Abstract
OBJECTIVE
Higher serum uric acid (SUA) is associated with diabetic kidney disease (DKD). Preventing Early Renal Loss in Diabetes (PERL) evaluates whether lowering SUA with allopurinol slows glomerular filtration rate (GFR) loss in people with type 1 diabetes (T1D) and mild to moderate DKD. We present the PERL rationale, design, and baseline characteristics.
RESEARCH DESIGN AND METHODS
This double-blind, placebo-controlled, multicenter trial randomized 530 participants with T1D, estimated GFR (eGFR) of 40–99.9 mL/min/1.73 m2, SUA ≥4.5 m/dL, and micro- to macroalbuminuric DKD or normoalbuminuria with declining kidney function (NDKF) (defined as historical eGFR decline ≥3 mL/min/1.73 m2/year) to allopurinol or placebo. The primary outcome is baseline-adjusted iohexol GFR (iGFR) after 3 years of treatment plus a 2-month washout period.
RESULTS
Participants are 66% male and 84% white. At baseline, median age was 52 years and diabetes duration was 35 years, 93% of participants had hypertension, and 90% were treated with renin-angiotensin system inhibitors (median blood pressure 127/71 mmHg). Median HbA1c was 8%, SUA 5.9 mg/dL, iGFR 68 mL/min/1.73 m2, and historical eGFR slope −3.5 mL/min/1.73 m2/year. Compared with participants with albuminuria (n = 419), those with NDKF (n = 94) were significantly older (56 vs. 52 years), had lower HbA1c (7.7 vs. 8.1%) and SUA (5.4 vs. 6.0 mg/dL), and had higher eGFR (82 vs. 74 mL/min/1.73 m2) and historical eGFR loss (−4.7 vs. −2.5 mL/min/1.73 m2/year). These differences persisted when comparing groups with similar rates of historical eGFR loss.
CONCLUSIONS
PERL will determine the effect of allopurinol on mild to moderate DKD in T1D, with or without albuminuria. Participants with normoalbuminuria and rapid GFR loss manifested many DKD risk factors of those with albuminuria, but with less severity.
Introduction
Current, widely accepted treatments to prevent or slow progression of diabetic kidney disease (DKD), including intensive glycemic control and renin-angiotensin system (RAS) inhibition, were added to the clinical repertoire more than two decades ago. Despite these advances, over the past several decades, the rising incidence and prevalence of diabetes has led to a growing number of people with DKD (1). The prevalence of end-stage renal disease (ESRD) related to DKD continues to increase (2,3), and DKD remains a strong risk factor for cardiovascular and all-cause mortality (4,5). Thus, new DKD treatments are urgently needed.
Efforts to develop novel DKD treatments must take into account the changing DKD phenotype. In addition to the classic clinical DKD phenotype of albuminuria followed by glomerular filtration rate (GFR) decline, DKD is now known to also include GFR decline with persistent normoalbuminuria (6). To prevent DKD progression, it is important to include both phenotypes in clinical trials, preferably at early stages of DKD, before significant GFR loss has occurred. This approach could enable an improved understanding of the underlying pathophysiological mechanisms and identify effective interventions for normoalbuminuric versus albuminuric DKD (6).
Serum uric acid (SUA) is a promising therapeutic target in DKD. Multiple lines of evidence from animal models, observational studies, and small clinical trials have implicated higher SUA levels as a pathophysiologically relevant and modifiable risk factor for chronic kidney disease (CKD) in general and for DKD more specifically, as previously reviewed (7). Higher baseline SUA, even within the normal range, is a strong and independent predictor of albuminuria and early GFR loss in populations with type 1 diabetes (T1D). Moreover, SUA reduction slowed GFR decline in two small clinical trials in participants with moderate CKD (roughly one-third with diabetes) with or without hyperuricemia. High SUA is believed to promote kidney injury in animal models through dysregulation of the nitric oxide pathway, induction of inflammatory cytokines, and oxidative stress (reviewed in Maahs et al. [7]). In addition, as shown by studies with the SUA-lowering drug allopurinol, high SUA could induce an increase in RAS and transforming growth factor-β (8,9). Notably, association between SUA and CKD progression was observed in early, but not advanced, CKD (10), suggesting that the optimal timing for SUA lowering may be early DKD. Such early intervention would also maximize the delay of progression to ESRD that can be achieved by slowing GFR decline.
The Preventing Early Renal Loss in Diabetes (PERL) study (reg. no. NCT02017171, ClinicalTrials.gov) is an ongoing, 3-year, multisite, international, double-blind, randomized clinical trial supported by the National Institutes of Health and JDRF that examines the hypothesis that SUA reduction with allopurinol can prevent or slow DKD progression in individuals with T1D and mild to moderate DKD. Participants in PERL were selected to have an SUA ≥4.5 mg/dL, a level associated with an ∼2.4-fold increase in risk of early GFR loss compared with SUA <4.5 mg/dL (11), because such participants are most likely to benefit from SUA lowering.
Unique to PERL is the deliberate inclusion of participants with albuminuric DKD as well as those with normoalbuminuria and declining kidney function (NDKF) at early stages of DKD. Thus, this study will determine the effect of the xanthine oxidase inhibitor allopurinol, as well as the magnitude of SUA reduction caused by this drug, on DKD progression in the study overall and in both DKD phenotypes. The purpose of this report is to describe the study design and baseline characteristics of the PERL participants and to compare the features of albuminuric DKD with those of NDKF.
Research Design and Methods
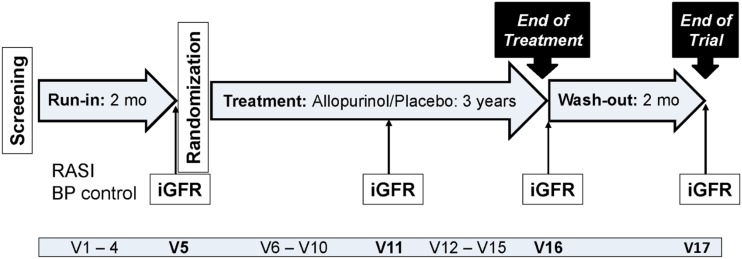
This double-blind, placebo-controlled, parallel-group, randomized clinical trial includes 530 participants with T1D who are at high risk of GFR loss on the basis of albuminuria or rapidly declining GFR but who have only mildly to moderately reduced kidney function. After an up to 2-month run-in period during which blood pressure was normalized and treatment with RAS inhibitors (RASIs) standardized, PERL participants were randomized to 3 years of masked treatment with the xanthine oxidase inhibitor allopurinol (200–400 mg daily depending on GFR levels) or placebo followed by a 2-month drug washout period (end of trial) (Fig. 1 and Supplementary Table 1). The primary outcome is GFR directly measured by iohexol plasma disappearance (iGFR) at the end of the trial (after the 2-month washout), adjusted for baseline iGFR. The study is powered to detect a 3 mL/min/1.73 m2 difference between treatment groups in this outcome, equivalent to a 1 mL/min/1.73 m2 difference in the yearly rate of GFR loss. Secondary outcomes include iGFR at the end of treatment (before the 2-month washout), adjusted for baseline iGFR; trajectories of iGFR and eGFR; and time to serum creatinine doubling or ESRD. The main study results are expected by the end of 2019.
