Abstract
Background: Despite major advances in the management of newborn infants, neonatal sepsis (NS) remain important causes of neonatal morbidity and mortality in the newborn, mainly among preterm and low birth weight infants. Objective: The aim of this study was to investigate the usefulness of neutrophil CD64 expression alone and together with other infection markers in NS. Methods: Peripheral blood samples were taken from 109 neonates, who were categorized into three groups: proven or clinical sepsis (n=35); disease without infection (n=42); and healthy controls (n=32). Complete blood count with differential, interleukin‐6 (IL‐6), C‐reactive protein (CRP), and cell surface expression of CD64 on neutrophils have been evaluated in a prospective manner as a diagnostic aid for NS. Results: Expression of CD64 was significantly enhanced in neonates with sepsis compared with newborns with disease without infection and healthy controls (P=0.001 and P=0.001, respectively). Cutoff values of IL‐6, CRP, CD64MFI, and CD64i were 24.9 pg/ml, 4.05 mg/l, 87.7, and 4.39, respectively. Sensitivity–negative predictive values of IL‐6, CRP, and CD64MFI/CD64i were 80.0–90.6%, 80.0–88.8%, and 88.6–94.0%, respectively. Combining all three tests increased the sensitivity to 100%; however, specificity and positive predictive value decreased to 62.1 and 55.5%, respectively. Conclusions: CD64 might be used either alone or combined with IL‐6 and CRP for early diagnosis of NS. The advantages of CD64 when compared with IL‐6 and CRP are rapid quantitation, very small blood volume required, and easy handling. J. Clin. Lab. Anal. 24:363–370, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: newborn, sepsis, early diagnosis, CD64, flow cytometry, neutrophil
INTRODUCTION
Despite major advances in the management of newborn infants, neonatal sepsis (NS) remain important causes of neonatal morbidity and mortality in the newborn, mainly among preterm and low birth weight infants 1, 2. The current gold standard for confirming the diagnosis of NS is isolation of the causal microorganism by blood culture in the presence of clinical signs and symptoms. However, blood culture results are not available until 24–48 hr after starting the culture, and they are often negative in cases of pneumonia and meningitis, or even in fatal generalized bacterial infection 3. Hence, there is much interest in studying markers of infection that can reliably differentiate between infected and noninfected infants.
With increasing understanding of the inflammatory cascade of sepsis and rapid advances in diagnostic technologies, many potential infection markers have been investigated.
FcγRI (CD64) is constitutively expressed with high density on monocytes and macrophages, less so on eosinophils, but only to a very low extent on resting neutrophils 4. Microbial cell wall components, such as lipopolysaccarides, endogenous complement split products, and cytokines, such as interferon‐γ and tumor necrosis factor‐α, are some of the activators of CD64. It was shown that CD64 had high sensitivity (95–97%) and specificity (88–90%) at the onset and up to 24 hr after the initial clinical presentation for diagnosing both early and late‐onset bacterial infection 5, 6. There is limited data on the usefulness of the determination of CD64 expression combined with other diagnostic markers in NS 2, 7. Therefore, we planned this study to evaluate the sensitivity and specificity of enhanced expression of CD64 combined with interleukin‐6 (IL‐6) and C‐reactive protein (CRP) for the early diagnosis of NS.
SUBJECTS AND METHODS
Subjects and Setting
This prospective observational study was conducted in the busy neonatal intensive care unit (NICU) of Zekai Tahir Burak Maternity Teaching and Research Hospital, which is a level III hospital in Turkey. Proven NS incidence was 5% in 2008 in our NICU.
During the study period of 12 months (April 2008–May 2009), 4,469 neonates were admitted and followed up in our NICU. Among them, 199 (4.4%) neonates proved to have NS.
Consecutive patients undergoing sepsis evaluation were enrolled for the study. The exclusion criteria were antibiotic change before blood sampling, undergoing surgery in the previous week, chromosomal abnormality, lack of informed consent from the parents, and inadequate blood sampling for all tests. A total of 38 infants with suspect NS were included in the study. Nearly similar number of gestational age and gender‐matched infants with disease without infection (n=42) were included as patient control. Gender‐matched healthy infants (n=32), without any disease or suspicion of sepsis followed up at the obstetrics yard, were included as healthy controls.
Three of the infants whose blood sample haemolysed were excluded from the study. Finally, 109 infants completed the study.
