Abstract
The association of celiac disease with type 1 diabetes mellitus is known, but the evolution of celiac disease is most frequently asymptomatic, without any clinical signs. Thus, diagnosis is impossible to make in the absence of serological tests. Our study aimed to determine the prevalence and the efficiency of IgA antitissue transglutaminase antibodies in the screening of celiac disease in children with type 1 diabetes mellitus. Method: During the course of 2008–2009, we performed an analytical clinical study that included the determination of IgA antitissue transglutaminase antibodies in a group of 119 children with type 1 diabetesmellitus. Fifty‐seven percent of the subjects were male and 43% were female, with a mean age of 11±4 years. Results: By evaluating IgA antitissue transglutaminase antibodies, we obtained a prevalence of 9.2% in children with type 1 diabetes mellitus, with a sensitivity and specificity of 80 and 82.6%, respectively. Conclusions: There is an increased prevalence of IgA antitissue transglutaminase antibodies, which suggests the need to use this method as an effective first‐line test in the screening of celiac disease in children with type 1 diabetes mellitus. J. Clin. Lab. Anal. 25:156–161, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: type 1 diabetes mellitus, celiac disease, IgA antitissue transglutaminase antibodies
INTRODUCTION
Celiac disease or gluten enteropathy is characterized by an abnormality of cell immunity at the level of the small intestine, caused by a diet of protein and gluten, which is found particularly in wheat, rye, and barley. Paraclinical diagnosis is classically based on specific histological changes: villous atrophy and jejunal crypt hyperplasia, which makes diagnosis difficult because of the long duration required for the release of results and the discomfort in using this method in children. Today, owing to the screening tests available, an increasingly greater number of celiac disease cases are diagnosed.
However, the clinical symptoms of gluten enteropathy can be interpreted as derived from other autoimmune diseases, so the diagnosis of celiac disease and the introduction of a gluten‐free diet, which improves the patient's quality of life 1, may be delayed. It is known that patients with type 1 diabetes mellitus are more prone to celiac disease 2. The association of type 1 diabetes mellitus with celiac disease seems to pose many diagnostic, therapeutic, and psychological problems 3, as patients need a diet that controls both diseases. Children with type 1 diabetes mellitus and celiac disease have a higher frequency of gastrointestinal symptoms than children with diabetes mellitus and negative serology for celiac disease 4. Type 1 diabetes mellitus and celiac disease are examples of health problems in childhood, with an increased risk for other associated diseases that occur later in life 5. Because patients with untreated celiac disease can develop severe complications, the early diagnosis of celiac disease in groups at risk, and particularly in those with type 1 diabetes mellitus is extremely important, consequently the importance of serological tests increases 6.
Aim of the Article
The main objective of this study was to evaluate the serological immunoenzymatic tests used in the screening of celiac disease in patients with type 1 diabetes mellitus, i.e., IgA antitissue transglutaminase antibodies (TgA‐IgA), IgA+IgG antideamidated gliadin peptide antibodies (DGP‐IgA), IgA antigliadin antibodies (AGA‐IgA), and IgG antigliadin antibodies (AGA‐IgG), using indirect immunofluorescence for the detection of IgA antiendomysium antibodies (EmA‐IgA) as the gold standard. The secondary objectives of the study were to establish correlations between the positive TgA‐IgA values and the age of subjects, as well as to evaluate TgA‐IgA differences between patients with diabetes mellitus without complications and with other manifestations. The study also aimed to analyze the differences in glycosylated hemoglobin (HbA1c) between patients with negative TgA‐IgA values, with TgA‐IgA values lower than 100 U/ml, and patients with TgA‐IgA values higher than 100 U/ml.
MATERIAL AND METHOD
Patients
The study group comprised 119 children with type 1 diabetes mellitus that were included in an analytical clinical study in the period 2008–2009, which involved serological screening tests specific for celiac disease. The sex distribution of the group was: 57% boys and 43% girls. We mention that for economic efficiency, the screening of celiac disease was performed with TgA‐IgA, the dosage of EmA‐IgA being only initiated in suspect cases, whereas the other serological tests, DGP‐IgA+IgG, AGA‐IgA, AGA‐IgG, were only carried out in patients positive for one or both tests.
