Abstract
Purpose
Prostate-specific membrane antigen (PSMA) PET/CT is increasingly used in patients with biochemically recurrent prostate cancer (BCR), mostly using gallium-68 (168Ga)-labelled radiotracers. Alternatively, fluorine-18 (18F)-labelled PSMA tracers are available, such as 18F-DCFPyL, which offer enhanced image quality and therefore potentially increased detection of small metastases. In this study we evaluate the lesion detection efficacy of 18F-DCFPyL PET/CT in patients with BCR and determine the detection efficacy as a function of their PSA value.
Methods
A total of 248 consecutive patients were evaluated and underwent scanning with 18F-DCFPyL PET/CT for BCR between November 2016 and 2018 in two hospitals in the Netherlands. Patients were examined after radical prostatectomy (52%), external-beam radiation therapy (42%) or brachytherapy (6%). Imaging was performed 120 min after injection of a median dose of 311 MBq 18F-DCFPyL.
Results
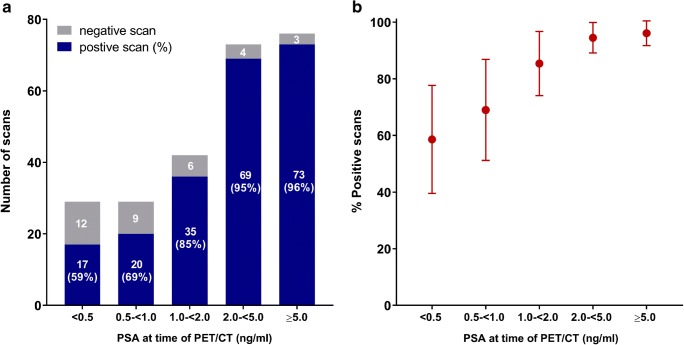
In 214 out of 248 PET/CT scans (86.3%), at least one lesion suggestive of cancer recurrence was detected (‘positive scan’). Scan positivity increased with higher PSA values: 17/29 scans (59%) with PSA values <0.5 ng/ml; 20/29 (69%) with PSA 0.5 to <1.0 ng/ml; 35/41 (85%) with PSA 1.0 to <2.0 ng/ml; 69/73 (95%) with PSA 2.0 to <5.0 ng/ml; and 73/76 (96%) with PSA ≥5.0 ng/ml. Interestingly, suspicious lesions outside the prostatic fossa were detected in 39–50% of patients with PSA <1.0 ng/ml after radical prostatectomy (i.e. candidates for salvage radiotherapy).
Conclusion
18F-DCFPyL PET/CT offers early detection of lesions in patients with BCR, even at PSA levels <0.5 ng/ml. These results appear to be comparable to those reported for 68Ga-PSMA and 18F-PSMA-1007, with potentially increased detection efficacy compared to 68Ga-PSMA for patients with PSA <2.0.
Electronic supplementary material
The online version of this article (10.1007/s00259-019-04385-6) contains supplementary material, which is available to authorized users.
Keywords: 18F-DCFPyL PET/CT, Biochemically recurrent, Prostate cancer, PSMA
Introduction
Prostate cancer (PCa) is the most common cancer in men in the Western world [1, 2]. Initial therapy includes local intervention with curative intent, such as radical prostatectomy (RP), external-beam radiation therapy (EBRT), or brachytherapy (BT). However, between 28% and 53% of all such treated patients will develop biochemically recurrent prostate cancer (BCR) [3]. BCR is defined as two consecutive prostate-specific antigen (PSA) values ≥0.2 ng/ml after RP, or any PSA increase of 2.0 ng/ml above the nadir following EBRT and BT [4–6]. Accurate imaging studies are important for patients with BCR, as early lesion localisation directs further treatment, which might include stereotactic metastasis-directed radiotherapy, salvage radiotherapy, salvage lymph node dissection, or the initiation of systemic treatment [3].