All newborns were evaluated by two clinicians who were blind to the results of the three diagnostic tests assessed before the categorization of the patients. The subjects were classified into one of the following three groups: Group 1—proven or clinical sepsis (n=35), (Group 1a: proven sepsis (n=23), Group1b; clinical sepsis (n=12)); Group 2—disease without infection (n=42), these neonates with the final diagnosis of transient tachypnea of the newborn (TTN) (n=22), respiratory distress syndrome (RDS) (n=18), bronchopulmonary dysplasia (BPD) 3, necrotizing enterocolitis (<grade II°) (n=1), and perinatal asphyxia (n=1); Group 3—healthy newborns (n=32) from whom blood sample had been taken because of suspicion of other conditions with no evidence of sepsis.
Sepsis Evaluation
Suggested diagnostic criteria for sepsis in neonates (two or more of the following clinical features) were used to identify patients for sepsis evaluations 8, 9: (1) respiratory compromise, that is, tachypnea, increased apnea, severe apnea, increased ventilatory support, or desaturation; (2) cardiovascular compromise, that is, bradycardia, pallor, decreased perfusion, or hypotension; (3) metabolic changes, that is, hypothermia, hyperthermia, feeding intolerance, glucose instability, or metabolic acidosis; or (4) neurologic changes, that is, lethargy, hypotonia, or decreased activity. These clinical features were validated in an earlier study to be strongly suggestive of infection 10.
As part of the evaluation, blood was drawn for a complete blood count, including manual differential. The following earlier validated hematologic criteria 8, 10, 11, 12 were used as indicators for sepsis: (1) Leucocytosis >34,000 or leucopenia <5,000 cells/mm3, (2) absolute neutrophil count (ANC) of <7,500 or >14,500 cells/mm3, (3) immature/total neutrophil ratio of >0.2, and (4) platelet count of <100–150,000 cells/mm3.
In addition to clinical criteria, if two or more parameters were abnormal, it was considered as a positive screen and the neonate was started on antibiotics. If the screen was negative but clinical suspicion persisted, it was repeated within 12 hr. If the screen was still negative, sepsis could be excluded with reasonable certainty.
Infants whose culture results were positive were diagnosed as having proven sepsis.
Most (86%) of the infants were not on antibiotics when enrolled in this study. However, antibiotic was used if infants were at risk of NS, such as maternal fever, prolonged rupture of membranes, and RDS, according to our NICU protocol. Blood samples of these infants were taken in the first 72 hr of life. If NS developed despite empiric antibiotic treatment, blood specimens were recollected and consequently antibiotic change was performed.
METHODS
Blood Samples
Peripheral blood samples were obtained for performing IL‐6, CRP, and the expression of CD64 when infection was suspected in all cases during the first 24 hr of clinical suspicion of NS. Samples from Group 3 were taken at the time of blood drawn for the laboratory tests and within the first week of life. Blood samples were immediately transported to the laboratory and processed upon arrival.
Blood Culture
Blood cultures were performed on infants when NS was suspected. In Group 2, blood culture was taken because of TTN, RDS, BPD, necrotizing enterocolitis, or perinatal asphyxia where NS could not be excluded. Blood culture was not taken from healthy controls. The Bactec microbial detection system (Becton‐Dickinson, Sparks, MD) was used to detect positive blood cultures.
Determination of Serum Levels of CRP
Serum concentrations of CRP were measured by a Tinaquant CRP (Latex) high sensitive immunoturbidimetric assay on the Roche Modular P analyzer according to the manufacturer's instructions (CRP latex HS, Roche kit, Roche Diagnostics, GmbH, D‐68298 Mannheim, Germany).
Determination of Plasma Levels of IL‐6
Plasma levels of IL‐6 were analyzed by IL‐6 solid phase, enzyme labeled, chemiluminescent sequential immunometric assay on IMMULITE 1000 analyzer, according to the manufacturer's instructions (Siemens Diagnostic Product Corporation, Los Angeles, CA).
Determination of CD64 Index
Briefly, 50 μl of whole blood was incubated for 15 min at room temperature with saturating amounts of fluorescein isothiocyanate (FITC)‐conjugated murine monoclonal antibody against 64 (Immunotech, Beckman‐Coulter, 13009 Marseille, France) or isotype control murine antibody followed by red blood cell lysis with a diethylene glycol red cell lysis solution (Becton‐Dickinson, Biosciences, San Jose, CA). The cell suspension was washed twice with 0.2% BSA/PBS at 400 g for 4 min.
