Abstract
Indoxyl sulfate and p‐cresylsulfate was associated with poor clinical outcome of uremia. We explored the relationship between the two toxins and renal function in chronic kidney disease (CKD) patients. This study enrolled 103 stable CKD patients (stage 3–5 and hemodialysis (HD) patients). Serum levels of indoxyl sulfate and p‐cresylsulfate were measured using ultra performance liquid chromatography. General laboratory results and patient background were also checked. Patients with advanced CKD had higher serum indoxyl sulfate, p‐cresylsulfate based on ANOVA test. There were significant correlation between indoxyl sulfate and p‐cresylsulfate and serum creatinine after multivariate regression analysis (B=3.59, P<0.01; B=0.93, P=0.04, respectively). In addition, there was a positive correlation between indoxyl sulfate and p‐cresylsulfate level (r=0.61, P<0.01). Indoxyl sulfate and p‐cresylsulfate level increased gradually while renal function declined and reached the peak at the stage of HD. Serum indoxyl sulfate level was closely associated with p‐cresylsulfate level in CKD patients. J. Clin. Lab. Anal. 25:191–197, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: protein‐bound uremic toxin, p‐cresylsulfate, indoxyl sulfate, chronic kidney disease
INTRODUCTION
Chronic kidney disease (CKD) has become a global and important health issue as the population increases annually. Uremic solutes are accumulated in CKD patients while renal function declined. Some of the solutes have high affinity for protein and have various cytotoxic effects in vivo 1. Recently, such protein‐bound toxins are receiving the attention from clinicians who are devoted to improving the clinical outcomes in CKD and hemodialysis (HD) patients.
Indoxyl sulfate and p‐cresylsulfate, the uremic toxin of this group, were well studied in the past several years 2. There was a report in the animal study 3 that indoxyl sulfate resulted in decreased renal function and increased glomerular sclerosis. Indoxyl sulfate also induced oxidative stress in endothelial cell and played a role in inhibition of endothelial proliferation and wound repair 4. In addition, indoxyl sulfate was thought to be involved in the pathogenesis of atherosclerosis in dialysis patients 5. However, two reports showed p‐cresol exist predominantly in vivo as conjugated p‐cresylsulfate; p‐cresylglucuronate and unconjugated p‐cresol (free form p‐cresol) were not detectable 6, 7. Many studies demonstrated the various toxic effects of p‐cresol in vitro 8, 9, 10. Their emphasis was placed on p‐cresol because the determination methods were based on deproteinization by acidification, leading to the disintegration of conjugates by hydrolysis 11. One study indicated that p‐cresylsulfate stimulate baseline leukocyte activity, whereas the p‐cresol essentially inhibit activated leukocyte function 12. Recently, free p‐cresol concentrations also have been reported to be associated with the poor clinical outcomes in HD patients 13, 14, 15.
These findings show the importance of IS in HD patients. However, the aim of our study was to investigate the relationship between serum levels of indoxyl sulfate, p‐cresylsulfate and renal function in CKD and HD patients.
MATERIALS AND METHODS
Subjects
One hundred and three stable patients with CKD stage 3, 4, 5 and HD were recruited from the outpatient department. Patients with acute infection and cardiovascular events in the last 3 months, malignancy, or those who were younger than 18 years were excluded.
The causes of CKD in the total number of patients included type 2 diabetic nephropathy, CGN, polycystic kidney disease, or lupus nephritis. For HD patients, they were on 4 hr maintenance dialysis three times a week using a synthetic dialysis membrane (polysulfone or polyamide). Dialyzers were not reused for these patients. Dialysis efficiency was evaluated according to the Kidney Disease Outcomes Quality Initiative guidelines. The study was performed according to the principles of the Declaration of Helsinki and approved by the ethics committee of the Mackay Memorial Hospital. Informed consent was signed for all patients.