Figure 1.
PERL study design. BP, blood pressure; mo, months; V, visit.
Study Population
Patients were eligible for the study if they had T1D for ≥8 years, eGFR between 40 and 99.9 mL/min/1.73 m2 by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation (12), SUA ≥4.5 mg/dL, and evidence of either 1) a history of micro- or moderate macroalbuminuria during the prior 2 years or, in the absence of a history of albuminuria, 2) a rapid eGFR decline (≥3 mL/min/1.73 m2/year) before study enrollment (11) (Table 1). In the absence of treatment with RASIs, history of albuminuria was defined as at least two out of three consecutive urine albumin excretion rate (UAER) or urine albumin-to-creatinine ratio (UACR) measurements during the prior 2 years of 20–3,333 μg/min or 30–5,000 mg/g, respectively. For participants on RASIs, the lower threshold was reduced to 12 μg/min for UAER and 18 mg/g for UACR. Rapid eGFR decline was defined as an eGFR decline ≥3 mL/min/1.73 m2/year, estimated from the slope derived from all available serum creatinine measurements (minimum of three) over the preceding 3–5 years. Exclusion criteria were screening systolic blood pressure >160 mmHg or diastolic blood pressure >100 mmHg, clinical indication for SUA lowering, known allergy to xanthine oxidase inhibitors or iodine-containing substances, serious comorbidities, ongoing pregnancy or breastfeeding, or the finding of HLA B*58:01 positivity, a risk factor for allopurinol-associated Stevens-Johnson syndrome (13) (Table 1).
Table 1.
Eligibility criteria
| Inclusion criteria |
| 1. Male or female participant with T1D |
| 2. Diabetes continuously treated with insulin within 1 year of diagnosis. (If onset before age 35 years, must have documentation of circulating T1D-associated autoantibodies, plasma C-peptide below limit of detection with concurrent blood glucose >100 mg/dL, or history of hospitalization for DKA.) |
| 3. Diabetes duration ≥8 years |
| 4. Age 18–70 years |
| 5. Evidence of kidney disease by at least one of the following criteria: |
| a. Micro- or macroalbuminuria: at least two out of three consecutive UACR >30–5,000 mg/g or UAER 20–3,333 μg/min if not on RAS blockade or 18–5,000 mg/g or 12–3,333 μg/min, respectively, if on RAS blockade at any time over 2 years before screening or at screening. |
| b. GFR (CKD-EPI) decline ≥3.0 mL/min/1.73 m2/year, estimated from the slope derived from all the available serum creatinine measurements (including the one at screening assessment) from the previous 3 years. If at least three serum creatinine measures are not available in the previous 3 years, then the slope can be derived from creatinine values from the previous 5 years. |
| 6. eGFR (CKD-EPI) of 40–99.9 mL/min/1.73 m2 at screening. The upper and the lower limits should be decreased by 1 mL/min/1.73 m2 for each year over age 60 years (with a lower limit of 35 mL/min/1.73 m2) and by 10 mL/min/1.73 m2 for strict vegans. |
| 7. SUA ≥4.5 mg/dL at screening |
| 8. Valid baseline (visit 4) iGFR measurements (R2 for the slope of plasma iohexol levels ≥0.9), or |
| 9. Participant in the PERL pilot study |
| Exclusion criteria |
| 1. History of gout or xanthinuria or other indications for uric acid–lowering therapy |
| 2. Recurrent renal calculi |
| 3. Use of urate-lowering agents within 2 months before screening |
| 4. Current use of azathioprine, 6-mercaptopurine, didanosine, warfarin, tamoxifen, amoxicillin/ampicillin, or other drugs interacting with allopurinol |
| 5. Known allergy to xanthine oxidase inhibitors or iodine-containing substances |
| 6. HLA B*58:01 positivity |
| 7. Renal transplant |
| 8. Non-DKD |
| 9. SBP >160 mmHg or DBP >100 mmHg at screening or SBP >150 mmHg or DBP >95 mmHg at the end of the run-in period |
| 10. Cancer treatment (excluding nonmelanoma skin cancer treated by excision) within 2 years before screening |
| 11. History of clinically significant hepatic disease, including hepatitis B or C and/or persistently elevated serum liver enzymes at screening and/or history of hepatitis B virus/hepatitis C virus positivity |
| 12. History of AIDS or HIV infection |
| 13. Hemoglobin concentration <11 g/dL (males) or <10 g/dL (females) at screening |
| 14. Platelet count <100,000/mm3 at screening |
| 15. History of alcohol or drug abuse in the past 6 months |
| 16. Blood donation in the 3 months before screening |
| 17. Breastfeeding or pregnancy or unwillingness to be on contraception throughout the trial |
| 18. Poor mental function or any other reason to expect patient difficulty in complying with the requirements of the study |
| 19. Serious preexisting medical problems other than diabetes (e.g., congestive heart failure, pulmonary insufficiency) |
DBP, diastolic blood pressure; DKA, diabetic ketoacidosis; SBP, systolic blood pressure.
Thirty-one participants were randomized for a 24-month pilot study at two study sites (Joslin Diabetes Center, Boston, MA, and Steno Diabetes Center, Copenhagen, Denmark), with procedures comparable to the main PERL study. Pilot participants who consented were transitioned to the pivotal PERL study before the end of the pilot study (n = 26). In the PERL study, 28 patients with minor deviations from eligibility criteria (e.g., hemoglobin or platelet count slightly below study limits but deemed clinically safe) (see Supplementary Table 2) were granted waivers to enroll at the request of the local site directors by the PERL exemption committee (comprising the two PERL co–principal investigators, the two data coordinating center co-directors, and 4 of the 16 main site directors) if failure to meet the safety criterion posed no substantial risk or a single laboratory eligibility criterion was borderline. In a retrospective review, 17 of the randomized patients (all to be included in the intention-to-treat analysis) were found to be ineligible as a result of site errors: 1 because of the use of UAER values outside the allowed time window; 5 because of the use of a serum creatinine value from the local rather than the central laboratory to estimate baseline eGFR, resulting in baseline eGFR values slightly exceeding the upper limit of 99.9 mL/min/1.73 m2; 2 because of site oversights concerning blood pressure values at the end of follow-up, which were over the protocol limit of 150/95 mmHg; and 9 because of site errors in the calculation of eGFR slopes.