The expression of CD64 in neutrophils was analyzed by flow cytometry. Flow cytometric analysis was performed using FACSCalibur (Becton Dickinson, Biosciences, Erembodegem, Belgium) to collect log green fluorescence, log right‐angle light scatter, and forward light scatter signals. The neutrophils were gated on the basis of their side‐ and forward‐scatter characteristics and 10,000 cells were analyzed in each sample. Data analysis was performed using light scatter gating to define the neutrophils population and the intensity of CD64 expression was quantified as mean equivalent soluble fluorescence units (MESF). The PMN CD64 expression of neutrophils in MESF units was corrected for any nonspecific antibody binding by subtracting values for the isotype control using Cell Quest Pro software version 4.2 (BD Biosciences Clontech, Palo Alto, CA). An index of expression of CD64 (CD64i) was calculated by the ratio of mean fluorescence intensities (CD64MFI) of the study population to the fluorescence signal of isotype control.
ETHICS
This study was performed with permission from the local Ethics Committee of Zekai Tahir Burak Maternity Teaching and Research Hospital, Ankara, Turkey. The blood samples were collected after taking informed consent from the parents.
STATISTICAL ANALYSIS
SPSS 17.0 (SPSS, Chicago, IL) was used for statistical analysis. Data are expressed as the arithmetic mean±standard deviation or median (min–max), as appropriate. Differences among three groups were analyzed by one‐way ANOVA or Kruskal–Wallis tests. χ2 test was performed for categorical variables. Spearman test was used to analyze correlation between variables. The diagnostic cutoff values were defined using the receiver operating curve (ROC) curve analysis. Sensitivity, specificity, and positive and negative predictive values of the diagnostic tests were also calculated. A combination of tests was considered positive if any one of the selected markers exceeded their respective cutoff values. The level of significance was set at 5% for all comparisons.
RESULTS
The mean gestational age and birth weight of all subjects were 32.7±4.7/week and 2013±961 g. Male to female ratio was 1.6:1.0. As stated above, the 109 neonates studied were classified into three groups. Table 1 shows the characteristics of the study subjects according to groups. Expectedly, the healthy newborns had significantly higher gestational age and birthweight compared with infants in other groups. Postnatal ages of the healthy newborns were lower than those of the others because they usually required fewer hospitalization days after delivery. When divided into subgroups, it was found that gestational age and birthweight were significantly lower in neonates with proven sepsis compared with ones with clinical sepsis (Table 2).
Table 1.
Characteristics of the Study Subjects According to Groupsa
| Group 1 (n=35) | Group 2 (n=42) | Group 3 (n=32) | P value | |
|---|---|---|---|---|
| Gestational age, week (mean±SD) | 31.2±4.8 | 31.5±4.8 | 37±2.8 | 1–2: 0.59 |
| 2–3: 0.001 | ||||
| 1–3: 0.001 | ||||
| Birth weight (g) (mean±SD) | 1,710±871 | 1,555±783 | 2,843±843 | 1–2: 0.67 |
| 2–3: 0.001 | ||||
| 1–3: 0.001 | ||||
| Male gender, n(%) | 23 (65.7) | 26 (61.9) | 18 (56.3) | 0.72 |
| Age at sepsis evaluation, day (median, range) | 8 (1–28) | 12 (1–29) | 1 (1–20) | 0.001 |
| Antenatal steroid, n(%) | 12 (34.3) | 16 (38.1) | 4 (12.5) | 0.03 |
| Cesarean delivery, n(%) | 25 (71.4) | 32 (76.2) | 24 (75.0) | 0.88 |
| Premature rupture of membranes >24 hr, n(%) | 7 (20.0) | 9 (21.4) | 5 (15.6) | 0.81 |
| 5 min Apgar score (<7), (median, range) | 5 (14.3) | 7 (16.7) | 2 (6.3) | 0.35 |
| Length of hospital, day (median, range) | 27 (0–87) | 21 (0–91) | 1 (1–27) | 0.001 |
| Mortality, n(%) | 7 (20.0) | 0 (0.0) | 0 (0.0) | 0.001 |
aGroup 1: newborns with proven or clinical sepsis. Group 2: newborns with disease without infection. Group 3: healthy newborns.
Table 2.
Characteristics of the Study Subjects According to Subgroupsa
| Group 1a (n=23) | Group 1b (n=12) | P value | |
|---|---|---|---|
| Gestational age, week | 29.7±4.3 | 34±4.5 | 0.01 |
| (mean±SD) | |||
| Birth weight (g) (mean±SD) | 1,448±691 | 2,212±985 | 0.02 |
| Male gender, n (%) | 17 (73.9) | 6 (50.0) | 0.29 |
| Age at sepsis evaluation, day (median, range) | 9 (1–28) | 8 (1–26) | 0.74 |
| Antenatal steroid, n(%) | 7 (30.4) | 5 (41.7) | 0.70 |
| Cesarean delivery, n(%) | 17 (73.9) | 8 (66.7) | 0.70 |
| Premature Rupture of membranes >24 hr, n(%) | 6 (26.1) | 1 (8.3) | 0.38 |
| 5 min Apgar score, <7 median (range) | 4 (17.4) | 1 (8.3) | 0.78 |
| Length of hospital, day (median, range) | 29 (1–87) | 17 (1–64) | 0.18 |
| Mortality, n (%) | 6 (26.1) | 1 (8.3) | 0.38 |
aGroup 1a: newborns with proven sepsis. Group 1b: newborns with clinical sepsis.
