Abstract
Cancer-related cognitive impairment (CRCI) adversely affects cancer patients. We had previously demonstrated that the BDNF Val66Met genetic polymorphism is associated with lower odds of subjective CRCI in the multitasking and verbal ability domains among breast cancer patients receiving chemotherapy. To further assess our previous findings, we evaluated the association of BDNF Val66Met polymorphism with subjective and objective CRCI in a temporally separate cohort of patients and pooled findings from both the original (n = 145) and current (n = 193) cohorts in a meta-analysis. Subjective CRCI was assessed using FACT-Cog. Objective CRCI was evaluated using computerized neuropsychological tests. Genotyping was carried out using Sanger sequencing. The association of BDNF Val66Met genotypes and CRCI was examined with logistic regression. A fixed-effect meta-analysis was conducted using the inverse variance method. In the meta-analysis (n = 338), significantly lower odds of CRCI were associated with Met allele carriers based on the global FACT-Cog score (OR = 0.52, 95% CI 0.29–0.94). Furthermore, Met allele carriers were at lower odds of developing impairment in the domains of memory (OR = 0.34, 95% CI: 0.17–0.70), multitasking (OR = 0.33, 95% CI: 0.18–0.59), and verbal ability (OR = 0.46, 95% CI: 0.24–0.88). Consistent with the previous study, lower odds of subjective CRCI among patients with the BDNF Met allele was observed after adjusting for potential confounders in the multitasking (OR = 0.30, 95% CI: 0.14–0.67) domain. In conclusion, carriers of the BDNF Met allele were protected against global subjective CRCI, particularly in the domains of memory, multitasking, and verbal ability. Our findings further contribute to the understanding of CRCI pathophysiology.
Electronic supplementary material
The online version of this article (10.1007/s12035-018-1410-4) contains supplementary material, which is available to authorized users.
Keywords: Cancer-related cognitive impairment, BDNF, Genetic polymorphism, Breast cancer, Chemotherapy
Introduction
Commonly known in literature as “chemobrain” or “chemofog,” subtle yet notable alterations in cognitive function are often observed in breast cancer patients receiving chemotherapy [1]. Manifesting as both patient-reported subjective complaints and objective changes detected by neuropsychological tests, cancer-related cognitive impairment (CRCI) has been reported to include memory loss, concentration deficit, and the decreased ability to multitask [2]. Evidence has shown that CRCI negatively affects the quality of life of cancer patients and the ability to cope with demands in their daily lives [3]. As its etiology is not yet fully understood, CRCI remains a subject of significant research. Ongoing work has suggested possible factors that may influence the risk of CRCI, such as pro-inflammatory cytokines, psychosocial determinants including anxiety and fatigue, and numerous genetic markers [4–6]. Among candidate genes that have been investigated are COMT, APOE, and BDNF [6, 7].
The BDNF gene expresses brain-derived neurotrophic factor (BDNF), which is a neurotrophic factor vital for neuronal survival, growth, and neural circuit maintenance [8]. The BDNF Val66Met single nucleotide polymorphism (SNP), which leads to substitution of valine with methionine at codon 66, is a functional polymorphism widely studied in neurological conditions such as schizophrenia and Parkinson’s disease [9, 10]. Our research group has discovered that the BDNF Val66Met polymorphism is associated with a lower risk of developing self-perceived CRCI in breast cancer patients [7], where carriers of the Met allele had lower odds of reporting subjective CRCI in the cognitive domains of verbal ability (OR = 0.34, 95% CI = 0.12–0.90) and multitasking (OR = 0.37, 95% CI = 0.15–0.91). However, other studies have suggested that carrying the Met allele may be associated with poorer perseveration, verbal memory abilities, and task switching [11]. Therefore, whether carriers of the Met allele are truly protected against cognitive decline remains controversial, implying that the BDNF Val66Met polymorphism may contribute to varying cognitive function [11].
As false positives are commonly observed in genetic association studies [12], further replication attempts are required to confirm associations that were initially observed, in order to provide stronger evidence on the impact of genetic determinants on CRCI. A deeper understanding of these genetic factors will allow the identification of cancer patients at a higher risk of CRCI for potential interventions. Therefore, in this study, we aim to evaluate the association of BDNF Val66Met polymorphism with subjective and objective CRCI in a temporally separate cohort of patients and pool findings from both the original and current cohorts in a meta-analysis.
Methods
Study Design
This was a multicenter, prospective cohort study conducted at three ambulatory cancer centers between February 2014 and December 2017 in Singapore. This study was approved by SingHealth Institutional Review Board (CIRB2014/754/B) and written informed consent was obtained from all patients.
Study Population
Eligible participants must fulfill the following inclusion criteria: (i) at least 21 years old, (ii) diagnosed with stages I to III breast cancer, (iii) scheduled to receive chemotherapy, (iv) has no prior history of chemotherapy and/or radiotherapy, (v) able to read and understand either English or Mandarin, and (vi) has Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1.
Patients were excluded from the study if they were (i) incapable of providing verbal/written consent or (ii) diagnosed with neuropsychiatric disorders and/or brain metastasis that might result in poor cognitive function.