Positron emission tomography/computed tomography (PET/CT) using radiotracers that bind to the prostate-specific membrane antigen (PSMA) is increasingly used for PCa diagnostics. PSMA is a class II transmembrane glycoprotein that provides a valuable target for radiolabelled imaging, as it is significantly overexpressed in malignant prostate cells [7]. So far, studies have primarily investigated gallium-68 (68Ga)-labelled PSMA tracers (68Ga-PSMA-HBED-CC), which have demonstrated promising results in patients with BCR [8]. Alternatively, fluorine-18 (18F)-labelled PSMA tracers are available, most notably 18F-DCFPyL [9, 10] and 18F-PSMA-1007 [11]. Because of a shorter positron range and higher positron yield, the 18F radionuclide provides higher PET image resolution than 68Ga, which may improve the detection of small metastases (e.g. at low PSA values) [12]. Although 18F-DCFPyL PET/CT is increasingly used in clinical practice, only minimal data are yet available on the diagnostic efficacy in patients with BCR [13]. Hence, the aim of this study was to determine the lesion detection efficacy of 18F-DCFPyL PET/CT in patients with BCR and establish the efficacy as a function of patients’ current PSA values.
Material and methods
This retrospective analysis consists of 248 patients with BCR consecutively scanned with 18F-DCFPyL PET/CT from November 2016 until December 2018 in two Dutch hospitals (Amsterdam University Medical Centres, VU University; Noordwest Ziekenhuisgroep, Alkmaar). Patients with BCR were included regardless of prior curative treatment (RP, EBRT, BT). Patients with persistent PSA after curative treatment or patients with castration-resistant prostate cancer were excluded. If multiple 18F-DCFPyL PET/CT scans for BCR were performed for a patient, only the first examination was included. No exclusion criteria were deployed other than the coexistence of other malignancies. Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics
| Characteristics | n = 248 | |
|---|---|---|
| Age, years (median, IQR) | 71 (67–75) | |
| n (%) | ||
| PSA at PET/CT (ng/ml) | <0.5 | 29 (12%) |
| 0.5–1 | 29 (12%) | |
| 1–2 | 41 (17%) | |
| 2–5 | 73 (29%) | |
| >5 | 76 (31%) | |
| PSA doubling time (months) (median, IQR) | 6 (3–12) | |
| Gleason score | 6 | 33 (13%) |
| 7 | 97 (39%) | |
| 8 | 36 (15%) | |
| 9–10 | 48 (19%) | |
| Unknown | 34 (14%) | |
| Tumour stage | T1c | 16 (6%) |
| T2 | 82 (33%) | |
| T3 | 109 (44%) | |
| T4 | 7 (3%) | |
| Unknown | 34 (14%) | |
| Initial therapy | Radical prostatectomy | 128 (52%) |
| External-beam radiation | 105 (42%) | |
| Brachytherapy | 15 (6%) | |
| ADT at PET/CT | 20 (8%) | |
| Prior salvage radiation therapy | 41 (17%) | |
IQR = interquartile range
Imaging
18F-DCFPyL was synthesised under good manufacturing practice (GMP) conditions at the on-site cyclotron facilities of both hospitals [14]. PET acquisitions were made 120 min after injection of a median dose of 311 MBq 18F-DCFPyL (interquartile range 284–325 MBq). Imaging was performed with a Philips Ingenuity TF (Philips Healthcare, the Netherlands/USA) and a Siemens Biograph TruePoint-16 (Siemens Healthineers, Germany) PET/CT scanner. The scan trajectory included mid-thigh to skull vertex, with 4 min (Philips scanner) and 5 min (Siemens scanner) per bed position. PET acquisitions were combined with a low-dose CT or contrast-enhanced CT scan (30–110 mAs, 110–130 kV). All images were corrected for decay, scatter, and random coincidences; photon attenuation correction was performed using CT images.
Images were reconstructed with the vendor-provided BLOB-based ordered-subset expectation maximisation algorithm on the Philips system (3 iterations; 33 subsets) [15] and the ordered-subset expectation maximisation algorithm on the Siemens system (4 iterations; 16 subsets, including a 5-mm Gaussian filter). The reconstructed images had a maximum image matrix size of 256 × 256, voxel size 2.67 × 2.67 × 4 mm (Siemens data) and matrix size 288 × 288, voxel size 2 × 2 × 2 mm (Philips data).