There were 23 proven sepsis episodes with positive culture results: Causative organisms were: Coagulase–Negative Staphylococcus spp (n=9), Klebsiella spp (n=5), Enterococcus spp. (n=5), Candida spp. (n=2), Pseudomonas spp. (n=1), and Streptoccus pneuomonia (n=1).
Table 3 shows the characteristics of the results of the sepsis evaluation, according to the three groups. Immature/total neutrophil ratio (>0.2), IL‐6, and CRP values were significantly higher, but platelet levels were lower in Group 1 compared with other two groups. Group with proven sepsis had higher neutrophil CD64MFI and CD64i values compared with infants with disease without infection and healthy newborns (P=0.001 and P=0.001, respectively).
Table 3.
Characteristics of the Sepsis Evaluation According to Groupsa
| Group 1 (n=35) | Group 2 (n=42) | Group 3 (n=32) | P value | |
|---|---|---|---|---|
| White blood cell count, cells per mm3, (mean±SD) | 11.6±7.0 | 13.0±4.2 | 14.3±4.8 | 0.13 |
| Segmented neutrophils, n(%) | 56.9±19.2 | 51.9±13.8 | 59.2±13.3 | 0.12 |
| Absolute neutrophil count, cells per mm3, (mean±SD) | 7.6±6.0 | 7.1±3.3 | 8.2±4.1 | 0.63 |
| Immature/total neutrophil ratio (>0.2), n (%) | 15 (42.9) | 4 (9.5) | 0 (0.0) | 0.001 |
| Platelet count, cells per mm3, (mean±SD) | 181±95 | 283±125 | 283±85 | P=0.001 |
| 1–2=0.001 | ||||
| 1–3=0.001 | ||||
| 2–3=1.0 | ||||
| IL‐6 (pg/ml) (median, range) | 115 (4.2–1000) | 6.3 (2–141) | 7.9 (2.4–62) | P=0.001 |
| 1–2=0.001 | ||||
| 1–3=0.001 | ||||
| 2–3=0.19 | ||||
| CRP (mg/l) (median, range) | 22.8 (0.5–41.6) | 0.49 (0.09–24) | 0.9 (0.01–32.9) | P=0.001 |
| 1–2=0.001 | ||||
| 1–3=0.001 | ||||
| 2–3=0.16 | ||||
| CD64 (MFI) (median, range) | 207.1 (50.2–524.2) | 58.9 (27.5–165) | 67.6 (29.6–309.8) | 0.001 |
| 1–2=0.001 | ||||
| 1–3=0.001 | ||||
| 2–3=0.12 | ||||
| CD64index | 10.3 (2.5–26.2) | 2.9 (1.3–8.2) | 3.3 (1.4–15.4) | P=0.001 |
aGroup 1: newborns with proven or clinical sepsis. Group 2: newborns with disease without infection. Group 3: healthy newborns.
When analyzed for subgroups, immature/total neutrophil ratio (>0.2) and IL‐6 values were higher neonates with proven sepsis compared with ones that had clinical sepsis (Table 4).
Table 4.
Characteristics of the Sepsis Evaluation According to Subgroupsa
| Group 1a (n=23) | Group 1b (n=12) | P value | |
|---|---|---|---|
| White blood cell count, cells per mm3, (mean±SD) | 11.8±8.1 | 11.4±4.4 | 0.61 |
| Segmented neutrophils, n(%) | 56.8±19.1 | 57.0±20.2 | 0.98 |
| Absolute neutrophil count, cells per mm3, (mean±SD) | 7.5±6.7 | 7.6±4.7 | 0.54 |
| Immature/total neutrophil ratio (>0.2), n(%) | 14 (60.9) | 1 (8.3) | 0.004 |
| Platelet count, cells per mm3, (mean±SD) | 173±105 | 195±74.5 | 0.48 |
| IL‐6 (pg/ml) (median, range) | 242 (16.5–1,000) | 27.3 (4.2–1,000) | 0.002 |
| CRP (mg/l) (median, range) | 23.9 (1.2–41.6) | 9.3 (0.6–39.9) | 0.15 |
| CD64 (MFI) (median, range) | 226 (50.5–425) | 177 (50.2–524) | 0.19 |
| CD64 index | 3.3 (0.7–6.3) | 2.6 (0.7–7.7) | 0.19 |
aGroup 1: newborns with proven or clinical sepsis. Group 2: newborns with disease without infection. Group 3: healthy newborns.
