Short abstract
Background
The role of allergy in breast cancer (BC) development remains inconclusive. A comprehensive review article is required to present and discuss all findings on this topic and to clarify the association between allergic disorders and the risk of BC.
Objective
We aimed to explain the association between atopy, different types of allergic disorders, and the risk of BC. Moreover, we explored the immunological mechanism behind this association.
Methods
We electronically reviewed publications in PubMed from 1979 to 2018 relating to atopy, allergy, asthma, atopic dermatitis, allergic rhinitis, food allergy, drug allergy, immunoglobulin E (IgE) or prick test, and BC.
Results
Most of the identified studies demonstrated nonsignificant results. However, the pattern of the results indicated an increased risk of BC in individuals with a history of allergies. The majority of studies reported higher prevalence of atopic dermatitis and allergic rhinitis among individuals with BC compared to the control groups. Similarity, most of the studies revealed an increased risk of BC among people with a positive history of atopic using IgE specific or prick test. However, a null association was reported in most of the asthmatic studies, and controversial results were detected in the individuals with history of food and drug allergies.
Conclusion
The majority of findings were not statistically significant. Moreover, bias and other methodological problems are the major issues, which make it challenging to compare the findings of different studies and reach a strong conclusive result. However, the pattern of the results from most studies indicated that allergic diseases might be associated with an increased risk of BC. Skewed immune system toward T-helper 2 might have an important role in this association.
Keywords: breast cancer, allergy, atopy, asthma, atopic dermatitis, allergic rhinitis, immunoglobulin E
Introduction
The dual role of allergy in cancer prevention or cancer development is highly controversial over the past few decades. Several hypotheses have been put forward to explicit the controversial findings.
It is proposed that allergic disorders could protect the body against cancer development via promoting the “immune surveillance,”1 which states that the immune system is able to detect and eliminate malignant cells more effectively in a hypersensitive state such as allergy. In immunoglobulin E (IgE)-mediated allergies, high level of IgE could bind to the tumor-specific antigens and facilitate the antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis mechanisms.2 Moreover, IgE could stimulate the production of reactive oxygen metabolites and nitric oxide, which are important in tumor eradication.3
Another theory which declares the protective role of allergic disorders in cancer development is prophylaxis theory.1,4 This theory explains that allergic reactions could prophylactically expel toxins, microorganisms, or environmental particles that may contain carcinogens from the body.5 The decreased risk of brain and lung cancers in some epidemiological studies supports this hypothesis.6–8
In contrast to the previous theories, the antigenic stimulation theory supports a positive association between history of allergic disorders and the risk of cancer. It suggests that inflammatory conditions associated with the allergic diseases may induce oxidative damage.9 The oxidative damage cause mutations in the genes that are involved in cell cycle, DNA damage repair, and apoptosis, increasing the risk of cancer development.1,10,11 Another recent hypothesis proposes that the recurrent injury and repair processes in the chronic inflammatory condition associated with an allergy could eventually skew T-lymphocyte response from T-helper 1 (Th1) toward Th2, which implies an important role in carcinogenesis.10 Th1 cells are associated with the suppression and eradication of tumor disease, and Th-2 cells are involved in the allergy and parasite diseases. Skewed immune system toward Th2 in the long term is associated with the suppression of immune system, which is involved in the tumor suppression. Th2 polarization could increase the systemic level of types 2 cytokines such as interleukin (IL)-4, IL-5, and IL-13 or cause the systemic suppression of interferon-γ, IL-12, and IL-18, which are important in tumor eradication.12
Recent studies demonstrated that the association between history of allergic disorders and the risk of cancer varied by types of cancer.13–16 An inverse association between allergy markers and risk of colorectal cancer, pancreatic cancer, and larynx cancer; a direct association for lymphoma, prostate cancer, and myeloma; and controversial results in breast cancer (BC) and lung cancer have been reported in several studies.10,16–18
In addition, the complex findings show that the allergy–cancer relationship is not only governed by the specific cancer site but also with the specific allergic disorders. For instance, the risk of lung cancer significantly increased in patients with asthma-specific history, but no strong evidence was reported between lung cancer and the other types of allergic conditions.13 Therefore, the role of different types of allergic disorders in each cancer type should be investigated specifically.
BC is the most commonly diagnosed cancer among women worldwide,19,20 which imposes high financial burden to health-care systems.21 A negative association,22,23 a positive association,24–26 and no association27 relating to the allergic disorders and risk of BC have been reported in the several studies. These conflicting results may be due to the pleiotropic nature of allergic immunity or different methods of measurement in the epidemiological studies. To best of our knowledge, no comprehensive study had been conducted to clarify the association between different types of allergic disorders and the risk of BC. Therefore, this review was designed to be more focus on BC and present and discuss all published epidemiological studies concerning the effect of atopy and different types of allergic disorders in the risk of BC. Moreover, we have tried to explore the immunological mechanism, mainly, the role of Th2-type immune system, behind this association.
History of Any Allergic Condition
A positive association between combined history of any allergic condition and risk of BC has been observed consistently in the cohort studies,25,26,28 although the results did not reach statistically significant (Table 1). The cohort of Sweden reported a history of any allergy could increase the risk of BC by 50% (standardized incidence rate [SIR]: 1.5; 95% confidence interval [CI], 0.8–2.6).28 Similarity, a slightly elevated risk of BC was detected in 2 cohort studies conducted in the United States.25,26
Table 1.
Studies of the Association Between History of Any Types of Allergic Disorder and the Risk of Breast Cancer.