Image interpretation
Scan interpretation was performed in the participating centres by four nuclear medicine physicians in total, with ample experience in PCa PET reading (>200 scans). Dual-reading was performed for all scans, the final conclusion was drawn up in consensus, recording the localisation of detected lesions (i.e. prostate/prostatic fossa, loco-regional lymph nodes, distant lymph nodes, bones, visceral organs). A scan was considered ‘positive’ if at least one lesion suggestive of PCa recurrence was detected. Prostate lesions and lymph nodes were considered positive when the activity in those lesions exceeded blood pool activity. Bone lesions were considered positive if the activity was higher than general bone marrow activity, without CT findings clearly demarcating benign lesions such as hemangioma.
Statistical analysis
Numerical variables were summarised as medians and interquartile ranges; categorical variables with proportions (%). Scan positivity was calculated for the following PSA strata (<0.5; 0.5 to <1.0; 1.0 to <2.0; 2.0 to <5.0; ≥5.0 ng/ml) and includes a 95% confidence interval (CI). Binary logistic regression analyses were performed to identify predictors of scan positivity (e.g. PSA value at the time of PET/CT, PSA doubling time, Gleason score, tumour stage, use of androgen deprivation therapy (ADT) at the time of PET/CT). Differences in the distribution of detected lesions (e.g. local recurrence, regional lymph nodes) between PSA strata were tested using Fisher’s exact test with Holm–Bonferroni correction.
Results
In 214 out of 248 18F-DCFPyL PET/CT scans (86.3%, 95% CI 82.0–90.6%), at least one lesion suggestive of PCa recurrence was detected (positive scan). Lesion detection increased with higher PSA values, with a detection efficacy of 59% (17/19 scans), 69% (20/29), 85% (35/41), 95% (69/73) and 96% (73/76), respectively, for scans at PSA values <0.5, 0.5 to <1.0, 1.0 to <2.0, 2.0 to <5.0 and ≥ 5.0 ng/ml (Figs. 1, 2). Excluding patients with incomplete clinical history (e.g. Gleason score, see Table 1) for the evaluation revealed similar results (Supplementary data 1). Aside from current PSA value, no other parameter (Gleason score, tumour stage, current ADT use, PSA-doubling time, previous salvage radiotherapy) was a significant predictor of 18F-DCFPyL PET/CT scan positivity in regression analysis (Supplementary data 2).
Fig. 1.
(a) Detection efficacy of 18F-DCFPyL PET/CT in patients with BCR and (b) the proportion of positive scans (including 95% confidence intervals)
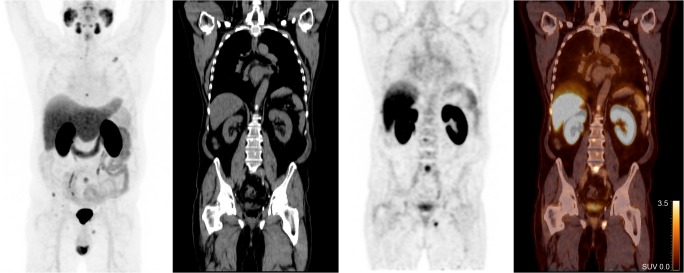
Fig. 2.
Illustration of detection of bone metastases in a patient with BCR after RP and low PSA value (0.7 ng/ml). Left: maximum-intensity projection. Right: coronal CT, PET and fusion images
Scans were most frequently positive due to detection of regional lymph node metastases (136/248 scans, 54.8%), followed by local recurrence in the prostate or prostatic fossa (92/248 scans, 37.1%); bone metastases (73/248, 329.4%); distant lymph node metastases (49/248, 19.8%); and visceral metastases (12/248, 4.8%) (Fig. 3, Supplementary data 3). The relative distribution of detected lesions was not significantly different at the various PSA strata (p = 0.22 to > 0.9, Supplementary data 4).
Fig. 3.
(a) Characteristics of the detected lesions in all patients with BCR (b) Characteristics of detected lesions in patients with BCR after radical prostatectomy, not yet treated with salvage radiotherapy
A subgroup analysis was performed for patients with BCR after RP and low PSA values (<1.0 ng/ml) (n = 43), as they may be candidates for curative salvage radiotherapy to the prostate bed [3]. In our cohort, 18F-DCFPyL PET/CT revealed suspicious lesions outside of the prostatic fossa in 39–50% of these patients (Figs. 2, 3).