Laboratory Assessment
At inclusion, fasting blood samples were obtained for CKD patients (predialysis) and were collected before the midweek HD session for HD patients. Estimated glomerular filtration rate (eGFR) was calculated using modified Modification of Diet in Renal Disease equation: 175*Scr⁁−1.154*Age⁁−0.203*0.742 (if female). The following data were collected: BUN (md/dL), creatinine (mg/dL), hemoglobin (g/dL), hematocrit (%), calcium (mg/dL), phosphate (mg/dL), albumin (g/dL), total indoxyl sulfate (mg/L), indoxyl sulfate (mg/L), and p‐cresylsulfate (mg/L). The bromocresol green method was used for the determination of albumin. The serum levels of C‐reactive protein were measured using a Behring Nephelometer II (Dade Behring, Tokyo, Japan).
Serum indoxyl sulfate and p‐cresylsulfate was analyzed with ultra‐performance liquid chromatography (UPLC). Briefly, serum samples were prepared and deproteinized by heat denaturation. UPLC (ACQUITY UPLC®) was performed at room temperature using a BEH phenyl column (2.1×100 mm) and a photodiode array detector at 280 nm. The buffers used were (A) 10 mM NH4H2PO4 (pH=4.0) and (B) 100% acetonitrile. The flow rate was 0.4 mL/min with a 9 min gradient cycling from 82.5% A/17.5% B to 55% A/45% B.
Under these conditions, both p‐cresylsulfate and indoxyl sulfate eluted at 2.75 and 1.4 min, respectively. Standard curves for p‐cresylsulfate and indoxyl sulfate were set at 0.5, 1, 2.5, 5, and 10 mg/L; both were processed in the same manner as the serum samples and correlated with the serum samples with average r 2 values of 0.999±0.001. Quantitative results were obtained and calculated as concentrations (mg/L). The sensitivity of this assay was 0.425 mg/L for p‐cresylsulfate and 0.225 mg/L for indoxyl sulfate.
Statistical Analysis
The demographic data were expressed as the mean±standard deviation. All study patients were divided into four groups based on eGFR (CKD stage 3, 4, 5 and HD). Intergroup comparisons were performed using one‐way ANOVA for continuous variables and χ2 test for categorical variables. The relationship between eGFR and indoxyl sulfate and p‐cresylsulfate were analyzed by linear regression analysis. Pearson's correlation coefficient or Spearman's rank correlation were used to analyze the relationship between serum indoxyl sulfate and p‐cresylsulfate and selected clinical or biochemical variables. All variables with a statistically significant P‐value in the univariate analysis were included in multivariate linear regression analysis. Serum concentrations of the two toxins in different cohorts were compared by t‐test (Asian vs. Caucasians). A value of P less than 0.05 was considered to be statistically significant. All statistical analyses were conducted by using the SPSS Version 17.0 software program (SPSS, Chicago, IL).
RESULTS
One hundred and three stable CKD patients were recruited to the study and the demographic and clinical characteristics of patients are given in Table 1. This study population consisted of 56 males (54.4%) and 47 females (45.6%), with a mean age of 60.5±9.6 years. Thirty‐six patients had diabetes mellitus (35.7%), 57 patients had hypertension (56.1%), and 24 patients had cardiovascular disease (23.6%). Among them, 24 patients (23.3%) were classified as CKD stage 3, 21 patients (20.4%) were classified as CKD stage 4, 22 patients (21.4%) were classified as CKD stage 5, and 36 patients (34.9%) were classified as CKD stage 5 on HD.
Table 1.