Study Procedures
Upon recruitment, demographic data and clinical information of participants were collected via patient interviews and from electronic medical records. Participants were prospectively evaluated at three time points: before start of chemotherapy (T1), 6 weeks after start of chemotherapy (T2), and 12 weeks after start of chemotherapy (T3). At each time point, participants completed assessments of both subjective and objective CRCI. In addition, health-related quality of life, anxiety, and fatigue were assessed using self-administered questionnaires. English and Chinese versions of each study tool were available. All assessments took approximately 45 min to complete and were conducted by trained interviewers.
Assessment of Subjective Cognitive Impairment
The Functional Assessment of Cancer Therapy–Cognitive Function (FACT-Cog) version 3 was used to evaluate patients’ self-perceived CRCI within the past 7 days [13]. FACT-Cog comprises 37 items in 6 domains of cognitive disturbances, which are mental acuity, concentration, memory, verbal ability, functional interference, and multi-tasking ability. Each item is evaluated on a 5-point Likert scale. Both English and Chinese versions of FACT-Cog have been validated and demonstrated satisfactory psychometric properties [14].
Subjective CRCI is defined as a reduction of at least 10.6 points in the FACT-Cog total score at T2 or T3 compared to baseline based on a previously determined minimal clinically important difference (MCID). Decline in a particular cognitive domain is defined as a reduction of at least 15% from a participant’s baseline score at T2 or T3 [15].
Assessment of Objective Cognitive Impairment
Objective cognitive assessment was carried out using the Cambridge Neuropsychological Test Automated Battery (CANTAB), a language-independent neuropsychological testing research software. In this study, the CANTAB test battery contained five tests: reaction time (RTI), paired associates learning (PAL), spatial working memory (SWM), attention switching task (AST), and rapid visual information processing (RVP) that assessed response speed, learning and memory, working memory, multitasking, and sustained attention, respectively, yielding a total of nine measures. The direction of one measure, A′, was reversed so higher scores indicate poorer cognitive performance for all measures. These tests have been validated and have shown sensitivity to capturing alterations in neuropsychological performance [16–18].
Reliable change indices (RCI) were computed to reflect cognitive changes in participants. RCI were obtained by subtracting CANTAB scores at T2 or T3 from baseline scores, adjusting for practice effects and dividing by the standard error of difference. Practice effects and standard error of difference were estimated from a control population using similar testing intervals. Objective cognitive decline is defined as an RCI of less than − 2 at either T2 or T3.
Assessment of Fatigue
Fatigue was evaluated with the Brief Fatigue Inventory (BFI) [19]. BFI measures the severity of fatigue and the impact of fatigue on daily functioning in the past 24 h on a numerical scale of 0 to 10. Six aspects of daily functioning were assessed: general activity, mood, walking ability, normal work, relations with other people, and enjoyment of life. A higher score indicates greater level of fatigue.
Assessment of Anxiety
The Beck Anxiety Inventory (BAI) was employed to measure anxiety in participants [20, 21]. BAI is a validated questionnaire consisting of 21 items describing subjective, somatic, or panic-related symptoms of anxiety on a scale of 0 to 3. A higher total score indicates greater level of anxiety.
Assessment of Insomnia
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) assesses health-related quality of life (HRQoL). In this study, we focused on the single-item scale rating insomnia, which is measured on a 4-point Likert scale. A higher score indicates increased severity of insomnia. Both English and Chinese versions of QLQ-C30 have been validated for use in cancer patients in Singapore [22, 23].
Genotyping
At baseline, a 10-ml blood sample was collected from participants in an ethylene diamine tetraacetic acid (EDTA) tube and centrifuged at 2500 rpm for 10 min within 40 min of collection. The buffy coat was extracted and at stored at − 80 °C until analysis.
Using QIAamp DNA Blood Mini Kit (QIAGEN), genomic DNA from the buffy coat was isolated. The region with the BDNF Val66Met polymorphism was amplified via polymerase chain reaction (PCR) using the following specific and optimized primers: 5′-GGACTCTGGAGAGCGTGAA-3′ (forward) and 5′-CGTGTACAAGTCTGCGTCCT-3′ (reverse). Genotyping of the PCR products was subsequently conducted by AITbiotech employing automated Sanger sequencing with a 3730xl DNA Analyzer (Applied Biosystems). AITbiotech was blinded to clinical outcomes of participants. To ensure quality control, genotyping was done for both the forward and reverse DNA strands.
Statistical Analysis
All statistical analyses were conducted with STATA Version 15 (StataCorp 2017). Descriptive statistics were used to summarize demographics and clinical characteristics of participants. Deviation of genotypes from Hardy-Weinberg equilibrium was assessed using chi-squared test with one degree of freedom. Evaluation of the associations between the BDNF Val66Met polymorphism and CRCI was done using logistic regression assuming a dominant model. Potential confounders age, race, menopausal status, chemotherapy regimens, years of education, and additionally for subjective CRCI, anxiety, depression, and insomnia were adjusted for [24, 25]. Sensitivity analyses was performed assuming a general genetic model with each genotype classified as a distinct class. To examine the relationship between anxiety and fatigue with BDNF genotype, linear mixed-effect models were employed with the presence of Met allele and time incorporated as fixed effects and intercepts varied as a random effect by each subject. To combine findings from both the original and current cohort, adjusted odds ratios from both studies were pooled in a fixed-effect meta-analysis using the inverse variance method. All statistical tests were two-sided, and p values less than 0.05 were considered statistically significant.
