Abstract
Background
Diabetic macular edema (DME) is an important cause of vision loss and despite the anatomical and functional improvement achieved with treatment, there are reports of persistent DME regardless of continuous anti-VEGF therapy. The purpose of this study is to examine the effect of patients with DME previously treated with other anti-VEGF agents who are transitioned to intravitreal aflibercept (IAI) on a fixed dosing regimen.
Methods
This prospective study included 20 patients presenting with DME with a history of previous anti-VEGF treatment with ranibizumab or bevacizumab. Patients received a 2 mg (0.05 mL) IAI every 4 weeks until no evidence of fluid by optical coherence tomography (OCT) followed by a fixed dosing schedule of 2 mg IAI once every 8 weeks through 24 months. There was a pre-planned interim analysis of the mean absolute change from baseline central foveal thickness at month 6 as measured by OCT. Secondary outcomes included mean change from baseline in ETDRS visual acuity and anatomic parameters. Optical Coherence tomography angiography (OCTA) capillary perfusion density (CPD) after transitioning to IAI therapy were also reported.
Results
Average central subfield thickness on OCT at baseline was 419.7 ± 92.0 and improved to 303.8 ± 73.1 at 6-months (p < 0.001). At 6 months after IAI treatment, BCVA increased + 1.5 letters from baseline (p = 0.38). OCTA CPD analysis revealed significant increase from baseline in the foveal avascular zone in non-proliferative diabetic retinopathy group (p = 0.02).
Conclusions
Patients with prior anti-VEGF therapy who were transitioned to IAI therapy revealed significant anatomic improvements through 6 months.
Trial registration Treatment of Diabetic Macular Edema With Aflibercept in Subjects Previously Treated With Ranibizumab or Bevacizumab (SwapTwo), Trial registration number: NCT02559180. Date of registration: September 24, 2015.https://clinicaltrials.gov/ct2/show/NCT02559180
Introduction
Diabetes mellitus is one of the most common chronic diseases and continues to rise in numbers and significance. Late estimates show a global prevalence of 382 million patients, expected to rise to 592 million by 2035 [1]. Diabetic macular edema (DME) is an important cause of vision loss in diabetic patients present in 20% of patients with younger onset versus approximately 40% in older onset diabetes [2, 3].
Anti-vascular endothelial growth factor (VEGF) has become the first-line treatment for DME [4]. Three VEGF inhibitors are commonly used: aflibercept (Eylea, Regeneron Pharmaceuticals, Tarrytown, NY, USA) and ranibizumab (Lucentis, Genentech, South San Francisco, California, USA) are Food and Drug Administration (FDA) approved treatment for DME, while bevacizumab (Avastin, Genentech, South San Francisco, California, USA) is used off-label. Despite the anatomical and functional improvement achieved with treatment, there are reports of persistent DME regardless of continuous anti-VEGF therapy. RISE and RIDE trials reported that, after 24 months of ranibizumab therapy, 27–46% of eyes had vision of 20/40 or worse and 19–26% of eyes still had central subfield thickness (CST) greater than 250 µm [5]. Protocol I reported persistent DME, defined as never having CST below 250 µm, in around 40% of eyes receiving monthly ranibizumab after 6 months [6]. Therefore, switching to a drug with different VEGF affinity and that can block other cytokine pathways maximizing therapeutic potential has become a common practice among retinal specialists [7]. Most physicians (77.5%) consider switching anti-VEGF agent due inadequate response after three to six injections, and the majority (59%) will have noticed visual improvements after the switching [4]. However, there is a lack of well-designed, prospective, randomized clinical trials assessing the efficacy of switching anti-VEGF on DME outcomes.
DRCR Network Protocol T, a multicenter, randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab in patients with center-involved DME, reported better visual and anatomical outcomes from baseline to 1 year with aflibercept, especially in patients with visual acuity ≤ 20/50 [8]. However, Protocol T used a rigid inclusion criterion which required patients not to have anti-VEGF therapy for a minimum of 12 months prior to entry. While most studies employ a fixed monthly dosing schedule, the majority of retina specialists do not treat using a monthly dosing pattern, instead less burdensome treatment paradigms, such as as-needed (pro ne rata, PRN) or treat-and-extend (TAE) dosing are used. However, the extension studies of RESTORE, RISE and RIDE trials demonstrate visual acuity (VA) decline in subjects transitioned to a more flexible treatment from a fixed dosing scheme [9, 10].
This study aims to evaluate the effects of switching patients with DME previously treated with other anti-VEGF agents to intravitreal aflibercept (IAI), and further placing them on a fixed dosing regimen.
Methods
The SWAP-TWO Study is a prospective, interventional, single arm study performed at the Cole Eye Institute, Cleveland, Ohio, USA. The study received approval from the Cleveland Clinic Investigational Review Board (IRB), and all study-related procedures were performed in accordance with good clinical practice (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) E6), applicable FDA regulations, and the Health Insurance Portability and Accountability Act. All patients signed an informed consent for their participation in the study.