| Studies | Country | Study Design | Sample Size | How Allergies Are Defined | RR/OR/SIR/HR/SMR (95% CI) | Main Finding | Reference Group | Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| Shirkhoda et al.29 | Iran | Case–control | Case (168)Control (165) | Physician-diagnosed or ISAAC questionnaire | OR: 1.0 (0.6–1.7) | No association | No history of allergy | Yesa |
| Lowcock et al.23 | Canada | Case–control | Case (3101)Control (3471) | Physician-diagnosed | All participant: OR: 0.9 (0.8–1.0)Premenopausal: OR: 0.9 (0.7–1.1)Postmenopausal: OR: 0.8 (0.7–1.0) | All participant: reduced riskPremenopausal: no associationPostmenopausal: reduced risk | No history of allergy | Yesb |
| Wang et al.16 | Germany | Case–control | Case (381)Control (2367) | Physician-diagnosed | OR: 1.1 (0.8–1.6) | No association | No atopy (no specific/total IgE positive) | Yesc |
| Hedderson et al.30 | USA | Case–control | Cases (723) Controls (958) | Physician-diagnosed | Overall: OR: 0.9 (0.8–1.1)Age <35 years: OR: 1.3 (0.9–1.8)Age >35 years: OR: 0.8 (0.6–0.1) | Overall: no associationAge <35 years: increased riskAge >35 years: reduced risk | No history of allergy | Age, smoking and education |
| Eriksson et al.28 | Sweden | Cohort | Participant with any allergy (N/S) | Self-reported symptoms | SIR: 1.5 (0.8–2.6) | Increased risk | Incidence rate in the general population in the country | No |
| Mills et al.26 | USA | Cohort | Participant with any allergy (N/S) | Self-reported | RR: 1.2 (0.9–1.6) | Increased risk | No history of allergy | Yesd |
| McWhorter25 | USA | Cohort | Participant with any allergy (N/S) | Physician-diagnosed | ROR: 1.2 (0.6–2.4) | Increased risk | No history of allergy | Yese |
Abbreviations: CI, confidence interval; HR, hazard ratio; IgE, Immunoglobulin E; ISAAC, International Study of Asthma and Allergies in Childhood; N/S, not specified; OR, odds ratio; ROR, risk OR; RR, relative risk; SIR, standardized incidence rate; SMR, standardized mortality ratio.
Bold values indicate statistical significance.
aAge, breast-feeding time (month), parity, family history of breast and ovarian cancer, and smoking.
bBody mass index, smoking, physical activity, education, income, country of birth, and ethnicity.
cAge, education, body mass index, family history of cancer, cigarette smoking and alcohol consumption, menopausal status, use of hormone replacement, and age at first full-term pregnancy.
dAge, age at menarche, first pregnancy, menopause, education, maternal history of breast cancer, smoking, and time since last physician contact.
eAge, race, sex, and smoking status; number of pregnancies; and Quetelet’s index.
In contrast, case–control studies did not clearly indicate a history of any allergic condition as a risk factor for BC developments. A case–control study designed in Canada found a significant small reduction in the risk of BC (odds ratio [OR]: 0.9; 95% CI, 0.8–1.0) among participant with history of any types of allergic disorders.23 The other case–control studies reported no association between asthma and the risk of BC.16,29,30
History of Asthma
With 1 exception,29 the epidemiological studies reported no strong association or a positive association between history of asthma and the risk of BC (Table 2).23,27,31–36
Table 2.
Studies of the Association Between History of Asthma and the Risk of Breast Cancer.
| Studies | Country | Study Design | Sample Size | How Allergies Are Defined | RR/OR/SIR/HR/SMR (95% CI) | Main Finding | Reference Group | Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| Shirkhoda et al.29 | Iran | Case–control | Case (168)Control (165) | Physician-diagnosed or ISAAC questionnairea | OR: 0.5 (0.2–1.0) | Reduced risk | No history of asthma | Yesb |
| Lowcock et al.23 | Canada | Case–control | Case (3101)Control (3471) | Physician-diagnosed | All participant: OR: 1.0 (0.9–1.2)Premenopausal: OR: 0.7 (0.5–1.0)Postmenopausal: OR: 1.1 (0.9–1.4) | All participant: no associationPremenopausal: reduced riskPostmenopausal: no association | No history of asthma | Yesc |
| Hwang et al.31 | Taiwan | Cohort | Participant with asthma (107 601) | Physician-diagnosed | SIR: 1.0 (0.9–1.1) | No association | Incidence rate in the general population in the country | Age |
| Ji et al.32 | Sweden | Cohort | Participant with asthma (140 425) | Subjects who had hospital admission for asthma | SIR: 1.0 (1.0–1.1) | No association | Incidence rate in the general population in the country (excluding asthmatic) | Yesd |
| Wang et al.16 | Germany | Case–control | Case (381)Control (2367) | Physician-diagnosed | OR: 1.2 (0.7–2.2) | Increased risk | No atopy (no specific/total IgE positive) | Yese |
| González-Pérez et al.33 | UK | Case–control | Case (827)Control (10 092) | Physician-diagnosed | OR: 0.9 (0.8–1.1) | No association | Incidence rate in the general population in the country (excluding asthmatic and COPD) | Age, BMI, smoking, alcohol intake, and certain comorbidities |
| Turner et al.34 | USA | Cohort | Participant with asthma (26 097) | Self-reported | RR: 1.1 (0.9–1.3) | No association | No history of asthma and allergic rhinitis | Yesf |
| Talbot-Smith et al.27 | Australia | Cohort | Women with asthma (155) | Physician-diagnosed | HR: 1.1 (0.5–2.6) | No association | N/S | Yesg |
| Eriksson et al.28 | Sweden | Cohort | Participant with asthma (2512) | Self-reported symptoms | SIR: 1.4 (0.6–2.7) | Increased risk | Incidence rate in the general population in the country | No |
| Vesterinen et al.35 | Finland | Cohort | Participant with asthma (78 000) | Physician-diagnosed | SIR: 1.0 (0.9–1.1) | No association | Incidence rate in the general population in the country | Age |
| Mills et al.26 | USA | Cohort | Participant with asthma (N/S) | Physician-diagnosed | RR: 1.2 (0.7–2.0) | Increased risk | No history of allergy | Yesh |
| Vena et al.36 | USA | Case–control | Case (1835)Control (2500) | Physician-diagnosed | OR: 1.0 | No association | N/S | Age and smoking |
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IgE, Immunoglobulin E; ISAAC, International Study of Asthma and Allergies in Childhood; N/S, not specified; OR, odds ratio; RR, relative risk; SIR, standardized incidence rate; SMR, standardized mortality ratio.
Bold values indicate statistical significance.
a“Have you had wheezing or whistling in the chest in the last 12 months?” OR “Did a doctor ever tell you that you had respiratory allergies?”
bAge, breast-feeding time (month), parity, family history of breast and ovarian cancer, and smoking.
cBMI, smoking, physical activity, education, income, country of birth, and ethnicity.
d5-year age, gender, period (5-year group), socioeconomic status, and residential area.
eAge, education, BMI, family history of cancer, cigarette smoking and alcohol consumption, menopausal status, use of hormone replacement, and age at first full-term pregnancy.
fRace, smoking, education, BMI, exercise, alcohol drinking, use of oral contraceptives, estrogen replacement therapy, age at first birth, age at menarche, age at menopause, height, and family history of breast cancer.
gAge, smoking status, BMI, number of pregnancies, and menopausal status.
hAge, age at menarche, first pregnancy, menopause, education, maternal history of breast cancer, smoking, and time since last physician contact.