Sample Size Calculation
Sample size calculation was performed using Quanto 1.2.4. In our original study, statistically significant association of CRCI with BDNF genotype was observed for the cognitive domains of verbal ability (OR = 0.34) and multitasking (OR = 0.37) [7]. The latter, which yielded a smaller effect size, was used for sample size estimation in this study. Based on an expected allelic frequency of 0.5 in a dominant model and predicted prevalence of impairment at 0.3 [7], a total of 167 participants is required to yield statistical power of 80% and type 1 error of 5%. Anticipating an attrition rate of 20%, a final sample size of 209 was targeted.
Results
Patient Characteristics
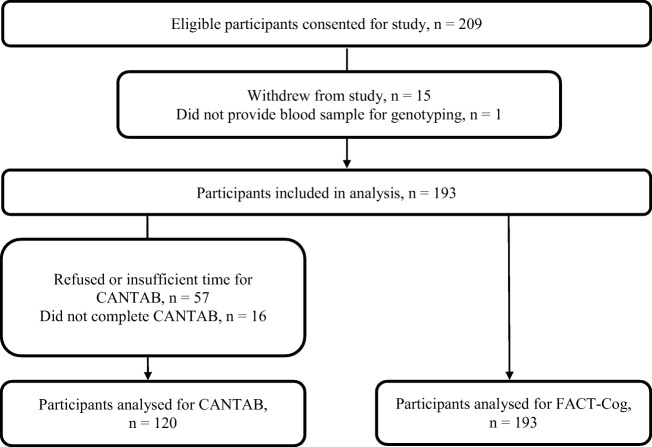
A total of 209 patients were recruited. However, 15 participants withdrew from the study (2 patients refused chemotherapy and 13 declined to complete study procedures) and 1 patient did not provide blood samples for genotyping. Therefore, 193 participants were included in the final analysis (Fig. 1). The demographic characteristics of patients who dropped out and those who remained in the study did not differ significantly. The mean (±SD) age of participants was 51.9 ± 8.9 years old. Majority of the participants were of Chinese ethnicity (79.8%) and had at least high school education (84.5%). More than half received radiotherapy (66.3%), underwent mastectomy (63.2%), and completed anthracycline-based chemotherapy (64.8%). Demographic and clinical characteristics of participants in both the current and original cohorts are comparable (Table 1).
Fig. 1.
Study flow diagram
Table 1.
Comparison of demographic and clinical characteristics of participants in the current cohort and original cohort
| Current cohort | Original cohort | |
|---|---|---|
| n = 193 | n = 145 | |
| Demographic characteristics | ||
| Age in years, mean (SD) | 51.9 (8.9) | 50.8 (8.8) |
| Ethnicity, n (%) | ||
| Chinese | 154 (79.8) | 119 (82.1) |
| Malay | 19 (9.8) | 15 (10.3) |
| Indian | 13 (6.7) | 7 (4.8) |
| Others | 7 (3.6) | 4 (2.8) |
| Education level, n (%) | ||
| Primary school | 29 (15.0) | 22 (15.2) |
| High school | 90 (46.6) | 70 (48.3) |
| Pre-university | 35 (18.1) | 29 (20.0) |
| Graduate/postgraduate | 38 (19.7) | 24 (16.6) |
| Unknown | 1 (0.5) | 0 (0.0) |
| Menopausal status, n (%) | ||
| Premenopausal | 95 (49.2) | 74 (51.0) |
| Postmenopausal | 98 (50.8) | 71 (49.0) |
| Clinical characteristics | ||
| Cancer staging, n (%) | ||
| Stage I | 27 (14.0) | 32 (22.1) |
| Stage II | 127 (65.8) | 71 (49.7) |
| Stage III | 39 (20.2) | 41 (28.3) |
| Radiotherapy, n (%) | 128 (66.3) | Not reported |
| Surgery, n (%) | Not reported | |
| Lumpectomy | 71 (36.8) | |
| Mastectomy | 122 (63.2) | |
| Chemotherapy, n (%) | ||
| Anthracycline-based | 125 (64.8) | 94 (64.8) |
| Non anthracycline-based | 68 (35.2) | 51 (35.2) |
| Behavioral symptoms | ||
| Baseline fatigue, mean (SD) | 1.6 (1.9) | 1.6 (1.7) |
| Baseline anxiety, mean (SD) | 6.9 (7.2) | 6.7 (6.1) |
| Baseline insomnia, mean (SD) | 22.8 (26.8) | 23.1 (26.9) |
Genotype and Allele Frequencies
All participants included in the final analysis were successfully genotyped for the BDNF Val66Met polymorphism. Val/Val and Met/Met homozygous genotypes accounted for 26.9% and 20.7% of the observed genotypes, respectively, while the remaining 52.3% comprised the heterozygous genotype. Val and Met allele frequencies were approximately equivalent. No deviation from Hardy-Weinberg equilibrium was detected whether allele frequencies were pooled or stratified by ethnicity (Table 2).
Table 2.
