Abstract
Oleander poisoning can be detected by digoxin immunoassays and for last two decades the fluorescence polarization immunoassay (FPIA) has been used for rapid detection of oleander poisoning in clinical laboratories. Recently, Abbott Laboratories (Abbott Park, IL) discontinued this assay. Therefore, we explored the possibility of using another digoxin assay (Dimension Vista Flex Reagent Cartridge, Tina Quant, EMIT 2000 and old FPIA assay for comparison) for rapid detection of oleander poisoning. When aliquots of drug‐free serum pools were supplemented with pure oleandrin or oleander extract, we observed the highest apparent digoxin values using Dimension Vista digoxin assay (Flex Reagent Cartridge). We also observed significant apparent digoxin values in vivo in sera of mice both 1 and 2 hr after feeding with oleander extract. When a serum pool prepared from patients taking digoxin was further supplemented with various amounts of oleander extract, the highest falsely elevated digoxin values were observed with Dimension Vista digoxin assay. Monitoring free digoxin using Dimension Vista digoxin assay (Flex Reagent Cartridge) did not eliminate this interference. Digibind neutralized digoxin‐like factors of oleander extract and such effect can be monitored by observing significant reduction in apparent free digoxin levels in the presence of Digibind as measured in the protein‐free ultrafiltrate using Dimension Vista digoxin assay (Flex Reagent Cartridge). J. Clin. Lab. Anal. 25:105–109, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: oleander, digoxin, interference, dimension vista Digoxin Assay
Oleanders (Nerium oleander) are evergreen ornamental shrubs with various colors of flowers that grow in the Southern parts of the United States from Florida to California, Australia, India, Sri Lanka, China and also other parts of the world. All parts of oleander plants are toxic. Human exposure to oleander occurs by accidental exposure, ingestion by children, administration in food or drink and for medicinal (as herbal supplements) as well as criminal poisoning 1, 2, 3, 4, 5. A report described how a 41‐year‐old man purchased oleander seeds through the Internet for intentional poisoning 6. Boiling or drying the plant does not inactivate oleandrin, the major toxin of the oleander. Death from drinking herbal tea containing oleander has been reported 7. Ingestion of oleander may cause nausea, vomiting, abdominal pain, diarrhea, dysthymias, and hyperkalemia. Clinical management of poisoning involves the administration of activated charcoal and supportive care. Digoxin‐specific Fab fragment (Digibind) is also effective in treating acute oleander poisoning 8.
Oleandrin, the major glycoside in oleander, can be detected in blood by high‐performance liquid chromatography and mass spectrometry (HPLC/MS) 9. However, this technique is complex and expensive. Therefore, this analyzer is not available in many hospital laboratories. Moreover, due to complex method of analysis (extraction of oleandrin from blood, derivatization and analysis), HPLC/MS methods require significant time and results are not available on the STAT basis. Cheung et al. reported the detection of poisoning by substances of plant origin (including oleander) using the fluorescence polarization immunoassay for digoxin (FPIA) and the TDx analyzer 10. Osterloh et al. reported an apparent digoxin level of 5.8 ng/ml after suicidal ingestion of oleander tea in a patient with no history of taking any cardioactive drug. The person eventually died from oleander toxicity 11. Eddleston et al. reported a mean apparent serum digoxin concentration of 1.49 nmol/l (1.16 ng/ml) using the FPIA digoxin assay in patients who were poisoned with oleander but eventually discharged from the hospital 12. We also reported the highest cross‐reactivity of oleandrin with the FPIA assay and moderate cross‐reactivity with Beckman SYNCHRON digoxin assay 13. Recently, Abbott Laboratories have discontinued the FPIA digoxin assay. Therefore, we explored the possibility of rapidly detecting oleander poisoning using another commercially available digoxin immunoassay. In this study, we used four different immunoassays: Flex Reagent Cartridge digoxin assay, FPIA, EMIT, and Tina Quant. In our previous report, we used Beckman SYNCHRON digoxin assay, MEIA (Microparticle Enzyme Immunoassay for digoxin for application on the AxSYM analyzer, Abbott Laboratories), FPIA, a chemiluminescent assay as well as turbidimetric digoxin assay on the ADVIA analyzer (Bayer Diagnostics, now a part of Siemens Diagnostics) 13. In this study, we used FPIA assay (as also used in our previous study) for comparison purpose only because we observed highest cross‐reactivity with oleandrin using FPIA consistent with other published reports in the literature as cited earlier. However, cross‐reactivities of oleander extract and active ingredient oleandrin with EMIT, Tina Quant, and Flex Reagent Cartridge digoxin assay have never been reported before and in this study we compared cross‐reactivities of oleander extract and oleandrin with these assays. In this study, we report our findings on rapid detection of oleander poisoning using the Flex Reagent Cartridge digoxin assay using Dimension Vista 1500 analyzer.
