Abstract
Background: Inhibition studies on PON1 as an organophosphate‐hydrolyzing and atheroprotective enzyme could be useful in elucidating the function of PON1. This study is aimed at examining the in vitro effects of the flavonoid naringenin on PON1 activity in human serum and purified enzyme. Methods: The inhibition kinetics of the interaction of naringenin with human PON1 in serum and purified enzyme was determined spectrophotometrically using paraoxon and phenylacetate as the substrates. Results: Naringenin could be introduced as an effective inhibitor on purified human PON1 activity for phenylacetate as the substrate with an IC50 value of 10 µM. Paraoxonase and arylesterase activities of PON1, in the serum assay, were also inhibited by naringenin with IC50 values of 37.9 and 34.6 µM, respectively. PON1, according to acompetitive‐type inhibition pattern, was inhibited by naringenin with K i constant of 14.5 µM for serum paraoxonase activity. The results were compared with a known inhibitor of PON1, 2‐hydroxyquinoline. We believe (to our knowledge) that this is the first reported study for kinetic parameters of PON1 inhibition by naringenin. Conclusions: Lipophilic property appears to be an important feature of the structure in evaluating the inhibitor potential. Comparison of our findings and other authors showed that the induction of PON1 gene by naringenin and its inhibitory effects on the enzyme protein are probably two different mechanisms by which the flavonoid affects PON1. The in vitro data reported in this study could be useful in the development of structure–activity relationship for PON1 inhibition. J. Clin. Lab. Anal. 25:395–401, 2011. © 2011 Wiley Periodicals, Inc.
Keywords: serum paraoxonase, flavonoids, naringenin, inhibition, kinetic parameters
Introduction
Serum paraoxonase (PON1, EC 3.1.8.1) is a serum enzyme belonging to the class of esterases 1, and it is physically bound to high‐density lipoprotein, HDL 2, 3. The enzyme's name derives from its ability to hydrolyze paraoxon, an organophosphate that is metabolically produced from the insecticide parathion 4. Paraoxon and phenylacetate as the substrates were routinely used for evaluating PON1 activity. There is another form of the paraoxonase enzyme in serum that is paraoxonase 3, PON3 5. PON3 has almost no paraoxonase activity with paraoxon as the substrate 2. Also, PON3 exhibit very limited arylesterase activity with phenylacetate as the substrate 2. Some studies suggested that the role of PON3 may be different from PON1 2. In addition, serum human PON3 is less abundant than PON1 3. PON1 is one of the main enzymes of HDL that may account in part for an important part of the antioxidative properties of HDL 3, 4. Studies have indicated that HDL protects low‐density lipoprotein (LDL) and cell membranes against oxidative damage 4. It has been shown that oxidized LDL plays an important role in the development of atherosclerotic lesions and HDL can protect LDL from oxidation and therefore prevent the production of the oxidized LDL. This protective effect of HDL could be attributed to the enzymes associated with HDL including PON1 4, 5. Other physiological roles have been reported for PON1 including hydrolysis and inactivation of homocysteine thiolactone, a toxic metabolite of homocysteine 6. The role of this enzyme is also showed in the activation and inactivation of specific drugs 7. In addition, PON1 has been found to play a role in other diseases including diabetes, familial hypercholesterolemia, chronic renal failure, obesity, metabolic syndrome, and sepsis 3, 6, 8, 9.
Flavonoids are a large family of polyphenols, which are widely found in vegetables and fruits and make a part of the human diet 10, 11. Several studies have indicated that increased consumption of flavonoid‐rich foods lead to a lower risk of developing cardiovascular disease, stroke, some forms of cancer, and other chronic diseases 10, 11, 12, 13, 14, 15. Increased oxidative stress is related to these diseases 11. Dietary flavonoids exert their functions by antioxidant mechanisms and are able to scavenge free radicals including hydroxyl, peroxyl, and superoxide 11, 16. Naringenin is one of the major citrus flavanones, a subclass of flavonoids 17. This flavonoid has some antioxidant activities and other effects such as inhibition of some enzymes like hydroxy methylglutaryl‐coenzyme A reductase, HMG‐COA reductase 17. Involvement of naringenin in several biological activities, such as antiradical, antilipoperoxidation, and anti‐inflammatory, has been proven 18.