Genotype and allele frequencies of participants (n = 193)
| Genotype/allele | Ethnic subpopulation, n (%) | Pooled, n (%) | |||
|---|---|---|---|---|---|
| Chinese | Malay | Indian | Othersb | ||
| Total | 154 | 19 | 13 | 7 | 193 |
| Genotype | |||||
| GG (Val/Val) | 35 (22.7) | 10 (52.6) | 4 (30.8) | 3 (42.9) | 52 (26.9) |
| GA (Val/Met) | 84 (54.6) | 6 (31.6) | 7 (53.8) | 4 (57.1) | 101 (52.3) |
| AA (Met/Met) | 35 (22.7) | 3 (15.8) | 2 (15.4) | 0 (0.0) | 40 (20.7) |
| Allele | |||||
| G (Val) allele | 154 (50.0) | 26 (68.4) | 15 (57.7) | 10 (71.4) | 205 (53.1) |
| A (Met) allele | 154 (50.0) | 12 (31.6) | 11 (42.3) | 4 (28.6) | 181 (46.9) |
| p valuea | 0.26 | 0.24 | 0.71 | 0.29 | 0.48 |
ap values of Chi-square tests to assess deviation from Hardy-Weinberg equilibrium
b“Others” include Sri Lankan, Filipino, and Burmese
Prevalence of Subjective and Objective Cognitive Impairment
A total of 193 participants completed FACT-Cog evaluation and 60 patients (31.1%) reported subjective CRCI (Table 3). Among specific cognitive domain, the highest proportion of participants reporting cognitive decline was observed in the mental acuity (28.5%) domains, followed by concentration (28.0%), multi-tasking (25.9%), verbal ability (20.2%), functional interference (19.2%), and memory (17.6%).
Table 3.
Proportion of participants with CRCI
| Proportion of participants, n (%) | |
|---|---|
| Subjective CRCI (n = 193) | |
| Summation score | 60 (31.1) |
| Cognitive domains | |
| Memory | 34 (17.6) |
| Verbal ability | 39 (20.2) |
| Concentration | 54 (28.0) |
| Mental acuity | 55 (28.5) |
| Functional interference | 37 (19.2) |
| Multitasking | 50 (25.9) |
| Decline in at least 1 domain | 88 (45.6) |
| Objective CRCI (n = 120) | |
| Individual test measures | |
| RTI – Five choice reaction time | 17 (14.2) |
| PAL – Total error (adjusted) | 15 (12.5) |
| SWM – Between errors | 5 (4.2) |
| SWM – Strategy | 15 (12.5) |
| AST – Switching cost | 3 (2.5) |
| AST – Congruency cost | 3 (2.5) |
| AST – Reaction latency | 8 (6.7) |
| RVP – A′ | 8 (6.8) |
| RVP – Latency | 20 (16.7) |
| Cognitive domains | |
| Response speed | 17 (14.2) |
| Learning and memory | 15 (12.5) |
| Working memory | 17 (14.2) |
| Multitasking | 10 (8.3) |
| Sustained attention | 28 (23.3) |
| Decline in at least 1 domain | 59 (49.2) |
| Number of domains | |
| 1 | 40 (33.3) |
| 2 | 11 (9.2) |
| 3 | 7 (5.8) |
| 4 | 1 (0.83) |
A total of 120 participants completed CANTAB assessments. Participants who completed CANTAB assessments were younger, more likely to be pre-menopausal and attained higher education levels than those who did not. However, there was no difference in baseline anxiety and fatigue levels, proportion reporting subjective CRCI and BDNF Val66Met genotypic distribution between the two groups of patients (Supplementary Table S1). A total of 59 individuals, representing nearly half of the patients (49.2%) experienced decline in at least one cognitive domain (Table 3). The highest proportion of patients with cognitive decline was reported in the domain of sustained attention (23.3%), followed by response speed (14.2%), working memory (14.2%), learning and memory (12.5%), and multitasking (8.3%).
Association of BDNF Genotypes with Cognitive Impairment
After adjusting for potential confounders including anxiety and fatigue, Met allele carriers showed a consistent trend of decreasing odds of subjective CRCI across all domains; however, statistical significance was only observed in memory (OR = 0.24, 95% CI = 0.09–0.61); multitasking (OR = 0.30, 95% CI = 0.14–0.67); and mental acuity (OR = 0.46, 95% CI = 0.21–0.99). Apart for the mental acuity domain (unadjusted OR = 0.53, 95% CI = 0.27–1.04), adjusting for potential confounders did not alter the significance of association (Table 4). In contrast, no significant associations were detected in all cognitive domains investigated for objective CRCI in both adjusted and unadjusted analysis (Table 4). Analysis performed assuming a general genetic model yielded results with similar trends (Supplementary Table S2).
Table 4.