A nonsignificant increased risk of BC among people with a history of physician-diagnosed asthma was observed in a case–control study in Germany (OR: 1.2; 95% CI, 0.7–2.2).16 Likewise, a cohort study designed in Sweden demonstrated 40% increased incidence risk of BC (SIR: 1.4; 95% CI, 0.6–2.7).28 In this cohort study, individuals who reported symptoms such as dyspnea or wheezing on exposure to allergens were classified as bronchial asthmatic participants. Another study conducted in the United States reported a nonsignificant positive association between BC incidence rate and history of physician-diagnosed asthma (relative risk [RR]: 1.2; 95% CI, 0.7–2.0).26
The other epidemiological studies reported no association between asthma and risk of BC. The asthmatic participants who were diagnosed by physician were included in these studies.23,27,31–36
History of Allergic Rhinitis
The majority of the identified studies indicated a higher prevalence of allergic rhinitis among BC patients compared to the general population (Table 3).
Table 3.
Studies of the Association Between History of AR and the Risk of Breast Cancer.
| Studies | Country | Study Design | Sample Size | How Allergies Are Defined | RR/OR/SIR/HR/SMR (95% CI) | Main Finding | Reference Group | Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| Shirkhoda et al.29 | Iran | Case–control | Case (168)Control (165) | Physician-diagnosed or ISAAC questionnairea | OR: 1.5 (0.7–3.0) | Increased risk | No history of AR | Yesb |
| Kozłowska et al.37 | Poland | Case–control | Case (231)Control (754) | Laryngological examinations, and history of clinical symptoms according to ARIA guidelines | Significant OR | Reduced risk | N/S | N/S |
| Hwang et al.31 | Taiwan | Cohort | Participant with AR (225 315) | Physician-diagnosed | SIR: 1.2 (1.1–1.3) | Increased risk | Incidence rate in the general population in the country | Age |
| Wang et al.16 | Germany | Case–control | Case (381)Control (4271) | Physician-diagnosed | OR: 1.2 (0.8–1.8) | Increased risk | No atopy (no specific/total IgE positive) | Yesc |
| Talbot-Smith et al.27 | Australia | Cohort | Participant with AR (597) | Physician-diagnosed | HR: 0.9 (0.5–1.7) | No association | N/S | Yesd |
| Eriksson et al.28 | Sweden | Cohort | Participant with AR (5006) | Self-reported symptoms | SIR: 1.5 (0.8–2.6) | Increased risk | Incidence rate in the general population in the country | No |
| Mills et al.26 | USA | Cohort | Participant with AR (N/S) | Self-reported | RR: 1.3 (1.0–1.9) | Increased risk | No history of allergy | Yese |
| Vena et al.36 | USA | Case–control | Case (1835)Control (2500) | Physician-diagnosed | OR: 0.9 | No association | N/S | Age and smoking |
Abbreviations: AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; CI, confidence interval; HR, hazard ratio; IgE, Immunoglobulin E; ISAAC, International Study of Asthma and Allergies in Childhood; N/S, not specified; OR, odds ratio; RR, relative risk; SIR, standardized incidence rate; SMR, standardized mortality ratio.
Bold values indicate statistical significance.
aAnswer yes to both “In the past 12 months, have you had a problem with sneezing, or a runny, or blocked nose when you DID NOT have a cold or the flu?” AND “In the past 12 months, has this nose problem been accompanied by itchy-watery eyes?”
bAge, breast-feeding time (month), parity, family history of breast and ovarian cancer, and smoking.
cAge, education, body mass index, family history of cancer, cigarette smoking and alcohol consumption, menopausal status, use of hormone replacement, and age at first full-term pregnancy.
dAge, smoking status, body mass index, number of pregnancies, and menopausal status.
eAge, age at menarche, first pregnancy, menopause, education, maternal history of breast cancer, smoking, and time since last physician contact.
Two cohort studies conducted in Taiwan31 and the United States26 found a history of diagnosed-physician rhinitis to be associated with an increased incidence rate of BC. In the cohort of the United States, the association was borderline statistical significance (RR: 1.3; 95% CI, 1.0–1.9). A Swedish cohort study, which considered rhinitis symptoms rather than physician-diagnosed, found a 50% increased incidence risk of BC (SIR: 1.5; 95% CI, 0.8–2.6).28 However, it was not statistically significant. Similarity, in our recent case–control study, we found 50% increased odds of BC (OR: 1.5; 95% CI, 0.7–3.0).29 We designed the first study that used International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire to consider the different types of allergic symptoms for better identification of patients with allergic rhinitis. Another case–control study in Germany found that physician-diagnosed positive history is associated with nonsignificant increased risk of BC (OR: 1.2, 95% CI, 0.8–1.8).16
Inconsistent results appeared from the recent study conducted in Poland, reporting allergic rhinitis was significantly less prevalent in patients with BC.37 However, the authors did not adjust the potential confounding factors. In their study, allergic rhinitis was defined by laryngological examinations and history of clinical symptoms according to Allergic Rhinitis and its Impact on Asthma guidelines.
In addition, some studies found no association between history rhinitis and BC. In Busselton’s cohort study (hazard ratio [HR]: 0.9; 95% CI, 0.51–1.7)27 and U.S. case–control study (OR: 0.9),36 no correlation reported between physician-diagnosed positive history and risk of BC.
History of Atopic Dermatitis
With 1 exception, epidemiological studies indicated that individuals with a history of atopic dermatitis have an elevated risk of BC, although all results did not reach statistically significant (Table 4).
Table 4.
Studies of the Association Between History of AD and the Risk of Breast Cancer.
| Studies | Country | Study Design | Sample Size | How Allergies Are Defined | RR/OR/SIR/HR/SMR (95% CI) | Main Finding | Reference Group | Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| Shirkhoda et al.29 | Iran | Case–control | Case (168)Control (165) | Physician-diagnosed or ISAAC questionnairea | OR: 1.3 (0.5–3.1) | Increased risk | No history of AD | Yesb |
| Hwang et al.31 | Taiwan | Cohort | Participant with AD (34 263) | Physician-diagnosed | SIR: 1.2 (0.8–1.6) | Increased risk | Incidence rate in the general population in the country | Age |
| Wang et al.16 | Germany | Case–control | Case (381)Control (4271) | Physician-diagnosed | OR: 1.0 (0.5–1.9) | No association | No atopy (no specific/total IgE positive) | Yesc |
| Olesen et al.38 | Denmark | Cohort | Participant with AD (2030) | Physician-diagnosed | SMR: 1.4 (0.7–2.7) | Increased risk | Incidence rate in the general population in the country | N/S |
Abbreviations: AD, atopic dermatitis; CI, confidence interval; HR, hazard ratio; IgE, Immunoglobulin E; ISAAC, International Study of Asthma and Allergies in Childhood; N/S, not specified; OR, odds ratio; RR, relative risk; SIR, standardized incidence rate; SMR, standardized mortality ratio.