Assessment of Covariates and Outcome Variables
Demographic data (age, sex, race, ethnicity), age at diabetes onset, diabetes duration, smoking status, medication use, and other components of the medical history were obtained from the participants during the study visits. Height and weight were obtained at each visit. Hypertension was defined as one or more of the following: a diagnosis of hypertension from electronic health records, blood pressure >140/90 mmHg, and/or use of antihypertensive medications. Participant-reported cardiovascular disease was defined as a history of myocardial infarction, coronary revascularization, stroke, or amputation. Participant-reported diabetic retinopathy was defined as any diabetic retinopathy requiring laser treatment, intraocular injection, vitrectomy, or causing blindness. Systolic and diastolic blood pressures were measured at each visit after 5 min of rest in the right arm at seated position. Central laboratory measurements were performed at the University of Minnesota Advanced Research and Diagnostic Laboratory. HbA1c was assayed with the Tosoh G8 analyzer (Tosoh Bioscience, San Francisco, CA). SUA and serum creatinine were measured by enzymatic methods on the Roche Cobas 6000 analyzer (Roche Diagnostics, Indianapolis, IN) and urine albumin by an immunoturbidimetric method on the same instrument. Plasma iohexol was measured using high-performance liquid chromatography. eGFR was calculated from serum creatinine using the CKD-EPI equation (12). iGFR was calculated from the plasma disappearance of iohexol (14).
Analysis of Baseline Data
PERL participants were classified as having albuminuric DKD or NDKF according to the following algorithm. Participants recruited according to the albuminuria criterion (see study population) were classified as having albuminuric DKD regardless of whether they had evidence of albuminuria during the run-in period. Those enrolled by the eGFR decline criterion were classified as having albuminuric DKD or NDKF on the basis of the UAER measurements during the run-in period. Albuminuria was defined as at least two UAER values ≥20 μg/min for those with three UAER measurements, a UAER geometric mean ≥20 μg/min in those with two measurements, and a UAER >40 μg/min in those with only one measurement. Normoalbuminuria was defined as a UAER geometric mean <20 μg/min in participants with two consecutive measurements and UAER <10 μg/min in those with only one measurement. Participants with a single UAER value between 10 and 40 μg/min were categorized as indeterminate albuminuria status. Continuous variables were summarized by means and SDs if normally distributed or by median and interquartile range if not. Categorical variables were summarized by numbers and proportions. Comparisons between albuminuric and normoalbuminuric groups were conducted using Fisher exact tests for categorical variables and Student t tests or Wilcoxon rank sum tests for normally distributed and nonnormally distributed continuous variables, respectively. Relationships between baseline characteristics were assessed by means of multivariate linear regression analysis. A two-sided P < 0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Baseline Characteristics of the Overall Study Population
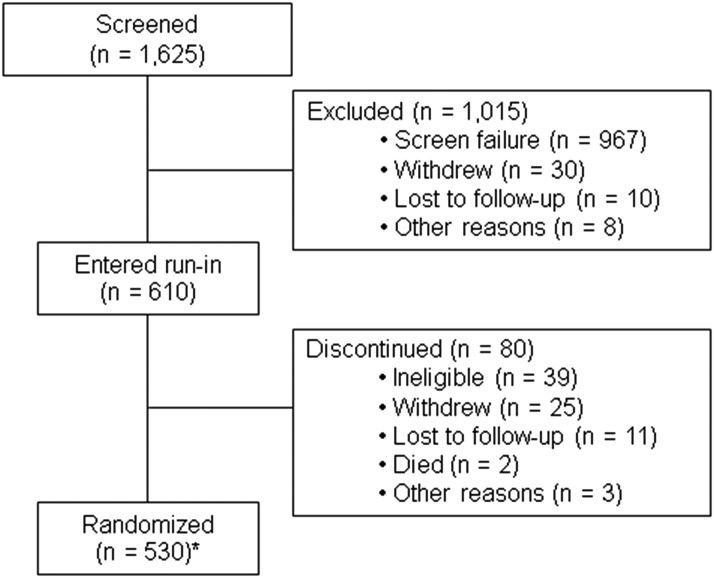
Of the 1,625 candidates screened, 1,015 were excluded before entering the run-in period, mostly because of screen failure; 80 discontinued during run-in; and 530 were randomized (Fig. 2). The median age of the randomized participants was 52 years; 66% were male, 84% were white, and 95% were non-Hispanic (Table 2). Median diabetes duration was 35 years and mean age at diabetes onset 14 years. A majority (79%) were overweight or obese (BMI ≥25 kg/m2). Most had hypertension (93%) and a self-reported history of diabetic retinopathy (68%), 21% self-reported prior cardiovascular disease, 39% were current or past smokers, 90% were treated with RASIs at a full or reduced dose (RASIs were contraindicated or not clinically indicated in the remaining participants), and 46% were treated with statins (Supplementary Table 3). Median systolic and diastolic blood pressures were 127 and 71 mmHg, respectively, and median HbA1c and SUA were 8.0% (64 mmol/mol) and 5.9 mg/dL, respectively. Median UAER was 42 μg/min, and the median slope of eGFR loss in the 3–5 years before enrollment was −3.5 mL/min/1.73 m2/year. Median eGFR and iGFR at baseline were 76 and 68 mL/min/1.73 m2, respectively.
Figure 2.
Diagram describing the enrollment for the PERL trial. *Of the 530 randomized participants, 17 were found to be ineligible for randomization in retrospective analyses.
Table 2.