Participants
This prospective study enrolled 20 eyes of 20 patients between December 2015 and August 2017. Eligible participants were aged ≥ 18 years with foveal-involving retinal edema secondary to diabetic retinopathy (DR) based on investigator review of clinical exam and spectral-domain optical coherence tomography (SD-OCT) with CST value of 325 µm, Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) of 80 (20/25) to 20 (20/400) in the study eye, and history of previous treatment with bevacizumab or ranibizumab with at least 4 previous injections in the last 6 months.
Patients were excluded if they had any prior or concomitant therapy with another investigational agent to treat DME in the studied eye, history of vitrectomy or panretinal photocoagulation in the study eye within 3 months, or previous intravitreal anti-VEGF therapy in the study eye within 30 days of enrollment. Patients with history of IAI were not allowed in the study. Prior systemic anti-VEGF therapy, investigational or FDA-approved, was only allowed up to 3 months prior to first dose. Additionally, individuals were excluded if they had any of the following concomitant ocular diseases at baseline evaluation: uncontrolled glaucoma, active intra-ocular or periocular infection in either eye, other causes of macular edema including pathologic myopia (spherical equivalent of –8 diopters or more negative, or axial length of 25 mm or more), presumed ocular histoplasmosis syndrome or any history of uveites, angioid streaks, choroidal rupture, choroidal neovascularization, age-related macular degeneration or multifocal choroiditis in the study eye. Epiretinal membranes were not considered an exclusion. Only one eye per subject was enrolled in the study. For patients who met eligibility criteria in both eyes, the investigator and patient designated the study eye. If a non-study eye required treatment for DME at study entry or during the subject’s participation in the study, the fellow eye could receive IAI, but it was not considered as an additional study eye. The frequency of fellow eye treatment was based on investigator discretion.
Visits and assessments
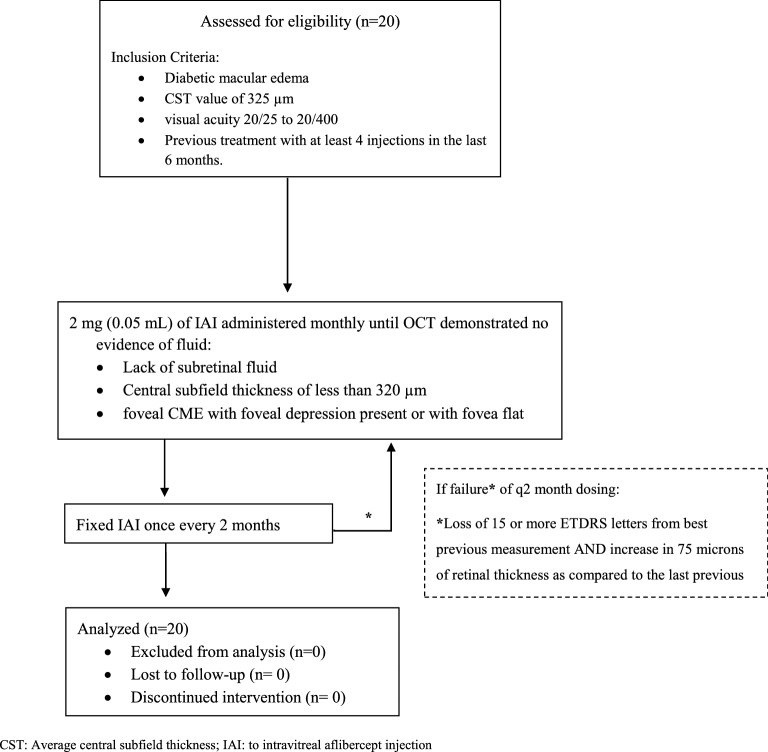
Patients were given 2 mg (0.05 mL) of IAI administered monthly until OCT demonstrated no evidence of fluid (defined as lack of subretinal fluid; central subfield thickness of less than 320 µm; extrafoveal cystoid macular edema (CME); or foveal CME with foveal depression present or with fovea flat) followed by fixed IAI once every 2 months (Fig. 1). IAI was supplied by Regeneron Pharmaceuticals, and was administered using the standard aseptic.
Fig. 1.
Flow chart
Study visits were scheduled every 28 ± 7 days. At each visit, the visual functions of both the eyes were assessed using the ETDRS chart (M&S Systems) and protocol visual acuity measurement consisting of BCVA testing and a forced choice paradigm [11]. A comprehensive eye examination and SD-OCT scanning were performed. The scanning protocol consisted of fast macular thickness maps as well as high definition 6.0 mm linear scans centered on the fovea using the Cirrus SD-OCT (Zeiss, San Leandro, California, USA) and a 3 × 3 mm (9 mm2) en face retinal map for vascular analysis using AngioVue Avanti RTVue XR (Optovue, Inc., Fremont, CA, USA).