MATERIALS AND METHODS
Oleander plant was obtained from a local nursery. Oleandrin was purchased from Sigma Chemical Company (St. Louis, MO). Digoxin concentrations were measured using the Dimension Vista digoxin assay (Flex Reagent Cartridge) on the Dimension Vista 1500 analyzer using manufacturer's protocol (Siemens Diagnostics, Deerfield, IL). We also used Tina Quant digoxin assay (on Modular P 800 platform), EMIT 2000 digoxin assay (Hitachi 917 Platform) both available from Roche Diagnostics (Indianapolis, IN) as well as FPIA digoxin assay (fluorescence polarization immunoassay on TDx/FLx platform) for comparison. During this study, we used a kit we already had in our laboratory. This assay is no longer commercially available from the Abbott Laboratories.
The FPIA digoxin assay requires a sample pretreatment. The assay was linear up to a serum digoxin concentration of 5.0 ng/ml and the detection limit of the assay is 0.20 ng/ml. Other digoxin assays require no specimen pretreatment. The analytical measurement range of Dimension Vista digoxin assay with Flex reagent Cartridge is 0.1–5.0 ng/ml.
We prepared the stock solution of pure oleandrin in ethyl alcohol (1 mg/ml). Then this stock solution was diluted further to prepare two working solutions of oleandrin (final concentrations; 0.1 and 0.01 mg/ml). An ethyl alcohol extract of oleander leaf was prepared by mixing 500 mg of dry weight of leaf with 5 ml of absolute ethyl alcohol followed by mixing in a blender. Then aliquots of drug‐free serum pools were supplemented with either oleander extract or pure oleandrin. In order to ensure that small amount of ethyl alcohol does not interfere with various digoxin immunoassays, we added microliter quantities of either oleandrin working solution or oleander extract in a dry test tube and then evaporated ethyl alcohol at room temperature under a gentle stream of nitrogen. Then the dry residue was reconstituted with either an aliquot of drug‐free serum or serum digoxin pool. Apparent digoxin levels were measured using all four digoxin assays. Each measurement was performed in triplicate, and values were expressed as the mean and one standard deviation.
The digoxin serum pool was prepared by combining serum specimens from patients receiving digoxin after removing patient identities. These specimens are routinely submitted to our laboratory for therapeutic drug monitoring. Left‐over specimens were used after performing and reporting all results to the ordering clinicians after holding these specimens for 1 week as required by our laboratory protocol. These specimens would otherwise be discarded.
In order to investigate whether the Dimension Vista digoxin assay can detect oleander poisoning in vivo, we used a mouse model experiments (a total five mice were used in this experiment). We fed mice with either 20 μg of pure oleandrin (dissolved in saline containing 10% ethyl alcohol) or the oleander extract used in Experiment 1 which was diluted 1:10 using water (reducing ethyl alcohol concentration to 10% by vol). Then 100 μl of diluted extract was introduced to mice orally by a gavage procedure. Each mouse weighs approximately 25 g (Balb‐C mouse; Harlan, Houston, TX). Blood was withdrawn 1 and 2 hr after feeding with oleander and apparent digoxin concentrations were measured using Dimension Vista assay (Flex Reagent Cartridge). No duplicate measurement was possible due to limited specimen volume.