The patients were tested at the Clinical Laboratory of Immunology and Gastroenterology of the Regional Center for the Management of Celiac Disease Cluj, organized within the structure of the Clinical Emergency Pediatric Hospital Cluj‐Napoca, Clinic of Pediatrics II, for Cluj, Bistriţa‐Năsăud, Maramureş, Suceava, Alba, Sălaj, Mureş counties (Table 1).
Table 1.
Geographical Distribution of Patients
| County | Absolute frequency | Relative frequency (%) | Cumulative relative frequency upward |
|---|---|---|---|
| Alba | 9 | 7.56 | 7.56 |
| Bihor | 1 | 0.84 | 8.40 |
| Bistriţa | 23 | 19.32 | 27.72 |
| Cluj | 59 | 49.57 | 77.29 |
| Galaţi | 1 | 0.84 | 78.13 |
| Iaşi | 1 | 0.84 | 78.97 |
| Maramureş | 14 | 11.76 | 90.73 |
| Mureş | 2 | 1.68 | 92.41 |
| Sălaj | 6 | 5.04 | 97.45 |
| Suceava | 3 | 2.55 | 100 |
| Total | 119 | 100 |
The clinical characteristics of our group mostly included not only patients without complications, but also patients with other manifestations (Table 2).
Table 2.
Clinical Characteristics of Patients
| Number of patients | |
|---|---|
| Type 1 diabetes wits ketoacidosis, without coma | 28 |
| Type 1 diabetes wits ketoacidosis, with lactic acidosis, without coma | 2 |
| Type 1 diabetes with poor control | 6 |
| Type 1 diabetes with unspecified complications | 2 |
| Type 1 diabetes without complications | 78 |
| Type 1 diabetes with background retinopathy | 1 |
| Type 1 diabetes with diabetic neuropathy | 1 |
| Type 1 diabetes with hypoglycemia | 1 |
| Total patients | 119 |
Type 1 insulin‐dependent diabetes mellitus is characterized by severe insulin deficiency, exogenous insulin being necessary for survival. It is the form of diabetes mellitus characteristic of the child. The diagnosis of type 1 diabetes mellitus in the studied group was based on the presence of constantly increased fasting glycemia values, higher than 110 mg%.
Method
The tests were performed using in vitro diagnostic kits, produced by Inova Diagnostics Inc. for the determination of TgA‐IgA, DGP‐IgA+IgG, AGA‐IgG, AGA‐IgA and EmA‐IgA in the serum, and Thermo Scientific (Finland) for the determination of HbA1c in whole blood.
Immunoenzymatic ELISA tests were carried out with the automated analyzer ChemWell 2910 Awareness Technology Inc., and indirect immunofluorescence tests were read with the Olympus CX31 fluorescence microscope (Japan). The determination of HbA1c was performed by immunoenzymatic turbidimetry on the Konelab 60i analyzer (Finland).
Statistical Analysis
Statistical analysis was performed using the SPSS software, version 16.0, and the data were collected and analyzed using Microsoft Excel, starting from contingency tables for the evaluation of a diagnostic procedure. The quality of the tests was assessed by calculating the following statistical indices: sensitivity (Se), specificity (Sp), false negative rate (FNR), false positive rate (RFP), Youden index (Y). The positive predictive value (PPV) and negative predictive value (NPV) were also calculated.
The study of the association between the serological tests was performed using Fisher's exact test. The differences in TgA‐IgA values between the groups of patients with diabetes mellitus without complications and diabetes mellitus with other manifestations were evaluated by the nonparametric Mann–Whitney test. The differences in HbA1c values between the groups of diabetes patients with vs. without complications were assessed using the parametric Student (t) test with equal variations (Fig. 1). The differences in HbA1c between patients with negative TgA‐IgA values, with TgA‐IgA values lower than 100 U/ml, and patients with TgA‐IgA values higher than 100 U/ml were analyzed using the parametric Brown–Forsythe test. The significance level was 0.05.
Figure 1.
