinol. Metab 97, 1114–1124 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Magitta NF, Pura M, Wolff ASB, Vanuga P, Meager A, Knappskog PM, Husebye ES, Autoimmune polyendocrine syndrome type 1 in Slovakia: Relevance of screening patients with autoimmune Addison’s disease. Eur. J. Endocrinol 158,705–709 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Zlotogora J, Shapiro MS, Polyglandular autoimmune syndrome type I among Iranian Jews. J. Med. Genet 29,824–826 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlova EM, Bukina AM, Kuznetsova ES, Kareva MA, Zakharova EU, Peterkova VA, Dedov II Autoimmune polyglandular syndrome type 1 in Russian patients: Clinical variants and autoimmune regulator mutations. Horm. Res. Paediatr 73,449–457 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Pearce SH, Cheetham T, Imrie H, Vaidya B, Barnes ND, Bilous RW, Carr D, Meeran K, Shaw NJ, Smith CS, Toft AD, Williams G, Kendall-Taylor P, A common and recurrent 13-bp deletion in the autoimmune regulator gene in British kindreds with autoimmune polyendocrinopathy type 1. Am. J. Hum. Genet 63,1675–1684 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myhre AG, Halonen M, Eskelin P, Ekwall O, Hedstrand H, Rorsman F, Kämpe O, Husebye ES, Autoimmune polyendocrine syndrome type 1 (APS I) in Norway. Clin. Endocrinol 54,211–217 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Podkrajsek KT, Bratanic N, Krzisnik C, Battelino T, Autoimmune regulator-1 messenger ribonucleic acid analysis in a novel intronic mutation and two additional novel AIRE gene mutations in a cohort of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. J. Clin. Endocrinol. Metab 90,4930–4935 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Stolarski B, Pronicka E, Korniszewski L, Poliak A, Kostrzewa G, Rowiñska E, Wlodarski P, Skórka A, Gremida M, Krajewski P, Ploski R, Molecular background of polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome in a Polish population: Novel AIRE mutations and an estimate of disease prevalence. Clin. Genet 70,348–354 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Valenzise M, Fierabracci A, Cappa M, Porcelli P, Barcellona R, De Luca F, Barollo S, Garelli S, Betterle C, Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy: Report of seven additional Sicilian patients and overview of the overall series from Sicily. Horm. Res. Paediatr 82,127–132 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Bruserud Ø, Oftedal BE, Landegren N, Erichsen MM, Bratland E, Lima K, Jorgensen AP, Myhre AG, Svartberg J, Fougner KJ, Bakke Á, Nedrebo BG, Mella B, Breivik L, Viken MK, Knappskog PM, Marthinussen MC, Lovâs K, Kämpe O, Wolff AB, Husebye ES, A longitudinal follow-up of autoimmune polyendocrine syndrome type 1. J. Clin. Endocrinol. Metab 101,2975–2983 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popler J, Alimohammadi M, Kämpe O, Dalin F, K Dishop M, Barker JM, Moriarty-Kelsey M, Soep JB, Deterding RR, Autoimmune polyendocrine syndrome type 1: Utility of KCNRG autoantibodies as a marker of active pulmonary disease and successful treatment with rituximab. Pediatr. Pulmonol 47,84–87 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Friedman TC, Thomas PM, Fleisher TA, Feuillan P, Parker RI, Cassorla F, Chrousos GP, Frequent occurrence of asplenism and cholelithiasis in patients with autoimmune polyglandular disease type I. Am. J. Med 91,625–630 (1991). [DOI] [PubMed] [Google Scholar]
- 24.Huibregtse KE, Wolfgram P, Winer KK, Connor EL, Polyglandular autoimmune syndrome type I—A novel AIRE mutation in a North American patient. J. Pediatr. Endocrinol. Metab 27,1257–1260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neufeld M, K Maclaren N, Blizzard RM, Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine 60, 355–362(1981). [DOI] [PubMed] [Google Scholar]
- 26.Orlova EM, Sozaeva LS, Kareva MA, Oftedal BE, Wolff ASB, Breivik L, Zakharova EY, Ivanova ON, Kampe O, Dedov II, Knappskog PM, Peterkova VA, Husebye ES, Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine syndrome type 1. J. Clin. Endocrinol. Metab 102,3546–3556(2017). [DOI] [PubMed] [Google Scholar]