Bold values indicate statistical significance.
aAnswered yes to both “Have you had itchy rash at any time in the last 12 months?” AND “Has this itchy rash at any time affected the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears or eyes?”
bAge, breast-feeding time (month), parity, family history of breast and ovarian cancer, and smoking.
cAge, education, body mass index, family history of cancer, cigarette smoking and alcohol consumption, menopausal status, use of hormone replacement, and age at first full-term pregnancy.
A cohort study conducted in Taiwan indicated a slightly increased risk of BC among subjects with a history of atopic dermatitis (SIR: 1.2; 95% CI, 0.8–1.6).31 In their study, dermatitis disease had been diagnosed by a specialist. Moreover, a Danish cohort population-based study demonstrated 40% increased cancer prevalence among hospitalized children with atopic dermatitis (standardized mortality ratio: 1.4; 95% CI, 0.7–2.7).38 A similar finding was observed in our recent case–control study.29 We found an increased risk of BC among subjects who were diagnosed dermatitis by a doctor or have dermatitis symptoms according to ISAAC quesionnire (OR: 1.3; 95% CI, 0.5–3.1).
In contrast, a population-based case–control study in Germany found no association between physician-diagnosed atopic dermatitis and BC (OR: 1.0; 95% CI, 0.5–1.9).16
History of Food and Drug Allergy
To date, few studies have investigated the association between history of food and drug allergies and the risk of BC, with inconsistency results (Table 5).
Table 5.
Studies of the Association Between History of Food or Drug Allergy and the Risk of Breast Cancer.
| Studies | Country | Study Design | Sample Size | How Allergies Are Defined | RR/OR/SIR/HR/SMR (95% CI) | Main Finding | Reference Group | Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| Food allergy | ||||||||
| Shirkhoda et al.29 | Iran | Case–control | Case (168)Control (165) | Physician-diagnosed or Self-reported | OR: 1.4 (0.7–3.0) | Increased risk | No history of food allergy | Yesa |
| Hedderson et al.30 | USA | Case–control | Cases (723)Controls (958) | Physician-diagnosed | Overall: OR: 0.8 (0.6–1.2)Age <35 years: OR: 1.2 (0.7–2.1)Age >35 years: OR: 0.7 (0.5–1.0) | Overall: reduced risk Age <35 years: increased riskAge >35 years: reduced risk | No history of allergy | Age, smoking and education |
| Drug allergy | ||||||||
| Shirkhoda et al.29 | Iran | Case–control | Case (168)Control (165) | Physician-diagnosed or Self-reported | OR: 0.6 (0.2–1.8) | Reduced risk | No history of drug allergy | Yesa |
| Hedderson et al.30 | USA | Case–control | Cases (723)Controls (958) | Physician-diagnosed | Overall: OR: 1.0 (0.8–1.3)Age <35 years: OR: 1.3 (0.9–2.1)Age >35 years: OR: 0.8 (0.6–1.2) | Overall: no associationAge <35 years: increased riskAge >35 years: reduced risk | No history of allergy | Age and education |
| Mills et al.26 | USA | Cohort | Participant with drug allergy (N/S) | Self-reported | RR: 1.0 (0.7–1.3) | No association | No history of allergy | Yesb |
Abbreviations: CI, confidence interval; HR, hazard ratio; N/S, not specified; OR, odds ratio; RR, relative risk; SIR, standardized incidence rate; SMR, standardized mortality ratio.
Bold values indicate statistical significance.
aAge, breast-feeding time (month), parity, family history of breast and ovarian cancer, and smoking.
bAge, age at menarche, first pregnancy, menopause, education, maternal history of breast cancer, smoking, and time since last physician contact.
A population-based case–control study in the United States found lower prevalence of doctor-diagnosed food allergy among BC patients compared to the control group (OR: 0.8; 95% CI, 0.6–1.2).30 However, a different pattern was observed among women 35 years or younger (OR: 1.2; 95% CI, 0.7–2.1) and women older than 35 years (OR: 0.7; 95% CI, 0.5–1.0) in the stratified analysis.30 The authors considered “individuals with no history of any allergy” as the reference group in the case–control analysis, which was different from our recent study conducted in Iran.29 In our case–control study, individuals with no history of food allergy was considered as the reference group, and we found that food allergy prevalence was higher among BC patients (OR: 1.4; 95% CI, 0.7–3.0), although it was not statistically significant, which might be due to small sample size.29
Of the identified studies, which investigate the relationship between a history of drug allergy and BC, 2 studies found no association26,30 and 1 study reported a nonsignificant decreased risk29 (Table 5).
A population-based case–control study30 and a population-based cohort study26 conducted in the United States reported no association between BC and physician-diagnosed drug allergy with OR: 1.0; 95% CI, 0.7–1.3 and RR: 1.0; 95% CI, 0.7–1.3, respectively. However, our recent case–control study in Iran found a history of drug allergy was associated with a nonsignificant decreased risk of BC (OR: 0.6; 95% CI, 0.2–1.8).29
Total IgE Level
The studies, which investigated the association between total IgE level and risk of BC, had varying outcomes (Table 6). Two studies found a positive association,29,39 1 study reported an inverse association,40 and 2 studies demonstrated no association.9,41
Table 6.
Studies of the Association Between Total IgE and the Risk of Breast Cancer.
| Studies | Country | Study Design | Sample Size | The Defined Cutoff | RR/OR/SIR/HR/SMR (95% CI) | Main Finding | Reference Group | Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| Shirkhoda et al.29 | Iran | Case–control | Case (168)Control (165) | 25 IU/mL | OR: 1.6 (0.9–2.7) | Increased risk | IgE level< 25 IU/mL | Yesa |
| Zhang et al.40 | China | Case–control | Case (102)Control (100) | 32.6 IU/mL | OR: 0.5 (0.3–0.7) | Reduced risk | IgE level< 32.6 IU/mL | N/S |
| Taghizadeh et al.9 | Netherland | Cohort | Participant with high total IgE level (N/S) | Continues variable | HR: 0.9 (0.5–1.6) | No association | N/S | Yesb |
| Vijayakumar et al.39 | India | Case–control | Case (166)Control (100) | Continues variable | Case: Mean ± SD: 65.4 ± 31.4 Control: Mean ± SD: 9.4 ± 8.4 | Increased risk | --- | No |
| Alsabti41 | Jordan | Cross-sectional | Case (31)Control (50) | Continues variable | Case: Mean ± SD: 29.5 ± 0.8Control: Mean ± SD: 31.2 ± 0.8 | No association | --- | No |
Abbreviations: CI, confidence interval; HR, hazard ratio; IgE, Immunoglobulin E; N/S, not specified; OR, odds ratio; RR, relative risk; SD, standard deviation; SIR, standardized incidence rate; SMR, standardized mortality ratio.