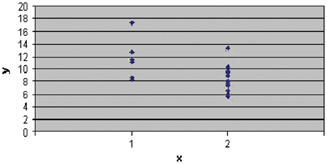
Evolution of HbA1c in patients with diabetes mellitus type I. Legend: x=patients with (1) vs. without complications (2); y=HbA1c values. Note HbA1c values increased in patients with complications (mean HbA1c=11.6) than patients without complications (mean HbA1c=8.12) and statistically significant differences (P=0.01<0.05) between these two groups.
RESULTS
TgA‐IgA Dosage
Global analysis shows us positive values of TgA‐IgA in 11 patients and a prevalence of 9.2% of TgA‐IgA. The analysis of positive TgA‐IgA values indicates a mean of 126.8 U/ml (Table 3) and the distribution of values in relation to age shows a higher frequency of these at the age of 8–10 years (Fig. 2).
Table 3.
TgA‐IgA Dosage
| TgA‐IgA | |
|---|---|
| MEAN case 1–12 | 126.81 |
| MEDIAN case 1–12 | 82 |
| SD case 1–12 | 118.57 |
| VALID_N case 1–12 | 11 |
| SUM case 1–12 | 1,395 |
| MIN case 1–12 | 22 |
| MAX case 1–12 | 390 |
| _25th % case 1–12 | 36.4 |
| _75th % case 1–12 | 168 |
Figure 2.
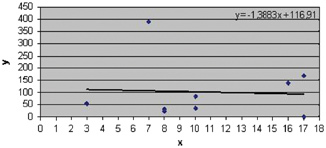
Distribution of positive values of TgA‐IgA relative to age. Legend: x=age (years); y=TgA‐IgA values (U/ml). Note a higher frequency of TgA‐IgA positive values (mean=126.8 U/ml) at the age of 8–10 years.
Evaluation of the Quality of Immunoenzymatic Tests Having IgA Antiendomysium Antibodies as Gold Standard
TgA‐IgA in the screening of celiac disease (Table 4)
Table 4.
Evaluation of TgA‐IgA
| TgA‐IgA | ||||
|---|---|---|---|---|
| EmA‐IgA | Yes | Not | Total | |
| Yes | Number of subjects | 12 | 3 | 15 |
| % of EmA‐IgA | 80 | 20 | 100 | |
| Not | Number of subjects | 9 | 43 | 52 |
| % of EmA‐IgA | 17.31 | 82.69 | 100 | |
| Total | Number of subjects | 21 | 46 | 67 |
| % of EmA‐IgA | 31.34 | 68.66 | 100 | |
On TgA‐IgA dosage, we obtained Se 80% (95% CI 51.9–95.6), PPV 57.1% (95% CI 34–78.1%) and Sp 82.6% (95% CI 69.9–91.7), and NPV 93.5% (95% CI 82.1–98.6). The FPR had a value of 17.4% and the FNR of 20%. The Youden index had a value of 2.62 and the K accuracy index of 8.78.
The association between TgA‐IgA and EmA‐IgA shows that because the significance level of Fisher's exact test (P=0.00<0.05) is statistically significant, the null hypothesis of the independence of the two variables TgA‐IgA and EmA‐IgA is rejected. Thus, there is an association between TgA‐IgA and EmA‐IgA (Table 5).
Table 5.
Association Between TgA‐IgA and EmA‐IgA
| Value | df | Asymp. Sig. (two‐sided) | Exact Sig. (two‐sided) | |
|---|---|---|---|---|
| Pearson chi‐square | 21.26 | 1 | 0.00 | |
| Fisher's exact test | 0.00 | |||
| 1 cells (25.0%) have expected count less than 5. The minimum expected count is 4.70 | ||||
DGP‐IgA+IgG, AGA‐IgA, AGA‐IgG in patients positive for TgA‐IgA (Tables 6, 7, 8)
Table 6.