Association of carrying BDNF Met allele with CRCI
| Variable | Unadjusted analysis | Adjusted analysis | ||
|---|---|---|---|---|
| OR | p value | OR | p value | |
| Subjective cognitive impairment | ||||
| (n = 193) | (n = 192)a | |||
| Total score | 0.63 (0.32–1.24) | 0.18 | 0.62 (0.29–1.30) | 0.21 |
| Memory | 0.45 (0.21–0.97) | 0.04b | 0.24 (0.09–0.61) | 0.003b |
| Multitasking | 0.43 (0.22–0.86) | 0.02b | 0.30 (0.14–0.67) | 0.003b |
| Verbal ability | 0.58 (0.28–1.24) | 0.16 | 0.57 (0.24–1.38) | 0.22 |
| Concentration | 0.98 (0.48–1.99) | 0.96 | 0.86 (0.38–1.90) | 0.70 |
| Mental acuity | 0.53 (0.27–1.04) | 0.07 | 0.46 (0.21–0.99) | 0.047b |
| Functional interference | 0.87 (0.40–1.93) | 0.74 | 0.69 (0.27–1.75) | 0.44 |
| Objective cognitive impairment | ||||
| (n = 120) | (n = 119)a | |||
| Response speed | 2.01 (0.54–7.49) | 0.30 | 3.02 (0.69–13.26) | 0.14 |
| Learning and memory | 1.10 (0.32–3.73) | 0.88 | 1.58 (0.36–6.87) | 0.54 |
| Working memory | 1.34 (0.40–4.43) | 0.64 | 1.32 (0.35–4.94) | 0.68 |
| Multitasking | 0.36 (0.10–1.33) | 0.12 | 0.32 (0.06–1.70) | 0.18 |
| Sustained attention | 2.12 (0.73–6.13) | 0.17 | 3.02 (0.88–10.35) | 0.08 |
aInsufficient covariate data for 1 participant
bp < 0.05
Trajectory of Fatigue and Anxiety and Association with BDNF Genotypes
Mean scores of BFI and BAI, indicating fatigue and anxiety, respectively, showed an increasing trend over time (Table 5). Baseline fatigue and anxiety were also shown to be significant predictors of subjective CRCI in univariate analysis (Supplementary Table S3); however, further analysis showed that anxiety and fatigue levels over time were not associated with BDNF Val66Met polymorphism.
Table 5.
Association of BDNF Met allele with fatigue and anxiety over time
| Mean scores (SD) | β | p value | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| Fatigue (BFI) | |||||
| All participants | 1.64 (1.89) | 1.91 (2.03) | 2.23 (2.05) | ||
| Met allele carrier | |||||
| No | 1.39 (1.56) | 2.15 (2.08) | 2.40 (2.04) | Reference | |
| Yes | 1.74 (1.99) | 1.81 (2.01) | 2.17 (2.05) | − 0.07 | 0.77 |
| Anxiety (BAI) | |||||
| All participants | 6.86 (7.23) | 8.22 (8.55) | 8.55 (7.66) | ||
| Met allele carrier | |||||
| No | 7.00 (8.79) | 8.87 (8.52) | 9.40 (7.42) | Reference | |
| Yes | 6.82 (6.60) | 7.98 (8.58) | 8.23 (7.74) | − 0.75 | 0.48 |
Meta-Analysis of Association between BDNF Genotypes and Subjective CRCI
Meta-analysis of odds ratios from the original (n = 145) and current (n = 193) cohorts showed comparable trends with consistent directions of association. Significantly lower odds of CRCI were associated with Met allele carriers in the domains of memory (OR = 0.34, 95% CI = 0.17–0.70); multitasking (OR = 0.33, 95% CI = 0.18–0.59); and verbal ability (OR = 0.46, 95% CI = 0.24–0.88) (Table 6). In addition, the pooled odds ratio of subjective CRCI based on total FACT-Cog score was also lower in Met allele carriers (OR = 0.52, 95% CI = 0.29–0.94) (Table 6). No significant heterogeneity was detected between the two studies for all domains (I2 = 0–34%).
Table 6.
Pooled odds ratios of CRCI among patients carrying BDNF Met allele (Val/Met or Met/Met) compared to Val/Val genotype
| Domain | Cohort | OR (95% CI) |
Weight | Pooled OR (95% CI) |
p value | I2 (%) |
|---|---|---|---|---|---|---|
| Summation | Previous | 0.40 (0.16–1.04) | 39.1 | 0.52 (0.29–0.94) | 0.03a | 0 |
| Current | 0.62 (0.29–1.30) | 60.9 | ||||
| Memory | Previous | 0.53 (0.19–1.53) | 45.7 | 0.34 (0.17–0.70) | 0.003a | 17 |
| Current | 0.24 (0.09–0.61) | 54.3 | ||||
| Multitasking | Previous | 0.37 (0.15–0.91) | 43.0 | 0.33 (0.18–0.59) | < 0.001a | 0 |
| Current | 0.30 (0.14–0.67) | 57.0 | ||||
| Verbal ability | Previous | 0.34 (0.12–0.90) | 43.0 | 0.46 (0.24–0.88) | 0.02a | 0 |
| Current | 0.57 (0.24–1.38) | 57.0 | ||||
| Concentration | Previous | 0.61 (0.23–1.59) | 40.9 | 0.75 (0.40–1.39) | 0.36 | 0 |
| Current | 0.86 (0.38–1.90) | 59.1 | ||||
| Mental acuity | Previous | 1.03 (0.37–2.86) | 36.5 | 0.62 (0.33–1.15) | 0.13 | 34 |
| Current | 0.46 (0.21–0.99) | 63.5 | ||||
| Functional interference | Previous | 0.38 (0.13–1.14) | 42.6 | 0.54 (0.26–1.09) | 0.08 | 0 |
| Current | 0.69 (0.27–1.75) | 57.4 |
ap < 0.05
Discussion
Findings in this well-powered study and pooled results from both the original and current studies show that carriers of the BDNF Met allele is associated with a trend towards lower odds of reporting self-perceived CRCI across different domains. This replicates the protective effect of BDNF Val66Met on subjective CRCI we have observed in our previous work. Consistent with our previous report, BDNFVal66Met was not associated with objective CRCI. Further meta-analysis of the original and the current cohort have also uncovered the protective effect between BDNFVal66Met and global subjective CRCI. This is a novel finding that has not been reported in the literature.