There was no statistically significant difference between preterm and term neonates in the median value of CD64MFI (70.7; 27.5–425.9 vs. 78.8; 29.6–524.2) (P=0.93). There was also no significant difference for median values of CD64 between sepsis episodes of gram‐negative or other microorganisms (193; 70–424 vs. 231; 50–425) (P=0.83).
Table 5 shows predictive values for hematologic data alone and in combination with CD64, according to cutoff points. Predictive values of CD64 index were similar to CD64MFI. Sensitivity and negative predictive value increased to 100%, but specificity and positive predictive value decreased 62.1 and 55.5%, respectively, when all three measurements were used for the diagnosis of NS. Cutoff points and area under curve (AUC) values obtained from ROC curve analysis are presented in Table 6 and Figure 1. CD64 had close but higher AUC value than those of IL‐6 and CRP.
Table 5.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value (Expressed as Percentage) for Hematologic Data Alone and in Combination with CD64, According to Cutoff Points
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|
| IL‐6 (pg/ml) | 80.0 | 91.8 | 82.3 | 90.6 |
| CRP (mg/l) | 80.0 | 75.6 | 60.8 | 88.8 |
| CD64 (MFI) | 88.6 | 85.1 | 73.8 | 94.0 |
| CD64 index | 88.6 | 85.1 | 73.8 | 94.0 |
| IL‐6 (pg/ml) | 97.1 | 77.0 | 66.6 | 98.2 |
| and CD64 (MFI) | ||||
| CRP(mg/l) | 97.1 | 68.9 | 59.6 | 98.0 |
| and CD64 )(MFI) | ||||
| IL‐6 (pg/ml), CRP (mg/l) and CD64 (MFI) | 100 | 62.1 | 55.5 | 100 |
Table 6.
Cutoff Points and AUC Values from Receiver Operating Characteristic Curves
| Cutoff value | AUC | P value | CI 95% Lower‐upper limits | |
|---|---|---|---|---|
| IL‐6 (pg/ml) | 24.9 | 0.89 | 0.001 | 0.81–0.96 |
| CRP (mg/l) | 4.05 | 0.87 | 0.001 | 0.81–0.94 |
| CD64 (MFI) | 87.7 | 0.90 | 0.001 | 0.83–0.96 |
| CD64 index | 4.39 | 0.90 | 0.001 | 0.83–0.96 |
Figure 1.
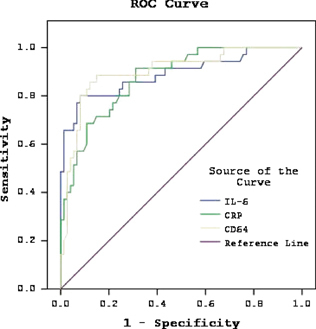
ROC curve analyze of hematologic parameters in neonatal sepsis episodes (n=35).
There was positive correlation between IL‐6 and CD64 values (P=0.001, r=0.58).
There was also positive correlation between CRP and CD64 values (P=0.001, r=0.48).
DISCUSSION
Early diagnosis of NS is extremely important because prompt institution of antimicrobial therapy improves outcomes. A clinically diagnostic marker must have a well‐defined cutoff value for differentiating infected from noninfected infants, and able to identify infected cases at an early stage 2, 13.
Among the various cytokines, most studies have confirmed the utility of IL‐6 as an early marker of NS. The cutoff values for IL‐6 to diagnose sepsis have ranged from 18 to 70 pg/ml 6, 8, 10, 14, 15. Laycesa‐Espinosa et al. showed that plasma levels of IL‐6 were significantly higher in newborns with proven sepsis 16. The diagnostic sensitivity, specificity, positive and negative predictive values for this cytokine was 47, 92, 78, and 75%, respectively. Ng et al. reported that high sensitivity, early in the course of infection (89% at 0 hr), decreased substantially to 67% by 24 hr and 58% by 48 hr after the onset of illness 17. In this study, at a cutoff value of 24.9 pg/ml, as defined by ROC analysis, sensitivity, specificity, the positive and negative predictive values of this cytokine for NS were 80.0, 91.8, 82.3, and 90.6%.