Bold values indicate statistical significance.
aAge, breast-feeding time (month), parity, family history of breast and ovarian cancer, and smoking.
bAge, body mass index (all at the first survey), and place of residence.
Two case–control studies conducted in India39 and Iran29 found an association between total IgE level and increased risk of BC. In the Indian study, total IgE level significantly elevated in BC and the levels were found to increase with clinical stage.39 In our recent study, total IgE level above 25 IU/mL was determined as borderline significantly associated with BC in univariate analysis (OR: 1.5; 95% CI, 1–2.3).29 However, the association was not significant in multivariate analysis.
Inconsistent results appeared from the study in China, indicating a significantly reduced risk of BC cancer among participant with high IgE level (OR: 0.5; 95% CI, 0.3–0.7).40 However, a cross-sectional study conducted in Jordan41 and a cohort study in Netherland9 detected no difference association between the level of total IgE and BC.
Specific IgE or Prick Test
Most studies revealed an increased risk of BC among people with a positive history of atopic using IgE specific or prick test (Table 7).
Table 7.
Studies of the Association Between Specific IgE and Prick Test and the Risk of Breast Cancer.
| Studies | Country | Study Design | Types of Allergen | Sample Size | The Defined Cutoff | RR/OR/SIR/HR/SMR (95% CI) | Main Finding | Reference Group | Confounding Factors |
|---|---|---|---|---|---|---|---|---|---|
| Specific IgE | |||||||||
| Skaaby et al.43 | Denmark | Cohort | 19 common inhalant allergens, mite, cat, grass, and birch | Patients with specific IgE positivity (14 849) | Specific IgE level ≥0.35 kU/l | HR: 0.3 (0.1–0.8) | Reduced risk | Mortality rate in the general population in the country | Yesa |
| Petridou et al.42 | Greece | Case–control | 12 most prevalent allergens in Greece | Case (103)Control (103) | Specific IgE level ≥ 0.35 kU/l | OR: 1.7 (1.0–3.1) | Increased risk | N/S | Yesb |
| Wang et al.16 | Germany | Case–control | Pollen of timothy, rye, birch and mugwort, house dust mite, cat, dog, and Cladosporium | Case (381)Control (2367) | Specific IgE level ≥ 0.35 kU/l | OR: 1.2 (0.9–1.7) | Increased risk | No atopy (no specific/total IgE positive) | Yesc |
| Patch/prick test | |||||||||
| Taghizadeh et al.9 | Netherland | Cohort | House dust, mixed pollen, epidermal products, and mixed molds | Participant with patch test positivity (N/S) | Positive patch test | Cancer hospitalization: HR: 1.0 (0.5–2.0) | Cancer hospitalization: no association | N/S | Yesd |
| Engkilde et al.44 | Denmark | Cohort | 23 allergens contained in the European Standard Screening Tray | Participant with patch test positivity (6065) | Positive patch test | OR: 0.8 (0.7–1.0) | Reduced risk | N/S | Age |
| Talbot-Smith et al.27 | Australia | Cohort | House dust mites, cat dander, cattle dander, 2 molds (Aspergillus fumigatus and Alternariae tenius), and 7 local pollens (rye grass, barley, capeweed, orchard grass, plantago, peppermint tree, couchweed grass, and wild oat) | Participant with prick test positivity (239) | Positive prick test | HR: 1.4 (0.6–3.4) | Increased risk | N/S | Yese |
| Eriksson et al.28 | Sweden | Cohort | Dermatophagoides pteronyssinus, horse, dog, cat, timothy, mugwort, birch, and Cladosporium | Participant with prick test positivity (2435) | Positive prick test | SIR: 2.5 (1.0–5.2) | Increased risk | Incidence rate in the general population in the country | No |
Abbreviations: CI, confidence interval; HR, hazard ratio; IgE, Immunoglobulin E; N/S, not specified; OR, odds ratio; RR, relative risk; SIR, standardized incidence rate; SMR, standardized mortality ratio.
Bold values indicate statistical significance.
aEducation, season of blood sample, physical activity, smoking habits, alcohol intake, body mass index, systolic and diastolic blood pressure, serum triglycerides, and total cholesterol.
bAge, education, height, age at menarche, parity, age at menopause, and alcohol consumption.
cAge, education, body mass index, family history of cancer, cigarette smoking and alcohol consumption, menopausal status, use of hormone replacement, and age at first full-term pregnancy.
dAge, body mass index (all at the first survey), and place of residence.
eAge, smoking status, body mass index, number of pregnancies, and menopausal status.
Two case–control studies designed in Greece42 and Germany16 found high risk of BC in relation to the positive IgE specific with OR of 1.7, 95% CI, 1.0–3.1 and 1.2, 95% CI, 0.9–1.7, respectively. However, 1 population-based prospective study in Denmark reported no association (HR: 1.00; 95% CI, 0.73–1.37).43 In all studies, the cutoff value for positivity of the specific IgE was considered as ≥0.35 kU/L.
In term of prick test, a cohort study designed in Sweden28 reported a significantly elevated risk of BC among individuals with positive patch test (SIR: 2.5; 95% CI, 1.0–5.2) (Table 7). Likewise, the cohort study in Australia reported nonsignificant high risk of BC (HR: 1.4; 95% CI, 0.6–3.4).27 However, a significantly reduced risk of BC was reported in the study conducted in Denmark (OR: 0.8; 95% CI, 0.7–1.0).44 Beside, a population-based cohort study in Netherlands showed no association between any types of atopic diseases and cancer hospitalization patients (OR: 1.0; 95% CI, 0.5–2.0).9
Discussion
Most of the identified studies demonstrated nonsignificant results. However, the pattern of the results indicated an increased risk of BC in individuals with history of allergies. The majority of studies reported a higher prevalence of atopic dermatitis and allergic rhinitis among individuals with BC compared to the control groups. Similarity, most of the studies revealed an increased risk of BC among people with a positive history of atopic using IgE specific or prick test. However, a null association was reported in most of the asthmatic studies, and controversial results were detected in the individuals with history of food and drug allergies.