To date, the only other studies investigating the effect of BDNF Val66Met polymorphism on self-perceived cognitive function have been carried out in healthy individuals and did not report lower odds of subjective CRCI among BDNF Met carriers [26, 27]. Therefore, we postulate that the protective effect of BDNF Val66Met polymorphism on cognitive impairment is conditional on the presence of active malignancy or ongoing cancer treatment, both which have been hypothesized as possible causes of CRCI [25]. This discrepancy may be explained by animal studies where the expression and release of BDNF have been shown to be heavily dependent on other physiological elements, such as stress and inflammation, which are elevated in cancer patients undergoing treatment [28]. It has also been demonstrated that as cancer patients undergo chemotherapy, changes in plasma BDNF levels differ between BDNF genotypes [29]. These observations indicate that the effect of genetic polymorphisms may be mediated by downstream mechanisms that vary in different disease states. To further elucidate the links between BDNF Val66Met polymorphism and CRCI, it will be useful to investigate and compare differences in gene and protein expression between BDNF genotypes in both healthy and cancer patient populations. This will not only enhance our understanding of how genetic factors influence the development of CRCI but also provide insights to the pathophysiology of CRCI.
Our earlier study showed a significant association of carrying the BDNF Met allele with decreased odds of self-perceived CRCI in the FACT-Cog domains of multitasking and verbal ability [7]. In this study, we were able to replicate our previous findings in multitasking ability but not in verbal ability although demographic and clinical characteristics of both the original and current cohorts were comparable. While similar directions of association were observed, the effect size of carrying the Met allele was smaller and did not achieve statistical significance (OR in this study = 0.57, OR in original study = 0.34). A possible explanation for this non-replication could be genetic heterogeneity, where impairment in the verbal ability domain may not be specific to the BDNF gene but also associated with other genes not covered in our studies. Another possibility is the phenomenon described as “winner’s curse” where effect sizes are often found to be overestimated in initial genetic association studies [12, 30]. Replication attempts subsequently yield smaller effect sizes and as a result, studies are underpowered to detect a significant impact of genetic polymorphisms. In contrast, our meta-analysis has detected a significant association between the genetic polymorphism and global subjective CRCI, which was not reported in the previous study. The original cohort was not adequately powered to evaluate the effect size, and this limitation has been overcome by the combined analysis of both cohorts.
In contrast to subjective CRCI, we did not detect any significant association between BDNF Val66Met polymorphism and objective CRCI, a trend which is consistent with findings from our previous work as well as other studies in breast and brain tumor patients [7, 31, 32]. The lack of agreement between trends and predictors of objective and subjective CRCI is counter-intuitive but has been commonly reported in literature [33]. Subjective reports of cognitive function are more reflective of the ability to complete daily activities, which require the coordination of different cognitive skills, some which may not have been measured by specific neuropsychological tests used to assess objective cognitive function. While these tests are widely acknowledged as the gold standard to assess cognitive function, the importance of subjective cognitive reports should not be dismissed as they portray the impact of impaired cognition on the daily functioning of patients. Future work in this area should emulate our study, incorporating both objective and subjective measures of cognitive function as both outcomes hold equal importance and are consistently shown to be poorly correlated with each other.
Past research has suggested that subjective cognitive impairment is closely linked to other chemotherapy-related symptoms such as anxiety, depression, and fatigue hence may be more indicative of emotional distress rather than compromised cognitive function [1]. One may therefore speculate that BDNF Val66Met polymorphism may be protective against these accompanying symptoms rather than CRCI, explaining the lack of agreement between the association of BDNF polymorphism with objective and subjective CRCI observed in our study. Nevertheless, anxiety and fatigue levels have been adjusted for in our analysis. Although we did not observe any significant associations in this study, other genetic association studies have also shown that unlike subjective CRCI, anxiety, fatigue, and depression are not ameliorated but worsened among BDNF Met carriers [34, 35]. Considering the combination of these facts, we are confident that our observations are due to true associations of BDNF Val66Met polymorphism with reduced odds of subjective cognitive decline rather than the confounding effects of fatigue or other psychosocial factors.
In genetic association studies, replication attempts are crucial to confirm initial findings. Past studies have suggested several genetic polymorphisms as possible predictors of CRCI [36, 37] but to the best of our knowledge, none have never been successfully replicated. For example, the effect of APOE ε4 allele was first observed in breast cancer and lymphoma survivors, but similar associations have not been replicated in similar patient populations and cognitive domains [32, 38, 39]. The association of COMT Val158Met with cognitive impairment in breast cancer survivors has only been successfully replicated in patients with brain tumors [31]. Thus, this study is essential as it replicates observed associations of similar direction and strength in the same cognitive domains and study population.