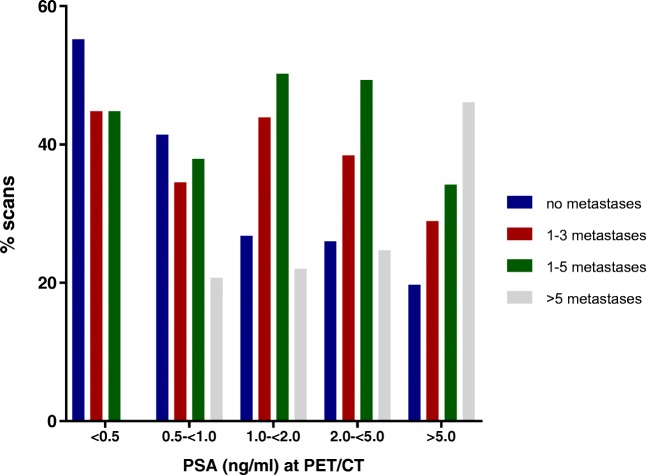
All patients who underwent 18F-DCFPyL PET/CT with a PSA <0.5 ng/ml revealed ≤3 metastases (‘oligometastatic disease’, Fig. 4). Conversely, in patients with a PSA ≥5.0 ng/ml, only 29% (22 out of 76) were diagnosed with 1–3 metastases (34% with 1–5 metastases) (Fig. 4).
Fig. 4.
Number of detected lesions per PSA stratum. Red and green bar indicating patients with oligometastases (defined as either ≤3 or ≤ 5 metastases, with or without a local recurrence). Grey bar representing patients with polymetastases (>5 metastases)
Discussion
In this study we evaluated the lesion detection efficacy of 18F-DCFPyL PET/CT in 248 patients with BCR. Suspected metastases were identified in the majority of PET examinations and even at PSA values <0.5 ng/ml, lesions were observed in 59% of patients. These results show clear improvement over the results with conventional imaging modalities, as well as choline PET/CT [16–18]. Compared to a recent meta-analysis on 68Ga-PSMA PET/CT [8], our study demonstrates equivalent results in patients with PSA values >2.0 ng/ml (i.e. 95% lesion detection with both tracers). Potentially improved detection is observed compared to 68Ga-PSMA in all PSA strata <2.0 ng/ml (most notably, 59% detection with 18F-DCFPyL when PSA <0.5 ng/ml vs. 45% with 68Ga-PSMA; Table 2) [8].
Table 2.
Comparison of results of various PSMA radiotracers
| Publication | Tracer | N | Detection rates per PSA strata (ng/ml) | |||
|---|---|---|---|---|---|---|
| <0.5 | 0.5 to <1.0 | 1.0 to <2.0 | ≥2.0 | |||
| Perera 2019 [8] | 68Ga-PSMA-11 | 4790 | 45% | 59% | 75% | 95% |
| <0.5 | 0.5 to <1.0 | 1.0 to <2.0 | ≥2.0 | |||
| Giesel 2018 [24] | 18F-PSMA-1007 | 251 | 62% | 74% | 91% | 94% |
| ≤0.5 | 0.51 to ≤1.0 | 1.1 to ≤2.0 | >2.0 | |||
| Rahbar 2018 [25] | 18F-PSMA-1007 | 100 | 86% | 89% | 100% | 100% |
| <0.5 | 0.5 to <1.0 | 1.0 to <2.0 | ≥2.0 | |||
| Present study 2019 | 18F-DCFPyL | 248 | 59% | 69% | 85% | 96% |
Recently revised European Association of Urology guidelines recommend performing PSMA PET/CT at BCR after RP if the PSA level is >0.2 ng/ml and if the results will influence subsequent treatment decisions [19]. PSMA PET/CT is recommended in case of BCR after EBRT in patients fit for curative salvage treatment. The guidelines also recommend offering a PSMA PET to men with a persistent PSA >0.2 ng/ml after prostatectomy to exclude metastatic disease. Those recommendations are in line with our findings that even at the lowest PSA values, sites of PCa recurrence could be detected with 18F-DCFPyL PET/CT in the majority of patients.