The relationship between paraoxonase enzyme and various flavonoids has been examined in several studies 16, 19, 20, 21, 22. In some the studies, the association between flavonoid‐containing foods and paraoxonase activity has been investigated in vivo 16, 19, 20. Others have focused on the effects of flavonoids on PON1 gene expression 21, 22. In general, in vitro studies about the relationship between serum paraoxonase and flavonoids including naringenin are limited and also in reported studies on the relationship between paraoxonase enzyme and flavonoids including naringenin as an important flavonoid, the effects of flavonoids on the enzyme itself have not been considered. Also, PON1 is a potential therapeutic target because it plays a role as an antiatherosclerotic and detoxifying enzyme 5, 8. In addition, there are limited data about activators and inhibitors of PON1. Therefore, this study was conducted to examine the in vitro effects of the flavonoid naringenin on human serum paraoxonase activity.
MATERIALS AND METHODS
Conditions of In Vitro Incubation
Incubation mixture consisted of human serum or purified human PON1 (ZeptoMetrix Corporation, Buffalo, NY) and naringenin (Sigma‐Aldrich, Germany) in 0.1 M Tris/HCl buffer, pH 8.0, containing 2 mM paraoxon (O,O‐diethyl‐O‐p‐nitrophenylphosphate; Sigma‐Aldrich) and 2 mM CaCl2 for the assay of paraoxonase activity and 1 mM phenylacetate (Fluka, Germany) and 1 mM CaCl2 for the determination of arylesterase activity. The same conditions were prepared with 2‐hydroxyquinoline (2‐HQ; Sigma‐Aldrich) to validate the assay of PON1 activity in serum. Concentrations of naringenin or 2‐HQ were added as methanol stocks to a final solvent concentration of 0.2% (v/v). The assays were run at a constant 0.2% methanol at all concentrations. In other words, all assays were set up to have a constant concentration of methanol, i.e. 0.2% in the assay cuvette. The incubation volume was 1 ml for the assay of paraoxonase activity and 3 ml for the determination of arylesterase activity.
PON1 Activity Assay in Serum
PON1 activity toward paraoxon was determined by the addition of serum to Tris/HCL buffer (0.1 M, pH 8.0) containing paraoxon (2 mM) and CaCl2 (2 mM). The rate of hydrolysis of paraoxon was measured spectrophotometrically (UV 1250, Shimadzu, Japan) by monitoring p‐nitrophenol libration at 412 nm. The enzyme activity was expressed in U/ml serum; 1 U of the paraoxonase activity produces 1 nmol of p‐nitrophenol per min 23, 24. Arylesterase activity was measured using phenylacetate as the substrate 7. Serum was added to the reaction mixture containing 1 mM phenylacetate and 1 mM CaCl2 in Tris/HCL buffer (0.1 M, pH 8.0). The increase in absorbance at 270 nm was recorded after the addition of serum. 1 U of arylesterase activity is defined as 1 µmol phenylacetate hydrolyzed/min/ml 7.
Assay of Purified PON1 Activity
The inhibitory effects of naringenin were examined on purified PON1 to establish and reveal that it works as an inhibitor on PON1 both in the serum and as a purified enzyme. Purified human PON1 was purchased from ZeptoMetrix Corporation (Cat. NO. 0801384). The assay of purified PON1 activity was performed with phenylacetate as the substrate similar to the measurement of PON1 activity in serum except purified PON1 used in the assay instead of serum.