Clinical and Biochemical Characteristics of the Study Population
| Characteristic | All patients (n=103) |
|---|---|
| Age (years) | 60.5±9.6 |
| Male (%) | 54.4% |
| Diabetes mellitus (%) | 35.7% |
| Hypertension (%) | 56.1% |
| CVD (%) | 23.6% |
| SBP (mmHg) | 142.1±17.9 |
| DBP (mmHg) | 76.8±12.4 |
| CKD stage (%) | |
| 3 | 23.3 |
| 4 | 20.4 |
| 5 | 21.4 |
| HD | 34.9 |
| Albumin (g/dL) | 4.06±0.34 |
| Hemoglobin (g/L) | 10.8±1.8 |
| Hematocrit (%) | 32±5.4 |
| BUN (mg/dL) | 55.1±25.9 |
| Creatinine (mg/dL) | 6.3±4.3 |
| eGFR (ml/min) | 17.1±15.3 |
| Calcium (mg/dL) | 9.1±0.6 |
| Phosphate (mg/dL) | 4.7±1.2 |
| Indoxyl sulfate (mg/L) | 21.1±20 |
| p‐Cresylsulfate (mg/L) | 12.8±11.5 |
CVD, cardiovascular disease; CKD, chronic kidney disease; HD, hemodialysis; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate.
Table 2 showed the clinical and biochemical characteristics of the study patients with different CKD stages. Based on the result of analysis, there was no significant difference of age, sex, diabetes, cardiovascular disease, systolic blood pressure, diastolic blood pressure, and calcium between different groups stratified by eGFR. Patients with advanced CKD had higher prevalence of hypertension (P<0.01). The level of hemoglobin and hematocrit was lower in advanced CKD (P<0.01, P=0.03, respectively). In addition, serum phosphate, indoxyl sulfate, and p‐cresylsulfate increased gradually and significantly, as renal function declined and reached the highest level in CKD stage 5 on HD stage (P<0.01, P<0.01, respectively).
Table 2.
Clinical and Biochemical Characteristics of the Study Patients With Different CKD Stages
| CKD classification | |||||
|---|---|---|---|---|---|
| Characteristic | Stage 3 (n=24) | Stage 4 (n=21) | Stage 5 (n=22) | Stage 5 HD (n=36) | P‐value |
| Age (years) | 60.8±8.6 | 59.4±9.3 | 60.3±12.4 | 61.0±8.9 | NS |
| Male (%) | 70.3% | 42.9% | 40.9% | 54.1% | NS |
| Diabetes mellitus (%) | 29.2% | 38.1% | 27.3% | 41.1% | NS |
| Hypertension (%) | 38.4% | 42.2% | 63.5% | 84.5% | P<0.01 |
| CVD (%) | 12.5% | 14.3% | 22.7% | 39.5% | NS |
| SBP (mmHg) | 130.8±16.5 | 135.6±15.9 | 145.0±17.3 | 151.4±19.2 | NS |
| DBP (mmHg) | 72.1±12.7 | 71.4±11.4 | 77.2±13.7 | 85.7±15.2 | NS |
| Albumin (g/cIL) | 4.2±0.2 | 4.1±0.2 | 3.8±0.2 | 3.9±0.3 | NS |
| Hemoglobin (g/L) | 11.6±1.7 | 11.9±1.8 | 9.8±1.6 | 10.4±1.5 | P<0.01 |
| Hematocrit (%) | 33.9±5.4 | 34.9±5.4 | 29.6±5.0 | 31.1±4.7 | P=0.03 |
| BUN (mg/dL) | 26.5±8.6 | 40.4±11.7 | 62.5±24.3 | 78.2±14.2 | P<0.01 |
| Creatinine (mg/dL) | 1.8±0.5 | 2.8±0.8 | 6.8±2.7 | 11.1±2.2 | P<0.01 |
| eGFR (ml/min) | 39.2±10.8 | 21.1±4.4 | 9.5±9.4 | 4.5±1.1 | P<0.01 |
| Calcium (mg/dL) | 9.2±0.3 | 9.1±0.4 | 8.9±0.7 | 9.0±0.7 | NS |
| Phosphate (mg/dL) | 4.1±0.5 | 4.3±0.6 | 4.9±0.9 | 5.3±1.5 | P<0.01 |
| Indoxyl sulfate (mg/L) | 3.2±3.0 | 5.4±3.6 | 19.9±10.5 | 42.5±15.6 | P<0.01 |
| p‐Cresylsulfate (mg/L) | 4.8±4.3 | 5.9±5.6 | 12.4±7.9 | 21.8±12.4 | P<0.01 |
Continuous variables are expressed in mean±SD and the categorical variables in percentage of cases (%). ANOVA was used for continuous variables and χ2 test for categorical variables. SBP, systolic blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure.