Further, 18F-DCFPyL PET/CT revealed lesions outside the prostatic fossa even at very low PSA levels (e.g. 39% with PSA <0.5 ng/ml) (Figs. 2, 3). These findings are clinically relevant, because patients with BCR after RP are candidates for local salvage radiation treatment to the prostatic fossa, and such treatment is likely to be ineffective in the presence of (lymph node) metastases. Indeed, Emmett et al. recently demonstrated that 68Ga-PSMA PET/CT may be effective for stratification of patients into groups with a high probability of response to salvage radiotherapy (negative PET or detection of local recurrence only) versus lower probability of response (detected lesions outside the prostatic fossa). In that study (n = 164), PSMA PET findings predicted salvage therapy outcomes better than established predictors, most notably current PSA values [20].
To our knowledge, there is only one prior study on 18F-DCFPyL PET/CT for patients with BCR, analysing n = 62 18F-DCFPyL PET/CT scans in direct comparison with 68Ga-PSMA PET/CT [13]. Dietlein et al. [13] found higher sensitivity with 18F-DCFPyL in patients with PSA values between 0.5–3.5 ng/ml (88% with 18F-DCFPyL versus 66% with 68Ga-PSMA, p = 0.042). Compared to Dietlein et al., we observed a higher detection efficacy, especially at PSA values <0.5 ng/ml (59% versus 13%, respectively). This difference might be due to the image interpretation protocol applied by Dietlein et al., which required corresponding findings on CT for all PET-positive lesions. Based on current standardised reporting systems, such a requirement is no longer applied for PSMA PET interpretation at our institutions [21, 22].
Recently, another 18F-labelled PSMA tracer was introduced: 18F-PSMA-1007 [23]. 18F-PSMA-1007 is only minimally excreted via the urinary tract, which potentially improves visualisation of tumour deposits adjacent to the urinary bladder (mainly local recurrence). Our results with 18F-DCFPyL PET/CT appear comparable to the outcomes of 251 18F-PSMA-1007 PET/CT scans analysed by Giesel et al. (e.g. 62% positive scans with 18F-PSMA-1007 when PSA <0.5 ng/ml [24]) (Table 2). Conversely, Rahbar et al. report notably more positive 18F-PSMA-1007 PET/CT scans compared to both Giesel et al. and the present study (86% positive scans when PSA <0.5 ng/ml; 100% positive scans when PSA >1.0 ng/ml [25]). Rahbar et al. included only 100 patients, however, and may have included more advanced PCa cases. For example, 38% of RP patients analysed by Rahbar et al. had already received salvage radiotherapy (compared to 17% in our cohort), and 27% of patients received ADT around the time of examination (compared to 8% in our cohort). In general, precise comparison of the results with different PSMA tracers (including 68Ga-PSMA) is difficult. Most studies are retrospective, and many factors other than the applied radiotracers may have influenced the diagnostic results (e.g. included patient population, PET scanner technique, image reconstruction methods, experience of the PET readers). In particular for cohorts including patients treated with EBRT, such as our cohort, somewhat higher detection rates are generally found in comparison with populations treated with RP only.
In terms of detection of local recurrence, no evident benefit of 18F-PSMA-1007 over 18F-DCFPyL is seen. Giesel et al. included only patients with BCR after RP and detected local recurrence in 25% of patients. Rahbar et al. noted local recurrence in 37% of all patients (treated with either EBRT or RP) [24, 25]. In our study, local recurrence was detected in 24% of all patients treated with RP and in 37% of the entire population.
Contrary to our finding that PSA at PET/CT is the only predictor of scan positivity, a recent study by Rauscher et al. found that concurrent ADT was also a predictor of a positive scan in multivariable analysis [26]. It should be noted that in our cohort, only 20 patients were receiving ADT at the time of PET/CT, the majority of whom received ADT in combination with EBRT; Rauscher et al. included only patients after RP, all with a PSA <1.0 ng/ml. Overall, the role of ADT in scan outcomes remains incompletely understood, for ADT may upregulate PSMA expression initially [27], but reduce lesion detection efficacy upon longer exposure [28]. Patients who received ADT after prostatectomy due to known metastatic disease without salvage options—in other words, in a palliative setting—were not included in this cohort. Although there is no definition as to where BCR ends in the course of prostate cancer progression, in our opinion, a cohort of patients with BCR should only include patients who are candidates for salvage options before PSMA PET/CT is performed.