In Vitro Inhibition Studies
The inhibition studies were performed by adding naringenin or 2‐HQ to the incubation mixture. Activity values of PON1 for three different concentrations of naringenin were measured by regression analysis using Microsoft Office 2003 Excel. PON1 activity without a concentration of naringenin was accepted as 100% activity. IC50 (the concentration at which 50% inhibition was observed) values were determined by activity % values of PON1 for three different concentrations of naringenin or 2‐HQ as the inhibitor. In the serum assay, in order to calculate K m and V max values of PON1 for paraoxon as the substrate the Lineweaver–Burk graph was plotted using six different substrate concentrations of paraoxon (0.25, 0.5, 0.8, 1, 2, and 4 µM). The K i value for naringenin was calculated by secondary plot of slope from the Lineweaver–Burk graph.
RESULTS
In this study, in vitro effects of naringenin were examined on PON1 activity in serum and purified enzyme. It was pleasing to detect PON1 activity in its native environment, thus experiments were performed in human serum as a source of native enzyme. To test whether naringenin had a direct effect on PON1, the compound was examined for inhibitory activity using the purified enzyme, instead of serum, in a similar manner. Paraoxon was used as the substrate for specific detection of PON1 activity and this allowed the evaluation of the enzyme activity in the presence of other serum enzymes. For determining the inhibitory effects of naringenin on arylesterase activity of PON1, phenylacetate was used as the substrate. Using of phenylacetate instead of paraoxon allowed less enzyme to be used in the assay, thus only arylesterase activity was determined for purified PON1.
Control experiments showed that PON1 activity in the serum was stable at room temperature for at least 90 min. Since naringenin to be assayed was dissolved in methanol, inhibitory effects of the solvent on PON1 activity were determined (Fig. 1). Our findings demonstrated that methanol at a concentration of 0.2% had no a significant inhibitory effect on PON1 activity. These results are consistent with a study by Eckerson et al. 25.
Figure 1.
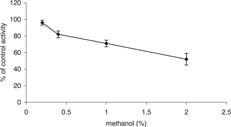
The tolerance of the enzymatic reaction for methanol. Percentage of control activities was determined at the indicated concentrations of methanol. Data points represent mean ± SD for incubations performed in triplicate determination.
2‐HQ was used as a known PON1 inhibitor to validate the assay of PON1 activity in serum. 2‐HQ was a potent inhibitor of PON1 activity. Paraoxonase and arylesterase activities of serum PON1 were inhibited up to 89 and 92%, respectively, by 2‐HQ at a concentration of 5 µM. Arylesterase activity of purified PON1 was also inhibited in the presence of 2‐HQ with IC50 value of <1 µM.
Naringenin had a significant inhibitory effect on PON1 activity. In the serum assay, inhibition type was determined by Lineweaver–Burk graph (Fig. 2). This graph was plotted using six different concentrations of paraoxon as the substrate (0.25, 0.5, 0.8, 1, 2, and 4 µM). The enzyme kinetic parameters were calculated by the graph for paraoxon as follows: K m = 0.9 mM and V max = 143.6 nmol/min/ml. The kinetic data showed that the inhibition of PON1 activity by naringenin was of the competitive type with K i value of 14.5 µM. Our findings showed that paraoxonase and arylesterase activities of serum PON1 were inhibited by the flavonoid at a concentration of 10 µM up to 25 and 32%, respectively. Naringenin had IC50 values in the serum assay of 37.9 and 34.6 µM for paraoxonase and arylesterase activities of PON1, respectively. Purified human PON1 was also inhibited by naringenin. Arylesterase activity of the purified PON1 was inhibited by the flavonoid with an IC50 value of 10 µM. The kinetic data on in vitro inhibition of PON1 by naringenin and 2‐HQ are compared in Table 1.
Figure 2.
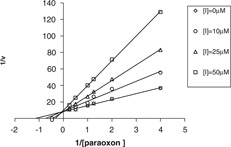
Lineweaver–Burk double‐reciprocal plot for PON1 inhibition in the presence of three different concentrations of naringenin (I). The slope of plots indicates that naringenin is a competitive inhibitor. V values have been expressed in nmol/min/ml and [paraoxon] values are expressed in mM. R 2 for [I] = 0, 10, 25, 50 are 0.995, 0.996, 0.997, and 0.999, respectively.