Figure 1 shows serum level of indoxyl sulfate (A) and p‐cresylsulfate (B), increased gradually with the decrease of renal function and reached the highest level in CKD stage 5 HD group. The linear regression analysis indicated a strong negative correlation of indoxyl sulfate and p‐cresylsulfate and eGFR in CKD population, including HD patients (r=−0.687, P<0.001 and r=−0.535, P<0.001, respectively) (Fig. 2). The result was not significantly different after excluding HD patients (Fig. 3).
Figure 1.
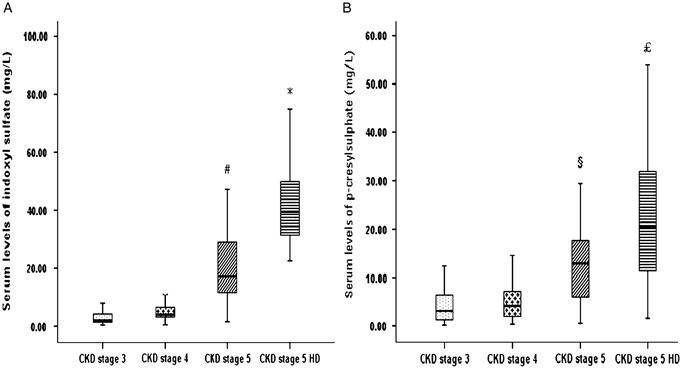
Serum levels of (A) indoxyl sulfate and (B) p‐cresylsulfate in patients with different CKD stages. ♯ P<0.05 vs. CKD stage 3 and 4; * P<0.05 vs. CKD stage 3, 4, and 5; § P<0.05 vs. CKD stage 3 and 4; £ P<0.05 vs. CKD stage 3, 4, and 5. Estimated GFR (mL/min/1.73 m2) of an individual patient was calculated using modified MDRD equation. Stage 3: 30–59 mL/min/1.73 m2; stage 4: 15–29 mL/min/1.73 m2; stage 5: <15 mL/min/1.73 m2; HD, hemodialysis.
Figure 2.
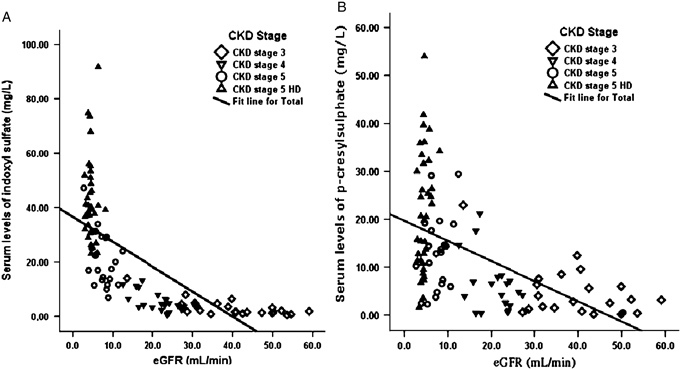
Linear regression curves. The relationship between (A) serum p‐cresylsulfate level (n=103) and (B) indoxyl sulfate (n=103) and the GFR in patients at different CKD Stages, 3–5 stage and HD (r=−0.687, P<0.001 and r=−0.535, P<0.001, respectively).
Figure 3.