As the window of opportunity for IL‐6 to detect infection was narrow, it should, therefore, be used in conjunction with a “late” and more specific marker, such as CRP, to improve its diagnostic capability in clinical practice 17. CRP reference intervals established by Chiesa et al. were at birth 95th percentile, 5.0 mg/l, at 24 hr 95th percentile, 14.0 mg/l, and at 48 hr of life 95th percentile, 9.7 mg/l 18. Haque suggested the cutoff value of CRP as 10mg/l or >2 SD above normal value for diagnosis of NS 8. Davis et al. reported that CRP had high sensitivity (88.2%) but low specificity (59.4%) 19. Similar to Davis et al.'s study, we found that CRP had high sensitivity (80.0%) but low specificity (75.6%). In our study, cutoff value of CRP (4.05 mg/l) was lower than the reported values.
A novel marker of infection, the CD64 score point, which incorporates the quantitative analysis of CD64 expression on both neutrophils and monocytes, has recently been introduced as a marker in adults which could distinguish between infection and healthy states 20. Furthermore, technological advances in flow cytometry have made it possible to quantitate neutrophil CD64 rapidly (<60 min), with precision, and, importantly for neonates, with minimal blood volumes (50 μl) 2. In this study, we found that CD64MFI was significantly higher in patients with NS compared with newborns with disease without infection and healthy ones. However, there was no significant difference for CD64MFI between proven and clinical sepsis cases.
Fjaertoft et al. showed that neutrophils from preterm newborn infants showed a moderately increased level of CD64 expression that, during their first month of life, was reduced to the level observed on neutrophils from term newborn infants and adults 21. However, expression of CD64 on neutrophils in preterm and term, noninfected newborn infants, although somewhat higher than on adult neutrophils, did not reach a level comparable with that found during bacterial infections 22, 23. In this study, median value of CD64 was not higher in preterms than that of term neonates.
It has been shown that the neutrophil expression of CD64 was neither affected by RDS nor PROM, nor by any other noninfective perinatal events 21. This finding was in contrast to what had earlier been found for the expression of CD11b, which was markedly affected by RDS 24. In this study, we have also shown that RDS or TTN alone did not increase CD64 expression. In adult patients, higher expression of CD64 on neutrophils was found in gram‐negative sepsis compared with gram‐positive sepsis 25. However, Shao et al. 26 had reported earlier we could not confirm this difference in our study. The cause for this difference between adults and neonates might be a less expressed neutrophil response to infection with gram‐negative bacteria in neonates. Furthermore, it has been shown that leucocytes from patients with streptococcal infection have an increased expression of this Fc receptor 27. Among our patients, streptococcus pneumonia was detected in one sepsis episode where CD64MFI value was very high (273.2).
Expression of CD64 has been reported to be a highly specific indicator of NS, although it had a low sensitivity in one study 16. Layseca‐Espinosa et al. found that this laboratory parameter showed a high specificity and positive predictive value for sepsis (96.8 and 88.8%, respectively), although with a low sensitivity (25.8%) and an intermediate negative predictive value (57.4%). Davis et al. reported CD64 had high diagnostic performance for sepsis (sensitivity 87.9%, specificity 71.2%) 19. Groselj‐Grenc et al. defined optimum diagnostic cutoff levels, AUCs, sensitivity and specificity of CD64MFI for neutrophils, and CD64 index as 72.0, 0.85, 65.5, 92.6%, and 2.45, 0.83, 65.5, 88.9%, respectively, for sepsis at the time of initial diagnosis 28. A large cohort study demonstrated that neutrophil CD64 was substantially up‐regulated and useful (sensitivity 96% and specificity 81%) for identifying term newborn infants with early‐onset clinical sepsis and pneumonia 5. The authors reported that additional use of CRP did not significantly improve the sensitivity of neutrophil CD64, but adversely affected the specificity of the test 5. Ng et al. showed that CD64 levels had high sensitivity (95–97%) and negative predictive value (97–99%) for early‐ and late‐onset infection in infants of very low birth weight, using cutoff values of 6,136 and 4,000 phycoerythrin molecules bound per cell, respectively 5, 6. Addition of IL‐6 or CRP levels enhanced the sensitivity and negative predictive value to 100% and had the specificity and positive predictive value exceeding 88 and 80%, respectively 6. In this study, cutoff value of CD‐64MFI for NS was found to be 87.7 by ROC curve analysis. Sensitivity and negative predictive value of CD64 alone were found to be 88.6 and 94.0% for NS. Sensitivity and negative predictive value increased to 100%, but specificity and positive predictive value decreased 62.1 and 55.5%, respectively, when IL‐6 CRP were combined with CD64.