Quantitative CPD analysis was performed with ReVue software version 2017.1.0.129 (Optovue, Inc, Fremont, CA). The built-in software calculates the capillary perfusion density (CPD) by computing the percentage area occupied by detected optical coherence tomography angiography (OCTA) vasculature. Vessels pixels area is ‘true’ while background/noise is ‘false’. This binary mask is then used to generate a 2D local density map and automatically calculated density values in grid sectors. Auto-segmentation was used to define anatomical borders for CPD analysis and for FAZ area demarcation. Manual corrections were made if any scan errors were identified.
Additional treatment with IAI could be applied if the subject experienced loss of 15 or more ETDRS letters from best previous measurement or increase in 75 µm of CST as compared to the best visit.
Outcome measures
SD-OCT central subfield thickness, ETDRS BCVA, and OCTA capillary perfusion density studies were monitored after enrollment, and CST data was also collected retrospectively for the 6 month period prior to enrollment. The primary outcome of the study was defined as the mean absolute change from baseline central foveal thickness at month 12 with pre-planned interim analysis as measured by SD-OCT (defined as the average thickness within the central 1 mm subfield) at month 6. Secondary outcomes included the efficacy of treatment outcomes by improvements in ETDRS BCVA from baseline, perfusion changes in OCTA before and after therapy, as well as safety and tolerability of IAI therapy by monitoring adverse events (AEs).
Safety analyses
Safety evaluations included ocular and non-ocular events reported by patients during or between study visits. AEs could also be detected through assessment and were recorded in case report forms. AEs were categorized according to severity (mild, moderate or severe) and relationship to study drug (related/not related).
Statistical methods
Measures were summarized using means, standard deviation (SD), median and range. Normality of measures was evaluated using the Shapiro–Wilk test. Since data were mostly normally distributed, comparisons with and between groups were performed using two-sided paired t-tests. Where appropriate, sensitivity analyses using nonparametric Wilcoxon signed rank tests were also performed. Analyses were performed using SAS software (V.9.2; Cary, North Carolina, USA). A significance level of 0.05 was assumed for all tests.
Results
Data was collected on 20 unique patients from baseline to 6 months. The mean age was 63.7 (range, 45–78) years, and 13 patients (65%) were female (Table 1). The average number of anti-VEGF treatments before transitioning was 4.25 (4–6) injections in the last 6 months with an average washout time of 44.4 days (± 21.2); 95% were injected with bevacizumab and 5% with ranibizumab prior to enrollment. No patient received more than one anti-VEGF drug prior to study enrollment. The mean baseline BCVA was 70 ± 7.2 (60–81) letters (≅ 20/40). The mean CST upon study entry was 419.7 ± 92 (328–585) µm. At baseline, 9 eyes (45%) were classified as mild/moderate non-proliferative diabetic retinopathy (NPDR), 5 (25%) severe NPDR, and 6 (30%) non-active proliferative diabetic retinopathy (PDR).
Table 1.
Baseline demographics and ocular characteristics
| Demographics | Baseline |
|---|---|
| Eyesa (right:left) | 20 (11:9) |
| Average age at screening | 63.7 (45–78) |
| Gender (female:male) | 13:7 |
| Average prior injectionsb | 4.25 |
| ETDRS scores: average (range) | |
| Study eye | 69.95 (60–81) |
| Fellow eye | 73.65 (37–85) |
| Diabetic retinopathy severity | |
| Mild | 0 |
| Moderate | 9 |
| Severe | 5 |
| PDR | 6 |
| OCT values | |
| Study eye | |
| CST [Mean (range)] | 419.7 (328–585) |
| Cube volume [Mean (range)] | 11.55 (9.1–13.9) |
| Cube average thickness [Mean (range)] | 320.7 (253–386) |
| Fellow eye | |
| CST [Mean (range)] | 300.40 (181–432) |
| Cube volume [Mean (range)] | 10.81 (8.8–12.8) |
| Cube average thickness [Mean (range)] | 300.20 (246–354) |
aOnly one eye per patient, bon the 6 month prior enrollment
CST central subfield thickness, ETDRS early treatment diabetic retinopathy study, OCT optical coherence tomography
Treatment frequency
During the study, the mean number of IAI treatments were 5.25 (4–6), over an average 5.3 (4–6) visits. An average of 2.15 (0–6) fellow eye injections were given in 65% patients. At month 6, 11 (55%) patients still required monthly treatment, while 9 (45%) were able to receive IAI every other month after an average of 3.3 injections prior change.
Anatomic outcomes
Table 2 demonstrates the differences between study variables at 6 months prior to enrollment, baseline, and month 6. CST, Cube Volume (CV), and Cube Average Thickness (CAT) were all significantly lower at month 6 (p < 0.001 for all variables) compared to baseline. CST improved from 419.7 ± 92.0 (328–585) µm at baseline to 303.8 ± 73.1 (198–485) µm at month 6 (27.63% reduction). CST measurements in the 6 month period prior to the baseline enrollment and drug switch were not statistically significant. Mean baseline CST at the visit 6 month visit prior to enrollment was 420.8 ± 100.5 and at baseline enrollment prior to drug switch was 419.7 ± 92.0 (p = 0.99).