One of the possible immunological mechanisms behind the role of allergy in cancer is related to the chronic inflammation in allergic disorders, which could directly or indirectly lead to cancer development and progression.45 In addition, the polarization of T cells toward Th2 in allergic diseases has been demonstrated to be pro-carcinogenesis. Previous studies reported that the epithelial cells in the atopic dermatitis secrete high level of thymic stromal lymphopoietin (TSLP).46 TSLP is an IL-17 like cytokine, which activates dendrite cells (DCs) migration to the draining lymph nodes, differentiates T naive cells into the inflammatory Th2, and initiates the secretion of type 2 cytokines.46,47 Studies demonstrated that TSLP fosters human breast tumor growth by promoting inflammation47,48 and provides a suitable environment for the establishment, growth, and metastasis of the primary BC cell line via Th2 polarization.49 Antibodies neutralizing TSLP or TSLP receptor inhibit tumor development and the secretion of IL-13 in the BC xenografts model. In addition, systemic increased types 2 cytokines such as IL-4, IL-5, and IL-13 due to Th2 polarization can contribute tumorgenesis in several ways. For instance, IL-13 induces myeloid cells to release transforming growth factor beta (TGF-β), which ultimately inhibits cytotoxic T cell activity.50
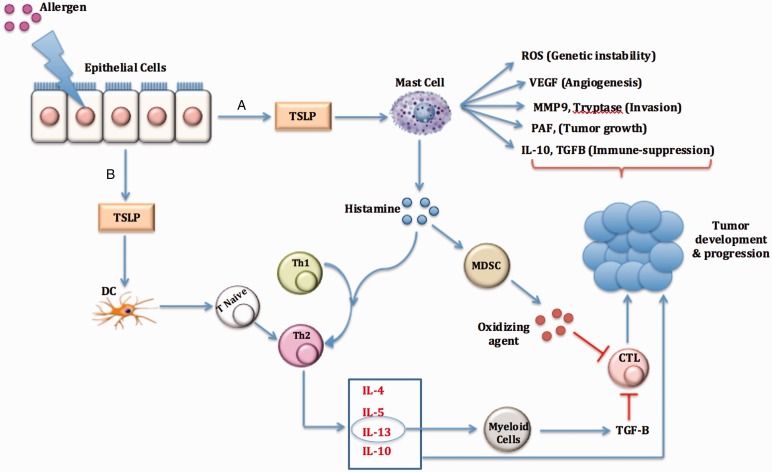
Another possible mechanism behind the association of allergy–cancer is mast cells. Mast cells are the prominent cells in the immediate-type allergic reactions that reside skin and mucous membranes.51,52 The role of mast cells in cancer development or protection is controversy over the studies. However, in vitro and in vivo studies support that mast cells could induce carcinogenicity through releasing several factors involved in gene instability (reactive oxygen species), angiogenesis (vascular endothelial growth factor), extracellular matrix degradation (Protease), immune suppressive (TGF-β and IL-10), and shifting immune system balance toward Th2 pathways (Figure 1).53 Effective mast cell targeting immunotherapy will shift the balance toward promoting the antitumor activities of mast cells.
Figure 1.
An overview on the role of allergy-related mechanisms in cancer development and progression. Epithelial cells in allergic disease secrete high level of TSLP, which lead to cancer initiation and progression by effecting mast cell (A) or DC (B). CTL, cytotoxic T cell; DC, dendrite cell; IL, interleukins; MDSC, myeloid-derived suppressor cells; MMP9, matrix metalloproteinase 9; PAF, platelet-activating factor; ROS, reactive oxygen species; TGF-β, transforming growth factor beta; Th, T-helper cells; TSLP, thymic stromal lymphopoietin; VEGF, vascular endothelial growth factor.
Moreover, mast cells release several mediators such as histamine, prostaglandins, and leukotrienes. Histamine, the main mediator in the allergic disorders, plays an important role in attracting DC and skewing immune system toward Th2.54 In the mammary gland, histamine plays a critical role in growth regulation, differentiation and functioning during development, pregnancy, and lactation.55–57 High level of histamine production and secretion has been found in human breast tumors and induced mammary tumors in rats.58–61 In addition, histamine elevates the production of IL-10 and reduces the secretion of IL-12,62 which have a beneficial effects on cancer development and progression.
In another route, mast cell–histamine promotes survival and proliferation of myeloid-derived suppressor cells (MDSCs).63 Histamine can alter the cytokine milieu, transcription factors, and signaling pathways important for MDSCs accumulation. The role of MDSCs in pro-tumorgenesis has been well defined before. MDSCs release small soluble oxidizers, impair T cell antigen recognition, and ultimately cause the suppression of T cell activity and cancer development.63–65
There are important issues in the interpretation of epidemiological studies, such as how the allergic disorders are defined in different studies (by a physician, by questionnaire, standardized, or self-reported?). In this review, we demonstrated that most of the identified studies reported no association between asthma and risk of BC, in contrast to the atopic dermatitis and allergic rhinitis. It is worth mentioning that the most impressive misclassification was predicted to appear in the asthma-specific studies due to overlapping between asthma and other chronic lung diseases. Moreover, the variable etiology of asthma, which may involve a number of immunologic and nonimmunologic factors, including allergy could affect the final results in the asthmatic studies.66
The other issue is immunosuppressive and anti-inflammatory treatment in many allergic disorders. Studies reported long-term risk of malignancy among patients treated with immunosuppressive.67 Moreover, animal models suggest that some antihistamines might be carcinogenic and increase the risk of cancer.68 This confounding factor was less considered in the epidemiological studies, as the treatment of allergy may be critically associated with the overall risk of cancer development in allergic people.
Moreover, the disparity in the methodology of epidemiological studies such as lack of adjustment for other possible confounding or inadequate statistical power and the design of study make it challenging to compare different studies and reach a strong conclusive result. No particular difference was found between the cohort finding and the case–control results. However, an important factor, which was neglected in the interpretation of results, is that the reference group in the statistically analysis is more variable among studies, and it affects the final results for the OR in the studied patients. Therefore, considerable caution is needed in interpreting the results of epidemiological studies.
In this article, we have tried to comprehensively review the studies reporting the associations between different types of allergy and BC. However, it is not systemic review and might be susceptible to bias. Further research should reduce the heterogeneity of the studies and include high-quality evidence in order to eliminate bias and other methodological problems.
Conclusion
Bias is considered as a major issue in the observational studies, therefore interpreting of the results should be done carefully. Moreover, there are different methodological problems in different studies, which make it challenging to compare the findings and reach a strong conclusive result. However, the pattern of the results from the most studies indicates that allergic diseases might be associated with an overall risk for BC, and skewed immune system toward Th2 implies an important role in this association.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by our institutional review board.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Statement of Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Statement of Informed Consent
There are no human subjects in this article and informed consent is not applicable.