Bhandari et al. observed that for all sepsis episodes, the CD64 index had an AUC in ROC analysis of 0.74, with a cutoff value of 2.30, the CD64 index in combination with the ANC had the highest negative predictive value (93%) for ruling out sepsis and 95% sensitivity for diagnosing sepsis 9. In this study, cutoff value of CD64i was found to be 4.39, AUC for CD64i was 0.90 with a sensitivity and specificity of 88.6 and 85.1%.
It was stated that the enhanced expression of CD64 seen in some infected infants might be related to an increase in the plasma levels of proinflammatory cytokines 16. We found that there was positive correlation between IL‐6 and CD64 expression. There was also positive correlation between CRP and CD64 expression.
Our study has several limitations. First, the definition of clinical sepsis with culture‐negative patients, particularly critically ill neonates, is still a matter of discussion. Second, the number of patients with culture‐positive sepsis was relatively small. On the other hand, some of the patients included in the study were receiving antibiotics at the time of enrollment. This is a potential confounder of the results as the pretreatment with antibiotics could potentially influence the cytokine values as well as positive blood cultures. Term and preterm infants have been grouped together in the cases and controls. This was another confounding variable. As inflammatory response to sepsis may be developmentally regulated, Group 3 babies can effectively only act as appropriate controls for bigger babies with the majority of samples drawn in the first day of life. There may be selection bias—as only 23/199 (11%) culture‐positive patients were recruited for the study. Despite these limitations, this study represents an important addition to the research literature by investigating the predictive values of a relatively novel marker of neutrophil CD64 combined with well known markers in the early diagnosis of sepsis.
CONCLUSION
Our results suggest that the enhancement of CD64 expression in peripheral blood is an immune parameter with a high sensitivity and negative predictive value for NS. CD64 might be used either alone or combined with IL‐6 and CRP for early diagnosis of NS. The advantages of CD64 when compared with IL‐6 and CRP are rapid quantitation, very small blood volume required, and easy handling.
Acknowledgements
The authors thank Professor Khalid N. Haque, reader in neonatal pediatrics, University of London and previously consultant neonatologist at Queen Mary Children Hospital in the UK, for his extremely helpful comments and remarks on this article. The skillful technical assistance of biologist Sibel Uslu, the staff at the Laboratory of Flow cytometry, Children's Hospital, and the staff at the Department of Neonatology is greatly appreciated.
REFERENCES
- 1. Klein JO, Remington JS. Current consepts of infections of the fetus and newborn infant In: Remington JS, Klein JO, editors. Infections Diseases of the Fetus and Newborn Infant. Philadelphia: W.B. Saunders Company; 1995. p 11–19. [Google Scholar]
- 2. Ng PC. Diagnostic markers of infection in neonates. Arch Dis Child Fetal Neonatal Ed 2004;89:F229–F235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panero A, Pacifico L, Rossi N, Mancuso G, Stegagno M, Chiesa C. Interleukin‐6 in neonates with early and late onset infection. Pediatr Infect Dis J 1997;16:370–375. [DOI] [PubMed] [Google Scholar]
- 4. Kolackova M, Kudlova M, Kunes P, et al. Early expression of FcgammaRI (CD64) on monocytes of cardiac surgical patients and higher density of monocyte anti‐inflammatory scavenger CD163 receptor in “on‐pump” patients. Mediators Inflamm 2008;2008: doi: 10.1155/2008/235461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng PC, Li G, Chui KM, et al. Neutrophil CD64 is a sensitive diagnostic marker for early‐onset neonatal infection. Pediatr Res 2004;56:796–803. [DOI] [PubMed] [Google Scholar]
- 6. Ng PC, Cheng SH, Chui KM, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C‐reactive protein in preterm very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1997;77:F221–F227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuppa AA, Calabrese V, D'Andrea V, et al. Evaluation of C reactive protein and others immunologic markers in the diagnosis of neonatal sepsis. Minerva Pediatr 2007;59:267–274. [PubMed] [Google Scholar]