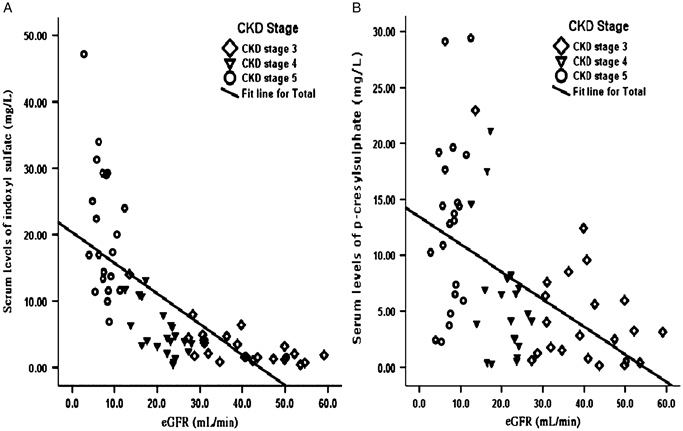
Linear regression curves. The relationship between (A) serum indoxyl sulfate level (n=69) and (B) p‐cresylsulfate level (n=69) and the GFR in patients at different CKD Stages, 3–5 stage (r=−0.708, P<0.001 and r=−0.511, P<0.001, respectively).
Table 3 revealed the correlations between serum indoxyl sulfate and p‐cresylsulfate level and clinical variables. Serum level of indoxyl sulfate and p‐cresylsulfate did not correlate to diabetes, sex, age, albumin, and calcium. Both indoxyl sulfate and p‐cresylsulfate correlated inversely with hemoglobin and hematocrit level (r=−0.23, P<0.05; r=−0.33, P<0.01; r=−0.22, P<0.05; r=−0.35, P<0.01; respectively). The two uremic toxins were positively associated with the serum level of BUN and creatinine (r=0.68, P<0.01; r=0.49, P<0.01; r=0.84, P<0.05; r=0.55, P<0.01; respectively) and negatively related to eGFR (r=−0.7, P<0.01; r=−0.54, P<0.01). However, after adjusting confounding factors by multivariate regression analysis, only serum creatinine had significant association with indoxyl sulfate and p‐cresylsulfate (B=3.59, P<0.01; B=0.93, P=0.04; respectively) (Table 4).
Table 3.
Correlations Between Serum Indoxyl Sulfate and p‐Cresylsulfate Level and Baseline Clinical and Biochemical Characteristics
| Indoxyl sulfate | p‐Cresylsulfate | |||
|---|---|---|---|---|
| Characteristic | r | P | r | P |
| DM | −0.02 | NS | 0.06 | NS |
| Sex | −0.04 | NS | 0.00 | NS |
| Age | 0.02 | NS | 0.09 | NS |
| Albumin | 0.02 | NS | 0.08 | NS |
| Hemoglobin | −0.23 | <0.05 | −0.33 | <0.01 |
| Hematocrit | −0.22 | <0.05 | −0.35 | <0.01 |
| BUN | 0.68 | <0.01 | 0.49 | <0.01 |
| Creatinine | 0.84 | <0.01 | 0.55 | <0.01 |
| eGFR | −0.70 | <0.01 | −0.54 | <0.01 |
| Calcium | −0.04 | NS | −0.19 | NS |
| Phosphate | 0.42 | <0.01 | 0.20 | 0.04 |
NS, no significance.
Table 4.
Multivariate Linear Regression Analysis for Evaluating the Relationship Between Independent Variables and Serum Indoxyl Sulfate and p‐Cresylsulfate in CKD Patients
| Multivariate linear regression analysis | ||||||
|---|---|---|---|---|---|---|
| Indoxyl sulfate | p‐Cresylsulfate | |||||
| Adjusted B | 95% CI | P‐value | Adjusted B | 95% CI | P‐value | |
| Hematocrit | −0.23 | −0.69–0.22 | NS | −0.38 | −0.78–0.01 | NS |
| Hemoglobin | −0.22 | −0.67–0.21 | NS | −0.37 | −0.77–0.01 | NS |
| BUN | 0.06 | −0.09–0.22 | NS | 0.03 | −0.10–0.16 | NS |
| Creatinine | 3.59 | 2.56–4.62 | <0.01 | 0.93 | 0.03–1.80 | 0.04 |
| eGFR | 0.01 | −0.32–0.33 | NS | −0.15 | −0.43–1.33 | NS |
| Phosphate | −0.43 | −2.69–1.83 | NS | −0.91 | −2.88–1.00 | NS |
NS, no significance.