Baseline characteristics of 530 randomized participants
| Albuminuria status |
|||||
|---|---|---|---|---|---|
| Variable | Total cohort (N = 530) | Indeterminate (n = 17) | Albuminuric DKD (n = 419) | NDKF (n = 94) | P value# |
| Age (years) | 52 (44, 59) | 54 (42, 60) | 52 (43, 59) | 56 (49, 62) | 0.002 |
| Male sex | 351 (66.2) | 7 (41.2) | 287 (68.5) | 54 (57.4) | 0.004 |
| Race | 0.1 | ||||
| White | 446 (84.2) | 14 (82.4) | 349 (83.3) | 83 (88.3) | |
| Black | 58 (10.9) | 2 (11.8) | 50 (11.9) | 6 (6.4) | |
| Asian | 6 (1.1) | 0 | 5 (1.2) | 1 (1.1) | |
| Other* | 20 (3.8) | 1 (5.9) | 15 (3.6) | 4 (4.3) | |
| Ethnicity | 0.1 | ||||
| Non-Hispanic | 504 (95.1) | 17 (100) | 395 (94.3) | 92 (97.9) | |
| Hispanic | 23 (4.3) | 0 | 22 (5.3) | 1 (1.1) | |
| Unknown | 3 (0.6) | 0 | 2 (0.5) | 1 (1.1) | |
| Diabetes duration (years) | 35 (25, 44) | 37 (30, 43) | 35 (25, 44) | 33 (25, 42) | 0.2 |
| Age at diabetes diagnosis (years) | 14 (9, 24) | 14 (10, 23) | 13 (8, 22) | 20 (11, 32) | 0.00002 |
| Hypertension | 491 (92.6) | 10 (58.8) | 409 (97.6) | 72 (76.6) | 2.6 × 10−14 |
| Prior self-reported CVD† | 103 (20.6) | 5 (33.3) | 85 (21.4) | 13 (14.9) | 0.2 |
| Self-reported diabetic retinopathy† | 337 (67.5) | 8 (50.0) | 294 (74.2) | 35 (43.8) | 2.2 × 10−10 |
| Smoking status | 0.05 | ||||
| Never | 322 (60.8) | 9 (52.9) | 246 (58.7) | 67 (71.3) | |
| Current | 58 (10.9) | 2 (11.8) | 51 (12.2) | 5 (5.3) | |
| Past | 150 (28.3) | 6 (35.3) | 122 (29.1) | 22 (23.4) | |
| RASI use‡ | 3.4 × 10−16 | ||||
| Full dose | 375 (70.8) | 6 (35.3) | 320 (76.4) | 49 (52.1) | |
| Reduced dose | 102 (19.3) | 3 (17.7) | 78 (18.6) | 21 (22.3) | |
| Contraindicated/not indicated | 48 (9.1) | 8 (47.1) | 17 (4.1) | 23 (24.5) | |
| No RASI | 5 (0.9) | 0 | 4 (1.0) | 1 (1.1) | |
| HMG-CoA reductase inhibitors† | 216 (46.2) | 6 (28.6) | 185 (49.5) | 25 (31.6) | 0.004 |
| BMI† | 29 (25, 33) | 27 (22, 31) | 29 (25, 33) | 30 (25, 34) | 0.4 |
| 25–29.9 kg/m2 (overweight) | 181 (34) | 5 (28) | 148 (35) | 28 (30) | 0.6 |
| ≥30 kg/m2 (obese) | 218 (41) | 6 (33) | 170 (41) | 42 (45) | |
| Blood pressure (mmHg)‡ | |||||
| Systolic | 127 (116, 137) | 123 (118, 131) | 128 (117, 138) | 121 (113, 131) | 0.003 |
| Diastolic | 71 (65, 79) | 73 (66, 76) | 71 (65, 80) | 69 (63, 76) | 0.003 |
| HbA1c (%)† | 8.0 (7.3, 8.8) | 7.2 (6.9, 8.0) | 8.1 (7.4, 9.0) | 7.7 (7.0, 8.5) | 0.0005 |
| SUA (mg/dL)‡ | 5.9 (5.1, 6.9) | 5.3 (4.9, 6.1) | 6.0 (5.2, 7.0) | 5.4 (4.7, 6.2) | 0.00003 |
| UAER (μg/min)†§ | By design | ||||
| Median | 42 (9, 207) | 3 (2, 15) | 84 (26, 310) | 3 (2, 5) | |
| <20 | 189 (36) | 14 (82) | 81 (19) | 94 (100) | |
| 20–199 | 203 (38) | 3 (18) | 200 (48) | 0 | |
| ≥200 | 136 (26) | 0 | 136 (33) | 0 | |
| Historical eGFR slope (mL/min/1.73 m2/year)†‖ | −3.5 (−5.8, 0) | −2.7 (−4.9, 0.8) | −2.4 (−5.6, 0.4) | −4.7 (−6.5, −3.6) | By design |
| Baseline eGFR (mL/min/1.73 m2)‡ | 76 (59, 90) | 87 (83, 96) | 74 (58, 88) | 82 (67, 90) | 0.02 |
| iGFR (mL/min/1.73 m2)†‡ | 68 (55, 80) | 82 (66, 87) | 67 (53, 78) | 76 (59, 87) | 0.002 |
| Recruitment modality¶ | |||||
| GFR slope ≥3 mL/min/1.73 m2/year | 126 (23.8) | 7 (41.2) | 25 (6.0) | 94 (100) | |
| Albuminuria | 394 (74.3) | 0 | 394 (94.0) | 0 | |
Data are median (interquartile range) or n (%). Hypertension is defined as one or more of the following: a diagnosis of hypertension from electronic medical records, blood pressure >140/90 mmHg, and/or use of antihypertensive medications. Self-reported cardiovascular disease (CVD) was defined as participant-reported previous myocardial infarction, coronary revascularization, stroke, or amputations. Self-reported diabetic retinopathy was defined as a self-reported diagnosis of any diabetic retinopathy or diabetic retinopathy requiring laser treatment, eye injection, or vitrectomy or causing blindness.
*In race, other is a combination of American Indian or Alaskan Native, Native Hawaiian or Other Pacific Islander, multirace, unknown, or unreported.
†Data missing for 30 participants for prior self-reported CVD, 31 for self-reported diabetic retinopathy, 62 for HMC-CoA reductase inhibitor use, 5 for BMI, 2 for HbA1c and UAER, 41 for historical eGFR slope, and 1 for iGFR.
‡Obtained during visit 4.
§Geometric mean of UAERs for visits 3 and 4.
‖Obtained during visit 1.
¶Ten participants did not qualify by albuminuria or eGFR criteria.
#P values refer to the comparison between albuminuric DKD and NDKF.