Because digoxin‐like factors of oleander are strongly protein bound 14, we studied the possibility of overcoming interference of oleander in the Dimension Vista assay (Flex Reagent cartridge) by measuring free digoxin in the protein‐free ultrafiltrate. For this purpose, aliquots of the digoxin pool were further supplemented with oleander extract and then both total and free digoxin concentration were measured. Protein‐free ultrafiltrate was prepared by centrifuging each specimen using Centrifree Micropartion System filter (Amicon, Danvers, MA) for 30 min at 1,500×g.
We also investigated the suitability of Dimension Vista digoxin assay (Flex Reagent Cartridge) to detect free apparent digoxin concentrations in specimens treated with Digibind. For this purpose, aliquots of another drug‐free serum pool were supplemented with oleander extract and then further supplemented with 0, 5, 10, and 20 μg/ml of Digibind (expected concentrations of Digibind in vitro in patients overdosed with digoxin and being treated with Digibind). Then specimens were incubated at 37°C for 30 min followed by the measurement of both total and free digoxin using Dimension Vista digoxin assay.
Statistical analyses were carried out using student t‐test, two tailed. We considered a difference significant only at 95% confidence interval or higher (P<0.05).
RESULTS
We observed the highest apparent digoxin concentrations using the Dimension Vista digoxin assay when aliquots of drug‐free serum were supplemented with pure oleandrin or oleander extract. The apparent digoxin values observed with the Dimension Vista digoxin assay (Flex Reagent Cartridge) were comparable to values obtained using the FPIA assay. For example, when an aliquot of drug‐free serum was supplemented with 250 ng/ml of oleandrin, the observed apparent digoxin concentrations obtained by Dimension Vista, FPIA, EMIT, and Tina Quant digoxin assays were 1.2, 1.3, 0.9, and none detected, respectively (Table 1). Because of the highest cross‐reactivity of the Dimension Vista digoxin assay with oleandrin and also with oleander extract where oleandrin is the active component, we used only this assay to investigate apparent digoxin concentrations in sera of mice after feeding with oleander extract. Small amount of blood available from each mouse prevented us to make parallel comparison of apparent digoxin concentrations using all four digoxin assays. As expected, when mice were fed with oleander significant apparent digoxin concentrations were observed 1 hr after feeding using the Dimension Vista digoxin assay (Flex Reagent Cartridge) (Table 2). The digoxin‐like factors present in sera of mice after feeding with oleander demonstrated a short half‐life of approximately 1 hr, but accurate calculation of half‐life is not possible because we did not directly determine the oleandrin concentration and cross‐reactivity of oleandrin with the Dimension vista digoxin assay (Flex Reagent Cartridge) did not show a linear correlation.
Table 1.
Apparent Digoxin Concentrations After Supplanting Aliquots of Drug‐Free Serum With Oleandrin or Oleander Extract
| Apparent digoxin, ng/ml, Mean (SD), n=3 | ||||
|---|---|---|---|---|
| Specimen | Vista (Flex Reagent Cartridge) | FPIA | EMIT | Tina Quant |
| Drug‐free serum | ND | ND | ND | ND |
| 50 ng/ml Oleandrin | 0.7 (0.00) | 0.6 (0.04) | 0.5 (0.04) | ND |
| 100 ng/ml Oleandrin | 0.9 (0.00) | 1.0 (0.03) | 0.6 (0.02) | ND |
| 250 ng/ml Oleandrin | 1.2 (0.06) | 1.3 (0.05) | 0.9 (0.03) | ND |
| 500 ng/ml Oleandrin | 1.6 (0.10) | 1.5 (0.02) | 1.2 (0.01) | 0.3 (0.00) |
| 1 μg/ml Oleandrin | 2.2 (0.06) | 2.3 (0.03) | 1.8 (.009) | 0.7 (0.06) |
| 5 μg/ml Oleandrin | 3.5 (0.06) | 3.6 (0.15) | 2.6 (0.07) | 1.0 (0.06) |
| 1 μl/ml Oleander | 0.9 (0.00) | 0.9 (0.03) | 0.8 (0.05) | ND |
| 2.5 μl/ml Oleander | 1.4 (0.10) | 1.3 (0.05) | 1.0 (0.06) | 0.2 (0.00) |
| 5 μl/ml Oleander | 1.6 (0.10) | 1.7 (0.05) | 1.3 (0.04) | 0.3 (0.10) |
Table 2.