A limitation of our study is that a different neuropsychological test battery, CANTAB, was employed to assess objective cognitive function as the Headminder system used in the original study was no longer commercially available. To reduce any potential discrepancy between Headminder and CANTAB, we ensured that all neuropsychological tests used were validated for similar cognitive domains. Furthermore, the RCI calculated to measure cognitive changes is standardized by dividing differences in test scores at two separate time points by the standard error of measurement. This ensures that score changes in both studies are comparable although different tests were utilized. Nevertheless, we acknowledge that the use of different cognitive assessment tools makes comparison between studies challenging. Furthermore, meta-analysis of findings on objective CRCI from both studies could not be performed as different neuropsychological tools were employed. In genetic association studies where replication attempts and meta-analyses are highly encouraged to increase the effective sample size for a more robust estimate of the genetic effect, it is imperative that similar tools to measure cognitive ability are used across different studies. It should also be noted that a proportion of participants did not complete CANTAB assessments. Given that patients who failed to complete CANTAB assessments were older, more likely to be post-menopausal, and received less education, the prevalence of objective cognitive decline may have been underestimated in this study. Nevertheless, we believe that this is unlikely to influence the lack of association that we observed between BDNF genotype and objective CRCI, as age, education level, and menopausal status were controlled for in our regression analysis. Furthermore, the BDNF Val66Met genotypic distribution between participants who had and had not completed CANTAB assessment were also found to be comparable.
In conclusion, carriers of the BDNF Met allele were protected against global subjective CRCI, particularly in the domains of memory, multitasking, and verbal ability. Similar trends towards reduced odds of subjective CRCI in all other cognitive domains were also observed in both original and current cohorts. We have also confirmed that no association could be detected between BDNF Val66Met polymorphism and objective cognitive function. As cancer- and treatment-related toxicities such as CRCI have been shown to have a devastating impact on cancer survivors, prediction models to estimate the risk of these toxicities should be established and tested for clinical use, so that survivors at risk can be targeted at an earlier stage for interventional measures to improve their daily functioning and quality of life. Genetic markers, such as BDNF Val66Met, should be incorporated in these prediction models once they have been validated in other cancer populations. Augmented with more findings from gene and protein expression studies, this work will contribute to current knowledge on the biochemical pathways that are involved with the development of CRCI. This allows us to identify potential drug targets, which can be further screened for candidates of pharmacological interventions to attenuate the negative impact of CRCI among cancer patients.
Electronic supplementary material
(DOCX 38 kb)
Funding
The authors acknowledge funding from the National Medical Research Council (NMRC/CIRG/1386/2014, NMRC/CIRG/1471/2017), National University of Singapore (R-148-000-233-114), and National Cancer Centre Singapore (NRFCB12131).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Ng T, Dorajoo SR, Cheung YT, Lam YC, Yeo HL, Shwe M, Gan YX, Foo KM, et al. Distinct and heterogeneous trajectories of self-perceived cognitive impairment among Asian breast cancer survivors. Psychooncology. 2018;27:1185–1192. doi: 10.1002/pon.4635. [DOI] [PubMed] [Google Scholar]
- 2.Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 3.Cheung YT, Shwe M, Tan YP, Fan G, Ng R, Chan A. Cognitive changes in multiethnic Asian breast cancer patients: a focus group study. Ann Oncol. 2012;23:2547–2552. doi: 10.1093/annonc/mds029. [DOI] [PubMed] [Google Scholar]
- 4.Cheung YT, Lim SR, Ho HK, Chan A. Cytokines as mediators of chemotherapy-associated cognitive changes: current evidence, limitations and directions for future research. PLoS One. 2013;8:e81234. doi: 10.1371/journal.pone.0081234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung YT, Lee HH-L, Chan A. Exploring clinical determinants and anxiety symptom domains among Asian breast cancer patients. Support Care Cancer. 2013;21:2185–2194. doi: 10.1007/s00520-013-1769-8. [DOI] [PubMed] [Google Scholar]
- 6.Ng T, Chan M, Khor CC, Ho HK, Chan A. The genetic variants underlying breast cancer treatment-induced chronic and late toxicities: a systematic review. Cancer Treat Rev. 2014;40:1199–1214. doi: 10.1016/j.ctrv.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Ng T, Teo SM, Yeo HL, Shwe M, Gan YX, Cheung YT, Foo KM, Cham MT, et al. Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neuro-Oncology. 2016;18:244–251. doi: 10.1093/neuonc/nov162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park H, Poo M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed AO, Mantini AM, Fridberg DJ, Buckley PF. Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: a meta-analysis. Psychiatry Res. 2015;226:1–13. doi: 10.1016/j.psychres.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 10.Altmann V, Schumacher-Schuh AF, Rieck M, Callegari-Jacques SM, Rieder CRM, Hutz MH. Val66Met BDNF polymorphism is associated with Parkinson’s disease cognitive impairment. Neurosci Lett. 2016;615:88–91. doi: 10.1016/j.neulet.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Toh YL, Ng T, Tan M, Tan A, Chan A (2018) Impact of brain‐derived neurotrophic factor genetic polymorphism on cognition: A systematic review. Brain Behav 8:e01009. 10.1002/brb3.1009 [DOI] [PMC free article] [PubMed]