Baseline Characteristics of Participants According to Albuminuria Status
Of the 530 randomized participants, 520 met the eGFR slope and/or albuminuria eligibility criteria. Of these, 419 (81%) had albuminuric DKD, 94 (18%) had NDKF, and 7 (1%) had indeterminate albuminuria status (Table 2). Compared with participants with albuminuric DKD, those with NDKF included more women and fewer within racial/ethnic minority groups (Table 2). Participants with NDKF had comparable diabetes duration to those with albuminuric DKD but were older at the time of diabetes onset and at enrollment. They had a lower prevalence of hypertension, lower systolic and diastolic blood pressures, lower HbA1c, and lower SUA. Fewer had self-reported diabetic retinopathy. Participants with NDKF were less often treated with RASIs (full or reduced dose) than those with albuminuric DKD. They also had higher baseline eGFR and iGFR and a greater historical rate of eGFR decline than those with albuminuria, which derived at least in part from the selection criteria.
Baseline Characteristics of Participants According to Albuminuria and Slope of eGFR Decline
Information on eGFR slope before enrollment in the study was available for 379 of the 419 (90%) participants with albuminuric DKD. Of these, 174 (46%) also had eGFR decline ≥3 mL/min/1.73 m2/year (Supplementary Table 4). These 174 participants were younger and had shorter diabetes duration and higher HbA1c and UAER than those with albuminuria alone. Compared with participants with albuminuria with rapid eGFR decline (i.e., ≥3 mL/min/1.73 m2/year), participants with NDKF were older at the time of diabetes onset and at enrollment and included fewer racial/ethnic minorities. They had a lower prevalence of hypertension and lower systolic and diastolic blood pressure, HbA1c, and SUA (Supplementary Table 4). They had less self-reported diabetic retinopathy and were less often treated with RASIs. They also had higher baseline iGFR and lower rates of historical eGFR loss than those with albuminuria and rapid eGFR decline.
Associations Between Baseline Characteristics According to Albuminuria Status
In multivariate analyses in both the albuminuric DKD and the NDKF groups, baseline iGFR was inversely associated with age (β = −0.2 and −0.4; P = 0.02 and 0.01, respectively), female sex (β = −8.8 and −12.0; P < 0.0001 and P = 0.0002, respectively), HbA1c (β = −1.9 and −2.9; P = 0.003 and 0.02, respectively), and SUA (β = −4.6; P < 0.0001 for both groups) (Supplementary Table 5). Also in both groups, baseline SUA was lower in women and in participants with lower BMI. Among participants with albuminuria, baseline UAER was positively associated with male sex (β = 0.4; P < 0.0001), HbA1c (β = 0.2; P < 0.0001), and systolic blood pressure (β = 0.01; P = 0.0002) and inversely associated with iGFR (β = −0.006; P = 0.02) and age (β = −0.02; P = 0.0001).
Baseline Characteristics of Participants According to Sex
Comparisons of the baseline characteristics of the women versus men within each DKD category are shown in Supplementary Table 6. In both the albuminuric DKD and the NDKF groups, men had higher baseline eGFR and measured GFR. Among participants with albuminuric DKD, men were older at diabetes onset and had shorter diabetes duration and higher diastolic blood pressure, albuminuria, and SUA. Some of the differences between men and women were also present among participants with NDKF, but these did not reach statistical significance likely in part because of the smaller sample size.
Conclusions
PERL is a double-blind, placebo-controlled, randomized multicenter international clinical trial examining the effect of SUA reduction with allopurinol on DKD progression in T1D. PERL enrolled 530 participants between the years 2013 and 2016. Follow-up will be completed in 2019. The trial is specifically targeted to a stage of DKD that is early enough to greatly increase the potential benefits of an intervention in terms of delay to ESRD but is sufficiently advanced to enrich the study population for GFR decliners who have already started to lose renal function and on whom the efficacy of the intervention can be tested in a clinical trial of practical size and length.
PERL is the first randomized clinical trial with adequate statistical power to test whether SUA reduction with allopurinol can slow progressive GFR loss in people with T1D, increased risk of progressive GFR decline, and higher SUA levels. For the purpose of power calculations and on the basis of the results in two small previous studies (11,15), treatment with allopurinol was postulated to reduce the rate of GFR decline from 3 to 2 mL/min/1.73 m2/year. If this effect is sustained over time, the PERL study population, with its baseline median iGFR of 68 mL/min/1.73 m2, would have a potential delay in progression to ESRD (defined as an iGFR <15 mL/min/1.73 m2) of nearly 9 years. This potential delay is substantially longer than what has been demonstrated for RASIs in T1D (16) or type 2 diabetes (T2D) (17).
While the majority of PERL participants have albuminuric DKD, almost one-fifth were enrolled in PERL because they had NDKF. Historically, DKD has been clinically characterized as the initial appearance and progressive worsening of albuminuria, followed by declining GFR. More recently, some people with T1D or T2D have been observed to develop GFR decline without albuminuria (i.e., to experience NDKF) (18,19). This observation may be partly due to better glycemic and blood pressure control, increased use of RASIs, or a treatment-independent change in the natural history secondary to changing demographics or longer survival with diabetes. However, the research kidney biopsies of participants with normoalbuminuria, T1D, and reduced GFR, who were not on RASIs and generally did not have good glycemic control, showed classical diabetic glomerulopathy lesions indistinguishable from those seen in individuals with T1D and classic albuminuric DKD (20,21).
The growing recognition of the NDKF phenotype raises the question of how best to clinically manage this group of patients. Because albuminuria has been deemed a predictor of GFR loss, DKD clinical trials have historically used albuminuria to identify participants at high risk of progression, and those without albuminuria have typically been excluded (22). Consequently, it is unclear whether current standards of care alter the disease course in patients with NDKF (23,24). PERL aims to address this limitation by including participants with both DKD phenotypes. In the absence of laboratory evidence for albuminuria, an eGFR loss ≥3 mL/min/1.73 m2/year was used to identify people with rapidly declining kidney function who are at higher risk of progressive kidney function loss (25,26). This criterion was based on studies indicating that in patients with T1D and normoalbuminuria, higher SUA is a strong and independent predictor of rapid GFR loss of this magnitude (11). Thus, it was logical to include people with NDKF who experienced GFR loss, despite the absence of albuminuria (27).