Table 2.
Differences between study variables from 6 months prior to enrollment, baseline and month 6
| Factor | 6 Months prior to enrollment | Baseline | Month 6 | 95% CI for changes | 6 Months prior to enrollment to baseline | Baseline to month 6 |
|---|---|---|---|---|---|---|
| [Mean ± SD] | [Mean ± SD] | [Mean ± SD] | P value | P value | ||
| ETDRS BCVA | 70.1 ± 7.7 | 70.0 ± 7.2 | 71.5 ± 8.9 | 1.55 (− 2.08, 5.18) | 0.95 | 0.38 |
| Central subfield thickness (µm) | 420.8 ± 100.5 | 419.7 ± 92.0 | 303.8 ± 73.1 | − 116.0 (− 150.8, − 81.15) | 0.99 | < 0.001 |
| Cube volume (mm3) | N/A | 11.5 ± 1.4 | 10.7 ± 1.2 | − 0.86 (− 1.06, − 0.65) | N/A | < 0.001 |
| Cube average thickness (µm) | N/A | 320.7 ± 38.6 | 297.2 ± 33.1 | − 23.50 (− 29.36, − 17.64) | N/A | < 0.001 |
| Foveal avascular zone (mm2) | N/A | 0.31 ± 0.13 | 0.33 ± 0.11a | − 0.03(− 0.11, 0.05) | N/A | 0.47 |
Statistically significant values are in bold italic
ETDRS early treatment diabetic retinopaty study, BCVA best corrected visual acuity, CI confidence interval
an = 18
Measurements of CST at consecutive study visits are outlined in Fig. 2. Each subsequent visit had statistically significant improvement in CST from baseline (p < 0.001 for all time-point comparisons). The average CV improved from 11.5 ± 1.4 (9.1–13.9) to 10.7 ± 1.2 (8.8–12.8) mm3 at month 6 (7.4% decrease). The mean CAT improved from 320.7 ± 38.6 (253–386) to 297.2 ± 33.1 (234 – 368) µm at month 6 (7.3% reduction). Throughout the study period, no change in DR severity was observed. Two patients had epiretinal membrane at baseline and no visually change was seen at 6 month (Fig. 3).
Fig. 2 .
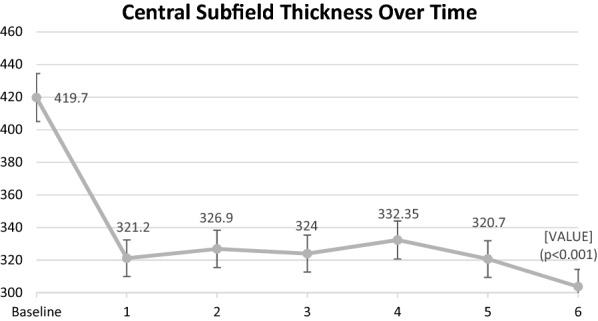
Visit-to-visit change in central subfield thickness
Fig. 3 .
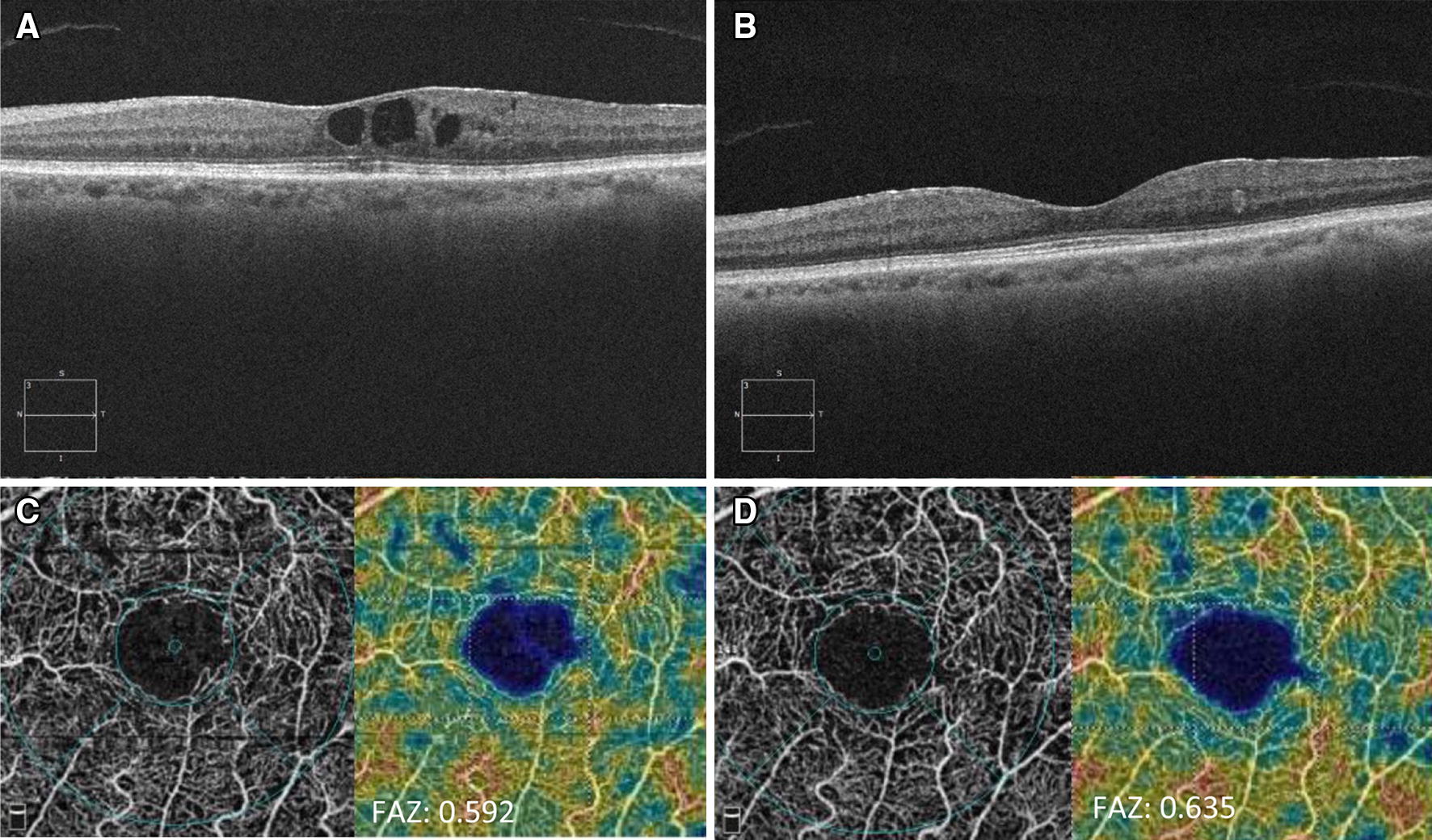