- 27.Alimohammadi M, Dubois N, Sköldberg F, Hallgren A, Tardivel I, Hedstrand H, Haavik J, Husebye ES, Gustafsson J, Rorsman F, Meloni A, Janson C, Vialettes B, Kajosaari M, Egner W, Sargur R, Pontén F, Amoura Z, Grimfeld A, De Luca F, Betterle C, Perheentupa J, Kämpe O, Carel JC, Pulmonary autoimmunity as a feature of autoimmune polyendocrine syndrome type 1 and identification of KCNRG as a bronchial autoantigen. Proc. Natl. Acad. Sci. U.S.A 106,4396–4401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.K Shum A, Alimohammadi M, Tan CL, Cheng MH, Metzger TC, Law CS, Lwin W, Perheentupa J, Bour-Jordan H, Carel JC, Husebye ES, De Luca F, Janson C, Sargur R, Dubois N, Kajosaari M, Wolters PJ, Chapman HA, Kämpe O, Anderson MS, BPIFB1 is a lung-specific autoantigen associated with interstitial lung disease. Sci. Transi. Med 5, 206ra139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushita T, Mizumaki K, Kano M, Yagi N, Tennichi M, Takeuchi A, Okamoto Y, Hamaguchi Y, Murakami A, Hasegawa M, Kuwana M, Fujimoto M, Takehara K, Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br. J. Dermatol 176,395–402 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Guan W.-j., Gao Y.-h., Xu G, Lin Z.-y., Tang Y, Gu Y.-y., Liu G.-h., Li H.-m., Chen R.-c., Zhong N.-s., Sputum matrix metalloproteinase-8 and −9 and tissue inhibitor of metalloproteinase-1 in bronchiectasis: Clinical correlates and prognostic implications. Respirology 20,1073–1081 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Bergin DA, Hurley K, Mehta A, Cox S, Ryan D, O’Neill SJ, Reeves EP, McElvaney NG, Airway inflammatory markers in individuals with cystic fibrosis and non-cystic fibrosis bronchiectasis. J. Inflamm. Res 6,1–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe K, Senju S, Toyoshima H, Yoshida M, Thickness of the basement membrane of bronchial epithelial cells in lung diseases as determined by transbronchial biopsy. Respir. Med 91,406–410 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Wolff ASB, Kärner J, Owe JF, Oftedal BE, Gilhus NE, Erichsen MM, Kämpe O, Meager A, Peterson P, Kisand K, Willcox N, Husebye ES, Clinical and serologic parallels to APS-I in patients with thymomas and autoantigen transcripts in their tumors. J. Immunol 193,3880–3890 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, Douek DC, Pittaluga S, Poliani PL, Lee YN, Notarangelo LD, Wang L, Alt FW, Kang EM, Milner JD, Niemela JE, Fontana-Penn M, Sinai SH, Malech HL, Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood 116,1263–1271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter JE, Rosen LB, Csomos K, Rosenberg JM, Mathew D, Keszei M, Ujhazi B, Chen K, Lee YN, Tirosh I, Dobbs K, Al-Herz W, Cowan MJ, Puck J, Bleesing JJ, Grimley MS, Malech H, De Ravin SS, Gennery AR, Abraham RS, Joshi AY, Boyce TG, Butte MJ, Nadeau KC, Balboni I, Sullivan KE, Akhter J, Adeli M, El-Feky RA, El-Ghoneimy DH, Dbaibo G, Wakim R, Azzari C, Palma P, Cancrini C, Capuder K, Condino-Neto A, Costa-Carvalho BT, Oliveira JB, Roifman C, Buchbinder D, Kumanovics A, Franco JL, Niehues T, Schuetz C, Kuijpers T, Yee C, Chou J, Masaad MJ, Geha R, Uzel G, Gelman R, Holland SM, Recher M, Utz PJ, Browne SK, Notarangelo LD, Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest 125,4135–4148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR, Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin. Infect. Dis 43,16–24 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Proekt I, Miller CN, Jeanne M, Fasano KJ, Moon JJ, Lowell CA, Gould DB, Anderson MS, DeFranco AL, LYN- and AIRE-mediated tolerance checkpoint defects synergize to trigger organ-specific autoimmunity. J. Clin. Invest 126,3758–3771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proekt I, Miller CN, Lionakis MS, Anderson MS, Insights into immune tolerance from AIRE deficiency. Curr. Opin. Immunol 49,71–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teh CE, Daley SR, Enders A, Goodnow CC, T-cell regulation by casitas B-lineage lymphoma (Cblb) is a critical failsafe against autoimmune disease due to autoimmune regulator (Aire) deficiency. Proc. Natl. Acad. Sci. U.S.A 107,14709–14714 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keir HR, Fong C, Crichton M, Scott G, Brady G, Dicker A, Chalmers J, Neutrophil extracellular trap formation in peripheral blood and airway neutrophils in bronchiectasis and CF. Eur. Respir. J 50, (2017). [Google Scholar]