Apparent Digoxin Concentrations (Measured by Dimension Vista Digoxin Assay; Flex Reagent Cartridge) After Feeding Mice With Oleander Extract
| Apparent digoxin concentration, ng/ml | ||
|---|---|---|
| Mouse ♯ (Dosage) | 1 hr after feeding | 2 hr after feeding |
| 1 (20 μg Oleandrin) | 2.0 | 1.2 |
| 2 (20 μg Oleandrin) | 1.8 | 0.9 |
| 3 (20 μg Oleandrin) | 2.2 | 1.4 |
| 4 (Diluted oleander extract) | 1.3 | 0.8 |
| 5 (Diluted oleander extract) | 1.1 | 0.6 |
Rigorous characterization of immunoassay interference due to a cross reactant should be performed in the presence of the primary analyte 15. Therefore, we studied cross‐reactivity of oleandrin with the digoxin immunoassays in the presence of the primary analyte digoxin. When aliquots of a digoxin pool were further supplemented with oleander extract, most significant positive interferences were observed using the Dimension Vista digoxin (Flex Reagent Cartridge) and FPIA assays. EMIT digoxin assay due to significant cross‐reactivity with oleandrin also showed both statistically and clinically significant increases in serum digoxin values in the presence of various amounts of oleandrin or oleander extract. Tina Quant assay was minimally affected. For example, when an aliquot of the serum digoxin pool was further supplemented with 100 ng/ml of oleandrin, the observed digoxin values using Dimension Vista (Flex Reagent Cartridge), FPIA, EMIT, and Tina Quant assay were 1.8, 1.9, 1.5, and 1.1 ng/ml, respectively, while the digoxin concentration in the original pool was 1.0 ng/ml (Table 3).
Table 3.
Effect of Oleander Extract on Serum Digoxin Values as Measured by the FPIA, Digoxin II and Digoxin III Assay
| Digoxin, ng/ml, Mean (SD), n=3 | ||||
|---|---|---|---|---|
| Specimen | Vista (Flex Reagent Cartridge) | FPIA | EMIT | Tina Quant |
| Digoxin Pool | 1.1 (0.06) | 1.0 (0.03) | 1.0 (0.16) | 1.0 (0.06) |
| +50 ng/ml Oleandrin | 1.6 (0.00) | 1.5 (0.05) | 1.3 (0.05) | 0.9 (0.06) |
| +100 ng/ml Oleandrin | 1.8 (0.00) | 1.9 (0.06) | 1.5 (0.13) | 1.1 (0.06) |
| +250 ng/ml Oleandrin | 2.0 (0.06) | 1.9 (0.04) | 1.7 (0.04) | 1.2 (0.06) |
| +500 ng/ml Oleandrin | 2.6 (0.00) | 2.7 (0.10) | 2.0 (0.06) | 1.2 (0.00) |
| +1 μg/ml Oleandrin | 3.0 (0.06) | 3.1 (0.07) | 2.5 (0.04) | 1.4 (0.06) |
| +5 μg/ml Oleandrin | 4.4 (0.06) | 4.3 (0.12) | 3.5 (0.07) | 1.5 (0.06) |
| +1 μl/ml Oleander | 1.2 (0.06) | 1.3 (0.03) | 1.2 (0.08) | 0.9 (0.00) |
| +2.5 μl/ml Oleander | 1.7 (0.06) | 1.7 (0.05) | 1.5 (0.06) | 1.1 (0.06) |
| +5 μl/ml Oleander | 2.4 (0.06) | 2.5 (0.07) | 1.9 (0.01) | 1.2 (0.06) |
Because digoxin‐like immunoreactive components of oleander are strongly protein bound, we studied the possibility of eliminating interference of oleander in Dimension Vista digoxin assay by monitoring free digoxin instead of total digoxin. Unfortunately due to high cross‐reactivity of oleandrin with the antibody used in the Dimension Vista digoxin assay (Flex Reagent Cartridge), elimination of this interference using monitoring free digoxin concentration is not feasible (Fig. 1).