Table 1.
Comparison of the IC50 Values and % Inhibition of PON1 Activity by Naringenin and 2‐HQ
| Serum PON1 IC50 (µM) | % Inhibition of serum PON1d | |||||
|---|---|---|---|---|---|---|
| Name of compound | Paraoxona | Phenylacetateb | Purified PON1 IC50 (µM)c | Paraoxona | Phenylacetateb | % Inhibition of purified PON1d |
| Naringenin | 37.9 | 34.6 | 10 | 25 | 32 | 50 |
| 2‐HQ | 1.8 | 1.4 | <1 | 89 | 92 | 94 |
2‐HQ, 2‐hydroxyquinoline.
aFor the assay of paraoxonase activity.
bFor the assay of arylesterase activity.
cIC50 values were determined by arylesterase activity.
dNaringenin and 2‐HQ were used at concentrations of 10 and 5 µM, respectively.
DISCUSSION
PON1 is an atheroprotective enzyme and also plays a role as a detoxifying enzyme in the inactivation of some organophosphate toxins 5. Therefore, a better understanding of PON1 modulators could be useful in finding potential therapeutic strategies 5, 8. In addition, potent compounds that affect PON1 activity in serum and as purified enzyme could be helpful research tools for different studies 26. PON1 is a potential therapeutic target for preventing cardiovascular disease 26, 27 and consumption of flavonoids is also related to lower incidence of heart disease 11, thus it seems to be important to understand various aspects of the relationship between the enzyme and flavonoids. Although the relationship between flavonoid‐rich foods and PON1 activity in vivo and also effects of flavonoids on PON1 gene expression have been reported in some studies, to our knowledge in vitro effects of flavonoids on the enzyme itself have not been examined. In this report, we focused on this aspect of the topic and the potential effects of the flavonoid naringenin on PON1 activity in human serum and purified enzyme were determined. In first, we performed a screening assay in serum to evaluate the effects of 11 flavonoids, including naringenin, myricetin, catechin, epicathchin, morin, daidzein, genistein, quercetin, hesperetin, hesperidin, and kampferol, on serum PON1 activity. Since the flavonoids except naringenin did not show a significant inhibitory effect on PON1 activity (data not shown), naringenin was selected to continue the inhibition studies.
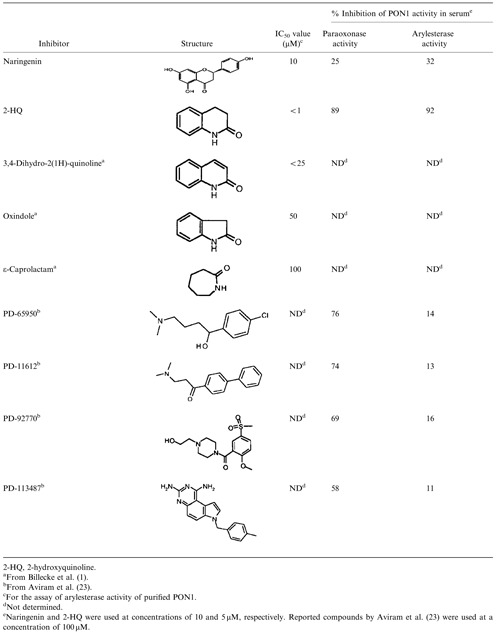
In this study, naringenin had a significant inhibitory effect on human serum paraoxonase activity. Table 2 shows the comparison of the IC50 values, % inhibition, and the structure of naringenin and 2‐HQ with some PON1 inhibitors that have been studied by Billecke et al. 1 and Aviram et al. 23. Compounds represented in this table are structurally unrelated. These compounds cannot be classified as analogs of known synthetic substrates for PON1, paraoxon, and phenylacetate. However, lipophilic property appears to be an important feature of the structure in evaluating inhibitor potential. Bargota et al. indicated that the aromatic nature of amino acids in active site of PON1 could describe why the enzyme prefers lipophilic substrates 28. Also, Josse et al. 29 demonstrated that the hydrophobic or aromatic feature of the amino acid in the PON1 active site is essential for serum paraoxonase activity.