Figure 4 shows that indoxyl sulfate level was closely related to p‐cresylsulfate level and a linear correlation between the two toxins (r=0.61, P<0.01). In addition, we also compared serum level of the two toxins with another cohort. It showed that patients with CKD in Asia had higher serum indoxyl sulfate than those in the West (P<0.01). On the contrary, serum p‐cresylsulfate level was higher in the West patients than in the Asian patients (P<0.01) (Table 5).
Figure 4.
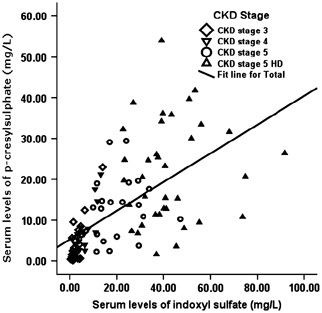
The relationship between serum p‐cresylsulfate level and indoxyl sulfate in patients at different CKD Stages, 3–5 and HD stage (r=0.61, P<0.01).
Table 5.
Comparisons of Serum Indoxyl Sulfate and p‐Cresylsulfate in Different Cohorts
| Asians* (n=103) | Caucasians♯ (n=139) | P‐value | |
|---|---|---|---|
| Indoxyl sulfate (mg/L) | 21.1±20 | 8.8±9.8 | <0.01 |
| p‐Cresylsulfate (mg/L) | 12.8±11.5 | 18.9±17.3 | <0.01 |
*Measured by UPLC, ♯Measured by RP‐HPLC. Both two cohorts containing predialysis and dialysis patients.
DISCUSSION
In our study, we found that both serum indoxyl sulfate and p‐cresylsulfate level increased gradually as renal function declined and reached a peak at the CKD stage 5 on HD. In addition, serum indoxyl sulfate level was closely associated with p‐cresylsulfate level in CKD patients.
Uremic toxins accumulate and lead to uremic syndrome as GFR declines. Indoxyl sulfate and p‐cresylsulfate, the two protein‐bound uremic toxins, have been demonstrated to have a strong association with poor clinical outcomes in dialysis patients 13, 14, 15. Owing to the high affinity of indoxyl sulfate and p‐cresol for serum albumin, these toxins cannot be effectively eliminated with regular dialysis. Subsequently, the accumulated toxins will result in adverse effects, including all‐cause mortality, cardiovascular events, and infection events. Previous studies were mainly focused on HD patients. However, we are interested in extending patient group from HD to CKD patients. In this study, we demonstrated that serum level of indoxyl sulfate and p‐cresylsulfate had significantly increased on CKD stage 5 HD. This indicated the importance of residual renal function on excretion of protein‐bound uremic toxins. It also reflected that patients, during the early stage of CKD, still had enough ability to excrete both toxins. Our results were consistent with the findings published recently by Dr Barreto 16 and Liabeuf 17. Thus, CKD patients with high serum levels of indoxyl sulfate and p‐cresol may have predisposition of having adverse clinical sequelae.
Nevertheless, clinicians are interested in how to reduce the concentrations of these two toxins in vivo. Preservation of residual function is the key factor to lower serum protein‐bound toxins. Bammens et al. reported that dialysis patients with convection had lower p‐cresol level than that those with high flux dialysis 18. Recently, one study reported that neither HD nor hemodiafiltration can effectively remove serum p‐cresylsulfate and indoxyl sulfate 19. In addition, serum level of the two toxins seemed to be lower in peritoneal than HD patients 20. The possible reasons could not be well explained, based on current evidences. Better preservation of residual renal function in peritoneal dialysis patients may partially explain this point.