Evaluation of DGP‐IgA+IgG
| DGP‐IgA+IgG | ||||
|---|---|---|---|---|
| EmA‐IgA | Yes | Not | Total | |
| Yes | Number of subjects | 2 | 3 | 5 |
| % of EmA‐IgA | 40 | 60 | 100 | |
| Not | Number of subjects | 1 | 3 | 4 |
| % of EmA‐IgA | 25 | 75 | 100 | |
| Total | Number of subjects | 3 | 6 | 9 |
| % of EmA‐IgA | 33.33 | 66.67 | 100 | |
Table 7.
Evaluation of AGA‐IgA
| AGA‐IgA | ||||
|---|---|---|---|---|
| EmA‐IgA | Yes | Not | Total | |
| Yes | Number of subjects | 3 | 1 | 4 |
| % of EmA‐IgA | 75 | 25 | 100 | |
| Not | Number of subjects | 2 | 1 | 3 |
| % of EmA‐IgA | 66.67 | 33.33 | 100 | |
| Total | Number of subjects | 5 | 2 | 7 |
| % of EmA‐IgA | 71.43 | 28.57 | 100 | |
Table 8.
Evaluation of AGA‐IgG
| AGA‐IgG | ||||
|---|---|---|---|---|
| EmA‐IgA | Yes | Not | Total | |
| Yes | Number of subjects | 3 | 1 | 4 |
| % of EmA‐IgA | 75 | 25 | 100 | |
| Not | Number of subjects | 0 | 3 | 3 |
| % of EmA‐IgA | 0 | 100 | 100 | |
| Total | Number of subjects | 3 | 4 | 7 |
| % of EmA‐IgA | 42.86 | 57.14 | 100 | |
On DGP‐IgA+IgG dosage, we obtained Se 40% (95% CI 5.27–85.3), PPV 67.6% (95% CI 9.43–95.1) and Sp 75% (95% CI 19.4–99.3), and NPV 50% (95% CI 11.8–88.1). FPR had a value of 25% and FNR of 60%. The Youden index had a value of 2.15 and the K accuracy index of 1.33.
On the AGA‐IgA dosage, we obtained Se 75% (95% CI 19.4–99.3), PPV 60% (95% CI 14.6–94.7) and Sp 33.3% (95% CI 0.84–90.5), and NPV 50% (95% CI 1.26–98.7). FPR had a value of 66.7% and FNR of 25%. The Youden index had a value of 2.08 and the K accuracy index of 1.02.
On the AGA‐IgG dosage, we obtained Se 75% (95% CI 19.4–99.3), PPV 100% (95% CI 29.2) and Sp 100% (95% CI 29.2), and NPV 75% (95% CI 19.4–99.3). FPR had a value of 0% and FNR of 25%. The Youden index had a value of 2.75 and the K accuracy index of 4.
The association between the presented tests and EmA‐IgA shows that because the significance level of Fisher's exact test is statistically insignificant (P>0.05), the null hypothesis of independence of the presented variables and EmA‐IgA cannot be rejected (so there is no association between GPD‐IgA+IgG, AGA‐IgA, AGA‐IgG, and EmA‐IgA).
TgA‐IgA Values in Patients With Type 1 Diabetes Mellitus Without Complications and With Other Manifestations in Relation to HbA1c
The monitoring of TgA‐IgA in dynamics, each child having an average of two determinations (Fig. 3), led to the extension of the database n=172, which allowed to focus on the secondary objectives of the study, i.e., the determination of correlations with the evolution of type 1 diabetes mellitus.
Figure 3.
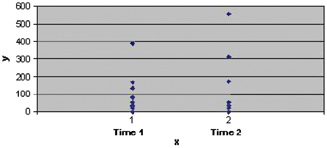
Dynamic of TgA‐IgA levels in patients with type 1 diabetes. Legend: x=pacients at Time 1 (initially, when the patient was diagnosed with celiac disease) and Time 2 (finally, TgA‐IgA first monitoring after starting a gluten‐free diet); y=TgA‐IgA values (U/ml). Note the higher values of TgA‐IgA at a Time 2 (mean=128.9 U/ml) compared with TgA‐IgA values at Time 1 (mean=102.1 U/ml) and statistically significant differences (P=0.049<0.05) between those two times. *We want to mention that two patients had only the first value of TgA‐IgA because they were recently diagnosed; therefore, they are not in this chart.