The baseline characteristics of the PERL study cohort largely reflect those of the general T1D population with declining kidney function (28). PERL participants are mostly white, reflecting the typical racial distribution of T1D. The majority are male, likely because of normally higher SUA levels in males (29) and due to the greater prevalence of DKD in men (30,31). In addition, the majority were overweight or obese, probably reflecting the high prevalence of these conditions in the general population and the positive association between higher body weight and higher SUA (an eligibility criterion for PERL) (32). The older median age and longer diabetes duration, compared with earlier DKD studies, is consistent with the delay of ESRD outcomes in T1D, which has been observed in the past two decades and attributed to improved control of risk factors such as hyperglycemia and hypertension and more widespread RASI use in later DKD stages (2,33). The high prevalence of hypertension and diabetic retinopathy is also consistent with the natural history of DKD in people with T1D. The prevalence of diabetic retinopathy in PERL may be underestimated because of reliance on self-report and lower prevalence of diabetic retinopathy in normoalbuminuric versus albuminuric DKD (34,35). The PERL population showed comparable or better control of risk factors (i.e., blood pressure, HbA1c, and RASI use) than has been reported for the general population with diabetes and DKD (36,37), likely because of the substantial recruitment from specialist diabetes centers and the requirement for optimization of medical therapy during the run-in period. While this might have generated differences between the PERL trial population and the larger population of people with T1D and DKD, it is important for trials evaluating the incremental effect of new interventions on complication outcomes to demonstrate efficacy in patients receiving the accepted standards of care.
The associations among baseline variables in the overall PERL population generally reflected the current understanding of DKD risk factors. The association of albuminuria with GFR in the participants with albuminuria is consistent with the well-documented inverse correlation between GFR and urine albumin excretion (38). The association of higher HbA1c and blood pressure with urine albumin excretion in people with T1D and albuminuria is also well described (39,40). Likewise, in most diabetes studies, female sex is associated with a lower likelihood of micro- and macroalbuminuria (41,42). Among PERL participants, female sex was associated both with lower SUA and lower iGFR at baseline. Women are known to have lower SUA than men (29), but the finding of lower iGFR in the women in both the DKD and NDKF groups was surprising given that the inverse correlation between SUA and iGFR might have predicted that female PERL participants would have higher iGFR. Although NDKF with low GFR may be more common in women than in men with T1D (21), in PERL, the iGFR differences by sex were present in both albuminuric DKD and NDKF groups. The reason for the lower iGFR in PERL female participants remains to be determined.
As in previous reports (21,43), PERL participants with NDKF were older and more often female and white. To be eligible for PERL, participants with normoalbuminuria were required to have a rate of GFR loss ≥3 mL/min/1.73 m2/year, and their actual rate of GFR loss over the 3–5 years before enrollment was nearly double that of the albuminuric group. This observation may be related, at least in part, to a selection bias because the rate of GFR decline was not an inclusion criterion for participants with albuminuric DKD. Interestingly, this rapid GFR loss was less strongly associated with established DKD risk factors. Specifically, compared with PERL participants with albuminuria, those with normoalbuminuria had substantially higher baseline iGFR; lower blood pressure, HbA1c, and SUA; and lower prevalence of self-reported diabetic retinopathy and hypertension. Of note, when PERL participants with NDKF were compared with those with albuminuria and similar rates of GFR decline, many of these differences were still present, suggesting that the observed differences were not entirely explained by the study selection criteria. It will be of great interest to see whether the higher rate of GFR decline among the participants with NDKF persists during the trial. Such an observation would suggest that people with T1D who experience rapid GFR loss despite normoalbuminuria and fewer established DKD risk factors may have an as-yet undescribed rapidly progressive DKD phenotype of which pathogenesis and natural history have different determinants than albuminuric DKD and therefore require intensive study.
The primary outcome of the PERL study is the final iGFR, measured after the 2-month washout period, adjusted for the baseline iGFR value. The rationale for selection of this primary outcome is threefold. First, measuring iGFR at the end of the washout period enables the study to differentiate the durable effects of allopurinol on DKD natural history independent from any transient effects it may have on GFR. One caveat is that allopurinol withdrawal has been reported to be associated with rebound hyperuricemia, hypertension, and accelerated GFR loss (8). Thus, a worsening GFR loss during the washout period may reflect either the transient nature of the allopurinol effect or rebound processes after allopurinol withdrawal. Second, examining the change in GFR (final GFR adjusted for baseline GFR) as the primary outcome enables assessment of the intervention effect in early DKD, whereas the customary focus on the late outcomes of serum creatinine doubling or ESRD would require a prohibitively long follow-up. Finally, measured GFR is more sensitive for the detection of GFR change than GFR estimated from serum creatinine, cystatin C, or both (44). PERL is one of the few clinical trials that both differentiates the permanent versus transient effects of the intervention on GFR and uses measured GFR rather than eGFR to improve precision in detecting group differences in change in kidney function as a primary outcome in a clinical trial. PERL will ultimately test whether the earlier studies (44) supporting the use of measured GFR can be confirmed and what the implications might be for the design of future clinical trials targeting reduction in early GFR loss.
In addition to lowering SUA and reducing uricosuria, allopurinol has been suggested to suppress RAS activity (45) and reduce urinary transforming growth factor-β (8). If PERL demonstrates a benefit of allopurinol on GFR loss, it will not be possible to definitively establish whether the benefit is due to the reduction in SUA per se and/or to other consequences of xanthine oxidase inhibition by allopurinol or other effects of the drug. However, the stored PERL biosamples, combined with the meticulously collected phenotype data, will enable further exploration of various potential underlying mechanisms of this treatment benefit. Furthermore, the planned secondary analyses evaluating the association between changes in SUA, or achieved SUA levels, during the trial and iGFR benefit could favor the treatment value of SUA reduction per se versus other possible allopurinol effects. Nonetheless, other trial designs may be needed to better answer this question. On the other hand, the establishment of allopurinol as a novel treatment to prevent or slow GFR loss in T1D would be a transformative finding regardless of the mechanisms through which this drug exerts its beneficial effect. Notably, people with T1D may have lower SUA levels than those with T2D (46), and this could possibly attenuate the effect of allopurinol on DKD progression in the overall T1D population. However, PERL participants were selected for having SUA levels above the median value for people with T1D. The reason for this decision was that patients with higher SUA had a greater likelihood of rapid GFR loss (7,10) and would thus benefit the most from SUA reduction. An additional effect of this selection criterion may be to increase the probability that the results from PERL will be relevant to other populations with similar SUA levels, such as people with T2D and early to moderate CKD. The fact that a majority of PERL participants were obese may make the findings in this study population additionally relevant to people with T2D.
In conclusion, the PERL study tests the hypothesis that treatment with allopurinol to lower SUA will reduce DKD progression in people with T1D, moderately elevated SUA, and mild to moderate DKD. The study is unique in its inclusion of participants with both albuminuric and normoalbuminuric reduction in kidney function, thus offering an opportunity to gain insight into the clinical features, natural history, and response to allopurinol in these distinct DKD phenotypes.