Figure 1.
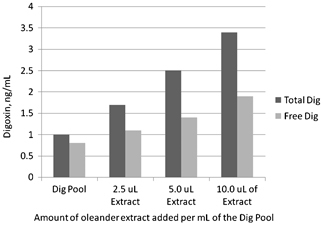
Total and free digoxin when aliquots of a digoxin pool are further supplemented with various amount of oleander extract. Both total and free digoxin concentrations were measured by using Dimension Vista digoxin assay (Flex Reagent Cartridge).
Digibind can neutralize free digoxin‐like components of oleander extract and such a process can be monitored by measuring free apparent digoxin concentration using the Dimension Vista digoxin assay (Flex Reagent cartridge). For example, when a drug‐free pool was supplemented with 20 μl of oleander extract to achieve an apparent digoxin concentration of 4.5 ng/ml, the observed free apparent digoxin concentration was 0.9 ng/ml. When an aliquot of this pool was supplemented with Digibind to achieve a Digibind concentration of 5.0 μg/ml, the apparent free digoxin concentration was significantly reduced to 0.3 ng/ml, indicating that digibind can neutralize free digoxin‐like components of oleander extract and such an effect can be monitored by measuring free digoxin. With higher 20 μg/ml of Digibind, no apparent digoxin concentration was observed in the protein‐free ultrafiltrate (Fig. 2).
Figure 2.
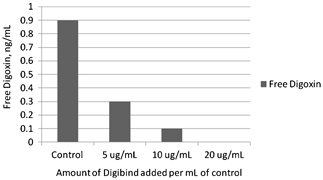
Effect of adding various amounts of Digibind in aliquots of oleander pool prepared by adding oleander extract to drug‐free serum on free digoxin concentrations. Free digoxin concentrations were measured by using Dimension Vista digoxin assay (Flex Reagent Cartridge).
DISCUSSION
Outbreaks of oleander poisoning in livestock are common 16. In Sri Lanka, oleander poisoning is a common method of suicide 17. Oleander poisoning was responsible for 7% of all admissions to the toxicology intensive‐care unit in a regional hospital in Tunisia 18. As mentioned earlier in the introduction section, FPIA has been used for past two decades for rapid detection of oleander poisoning. Roberts et al. used the FPIA digoxin assay for studying pharmacokinetics of oleander in poisoned patients 19. In recent years, the use of complementary and herbal medicines has become more popular with the general population and several herbal products also containing oleander extract. Because FPIA assay is no longer commercially available, an alternative digoxin assay for indirect but rapid detection of oleander poisoning would be helpful.
For our studies, we selected oleandrin concentrations based on oleandrin concentration used by other investigators in their studies for investigating how oleandrin cross‐reacts with various digoxin assays 10, 13, 14. Our investigation clearly indicates that Dimension Vista digoxin assay has comparable sensitivity as FPIA assay for rapidly detecting oleander poisoning. In addition, Dimension Vista digoxin assay (Flex Reagent Cartridge) does not require specimen pretreatment like the FPIA and is more rapid and easier to use than the FPIA digoxin assay. EMIT digoxin assay is also sensitive for rapid detection of oleander poisoning and demonstrated second best cross‐reactivity with oleandrin. In addition, Dimension Vista digoxin (Flex Reagent Cartridge) assay can also be used to monitor progress of Digibind therapy in a patient poisoned with oleandrin and being treated with Digibind. However, both Dimension Vista (Flex Reagent Cartridge) assay and EMIT digoxin assay are unsuitable for therapeutic drug monitoring of digoxin in a patient also receiving herbal supplement containing oleander extract.