Table 2.
Comparison of the IC50 Values, % Inhibition and the Structure of Naringenin With Some PON1 Inhibitors
|
Findings of Aviram et al. 23 showed that paraoxonase activity of serum PON1 was inhibited up to 58% by PD‐113487 and up to 76% by PD‐65950 (the most potent inhibitor) at a concentration of 100 µM. The compounds used in the study of the investigators had the inhibitory effects on paraoxonase activity of serum PON1 but had no a significant inhibitory effect (14% for PD‐65950) on arylesterase activity of the enzyme even at a high concentration, 100 µM. In this study, naringenin inhibited paraoxonase and arylesterase activities of serum PON1 up to 25 and 32%, respectively, at a concentration of 10 µM, i.e. 10‐fold less than the concentration used by Aviram et al. 23. Arylesterase activity of purified PON1 was inhibited by naringenin up to 50% at a concentration of 10 µM (IC50 value = 10 µM). In addition, in the serum assay, the IC50 values obtained for paraoxon (37.9 µM) and phenylacetate (34.6 µM) as the substrates are better than the reported IC50 values by Billecke et al. 1 for compounds such as oxindole (IC50 value = 50 µM) and ε‐caprolactam (IC50 value = 100 µM). Also, it should be noted that the reported inhibitors by Billecke et al. 1 and Aviram et al. 23 are synthetic compounds, but the introduced inhibitor in this study, naringenin, is a flavonoid found at high concentrations in citrus fruits 17.
The relationship between flavonoids, paraoxonase enzyme, and oxidative status has been investigated by other authors. Ustundag et al. 20 showed that soy isoflavones (genistein, daidzein and glycitin) stimulate paraoxonase activity in rats. However, in that study, effects of the isoflavones have been related to their powerful antioxidant characteristics 20. Ali et al. 30 showed that various doses of naringin can reduce hydrogen peroxide and lipid peroxidation and increase activity of antioxidant enzymes such as PON. However, the authors expressed that the mechanism of naringin effect is not obviously distinguished 30. Jeon et al. indicated that naringin increases the mRNA expression of antioxidant enzymes such as superoxide dismutase and catalase and reduces the hepatic mitochondrial H2O2 content 31. In a study by Lee et al. 32, naringenin enhanced the antioxidant capacity of erythrocytes and liver. Effects of flavonoids on the gene expression of PON1 have been reported in some other studies. Gong et al. 21 suggested that quercetin has antiatherogenic property by upregulating the gene expression of PON1. Gouedard et al. 22 evaluated directly the effects of naringenin on PON1 gene expression. These investigators indicated that PON1 mRNA increases following treatment with naringenin in human hepatoma cell line 22. It may be concluded from our findings and the reported results by Gouedard et al. that naringenin has an affect on PON1 by increasing gene expression, but its molecular structure can act as an inhibitor of the enzyme protein. Therefore, induction of PON1 gene by naringenin and its inhibitory effects on the enzyme protein are probably two different mechanisms by which the flavonoid affects PON1. However, additional studies are required to clarify in vivo effects of naringenin on PON1 gene expression and the enzyme protein. It should be noted that inhibition of PON1 by the flavonoid naringenin is not surprising because the previous studies have been indicated that flavonoids could inhibit various enzymes like hydrolases 33.
The data about bioavailability of flavonoids are limited 17. Bioavailability differs greatly for various flavonoids 34. Some studies have shown that plasma concentrations of flavonoids reach 15 µM after uptake of 400–760 ml of orange juice or grapefruit juice 17. The result is not surprising, because citrus fruits and juices contain high concentrations of the compounds, several hundred milligrams per liter 17. Also, Manach et al. 34 reported that plasma concentrations of naringenin approximately reach 6 µM with only 200 mg ingested as grapefruit juice. These are in part comparable to concentrations of naringenin used in this study. In addition, the greater intake of naringenin‐rich sources can increase its plasma concentrations. It has also been indicated that naringenin is bioavailable from some sources such as tomato paste notably used 17.