Supplementary Material
Article Information
Acknowledgments. The PERL study investigators thank all the study participants for enormous contributions made to this long and demanding trial. They are also grateful to the PERL data safety and monitoring board (Linda Fried, Ananda Basu, Melanie Blank, Tom Greene, Lawrence Holzman, Charity G. Moore Patterson, and John Sedor) and the National Institutes of Health officer Dr. Teresa Jones for guidance and to Green Mountain Pharmaceuticals and Belmar Pharmacy (Lakewood, CO) for the skilled preparation and distribution of the study drug. The PERL investigators also acknowledge all the PERL research coordinators whose daily commitment and effort has made this study possible (alphabetically): Daphne Adelman, Gayatri Anupindi, Cathy Bagne, Mary Bates, Karen Belanger, Emily Boone, Jane Bulger, Leslie Cham, Jing H. Chao, Theresa Christiansen, Mary Ann Clearwaters, Mary Jane Clifton, Hector Com, Debra Conboy, Kristie DeHaan, Tineke Dineen, Amy Dunlop, Sara Eischen, C. Marcelo Falappa, Lestat Feliciano, Birgit Fink, Benjamin Flagg, Victoria Gage, Jason Gensler, Anne Goodling, Vasundhara Goplani, Monica Hartmuller, Eric Henricks, Jessie Arman Hermann, Madeleine Hermans, Xinli Huang, Karen Hyams, Marla Inducil, Lone Jelstrup, Florence John, Yvette Kowalski, Vesta Lai, Lee-Ann Langkaas, Diane Larsen, Catherine Leiendecker Foster, Virginia Leone, Camilla Levister, Annie Lukkari, Dawn Lum, Caroline Lyster, Maria Maione, Elaine Massaro, Jennifer McCance, Christine Mendonca, Sara R. Meyers, Joan Milton, Cindy A. Murphy, Ariane Nguyen, Andrej Orszag, Cynthia Plunkett, Carmyn Polk, Laurentiu Pop, Danielle Powell, Chinmaya Rajderkar, Clementina Ramos Garrido, Carol Recklein, Marilyn Richardson, Nicole Robinson, Lauri Schafer, Michelle Smith, Trudy Strand, Anna-Kay Thompson, Lindsey Towers, Josephine Tse, Joanie Tsougrianis, Victoria Tully, Sara Vecchi, Katherine Wilder, Tanisha Wilma, and Brittany Williams.
Funding. The PERL study is funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R03-DK-094484, R34-DK-097808, and UC4-DK-101108) and JDRF (17-2012-377). The iohexol used for the iGFR measurements was a generous gift of GE Healthcare. Research reported in this publication was supported by the National Center for Advancing Translational Sciences, the NIDDK, and the National Institute on Aging (Claude Pepper Center grants) under award numbers P30-DK-036836, UL1-TR-002494, P30-DK-020572, UL1-TR-001422, UL1-TR-002556, UL1-TR-002319, UL1-TR-001105, UL1-TR-002319-02, P30-AG-08808, and P30-AG-024824.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, NIDDK, National Institute on Aging, JDRF, or GE Healthcare.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A., S.P., A.P., R.A., M.L.C., D.Z.C., J.P.C., I.H.d.B., T.G.E., A.T.G., A.B.G., J.S.H., I.B.H., A.B.K., I.L., D.M.M., J.B.M., M.E.M., B.A.P., R.P.-B., M.P., S.E.R., P.R., P.S., R.J.S., C.S., K.R.T., G.E.U., A.W., R.S.W., C.W., M.M., and A.D. designed the study, supervised its execution, and critically reviewed the manuscript and approved the submitted manuscript. M.A., S.P., A.P., M.M., and A.D. wrote the manuscript. A.T.G., C.S., C.W., and A.D. analyzed the data. M.M. and A.D. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0342/-/DC1.
*The full list of PERL study group members is available in the online Supplementary Data.
Contributor Information
Collaborators: PERL Study Group, Alessandro Doria, Michael Mauer, Ronnie Aronson, Maria Luiza Caramori, Jill P. Crandall, Ian H. de Boer, Alessandro Doria, John H. Eckfeldt, Thomas G. Elliott, Michael Flessner, Andrzej T. Galecki, Allison B. Goldfine, Irl B. Hirsch, Amy B. Karger, Ildiko Lingvay, David M. Maahs, Michael Mauer, Janet B. McGill, Mark E. Molitch, Helen Nickerson, Afshin Parsa, Bruce A. Perkins, Sarit Polsky, Rodica Pop-Busui, Marlon Pragnell, Sylvia E. Rosas, Peter Rossing, Peter Senior, Ronald J. Sigal, Catherine Spino, Katherine R. Tuttle, Guillermo E. Umpierrez, Andrzej T. Galecki, Massimo Pietropaolo, Catherine Spino, Yi-Miau Tsai, Chunyi Wu, John H. Eckfeldt, Amy B. Karger, William Robiner, Michael Flessner, Afshin Parsa, Helen Nickerson, Marlon Pragnell, Alessandro Doria, Allison B. Goldfine, Sylvia Rosas, Enrico Cagliero, Michael Thompson, Ruth S. Weinstock, Christina Gjerlev-Poulsen, Maria Lajer, Frederik Persson, Sascha Pilemann-Lyberg, Peter Rossing, Maria Luiza Caramori, Michael Mauer, Mary Frohauer, San Thida, Peter Gottlieb, David Maahs, Sarit Polsky, Viral Shah, Emily Schroeder, Michael McDermott, Lynn Ang, Frank C. Brosius, III, Nazanene H. Esfandiari, Kara Mizokami-Stout, Rodica Pop-Busui, Rachel Perlman, Arti Bhan, Davida Kruger, Wenyu Huang, Mark E. Molitch, Amisha Wallia, Matthew K. Abramowitz, Valentin Anghel, Erika Brutsaert, Jill P. Crandall, Nithya Mani, Divya Rajasekaran, Carol Levy, Melissa Katz, Naina Sinha, Nobuyuki Gregory, Shayan Bill Miyawaki, Ulrich K. Shirazian, David Schubart, Bruce A. Cherney, Lorraine L. Perkins, Andrew Lipscombe, Ronnie Advani, Ronald Aronson, Janet B. Goldenberg, Amy McGill, Maamoun Riek, Julie Salam, Ronald J. McKeen, Peter Sigal, Rose Senior, J. Sonya Yeung, Guillermo E. Haw, Bruce W. Umpierrez, Darin Bode, Maryam Olson, Ian H. Afkarian, Irl B. de Boer, Dace L. Hirsch, Grace Trence, Ildiko Lee, Radica Lingvay, Katherine R. Alicic, Tuttle, and Thomas G. Elliott
References
- 1.Afkarian M, Zelnick LR, Hall YN, et al. . Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 2016;316:602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krolewski AS, Bonventre JV. High risk of ESRD in type 1 diabetes: new strategies are needed to retard progressive renal function decline. Semin Nephrol 2012;32:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley RN, Collins AJ. The USRDS: what you need to know about what it can and can’t tell us about ESRD. Clin J Am Soc Nephrol 2013;8:845–851 [DOI] [PubMed] [Google Scholar]
- 4.Groop PH, Thomas MC, Moran JL, et al.; FinnDiane Study Group . The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010;53:2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugliese G. Updating the natural history of diabetic nephropathy. Acta Diabetol 2014;51:905–915 [DOI] [PubMed] [Google Scholar]