Optical coherence tomography and optical coherence tomography angiography of a patient with epiretinal membrane at baseline. A OCT from a patient with epiretinal membrane at baseline. B Patient 6 month after switching to IAI. C Example of En Face OCT and full retina capillary perfusion density analysis from same patient presented with ERM at baseline. D Example of En Face OCT and full retina capillary perfusion density analysis 6 month after switching to IAI
Foveal avascular zone (FAZ) area was 0.31 mm2 at baseline and increased to 0.33 mm2 at 6 months. However, change from baseline to month 6 was not statistically significant (p = 0.47). When broken down by RD severity, the NPDR group (n = 15) showed an area of 0.27 mm2 at baseline, which increased to 0.32 mm2 at month 6 (p = 0.02). NPDR group also showed a significant improvement in CST (− 88.1 µm, p = 0.004). PDR group (n = 5) presented an area of 0.34 mm2 at baseline with a non-significant increase to 0.35 mm2 at month 6 (p = 0.7). In contrast, CST had statistically significant improvement from baseline in PDR group (− 109.8 µm, p = 0.01). No significant changes to retina density were observed between baseline and 6-months (46.8 ± 5.4 vs. 45.3 ± 5.2, p = 0.37).
Visual acuity
Mean BCVA at the 6 month visit prior to enrollment and drug switch was 70.1 ± 7.7 and at baseline was 70.0 ± 7.2 (p = 0.95). BCVA increased minimally between the baseline visit and 6 months, [70.0 ± 7.2 (60–81) to 71.5 ± 8.9 (54–83) letters], but this change was not statistically significant (p = 0.38). BCVA measurements at consecutive visits are outlined in Fig. 4. At baseline, 65% (n = 13) patients were 20/40 or better, 35% (n = 7) patients were 20/50 or worse, and no patients were 20/200 or worse. By the end of month 6, 60% (12) patients were 20/40 or better, 40% (8) patients were 20/50 or worse, and no patients were 20/200 or worse. Figure 5 demonstrates the VA changes in patients noted by month 6.
Fig. 4.
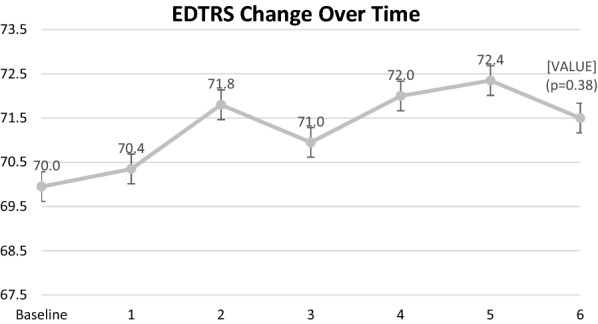
Visit-to-visit change in best correct visual acuity. ETDRS early treatment diabetic retinopathy study
Fig. 5.