- 41.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D, The cellular mechanism of Aire control of T cell tolerance. Immunity 23,227–239 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D, Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348,589–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuchiya Y, Takayanagi N, Sugiura H, Miyahara Y, Tokunaga D, Kawabata Y, Sugita Y, Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur. Respir. J 37,1411–1417 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Cheema GS, Quismorio FP Jr., Interstitial lung disease in systemic lupus erythematosus. Curr. Opin. Pulm. Med 6,424–429 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Papiris SA, Maniati M, Constantopoulos SH, Roussos C, Moutsopoulos HM, Skopouli FN, Lung involvement in primary Sjogren’s syndrome is mainly related to the small airway disease. Ann. Rheum. Dis 58,61–64 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camus P, Piard F, Ashcroft T, Gal AA, Colby TV, The lung in inflammatory bowel disease. Medicine 72,151–183 (1993). [PubMed] [Google Scholar]
- 47.Chase NM, Verbsky JW, Hintermeyer MK, Waukau JK, Tomita-Mitchell A, Casper JT, Singh S, Shahir KS, Tisol WB, Nugent ML, Rao RN, Mackinnon AC, Goodman LR, Simpson PM, Routes JM, Use of combination chemotherapy for treatment of granulomatous and lymphocytic interstitial lung disease (GLILD) in patients with common variable immunodeficiency (CVID). J. Clin. Immunol 33, 30–39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrison AP, Xu Z, George K, Lu L, Summers RM, Mollura DJ, Progressive and multi-path holistically nested neural networks for pathological lung segmentation from CT images, in Medical Image Computing and Computer Assisted Intervention, Descoteaux M, Maier-Hein L, Franz A, Jannin P, Collins D, Duchesne S, Eds. (Springer, 2017), vol. 10435, pp. 621–629. [Google Scholar]
- 49.Jin D, Xu Z, Harrison AP, George K, Mollura DJ, 3D convolutional neural networks with graph refinement for airway segmentation using incomplete data labels, in Machine Learning in Medical Imaging, Wang Q, Shi Y, Suk HI, Suzuki K, Eds. (Springer, 2018), vol. 10541, pp. 141–149. [Google Scholar]
- 50.George K, Harrison AP, Jin D, Xu Z, Mollura DJ, Pathological pulmonary lobe segmentation from ct images using progressive holistically nested neural networks and random walker, in Deep Learning in Medical Image Analysis and Multimodal Learning for Clinical Decision Support, Cardoso J, Arbel T, Carneiro G, Syeda-Mahmood T, Tavares JMRS, Moradi M, Bradley A, Greenspan H, Papa JP, Madabushi A, Nascimento JC, Cardoso JS, Belagiannis V, Lu Z, Eds. (Springer International Publishing, 2017), vol. 10533. [Google Scholar]
- 51.Depeursinge A, Vargas A, Platon A, Geissbuhler A, Poletti P-A, Müller H, Building a reference multimedia database for interstitial lung diseases. Comput. Med. Imaging Graph 36,227–238(2012). [DOI] [PubMed] [Google Scholar]
- 52.Simonyan K, Zisserman A paper presented at the International Conference on Learning Representations (ICLR), 2015.
- 53.Gao M, Bagci U, Lu L, Wu A, Buty M, Shin HC, Roth H, Papadakis GZ, Depeursinge A, Summers RM, Xu Z, Mollura DJ, Holistic classification of CT attenuation patterns for interstitial lung diseases via deep convolutional neural networks. Comput. Methods Biomech. Biomed. Eng. Imaging Vis 6,1–6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G, User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31,1116–1128 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur. Respir. J 2,561–585 (1989). [PubMed] [Google Scholar]
- 56.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW, QuPath: Open source software for digital pathology image analysis. Sci. Rep 7,16878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lionakis MS, Lim JK, Lee CC, Murphy PM, Organ-specific innate immune responses in a mouse model of invasive candidiasis. J. Innate Immun 3,180–199 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, Mikelis CM, Rose SR, Ferre EMN, Yockey L, Lemberg K, Kuehn HS, Rosenzweig SD, Lin X, Chittiboina P, Datta SK, Belhorn TH, Weimer ET, Hernandez ML, Hohl TM, Kuhns DB, Lionakis MS, CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLOSPathog 11, e1005293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.