References
- 1.Sherman PW, Holland E, Sherman JS. Allergies: their role in cancer prevention. Q Rev Biol. 2008; 83(4):339–362. [DOI] [PubMed] [Google Scholar]
- 2.Jensen-Jarolim E, Achatz G, Turner M, et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008; 63(10):1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ockert D, Schmitz M, Hampl M, Rieber EP. Advances in cancer immunotherapy. Immunol Today. 1999; 20(2):63–65. [DOI] [PubMed] [Google Scholar]
- 4.Hsiao J-R, Ou C-Y, Lo H-I, et al. Allergies and risk of head and neck cancer: an original study plus meta-analysis. PLoS One. 2013; 8(2):e55138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991; 66(1):23–62. [DOI] [PubMed] [Google Scholar]
- 6.Brenner AV, Linet MS, Fine HA, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002; 99(2):252–259. [DOI] [PubMed] [Google Scholar]
- 7.Castaing M, Youngson J, Zaridze D, et al. Is the risk of lung cancer reduced among eczema patients? Am J Epidemiol. 2005; 162(6):542–547. [DOI] [PubMed] [Google Scholar]
- 8.Wigertz A, Lönn S, Schwartzbaum J, et al. Allergic conditions and brain tumor risk. Am J Epidemiol. 2007; 166(8):941–950. [DOI] [PubMed] [Google Scholar]
- 9.Taghizadeh N, Vonk JM, Hospers JJ, et al. Objective allergy markers and risk of cancer mortality and hospitalization in a large population-based cohort. Cancer Causes Control. 2015; 26(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Josephs DH, Spicer JF, Corrigan CJ, Gould HJ, Karagiannis SN. Epidemiological associations of allergy, IgE and cancer. Clin Exp Allergy. 2013; 43(10):1110–1123. [DOI] [PubMed] [Google Scholar]
- 11.Zheng SL, Liu W, Wiklund F, et al. A comprehensive association study for genes in inflammation pathway provides support for their roles in prostate cancer risk in the CAPS study. Prostate. 2006; 66(14):1556–1564. [DOI] [PubMed] [Google Scholar]
- 12.Kool M, Hammad H, Lambrecht BN. Cellular networks controlling Th2 polarization in allergy and immunity. F100 Bio Rep. 2012; 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Y, Hill AW. Atopy and specific cancer sites: a review of epidemiological studies. Clin Rev Allergy Immunol. 2016; 51(3):338–352. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy. 2006; 60(9):1098–1111. [DOI] [PubMed] [Google Scholar]
- 15.Vojtechova P, Martin RM. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer Causes Control. 2009; 20(7):1091–1105. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Rothenbacher D, Löw M, Stegmaier C, Brenner H, Diepgen TL. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int J Cancer. 2006; 119(3):695–701. [DOI] [PubMed] [Google Scholar]
- 17.Olson SH, Hsu M, Satagopan JM, et al. Allergies and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Case-Control Consortium. Am J Epidemiol. 2013; 178(5):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prizment AE, Folsom AR, Cerhan JR, Flood A, Ross JA, Anderson KE. History of allergy and reduced incidence of colorectal cancer, Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2007; 16(11):2357–2362. [DOI] [PubMed] [Google Scholar]
- 19.Sadeghi F, Ardestani A, Hadji M, et al. Travel burden and clinical profile of cancer patients admitted to the Cancer Institute of Iran in 2012. Arch Iran Med. 2017; 20(3):147–152. [PubMed] [Google Scholar]
- 20.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 21.Ansaripour A, Zendehdel K, Tadayon N, Sadeghi F, Uyl-de Groot CA, Redekop WK. Use of data-mining to support real-world cost analyses: an example using HER2-positive breast cancer in Iran. PLoS One. 2018; 13(10):e0205079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalczewska ME, Brożyna A, Jóźwicki W, et al. Analysis of the involvement of cytokines in allergy and breast cancer association. Contemp Oncol. 2014; 18(6):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowcock EC, Cotterchio M, Ahmad N. Association between allergies, asthma, and breast cancer risk among women in Ontario, Canada. Cancer Causes Control. 2013; 24(5):1053–1056. [DOI] [PubMed] [Google Scholar]
- 24.Chae YK, Neagu S, Kim J, Smyrlis A, Gooptu M, Tester W. Association between common allergic symptoms and cancer in the NHANES III female cohort. PLoS One. 2012; 7(9):e42896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McWhorter WP. Allergy and risk of cancer. A prospective study using NHANESI followup data. Cancer. 1988; 62(2):451–455. [DOI] [PubMed] [Google Scholar]
- 26.Mills PK, Beeson WL, Fraser GE, Phillips RL. Allergy and cancer: organ site-specific results from the Adventist Health Study. Am J Epidemiol. 1992; 136(3):287–295. [DOI] [PubMed] [Google Scholar]
- 27.Talbot-Smith A, Fritschi L, Divitini ML, Mallon DF, Knuiman MW. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. Am J Epidemiol. 2003; 157(7):606–612. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson NE, Holmen A, Högstedt B, Mikoczy Z, Hagmar L. A prospective study of cancer incidence in a cohort examined for allergy. Allergy. 1995; 50(9):718–722. [DOI] [PubMed] [Google Scholar]
- 29.Shirkhoda M, Ghiasvand R, Sadeghi F, et al. Association between different types of allergy disorders, total immunoglobulin E and risk of breast cancer. Biol Forum Int J. 2017;9(2):280–286. [Google Scholar]
- 30.Hedderson MM, Malone KE, Daling JR, White E. Allergy and risk of breast cancer among young women (United States). Cancer Causes Control. 2003; 14(7):619–626. [DOI] [PubMed] [Google Scholar]
- 31.Hwang CY, Chen YJ, Lin MW, et al. Cancer risk in patients with allergic rhinitis, asthma and atopic dermatitis: a nationwide cohort study in Taiwan. Int J Cancer. 2012; 130(5):1160–1167. [DOI] [PubMed] [Google Scholar]
- 32.Ji J, Shu X, Li X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in hospitalised asthma patients. Br J Cancer. 2009; 100(5):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González-Pérez A, Fernández-Vidaurre C, Rueda A, Rivero E, García Rodríguez LA. Cancer incidence in a general population of asthma patients. Pharmacoepidemiol Drug Saf. 2006; 15(2):131–138. [DOI] [PubMed] [Google Scholar]
- 34.Turner MC, Chen Y, Krewski D, Ghadirian P, Thun MJ, Calle EE. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol. 2005; 162(3):212–221. [DOI] [PubMed] [Google Scholar]