- 7.Maahs DM, Caramori L, Cherney DZ, et al.; PERL Consortium . Uric acid lowering to prevent kidney function loss in diabetes: the Preventing Early Renal Function Loss (PERL) allopurinol study. Curr Diab Rep 2013;13:550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol 2007;27:435–440 [DOI] [PubMed] [Google Scholar]
- 9.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008;300:924–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS. Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis 2018;71:362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ficociello LH, Rosolowsky ET, Niewczas MA, et al. . High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care 2010;33:1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 2011;155:408]. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung JW, Song WJ, Kim YS, et al. . HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant 2011;26:3567–3572 [DOI] [PubMed] [Google Scholar]
- 14.Gaspari F, Perico N, Matalone M, et al. . Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol 1998;9:310–313 [DOI] [PubMed] [Google Scholar]
- 15.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 2009;58:1668–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group . The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 17.Lewis EJ, Hunsicker LG, Clarke WR, et al.; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 18.Costacou T, Ellis D, Fried L, Orchard TJ. Sequence of progression of albuminuria and decreased GFR in persons with type 1 diabetes: a cohort study. Am J Kidney Dis 2007;50:721–732 [DOI] [PubMed] [Google Scholar]
- 19.Molitch ME, Steffes M, Sun W, et al.; Epidemiology of Diabetes Interventions and Complications Study Group . Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 1994;43:1358–1364 [DOI] [PubMed] [Google Scholar]
- 21.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes 2003;52:1036–1040 [DOI] [PubMed] [Google Scholar]
- 22.Porrini E, Ruggenenti P, Mogensen CE, et al.; ERA-EDTA diabesity working group . Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol 2015;3:382–391 [DOI] [PubMed] [Google Scholar]
- 23.Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB. Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: results from the DEMAND study. Cardiorenal Med 2012;2:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauer M, Zinman B, Gardiner R, et al. . Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krolewski AS, Niewczas MA, Skupien J, et al. . Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 2014;37:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins BA, Ficociello LH, Ostrander BE, et al. . Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007;18:1353–1361 [DOI] [PubMed] [Google Scholar]
- 27.Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol 2006;17:339–352 [DOI] [PubMed] [Google Scholar]
- 28.de Boer IH, Afkarian M, Rue TC, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Renal outcomes in patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 2014;25:2342–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desideri G, Castaldo G, Lombardi A, et al. . Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci 2014;18:1295–1306 [PubMed] [Google Scholar]
- 30.de Hauteclocque A, Ragot S, Slaoui Y, et al.; SURDIAGENE Study group . The influence of sex on renal function decline in people with type 2 diabetes. Diabet Med 2014;31:1121–1128 [DOI] [PubMed] [Google Scholar]
- 31.Orchard TJ, Dorman JS, Maser RE, et al. . Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 1990;39:1116–1124 [DOI] [PubMed] [Google Scholar]
- 32.Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis 2001;11:401–406 [PubMed] [Google Scholar]
- 33.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas MC, Macisaac RJ, Jerums G, et al. . Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (National Evaluation of the Frequency of Renal Impairment Co-existing With NIDDM [NEFRON] 11). Diabetes Care 2009;32:1497–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigalleau V, Lasseur C, Raffaitin C, et al. . Normoalbuminuric renal-insufficient diabetic patients: a lower-risk group. Diabetes Care 2007;30:2034–2039 [DOI] [PubMed] [Google Scholar]
- 36.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 37.Hill CJ, Cardwell CR, Patterson CC, et al. . Chronic kidney disease and diabetes in the national health service: a cross-sectional survey of the U.K. national diabetes audit. Diabet Med 2014;31:448–454 [DOI] [PubMed] [Google Scholar]
- 38.Parving H-H, Mauer M, Fioretto P, Rossing P, Ritz E. Diabetic nephropathy. In The Kidney. 9th ed Brenner BM, Ed. Philadelphia, Elsevier, Saunders, Inc., 2012, p. 1411–1454 [Google Scholar]
- 39.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 2002;25:859–864 [DOI] [PubMed] [Google Scholar]
- 40.Steinke JM, Mauer M; International Diabetic Nephropathy Study Group . Lessons learned from studies of the natural history of diabetic nephropathy in young type 1 diabetic patients. Pediatr Endocrinol Rev 2008;5(Suppl. 4):958–963 [PubMed] [Google Scholar]
- 41.Hovind P, Tarnow L, Rossing P, et al. . Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 2004;328:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibley SD, Thomas W, de Boer I, Brunzell JD, Steffes MW. Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort: role of central obesity. Am J Kidney Dis 2006;47:223–232 [DOI] [PubMed] [Google Scholar]
- 43.Thorn LM, Gordin D, Harjutsalo V, et al.; FinnDiane Study Group . The presence and consequence of nonalbuminuric chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2015;38:2128–2133 [DOI] [PubMed] [Google Scholar]
- 44.Delanaye P, Melsom T, Ebert N, et al. . Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: why to measure glomerular filtration rate with iohexol? Clin Kidney J 2016;9:700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tani S, Nagao K, Hirayama A. Effect of febuxostat, a xanthine oxidase inhibitor, on cardiovascular risk in hyperuricemic patients with hypertension: a prospective, open-label, pilot study. Clin Drug Investig 2015;35:823–831 [DOI] [PubMed] [Google Scholar]
- 46.Maxwell SR, Thomason H, Sandler D, et al. . Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1997;27:484–490 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.