It may be concluded from this study that naringenin could be introduced as an effective inhibitor on paraoxonase and arylesterase activities of PON1 in vitro. This enzyme was inhibited by naringenin in a competitive‐type inhibition pattern. Comparison of our findings and other authors showed that naringenin increases PON1 gene expression, but it is an inhibitor of the enzyme protein in vitro. The in vitro data reported in this study could be useful in the development of structure–activity relationship for serum paraoxonase inhibition. It remains to be investigated whether naringenin as PON1 inhibitor using in vitro conditions will be the enzyme inhibitor in vivo. Additional researches are required to obtain more detailed biochemical knowledge on PON1 interrelations with flavonoids.
REFERENCES
- 1. Billecke S, Draganov D, Counsell R, et al. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab Dispos 2000;28:1335–1342. [PubMed] [Google Scholar]
- 2. Ng CJ, Shih DM, Hama SY, et al. The paraoxonase gene family and atherosclerosis. Free Radic Biol Med 2005;38:153–163. [DOI] [PubMed] [Google Scholar]
- 3. Draganov DI, La Du BN. Pharmacogenetics of paraoxonases: A brief review. Naunyn‐Schmiedeberg's Arch Pharmacol 2004;369:78–88. [DOI] [PubMed] [Google Scholar]
- 4. Mackness MI, Mackness B, Durrington PN. Paraoxonase and coronary heart disease. Atheroscler Suppl 2002;3:49–55. [DOI] [PubMed] [Google Scholar]
- 5. Harel M, Aharoni A, Gaidukov L, et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti‐atherosclerotic enzymes. Nat Struct Mol Biol 2004;11:412–419. [DOI] [PubMed] [Google Scholar]
- 6. Goswami B, Tayal D, Gupta N, Mallika V. Paraoxonase: A multifaceted biomolecule. Clin Chim Acta 2009;410:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicol Appl Pharmacol 2009;235:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aviram M, Rosenblat M. Paraoxonases and cardiovascular diseases: Pharmacological and nutritional influences. Curr Opin Lipidol 2005;16:393–399. [DOI] [PubMed] [Google Scholar]
- 9. Camps J, Marsillach J, Joven J. The paraoxonases: Role in human diseases and methodological difficulties in measurement. Crit Rev Clin Lab Sci 2009;46:83–106. [DOI] [PubMed] [Google Scholar]
- 10. Azuma K, Ippoushi K, Ito H, Horie H, Terao J. Enhancing effect of lipids and emulsifiers on the accumulation of quercetin metabolites in blood plasma after the short‐term ingestion of onion by rats. Biosci Biotechnol Biochem 2003;67:2548–2555. [DOI] [PubMed] [Google Scholar]
- 11. Lotito SB, Frei B. Consumption of flavonoid‐rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic Biol Med 2006;41:1727–1746. [DOI] [PubMed] [Google Scholar]
- 12. Vaya J, Mahmood S, Goldblum A, et al. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry 2003;62:89–99. [DOI] [PubMed] [Google Scholar]
- 13. Pietinen P, Rimm EB, Korhonen P, et al. Intake of dietary fiber and risk of coronary heart disease in a cohort of Finnish men. The alpha‐tocopherol, beta carotene cancer prevention study. Circulation 1996;94:2720–2727. [DOI] [PubMed] [Google Scholar]
- 14. Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. J Am Med Assoc 1996;275:447–451. [DOI] [PubMed] [Google Scholar]
- 15. Lloyd T, Chinehilli VM, Rollings N, et al. Fruit consumption, fitness, and cardiovascular health in female adolescents: The Penn State Young Women's Health Study. Am J Clin Nutr 1998;67:624–630. [DOI] [PubMed] [Google Scholar]
- 16. Fuhrman B, Aviram M. Preservation of paraoxonase activity by wine flavonoids: Possible role in protection of LDL from lipid peroxidation. Ann N Y Acad Sci 2002;957:321–324. [DOI] [PubMed] [Google Scholar]