- 35.Vesterinen E, Pukkala E, Timonen T, Aromaa A. Cancer incidence among 78,000 asthmatic patients. Int J Epidemiol. 1993; 22(6):976–982. [DOI] [PubMed] [Google Scholar]
- 36.Vena JE, Bona JR, Byers TE, Middleton E, Jr, Swanson MK, Graham S. Allergy-related diseases and cancer: an inverse association. Am J Epidemiol. 1985; 122(1):66–74. [DOI] [PubMed] [Google Scholar]
- 37.Kozłowska R, Bożek A, Jarząb J. Association between cancer and allergies. Allergy Asthma Clin Immunol. 2016; 12(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olesen AB, Engholm G, Storm HH, Thestrup-Pedersen K. The risk of cancer among patients previously hospitalized for atopic dermatitis. J Inves Dermat. 2005; 125(3):445–449. [DOI] [PubMed] [Google Scholar]
- 39.Vijayakumar T, Ankathil R, Remani P, Sasidharan VK, Vijayan KK, Vasudevan DM. Serum immunoglobulins in patients with carcinoma of the oral cavity, uterine cervix and breast. Cancer Immunol Immunother. 1986; 22(1):76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Guo G, Jianzhong C, Zheng Y. Decreased level of IgE is associated with breast cancer and allergic diseases. Med Sci Monit. 2016; 22:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alsabti EA. Serum immunoglobulins in breast cancer. J Surg Oncol. 1979; 11(2):129–133. [DOI] [PubMed] [Google Scholar]
- 42.Petridou ET, Chavelas C, Dikalioti SK, et al. Breast cancer risk in relation to most prevalent IgE specific antibodies: a case control study in Greece. Anticancer Res. 2007; 27(3B):1709–1713. [PubMed] [Google Scholar]
- 43.Skaaby T, Husemoen LLN, Thuesen BH, Hammer-Helmich L, Linneberg A. Atopy and cause specific mortality. Clin Exp Allergy. 2014; 44(11):1361–1370. [DOI] [PubMed] [Google Scholar]
- 44.Engkilde K, Thyssen JP, Menné T, Johansen JD. Association between cancer and contact allergy: a linkage study. BMJ Open. 2011: 1:e000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed AB, Zidi S, Sghaier I, et al. Common variants in IL-1RN, IL-1β and TNF-α and the risk of ovarian cancer: a case control study. Cent Eur J Immunol. 2017; 42(2):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y-J, Soumelis V, Watanabe N, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007; 25:193–219. [DOI] [PubMed] [Google Scholar]
- 47.Pedroza-Gonzalez A, Xu K, Wu T-C, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011; 208(3):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olkhanud PB, Rochman Y, Bodogai M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. 2011; 186(10):5656–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erdmann RB, Gartner JG, Leonard WJ, Ellison CA. Lack of functional TSLP receptors mitigates Th2 polarization and the establishment and growth of 4T1 primary breast tumours but has different effects on tumour quantities in the lung and brain. Scand J Immunol. 2013; 78(5):408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berzofsky JA, Terabe M. A novel immunoregulatory axis of NKT cell subsets regulating tumor immunity. Cancer Immunol Immunother. 2008; 57(11):1679–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang B, Lei Z, Zhang G-M, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008; 112(4):1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller HRP. Mucosal mast cells and the allergic response against nematode parasites. Vet Immunol Immunopathol. 1996; 54(1-4):331–336. [DOI] [PubMed] [Google Scholar]
- 53.Varricchi G, Galdiero MR, Loffredo S, et al. Are mast cells MASTers in cancer? Front Immunol. 2017; 8:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jelinek I, László V, Buzás E, et al. Increased antigen presentation and Th1 polarization in genetically histamine-free mice. Int Immunol. 2006; 19(1):51–58. [DOI] [PubMed] [Google Scholar]
- 55.Kierska D, Fogel WA, Maslinski C. Histamine concentration and metabolism in mouse mammary gland during the estrous cycle. Inflamm Res. 1997; 46(13):63–64. [DOI] [PubMed] [Google Scholar]
- 56.Wagner W, Ichikawa A, Tanaka S, Panula P, Fogel WA. Mouse mammary epithelial histamine system. J Physiol Pharmacol. 2003; 54(2):211–223. [PubMed] [Google Scholar]
- 57.Maslinski C, Kierska D, Fogel WA, Kinnunen A, Panula P. Histamine: its metabolism and localization in mammary gland. Comp Biochem Physiol C. 1993; 105(2):269–273. [DOI] [PubMed] [Google Scholar]
- 58.Rivera ES, Cricco GP, Engel NI, Fitzsimons CP, Martín GA, Bergoc RM. Histamine as an autocrine growth factor: an unusual role for a widespread mediator. Semin Cancer Biol. 2000; 10:15–23. [DOI] [PubMed] [Google Scholar]
- 59.Reynolds J, Akhter J, Magarey C, Schwartz P, Adams WJ, Morris DL. Histamine in human breast cancer. Br J Surg. 1998; 85(4):538–541. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Caballero M, Neugebauer E, Rodriguez F, Nuñez de Castro I, Vara-Thorbeck C. Histamine synthesis and content in benign and malignant breast tumours. Its effects on other host tissues. Surg Oncol. 1994; 3(3):167–173. [DOI] [PubMed] [Google Scholar]
- 61.Sieja K, Stanosz S, von Mach-Szczypiński J, Olewniczak S, Stanosz M. Concentration of histamine in serum and tissues of the primary ductal breast cancers in women. Breast. 2005; 14(3):236–241. [DOI] [PubMed] [Google Scholar]
- 62.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001; 108(12):1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin RK, Saleem SJ, Folgosa L, et al. Mast cell histamine promotes the immunoregulatory activity of myeloidygulator suppressor cells. J Leukoc Biol. 2014; 96(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12(4):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walankiewicz M, Grywalska E, Polak G, Kotarski J, Siwicka-Gieroba DJ, Roliński J. Myeloid-derived suppressor cells in ovarian cancer: friend or foe? Cen Euro J Immunol. 2017; 42(4):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002; 53(1):477–498. [DOI] [PubMed] [Google Scholar]
- 67.Kempen JH, Gangaputra S, Daniel E, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol. 2008; 146(6):802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brandes LJ, Warrington RC, Arron RJ, et al. Enhanced cancer growth in mice administered daily human-equivalent doses of some H1-antihistamines: predictive in vitro correlates. J Natl Cancer Ins. 1994; 86(10):770–775. [DOI] [PubMed] [Google Scholar]