Furthermore, the serum levels of indoxyl sulfate and p‐cresol were known to be influenced by intestinal microbial growth, dietary habit, and bowel habit. From our results, it showed that serum levels of p‐cresylsulfate and indoxyl sulfate were significantly different in Asians and Caucasians, which might reflect different dietary habits and analytic methods. The bowel habit was another possible source of influencing the two toxins absorption, which also changed with aging. Our previous study demonstrated that age was not an independent factor to affect serum p‐cresylsulfate and indoxyl sulfate 21. Currently, two main therapeutic strategies have been proposed: First, interventions that modulated intestinal bacterial growth, such as probiotics and prebiotics. One small study has revealed that urinary p‐cresol was reduced by a mixture of inulin and fructo–oligosaccharides in healthy volunteers 22. Another prospective phase I/II study indicated 4 weeks of oligofructose inulin significantly lowered serum p‐cresylsulfate concentration in HD patients 23. Second, use of adsorbent that reduces the adsorption of uremic toxins. AST‐120, a kind of oral charcoal absorbent, was reported to have the effect of lowering the serum indoxyl sulfate level 24. Sevelamer hydrochloride, a noncalcium‐based phosphate binder, has been shown to bind indole and p‐cresol in vitro 25. It was another potential absorbent, although it could not lower the serum level of indoxyl sulfate and p‐cresol in an animal study 26. Whether treatment with Sevelamer hydrochloride could reduce the two serum toxins level in vivo is still unknown.
In addition, based on our results, serum indoxyl sulfate had positive correlation with serum p‐cresylsulfate. The result did not change even in HD patients (data not shown). It is not difficult to understand this point, as the two toxins have similar origins and were removed mainly depended on the renal function. However, one study, with 75 HD patients published recently, revealed that serum Indoxl sulfate did not relate to the p‐cresylsulfate level 27. The definite reason is unclear. Further studies are required to elucidate this disparity.
In conclusion, our study indicates that both indoxyl sulfate and p‐cresylsulfate level increased as renal function declined. Serum indoxyl sulfate level was positively related to presylsulfate level in CKD patients.
REFERENCES
- 1. Vanholder R, De Smet R. Pathophysiologic effects of uremic retention solutes. J Am Soc Nephrol 1999;10:1815–1823. [DOI] [PubMed] [Google Scholar]
- 2. Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 2003;63:1934–1943. [DOI] [PubMed] [Google Scholar]
- 3. Niwa T, Nomura T, Sugiyama S, et al. The protein metabolite hypothesis, a model for the progression of renal failure: An oral sorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int 1997;52:S23–S28. [PubMed] [Google Scholar]
- 4. Dou L, Bertrand E, Cerini C, et al. The uremic solutes p‐cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 2004;65:442–451. [DOI] [PubMed] [Google Scholar]
- 5. Taki K, Tsuruta Y, Niwa T. Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am J Nephrol 2007;27:30–35. [DOI] [PubMed] [Google Scholar]
- 6. De Loor H, Bammens B, Evenepoel P, et al. Gas chromatographic‐mass spectrometric analysis for measurement of p‐cresol and its conjugated metabolites in uremic and normal serum. Clin Chem 2005;51:1535–1538. [DOI] [PubMed] [Google Scholar]
- 7. Martinez AW, Recht NS, Hostetter TH, et al. Removal of P‐cresol sulfate by hemodialysis. J Am Soc Nephrol 2005;16:3430–3436. [DOI] [PubMed] [Google Scholar]
- 8. Wratten ML, Tetta C, De Smet R, et al. Uremic ultrafiltrate inhibits platelet‐activating factor synthesis. Blood Purif 1999;17:134–141. [DOI] [PubMed] [Google Scholar]