- 17. Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res 2004;24:851–874. [Google Scholar]
- 18. Amaro MI, Rocha J, Vila‐Real H, et al. Anti‐inflammatory activity of naringin and the biosynthesized naringenin by naringinase immobilized in microstructured materials in a model of DSS‐induced colitis in mice. Food Res Int 2009;42:1010–1017. [Google Scholar]
- 19. Kleemola P, Freese R, Jauhiainen M, Pahlman R, Alfthan G, Mutanen M. Dietary determinants of serum paraoxonase activity in healthy humans. Atherosclerosis 2002;160:425–432. [DOI] [PubMed] [Google Scholar]
- 20. Ustundag B, Bahcecioglu IH, Sahin K, et al. Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steatohepatitis model. Dig Dis Sci 2007;52:2006–2014. [DOI] [PubMed] [Google Scholar]
- 21. Gong M, Garige M, Varatharajalu R, et al. Quercetin up‐regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem Biophys Res Commun 2009;379:1001–1004. [DOI] [PubMed] [Google Scholar]
- 22. Gouedard C, Barouki R, Morel Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor‐dependent mechanism. Mol Cell Biol 2004;24:5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo‐Parmo SL, La Du BN. Paraoxonase inhibits high‐density lipoprotein oxidation and preserves its functions: A possible peroxidative role for paraoxonase. J Clin Invest 1998;101:1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kural BV, Orem C, Uydu HA, Alver A, Orem A. The effects of lipid‐lowering therapy on paraoxonase activities and their relationships with the oxidant‐antioxidant system in patients with dyslipidemia. Coron Artery Dis 2004;15:277–283. [DOI] [PubMed] [Google Scholar]
- 25. Eckerson HW, Romson J, Wyte C, La Du BN. The human serum paraoxonase polymorphism: Identification of phenotypes by their response to salts. Am J Hum Genet 1983;35:214–227. [PMC free article] [PubMed] [Google Scholar]
- 26. Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomics of the paraoxonase (PON1) polymorphisms: Effects of on Pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med 2003;54:371–392. [DOI] [PubMed] [Google Scholar]
- 27. Durrington PN, Mackness B, Mackness MI. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler Thromb Vasc Biol 2002;22:1248–1250. [DOI] [PubMed] [Google Scholar]
- 28. Bargota RS, Akhtar M, Biggadike K, Gani D, Allemann RK. Structure‐activity relationship on human serum paraoxonase (PON1) using substrate analogues and inhibitors. Bioorg Med Chem Lett 2003;13:1623–1626. [DOI] [PubMed] [Google Scholar]
- 29. Josse D, Xie W, Masson P, et al. Tryptophan residue(s) as major components of the human serum paraoxonase active site. Chem Biol Interact 1999;119–120:79–84. [DOI] [PubMed] [Google Scholar]
- 30. Ali MM, Abdelkader MA. The influences of naringin on the oxidative state of rats with streptozotocin‐induced acute hyperglycaemia. Z Naturforsch 2004;59c:726–733. [DOI] [PubMed] [Google Scholar]
- 31. Joen S‐M, Bok S‐H, Jang M‐K, et al. Comparison of antioxidant effects of naringin and probucol in cholesterol‐fed rabbits. Clin Chim Acta 2002;317:181–190. [DOI] [PubMed] [Google Scholar]
- 32. Lee M‐K, Bok S‐H, Jeong T‐S, et al. Supplementation of naringenin and its synthetic derivative alters antioxidant enzyme activities of erythrocyte and liver in high cholesterol‐fed rats. Bioorg Med Chem 2002;10:2239–2244. [DOI] [PubMed] [Google Scholar]
- 33. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther 2002;96:67–202. [DOI] [PubMed] [Google Scholar]
- 34. Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81:230S–242S. [DOI] [PubMed] [Google Scholar]