Accurate identification of patients with oligometastatic disease is of current interest [29], although the definition of oligometastatic disease remains unclear (either ≤3 or ≤5 metastases). The clinical benefit of (oligo)metastases-directed treatment has not yet been established [3]. Nevertheless, it seems evident that any success of metastases-directed strategies will depend on accurate imaging studies, as well as adequate timing of diagnostic procedures. From our data it can be observed that performing 18F-DCFPyL PET/CT soon after diagnosis of BCR (i.e. at lower PSA values) favours detection of oligometastases (Fig. 4). At higher PSA levels (>5.0 ng/ml), more patients are diagnosed with polymetastases (>5). Yet even in these patients, a substantial proportion had oligometastastic disease on 18F-DCFPyL PET/CT (Fig. 4).
An important limitation of the present study, and studies on PSMA PET for BCR in general, is the lack of histopathologic confirmation of PET results [8, 12, 30]. PSMA PET-detected lesions are often smaller than 1 cm, making biopsy procedures difficult and burdensome for patients. In our study, histologic confirmation of PET findings (CT-guided biopsy, lymph node dissection, prostate biopsy) had been performed in only 15 of our patients, although follow-up of patients from outside our PET centres was often lacking. In 12 of these patients (80%), the malignant nature of detected lesions was confirmed. Overall, these numbers are insufficient to assess the extent of false-positive outcomes (the specificity) in our study. High specificity of PSMA PET has been demonstrated in primary PCa patients, however, where imaging findings can be correlated to histopathology from lymph node dissection (96% specificity with 68Ga-PSMA [30]; 96–99% specificity with 18F-DCFPyL [31]). We were also unable to evaluate the sensitivity of 18F-DCFPyL on a lesion basis, since the true number of PCa metastases remains unknown. PET is inherently limited by its image resolution to the detection of small metastases (<2–4 mm). We should therefore assume that 18F-DCFPyL PET/CT still underestimates the extent of disease.
Clinically, it is important to realise that improved detection of metastases is only of benefit to patients if followed by appropriate, proven-effective therapeutic strategies. The identification of additional metastases may delay treatment of local recurrences, trigger metastasis-directed treatment, or result in initiation of systemic therapy. None of the clinical outcomes of these (PSMA PET-based) decisions have yet been sufficiently studied in clinical trials [3, 29, 32].
Conclusion
18F-DCFPyL PET/CT appears effective for detecting metastases in patients with BCR, even at PSA values <0.5 ng/ml. The detection efficacy appears at least comparable to published results with 68Ga-PSMA and 18F-PSMA-1007.
Electronic supplementary material
(DOCX 36 kb)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective study was approved by the institutional review board of the Amsterdam Medical Centers (VU University) and Noordwest Ziekenhuisgroep; the need for written informed consent was waived (review number 2018.453).
Footnotes
This article is part of the Topical Collection on Oncology – Genitourinary.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. Wondergem and B.H.E. Jansen contributed equally to this work.
Contributor Information
M. Wondergem, Email: m.wondergem@nzw.nl
B. H. E. Jansen, Email: bh.jansen@vumc.nl
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165(4):1146–1151. doi: 10.1016/S0022-5347(05)66452-X. [DOI] [PubMed] [Google Scholar]
- 5.Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American urological association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177(2):540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 6.Roach M, 3rd, Hanks G, Thames H, Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Perner S, Hofer MD, Kim R, Shah RB, Li H, Moller P, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38(5):696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2019. 10.1016/j.eururo.2019.01.049. [DOI] [PubMed]
- 9.Chen Y, Pullambhatla M, Foss CA, Byun Y, Nimmagadda S, Senthamizhchelvan S, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pen tanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17(24):7645–7653. doi: 10.1158/1078-0432.CCR-11-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17(4):565–574. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016. 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed]