REFERENCES
- 1. Shawn D, Pearn J. Oleander poisoning. Med J Aust 1979;2:267–269. [DOI] [PubMed] [Google Scholar]
- 2. Blum LM, Reiders F. Oleander distribution in a fatality from rectal and oral Nerium oleander extract administration. J Anal Toxicol 1987;82:121–122. [DOI] [PubMed] [Google Scholar]
- 3. Saravanapavananthan N, Ganeshamoorthy J. Yellow oleander poisoning: A case study of 170 cases. Forensic Sci Int 1988;36:247–250. [DOI] [PubMed] [Google Scholar]
- 4. Brewster D. Herbal poisoning: A case report of fetal yellow oleander poisoning from the Solomon Island. Ann Trop Paediatr 1986;6:289–291. [DOI] [PubMed] [Google Scholar]
- 5. Langford S, Boor PJ. Oleander toxicity: An examination of human and animal toxic exposure. Toxicology 1999;109:1–13. [DOI] [PubMed] [Google Scholar]
- 6. Arachchillage DR, Hewapathirana N, Fernando DJ. The role of the Internet in facilitating yellow oleander poisoning and in providing effective treatment. Eur J Intern Med 2007;18:167. [DOI] [PubMed] [Google Scholar]
- 7. Haynes BE, Bessen HA, Wightman WD. Oleander tea: Herbal draught of death. Ann Emerg Med 1985;14:350–353. [DOI] [PubMed] [Google Scholar]
- 8. Bandara V, Weinstein SA, White J, Eddleston M. A review of natural history, toxinology, diagnosis and clinical management of Nerium oleander (common oleander) and Thevetia peruviana (yellow oleander) poisoning. Toxicon 2010;56:273–281. [DOI] [PubMed] [Google Scholar]
- 9. Tracqui A, Kintz P, Branche F, Ludes B. Confirmation of oleander poisoning by HPLC/MS. Int J Legal Med 1998; 111:32–34. [DOI] [PubMed] [Google Scholar]
- 10. Cheung K, Hinds JA, Duffy P. Detection of poisoning by plant origin cardiac glycosides with the Abbott TDx analyzer. Clin Chem 1989;35:295–297. [PubMed] [Google Scholar]
- 11. Osterloh J. Cross‐reactivity of oleander glycosides. J Anal Toxicol [Letter] 1988;12:53. [DOI] [PubMed] [Google Scholar]
- 12. Eddleston M, Ariaratnam CA, Sjostrom L, et al. Acute yellow oleander (Thevetia peruvica) poisoning: Cardiac arrhythmias, electrolyte disturbances, and serum cardiac glycoside concentrations on presentation to hospital. Heart 2000;83:310–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dasgupta A, Datta P. Rapid detection of oleander poisoning by using digoxin immunoassays: Comparison of five assays. Ther Drug Monit 2004;26:658–663. [DOI] [PubMed] [Google Scholar]
- 14. Jortani SA, Helm R, Valdes R. Inhibition of Na,K‐ATPase by oleandrin and oleandrigenin and their detection by digoxin immunoassays. Clin Chem 1996;42:1654–1658. [PubMed] [Google Scholar]
- 15. Miller JJ, Valdes R. Methods for calculating cross reactivity in immunoassays. J Clin Immunoassay 1992;15:97–100. [Google Scholar]
- 16. Soto‐Blanco B, Fontenele‐Neto JD, Silva DM, et al. Acute cattle intoxication from Nerium oleander pods. Trop Anim Health Prob 2006;38:451–454. [DOI] [PubMed] [Google Scholar]
- 17. Eddleston M, Gunnell D, Karunaratne A, et al. Epidemiology of intentional poisoning in rural Sri Lanka. Br J Psychiatry 2005;187:583–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamouda C, Amamou M, Thabet H, et al. Plant poisoning from herbal medication admitted to Tunisian toxicology intensive care unit 1983–1998. Vet Hum Toxicol 2000;42:137–141. [PubMed] [Google Scholar]
- 19. Roberts DM, Southcott E, Potter JM, et al. Pharmacokinetics of digoxin cross‐reacting substances in patients with acute yellow oleander (Thevetia peruviana) poisoning including the effect of activated charcoal. Ther Drug Monit 2006;29:784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]