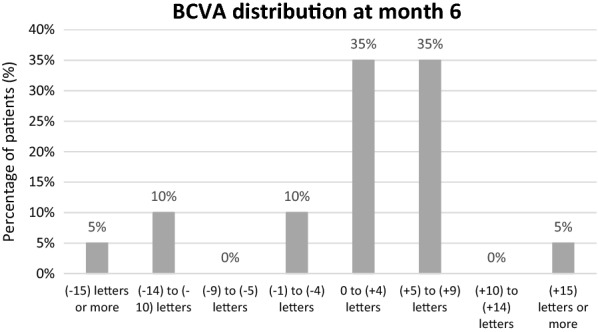
Visual acuity changes in patients noted by month 6. BCVA best-corrected visual acuity
Safety
Three serious AEs were registered during the follow-up. At month 6, one study subject experienced chest pain, and cardiac catheterization was performed. Another was hospitalized for bilateral leg cellulitis (Month 6), and a third patient was hospitalized for dehydration, hypertension, and hyperglycemia (Month 2). No serious ocular AEs, including endophthalmitis, uveitis, retinal detachment, retinal pigment epithelial tears, submacular hemorrhage, or sustained elevated intraocular pressure requiring intervention were observed.
Discussion
Results of this study demonstrated that, after switching to IAI, significant anatomical improvements were achieved. Several studies have assessed switching anti-VEGF in DME [12–25]. Retrospective studies with similar follow-up periods have reported significant CST improvement without BCVA gain [12, 20, 26]. The statistically significant anatomic improvement demonstrated after converting to IAI may be explained by several factors. IAI has been shown to bind VEGF with affinities 94 times stronger than ranibizumab, and 119 times greater than bevacizumab [27]. Additionally, IAI also binds VEGF-B and placental growth factor (PLGF) [27]. The concentration of PLGF plays a role in DR pathogenesis through increased levels of VEGF, and the activation of protein kinase which affects the blood retinal barrier [28]. The achievement of superior outcomes after conversion switching may not necessarily reflect only the drug superiority but also the benefit of a fixed-dosing paradigm in an FDA-approved manner over the possible undertreatment of variable-dosing regimens [9].
In contrast, Lim et al. reported distinct functional outcomes when assessing anti-VEGF switching to IAI from ranibizumab and/or bevacizumab in 21 eyes [16]. In their study, BCVA gain was significant at 5 months after switching, but not all patients included had persistent DME at baseline. Mira et al. also reported anatomical and functional gains 3 months following the transition to another anti-VEGF agent [19]. However, baseline BCVA in their study was 0.7 logmar (≅ . 20/100) which is considerably worse than the average baseline BCVA in this report, and in the general population. Since poor baseline BCVA is an important predictor of functional improvement in DME treatment that is probably a reason for the different outcomes reported [29]. Prospective data is also available and most studies reported no visual improvement despite a significant universal reduction in the CST [15, 17, 21]. A pos-hoc analysis of VIVID/VISTA trials reported that the rise in visual acuity is gradual, and visual acuity peak is only established after 6–9 months of treatment or longer [30].
This study reports no difference in FAZ area after 6 months treatment with IAI. Outcomes of other reports on effects of chronic anti-VEGF therapy on FAZ area are contradictory. Evidence regarding the ability of anti-VEGF agents to halt the progression of ischemic change is inconclusive. Some reports have asserted that continued anti-VEGF therapy can decrease the progression of the ischemic damage in patients with retinal microangiopathies [31, 32], while others have not notified any alterations [33, 34], and yet others have noted worsening of capillary drop out [35–38]. Even if anti-VEGF agents do reduce ischemic injury in the foveal area, this outcome might reach a ceiling effect with continuous treatment, and hence could explain why this study failed to show significant FAZ area changes in a patient population undergoing chronic anti-VEGF therapy.
Drawbacks of previous studies on the switching of patients to IAI include their retrospective design, variable follow up time, and a lack of protocol defined ETDRS BCVA and anatomic assessments. This prospective study allowed for entry criteria seen in clinical practice, and consistent evaluation of anatomical and visual outcomes. The limitations of this study include the small sample size, and lack of standardized treatment regimen prior to entry with other anti-VEGF drugs in addition to the short follow-up time.
Overall, this study begins to offer useful clinical insights into switching anti-VEGF medications followed by extended treatment interval IAI. Improved anatomic outcomes were achieved in this interim 6 month analysis, although visual outcomes were not statistically significant. The 12-month results of this study will be helpful in determining whether vision does indeed improve with continued treatment, and if fixed-dosing at a longer interval once macular fluid is resolved can sustain these positive effects.
Acknowledgements
Not applicable.