- 12.Rowe SP, Gorin MA, Allaf ME, Pienta KJ, Tran PT, Pomper MG, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016;19(3):223–230. doi: 10.1038/pcan.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietlein F, Kobe C, Neubauer S, Schmidt M, Stockter S, Fischer T, et al. PSA-stratified performance of 18F- and 68Ga-labeled tracers in PSMA-PET imaging of patients with biochemical recurrence of prostate cancer. J Nucl Med. 2016. 10.2967/jnumed.116.185538. [DOI] [PubMed]
- 14.Ravert HT, Holt DP, Chen Y, Mease RC, Fan H, Pomper MG, et al. An improved synthesis of the radiolabeled prostate-specific membrane antigen inhibitor, [(18) F]DCFPyL. J Labelled Comp Radiopharm. 2016;59(11):439–450. doi: 10.1002/jlcr.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popescu LM, Matej S, Lewitt RM, editors. Iterative image reconstruction using geometrically ordered subsets with list-mode data. In IEEE Symposium Conference Record Nuclear Science. 2004;6:3536-3540. 10.1109/NSSMIC.2004.1466649
- 16.Vargas HA, Martin-Malburet AG, Takeda T, Corradi RB, Eastham J, Wibmer A, et al. Localizing sites of disease in patients with rising serum prostate-specific antigen up to 1ng/ml following prostatectomy: how much information can conventional imaging provide? Urol Oncol. 2016;34(11):482 e5–482e10. doi: 10.1016/j.urolonc.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38(5):305–314. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 18.Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol) 2010;22(1):46–55. doi: 10.1016/j.clon.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Cornford P BJ, Bolla M, Briers E, De Santis M, Gross T. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer., https://uroweb.org/guideline/prostate-cancer/?type=summary-of-changes. 2019. [DOI] [PubMed]
- 20.Emmett L, van Leeuwen PJ, Nandurkar R, Scheltema MJ, Cusick T, Hruby G, et al. Treatment outcomes from (68)Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med. 2017;58(12):1972–1976. doi: 10.2967/jnumed.117.196683. [DOI] [PubMed] [Google Scholar]
- 21.Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59(3):469–478. doi: 10.2967/jnumed.117.198119. [DOI] [PubMed] [Google Scholar]
- 22.Rowe SP, Pienta KJ, Pomper MG, Gorin MA. Proposal for a structured reporting system for prostate-specific membrane antigen-targeted PET imaging: PSMA-RADS version 1.0. J Nucl Med. 2018;59(3):479–485. doi: 10.2967/jnumed.117.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44(4):678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giesel FL, Knorr K, Spohn F, Will L, Maurer T, Flechsig P, et al. Detection efficacy of [(18)F]PSMA-1007 PET/CT in 251 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2018. 10.2967/jnumed.118.212233. [DOI] [PMC free article] [PubMed]
- 25.Rahbar K, Afshar-Oromieh A, Seifert R, Wagner S, Schafers M, Bogemann M, et al. Diagnostic performance of (18)F-PSMA-1007 PET/CT in patients with biochemical recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(12):2055–2061. doi: 10.1007/s00259-018-4089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauscher I, Duwel C, Haller B, Rischpler C, Heck MM, Gschwend JE, et al. Efficacy, predictive factors, and prediction nomograms for (68)Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73(5):656–661. doi: 10.1016/j.eururo.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Wright GL, Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48(2):326–334. doi: 10.1016/S0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 28.Afshar-Oromieh A, Debus N, Uhrig M, Hope TA, Evans MJ, Holland-Letz T, et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(12):2045–2054. doi: 10.1007/s00259-018-4079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecouvet FE, Oprea-Lager DE, Liu Y, Ost P, Bidaut L, Collette L, et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC imaging group. Lancet Oncol. 2018;19(10):e534–ee45. doi: 10.1016/S1470-2045(18)30571-0. [DOI] [PubMed] [Google Scholar]
- 30.Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Meta-analysis of (68)Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med. 2018. 10.2967/jnumed.118.219501. [DOI] [PMC free article] [PubMed]
- 31.Progenics-Pharmaceuticals. Progenics Reports Results of Phase 2/3 Trial of PSMA PET Imaging Agent PyL for the Detection of Prostate Cancer. 2018. https://www.globenewswire.com/news-release/2018/10/05/1617353/0/en/Progenics-Reports-Results-of-Phase-2-3-Trial-of-PSMA-PET-Imaging-Agent-PyL-for-the-Detection-of-Prostate-Cancer.html
- 32.Fanti S, Minozzi S, Antoch G, Banks I, Briganti A, Carrio I, et al. Consensus on molecular imaging and theranostics in prostate cancer. Lancet Oncol. 2018;19(12):e696–e708. doi: 10.1016/S1470-2045(18)30604-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 36 kb)