- 9. Vanholder R, De Smet R, Waterloos MA, et al. Mechanisms of uremic inhibition of phagocyte reactive species production: characterization of the role of p‐cresol. Kidney Int 1995;47:510–517. [DOI] [PubMed] [Google Scholar]
- 10. Dou L, Cerini C, Brunet P, et al. P‐cresol, a uremic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney Int 2002;62:1999–2009. [DOI] [PubMed] [Google Scholar]
- 11. De Smet R, Van Kaer J, Van Vlem B, et al. Toxicity of free p‐cresol: A prospective and cross‐sectional analysis. Clin Chem 2003;49:470–478. [DOI] [PubMed] [Google Scholar]
- 12. Schepers E, Meert N, Glorieux G, et al. P‐cresylsulphate, the main in vivo metabolite of p‐cresol, activates leucocyte free radical production. Nephrol Dial Transplant 2007;22:592–596. [DOI] [PubMed] [Google Scholar]
- 13. Bammens B, Evenepoel P, Keuleers H, et al. Free serum concentrations of the proteinbound retention solute p‐cresol predict mortality in hemodialysis patients. Kidney Int 2006;69:1081–1087. [DOI] [PubMed] [Google Scholar]
- 14. Meijers BK, Bammens B, De Moor B, et al. Free p‐cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 2008;73:1174–1180. [DOI] [PubMed] [Google Scholar]
- 15. Lin CJ, Wu CJ, Pan CF, et al. Serum protein‐bound uremic toxins and clinical outcomes in hemodialysis patients. Nephrol Dial Transplant 2010;25:3693–3700. [DOI] [PubMed] [Google Scholar]
- 16. Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 2009;4:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liabeuf S, Barreto DV, Barreto FC, et al. Free p‐cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010;25:1183–1191. [DOI] [PubMed] [Google Scholar]
- 18. Bammens B, Evenepoel P, Verbeke K, et al. Removal of the protein‐bound solute p‐cresol by convective transport: a randomized crossover study. Am J Kidney Dis 2004;44:2278–2285. [DOI] [PubMed] [Google Scholar]
- 19. Krieter DH, Hackl A, Rodriguez A, et al. Protein‐bound uraemic toxin removal in haemodialysis and post‐dilution haemodiafiltration. Nephrol Dial Transplant 2010;25:212–218. [DOI] [PubMed] [Google Scholar]
- 20. Bammens B, Evenepoel P, Verbeke K, et al. Time profiles of peritoneal and renal clearances of different uremic solutes in incident peritoneal dialysis patients. Am J Kidney Dis 2005;46:512–519. [DOI] [PubMed] [Google Scholar]
- 21. Lin CJ, Wu CJ, Pan CF, et al. Serum concentration of p‐cresol and indoxyl sulfate in elderly hemodialysis patients. Int J Gerontol 2010; in press [Google Scholar]
- 22. De Preter V, Vanhoutte T, Huys G, et al. Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose‐enriched inulin on colonic nitrogen–protein metabolism in healthy humans. Am J Physiol Gastrointest Liver Physiol 2007;292:G358–G368. [DOI] [PubMed] [Google Scholar]
- 23. Meijers BK, De Preter V, Verbeke K, et al. P‐Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose‐enriched inulin. Nephrol Dial Transplant 2010;25:219–224. [DOI] [PubMed] [Google Scholar]
- 24. Fujii H, Nishijima F, Goto S, et al. Oral charcoal adsorbent (AST‐120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant 2009;24:2089–2095. [DOI] [PubMed] [Google Scholar]
- 25. De Smet R, Thermote F, Lamiere N. Sevelamer hydrochloride adsorbs the uremic compound indoxyl sulfate [abstract]. J Am Soc Nephrol 2003;14:206A. [Google Scholar]
- 26. Phan O, Ivanovski O, Nguyen‐Khoa T, et al. Sevelamer prevents uremiaenhanced atherosclerosis progression in apolipoprotein E‐deficient mice. Circulation 2005;112:2875–2882. [DOI] [PubMed] [Google Scholar]
- 27. Meijers BK, De Loor H, Bammens B, et al. P‐Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin J Am Soc Nephrol 2009;4:1932–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]