Abbreviations
- VEGF
vascular endothelial growth factor
- DME
diabetic macular edema
- FDA
food and drug administration
- CST
central subfield thickness
- PRN
pro ne rata
- TAE
treat-and-extend
- VA
visual acuity
- IAI
intravitreal aflibercept
- IRB
investigational review board
- DR
diabetic retinopathy
- SD-OCT
spectral-domain optical coherence tomography
- ETDRS
early treatment diabetic retinopathy study
- BCVA
best-corrected visual acuity
- CME
cystoid macular edema
- OCTA
optical coherence tomography angiography
- CPD
capillary perfusion density
- AEs
adverse events
- SD
standard deviation
- NPDR
non-proliferative diabetic retinopathy
- CV
cube volume
- CAT
cube average thickness
- FAZ
foveal avascular zone
- PLGF
placental growth factor
Authors’ contributions
ASB, RPS contributed to study concept and design. AR, AB, AY and RPS made substantial contributions to acquisition of data. TFC, FFC, contributed to the drafting of the manuscript. TFC, FFC, FQS contributed to the statistical analysis. ASB and RPS supervised the study. All authors contributed toχ the acquisition, analysis or interpretation of data and critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
Part of this study was supported by an unrestricted research grant from Regeneron Pharmaceuticals Inc.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Approved by Cleveland Clinic Investigational Review Board (IRB) and all patients signed an informed consent for their participation.
Consent for publication
Not applicable.
Competing interests
TFC, FFC, and FQS have no competing interest to disclosure; ASB: received grants from Regeneron, personal fee from VINDICO and MCME Global; AR: received personal fee from Alcon, Allergan, and Zeiss; AY: received grants from Regeneron; RPS: received grants and personal fees from Regeneron, Genentech/Roche, Apellis, Optos, Zeiss, Biogen, and Alcon/Novartis outside the submitted work.
Footnotes
Publisher's Note
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Med (United Kingdom) 2014;42(12):698–702. doi: 10.1016/j.mpmed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brit Y, Cunha-vaz JJ, Abreu JRFDE, et al. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975;59(11):649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol. 2010;88(3):279–291. doi: 10.1111/j.1755-3768.2008.01501.x. [DOI] [PubMed] [Google Scholar]
- 4.Trends G. American Society of Retinal Surgeons. Preferences and Trends (PAT) Survey. 2016.
- 5.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Diabetic Retinopathy Clinical Research Network, Writing Committee. Elman MJ, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banaee T, Ashraf M, Conti FF, Singh RP. Switching anti-VEGF drugs in the treatment of diabetic macular edema. Ophthal Surg Lasers Imagin Retin. 2017;48(9):748–754. doi: 10.3928/23258160-20170829-10. [DOI] [PubMed] [Google Scholar]
- 8.TDRCR N, Network TDRCR. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N Engl J Med. 2015;372(13):1193–1203. 10.1056/nejmoa1414264.aflibercept. [DOI] [PMC free article] [PubMed]
- 9.Boyer DS, Nguyen QD, Brown DM, Basu K, Ehrlich JS. Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology. 2015;122(12):2504–2513. doi: 10.1016/j.ophtha.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: The RESTORE extension study. Ophthalmology. 2014;121(5):1045–1053. doi: 10.1016/j.ophtha.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/S0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich R, Dan I, Deitch I, Axer-Siegel R, Mimouni K. The effectiveness of intravitreal ranibizumab in patients with diabetic macular edema who have failed to respond to intravitreal bevacizumab. Ophthalmologica. 2016;235(3):133–136. doi: 10.1159/000444103. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Lee WKKS. Short-term outcomes of switching to ranibizumab therapy. J Ocul Pharmacol Ther. 2016;32(10):659–664. doi: 10.1089/jop.2016.0074. [DOI] [PubMed] [Google Scholar]
- 14.Hanhart J, Chowers I. Evaluation of the response to ranibizumab therapy following bevacizumab treatment failure in eyes with diabetic macular edema. Case Rep Ophthalmol. 2015;6(1):44–50. doi: 10.1159/000375230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fechter C, Frazier H, Marcus WB, Farooq A, Singh HMD. Ranibizumab 0.3 mg for persistent diabetic macular edema after recent, frequent, and chronic bevacizumab: the ROTATE trial. Ophthal Surg Lasers Imagin Retin. 2016;47(11):1–18. doi: 10.3928/23258160-20161031-07. [DOI] [PubMed] [Google Scholar]
- 16.Lim LS, Wong EYMM, Yeo I, et al. Conversion to aflibercept for diabetic macular edema unresponsive to ranibizumab or bevacizumab. Clin Ophthalmol. 2015;9:1715–1718. doi: 10.2147/OPTH.S81523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood EH, Karth PA, Moshfeghi DM. Short-term outcomes of aflibercept therapy for diabetic macular edema in patients with incomplete response to ranibizumab and/or bevacizumab. Ophthal Surg Lasers Imagin Retin. 2015;46(9):950–954. doi: 10.3928/23258160-20151008-08. [DOI] [PubMed] [Google Scholar]