- 12.Kraft P. Curses—winnerʼs and otherwise—in genetic epidemiology. Epidemiology. 2008;19:649–651. doi: 10.1097/EDE.0b013e318181b865. [DOI] [PubMed] [Google Scholar]
- 13.Wagner LI, Sweet J, Butt Z, et al. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy—cognitive function instrument. J Support Oncol. 2009;7:W32–W39. [Google Scholar]
- 14.Cheung YT, Lim SR, Shwe M, Tan YP, Chan A. Psychometric properties and measurement equivalence of the English and Chinese versions of the functional assessment of cancer therapy-cognitive in Asian patients with breast cancer. Value Heal. 2013;16:1001–1013. doi: 10.1016/j.jval.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Cheung YT, Foo YL, Shwe M, Tan YP, Fan G, Yong WS, Madhukumar P, Ooi WS, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-cog) in breast cancer patients. J Clin Epidemiol. 2014;67:811–820. doi: 10.1016/j.jclinepi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dement Geriatr Cogn Disord. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 17.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-D. [DOI] [PubMed] [Google Scholar]
- 18.Morris RG, Downes JJ, Sahakian BJ, Evenden JL, Heald A, Robbins TW. Planning and spatial working memory in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:757–766. doi: 10.1136/jnnp.51.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1186::AID-CNCR24>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Ke Y, Ng T, Yeo HL, Shwe M, Gan YX, Chan A. Psychometric properties and measurement equivalence of the English and Chinese versions of the Beck Anxiety Inventory in patients with breast cancer. Support Care Cancer. 2017;25:633–643. doi: 10.1007/s00520-016-3452-3. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- 22.Luo N, Fones CSL, Lim SE, Xie F, Thumboo J, Li SC. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): validation of English version in Singapore. Qual Life Res. 2005;14:1181–1186. doi: 10.1007/s11136-004-4782-z. [DOI] [PubMed] [Google Scholar]
- 23.Cheung Y-B, Thumboo J, Goh C, Khoo KS, Che W, Wee J. The equivalence and difference between the English and Chinese versions of two major, cancer-specific, health-related quality-of-life questionnaires. Cancer. 2004;101:2874–2880. doi: 10.1002/cncr.20681. [DOI] [PubMed] [Google Scholar]
- 24.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore HCF. An overview of chemotherapy-related cognitive dysfunction, or “chemobrain”. Oncology. 2014;28:797–804. [PubMed] [Google Scholar]
- 26.Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, Harrington KD, Bourgeat P, et al. BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2013;34:2457–2464. doi: 10.1016/j.neurobiolaging.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy KM, Reese ED, Horn MM, Sizemore AN, Unni AK, Meerbrey ME, Kalich AG, Jr, Rodrigue KM. BDNF val66met polymorphism affects aging of multiple types of memory. Brain Res. 2015;1612:104–117. doi: 10.1016/j.brainres.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of bdnf gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng T, Lee YY, Chae J, Yeo AHL, Shwe M, Gan YX, Ng RCH, Chu PPY, et al. Evaluation of plasma brain-derived neurotrophic factor levels and self-perceived cognitive impairment post-chemotherapy: a longitudinal study. BMC Cancer. 2017;17:867. doi: 10.1186/s12885-017-3861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 31.Correa DD, Satagopan J, Cheung K, Arora AK, Kryza-Lacombe M, Xu Y, Karimi S, Lyo J, et al. COMT, BDNF , and DTNBP1 polymorphisms and cognitive functions in patients with brain tumors. Neuro-Oncology. 2016;18:1425–1433. doi: 10.1093/neuonc/now057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng H, Li W, Gan C, et al. The COMT (rs165599) gene polymorphism contributes to chemotherapy-induced cognitive impairment in breast cancer patients. Am J Transl Res. 2016;8:5087–5097. [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38:926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Koh MJ, Jeung H-C, Namkoong K, Chung HC, Kang JI. Influence of the BDNF Val66Met polymorphism on coping response to stress in patients with advanced gastric cancer. J Psychosom Res. 2014;77:76–80. doi: 10.1016/j.jpsychores.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Dooley LN, Ganz PA, Cole SW, Crespi CM, Bower JE. Val66Met BDNF polymorphism as a vulnerability factor for inflammation-associated depressive symptoms in women with breast cancer. J Affect Disord. 2016;197:43–50. doi: 10.1016/j.jad.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Small BJ, Rawson KS, Walsh E, Jim HSL, Hughes TF, Iser L, Andrykowski MA, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 37.Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 38.Vardy J, Dhillon HM, Pond GR, Rourke SB, Xu W, Dodd A, Renton C, Park A, et al. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann Oncol. 2014;25:2404–2412. doi: 10.1093/annonc/mdu448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koleck TA, Bender CM, Sereika SM, Ahrendt G, Jankowitz RC, McGuire KP, Ryan CM, Conley YP. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast Cancer. Oncol Nurs Forum. 2014;41:E313–E325. doi: 10.1188/14.ONF.E313-E325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 38 kb)