- 8. Haque KN. Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med 2005;6:S45–S49. [DOI] [PubMed] [Google Scholar]
- 9. Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics 2008;121:129–134. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez BE, Mercado CK, Johnson L, Brodsky NL, Bhandari V. Early markers of late‐onset sepsis in premature neonates: clinical, hematological and cytokine profile. J Perinat Med 2003;31:60–68. [DOI] [PubMed] [Google Scholar]
- 11. Smulian JC, Bhandari V, Campbell WA, Rodis JF, Vintzileos AM. Value of umbilical artery and vein levels of interleukin‐6 and soluble intracellular adhesion molecule‐1 as predictors of neonatal hematologic indices and suspected early sepsis. J Matern Fetal Med 1997;6:254–259. [DOI] [PubMed] [Google Scholar]
- 12. Buhimschi CS, Buhimschi IA, Abdel‐Razeq S, et al. Proteomic biomarkers of intra‐amniotic inflammation: relationship with funisitis and early‐onset sepsis in the premature neonate. Pediatr Res 2007;61:318–324. [DOI] [PubMed] [Google Scholar]
- 13. Ng PC. Clinical trials for evaluating diagnostic markers of infection in neonates. Biol Neonate 2005;87:111–112. [DOI] [PubMed] [Google Scholar]
- 14. Onal EE, Kitapci F, Dilmen U, Adam B. Interleukin‐6 concentrations in neonatal sepsis. Lancet 1999;353:239–240. [DOI] [PubMed] [Google Scholar]
- 15. Kantar M, Kultursay N, Kutukculer N, Akisu M, Cetingul N, Caglayan S. Plasma concentrations of granulocyte‐macrophage colony‐stimulating factor and interleukin‐6 in septic and healthy preterms. Eur J Pediatr 2000;159:156–157. [DOI] [PubMed] [Google Scholar]
- 16. Layseca‐Espinosa E, Pérez‐González LF, Torres‐Montes A, et al. Expression of CD64 as a potential marker of neonatal sepsis. Pediatr Allergy Immunol 2002;13:319–327. [DOI] [PubMed] [Google Scholar]
- 17. Ng PC, Cheng SH, Chui KM, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C‐reactive protein in preterm very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1997;77:F221–F227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiesa C, Signore F, Assumma M, et al. Serial measurements of C‐reactive protein and interleukin‐6 in the immediate postnatal period: reference intervals and analysis of maternal and perinatal confounders. Clin Chem 2001;47:1016–1022. [PubMed] [Google Scholar]
- 19. Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med 2006;130:654–661. [DOI] [PubMed] [Google Scholar]
- 20. Fjaertoft G, Håkansson LD, Pauksens K, Sisask G, Venge P. Neutrophil CD64 (FcgammaRI) expression is a specific marker of bacterial infection: a study on the kinetics and the impact of major surgery. Scand J Infect Dis 2007;39:525–535. [DOI] [PubMed] [Google Scholar]
- 21. Fjaertoft G, Håkansson L, Foucard T, Ewald U, Venge P. CD64 (Fcgamma receptor I) cell surface expression on maturing neutrophils from preterm and term newborn infants. Acta Paediatr 2005;94:295–302. [DOI] [PubMed] [Google Scholar]
- 22. de Haas M, Vossebeld PJM, Von dem Borne AEGKr, Roos D. Fcgamma receptors of phagocytes. J Lab Clin Med 1995;126:330–341. [PubMed] [Google Scholar]
- 23. Fjaertoft G, Håkansson L, Ewald U, Foucard T, Venge P. Neutrophils from term and preterm infants express the high affinity Fcgamma‐receptor I (CD64) during bacterial infections. Pediatr Res 1999;45:871–876. [DOI] [PubMed] [Google Scholar]
- 24. Nupponen I, Pesonen E, Andersson S, et al. Neutrophil activation in preterm infants who have respiratory distress syndrome. Pediatrics 2002;110:36–41. [DOI] [PubMed] [Google Scholar]
- 25. Herra CM, Keane CT, Whelan A. Increased expression of Fcγ receptors on neutrophils and monocytes may reflect ongoing bacterial infection. J Med Microbiol 1996;44:135–140. [DOI] [PubMed] [Google Scholar]
- 26. Shao J, Huang XW, Sun MY, DU LZ, Tang YM, Le YL. Expression of peripheral blood neutrophil CD64 in neonatal septicemia. Zhonghua Er Ke Za Zhi 2005;43:510–513. [PubMed] [Google Scholar]
- 27. Guyre Pm, Campbell As, Kniffin Wd, Fanger Mw. Monocytes and polymorphonuclear neutrophils of patients with streptococcal pharyngitis express increased numbers of type I IgG Fc receptors. J Clin Invest 1990;86:1892–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Groselj‐Grenc M, Ihan A, Derganc M. Neutrophil and monocyte CD64 and CD163 expression in critically ill neonates and children with sepsis: comparison of fluorescence intensities and calculated indexes. Mediators Inflamm 2008;2008:202646. [DOI] [PMC free article] [PubMed] [Google Scholar]