- 18.Klein KA, Cleary TS, Reichel E. Effect of intravitreal aflibercept on recalcitrant diabetic macular edema. Int J Retin Vitr. 2017;3(1):1–7. doi: 10.1186/s40942-017-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mira F, Paulo M, Henriques F, Figueira J. Switch to aflibercept in diabetic macular edema patients unresponsive to previous anti-VEGF therapy. J Ophthalmol. 2017. 10.1155/2017/5632634. [DOI] [PMC free article] [PubMed]
- 20.Rahimy E, Shahlaee A, Khan MA, et al. Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol. 2016;164(118–127):e2. doi: 10.1016/j.ajo.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Bahrami B, Hong T, Zhu M, Schlub TE, Chang A. Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefe’s Arch Clin Exp Ophthalmol. 2017;255(6):1133–1140. doi: 10.1007/s00417-017-3624-y. [DOI] [PubMed] [Google Scholar]
- 22.Do DV, Nguyen QD, Vitti R, et al. Intravitreal aflibercept injection in diabetic macular edema patients with and without prior anti-vascular endothelial growth factor treatment outcomes from the phase 3 program. Ophthalmology. 2016;123(4):850–857. doi: 10.1016/j.ophtha.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Katz G, Moisseiev E, Goldenberg D, et al. Ranibizumab for persistent diabetic macular edema after bevacizumab treatment. Eur J Ophthalmol. 2017;27(2):210–214. doi: 10.5301/ejo.5000838. [DOI] [PubMed] [Google Scholar]
- 24.Ashraf M, Souka AEH. Short-term effects of early switching to ranibizumab or aflibercept in diabetic macular edema cases with non-response to bevacizumab. Ophthal Surg Lasers Imagin Retin. 2017;48(3):230–236. doi: 10.1016/j.jenvman.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 25.Shah CP, Heier JS. Aflibercept for diabetic macular edema in eyes previously treated with ranibizumab and/or bevacizumab may further improve macular thickness. Ophthal Surg Lasers Imagin Retin. 2016;47(9):836–839. doi: 10.3928/23258160-20160901-06. [DOI] [PubMed] [Google Scholar]
- 26.Jampol LM, Glassman AR, Bressler NM, Wells JA, Ayala AR. Anti-vascular endothelial growth factor comparative effectiveness trial for diabetic macular edema. JAMA Ophthalmol. 2016;134(12):1429. doi: 10.1001/jamaophthalmol.2016.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto N, de Kozak Y, Jeanny JC, et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50(2):461–470. doi: 10.1007/s00125-006-0539-2. [DOI] [PubMed] [Google Scholar]
- 29.Lai I, Hsu W, Yang C, Hsieh Y. Prognostic factors of short-term outcomes of intravitreal ranibizumab in diabetic macular edema. Int J Ophthalmol. 2017;10(5):765–771. doi: 10.18240/ijo.2017.05.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziemssen F, Schlottman PG, Lim JI, Agostini H, Lang GE, Bandello F. Initiation of intravitreal aflibercept injection treatment in patients with diabetic macular edema: a review of VIVID-DME and VISTA-DME data. Int J Retin Vitr. 2016;2:1–7. doi: 10.1186/s40942-016-0041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill A, Cole ED, Novais EA, et al. Visualization of changes in the foveal avascular zone in both observed and treated diabetic macular edema using optical coherence tomography angiography. Int J Retin Vitr. 2017;3(1):19. doi: 10.1186/s40942-017-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121(9):1783–1789. doi: 10.1016/j.ophtha.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Michalska-Małecka K, Heinke Knudsen A. Optical coherence tomography angiography in patients with diabetic retinopathy treated with anti-VEGF intravitreal injections. Medicine (Baltimore) 2017;96(45):e8379. doi: 10.1097/MD.0000000000008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghasemi Falavarjani K, Iafe NA, Hubschman JP, Tsui I, Sadda SR, Sarraf D. Optical coherence tomography angiography analysis of the foveal avascular zone and macular vessel density after anti-VEGF therapy in eyes with diabetic macular edema and retinal vein occlusion. Investig Ophthalmol Vis Sci. 2017;58(1):30–34. doi: 10.1167/iovs.16-20579. [DOI] [PubMed] [Google Scholar]
- 35.Terui T, Kondo M, Sugita T, et al. Changes in areas of capillary nonperfusion after intravitreal injection of bevacizumab in eyes with branch retinal vein occlusion. Retina. 2011;31(6):1068–1074. doi: 10.1097/IAE.0b013e31820c83c2. [DOI] [PubMed] [Google Scholar]
- 36.Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013;120(4):795–802. doi: 10.1016/j.ophtha.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Sophie R, Hafiz G, Scott AW, et al. Long-term outcomes in ranibizumab-treated patients with retinal vein occlusion the role of progression of retinal nonperfusion. Am J Ophthalmol. 2013;156(4):693–705. doi: 10.1016/j.ajo.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feucht N, Schönbach EM, Lanzl I, Kotliar K, Lohmann CP, Maier M. Changes in the foveal microstructure after intravitreal bevacizumab application in patients with retinal vascular disease. Clin Ophthalmol. 2013;7(1):173–178. doi: 10.2147/OPTH.S37544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.