The global analysis evidenced no statistically significant differences in HbA1c values between patients with negative TgA‐IgA values, with TgA‐IgA values lower than 100 U/ml, and TgA‐IgA values higher than 100 U/ml, P=0.05>0.05, nonparametric Borwn–Forsythe test (mean HbA1c values 8.4 vs. 7.76 vs. 10.4) (Table 9).
Table 9.
Comparison of HbA1c Values With Different Stages of TgA‐IgA
| 95% confidence interval for the average population | |||||||
|---|---|---|---|---|---|---|---|
| HbA1c | Valid N case | Mean case | SD case | Lower limit | Upper limit | MIN case | MAX case |
| TgA‐IgA negative | 149 | 8.41 | 2.04 | 8.08 | 8.74 | 5.1 | 15.4 |
| TgA‐IgA<100 U/ml | 12 | 7.76 | 1.23 | 6.98 | 8.54 | 5.6 | 9.5 |
| TgA‐IgA>100 U/ml | 11 | 10.41 | 3.33 | 8.17 | 12.65 | 6 | 17.4 |
Through the analysis of positive TgA‐IgA values in subjects with type 1 diabetes mellitus without complications and patients with other manifestations of the disease, we accept that there are no statistically significant differences in TgA‐IgA, P=0.36>0.05, nonparametric Mann–Whitney test (mean TgA‐IgA values in subjects with type 1 diabetes mellitus without complications 111.8 vs. 183.5 U/ml in patients with type 1 diabetes mellitus with other manifestations) (Table 10). There are significant differences in HbA1c values between the groups of patients with diabetes mellitus without complications and patients with type 1 diabetes mellitus with other manifestations, P=0.01<0.05, parametric Student (t) test with equal variations (mean HbA1c values in subjects with type 1 diabetes mellitus without complications 8.12 vs. 11.60 U/ml in patients with type 1 diabetes mellitus with other manifestations) (Table 11).
Table 10.
Positive Values of TgA‐IgA in Children With Type 1 Diabetes Mellitus
| TgA‐IgA | N | Mean | Median | SD | SUM | 25th % | 75th % | MIN | MAX |
|---|---|---|---|---|---|---|---|---|---|
| Diabetes without complications | 17 | 111.8 | 56.90 | 113.6 | 1,900.6 | 36.4 | 148 | 22 | 390 |
| Diabetes with other manifestations | 6 | 183.5 | 145 | 192.1 | 1,101.5 | 53.7 | 168 | 30.8 | 559 |
Table 11.
HbA1c Values in Children With Type 1 Diabetes Mellitus
| 95% confidence interval for mean | ||||||||
|---|---|---|---|---|---|---|---|---|
| HbA1c | N | Mean | Median | SD | Lower bound | Upper bound | MIN | MAX |
| Diabetes without complications | 17 | 8.12 | 7.90 | 1.93 | 7.13 | 9.11 | 5.60 | 13.30 |
| Diabetes with other manifestations | 6 | 11.60 | 11.3 | 3.32 | 8.12 | 15.08 | 8.30 | 17.40 |
DISCUSSION
The analysis of the results obtained shows the presence of an association of TgA‐IgA with type 1 diabetes mellitus. The high mean TgA‐IgA value (126.8 U/ml) indicates the presence of an increasing number of chronic forms of type 1 diabetes mellitus associated with celiac disease, with a higher frequency of the distribution of values at the age of 8–10 years. The evaluation of serological tests evidences increased values of statistical TgA‐IgA parameters except for PPV. High AGA‐IgG parameters are found. The monitoring of the evolution of TgA‐IgA in dynamics shows a lower TgA‐IgA value at time one (initially) compared with the TgA‐IgA value at time two (finally), supporting the fact that the association of celiac disease in patients with type 1 diabetes mellitus remains an extremely important health problem.
The analysis of TgA‐IgA values during evolution in relation to HbA1c values does not reveal statistically significant differences, but important differences from the point of view of the interpretation of laboratory data are noted. Patients with negative TgA‐IgA values do not fully comply with the type 1 diabetes mellitus treatment, HbA1c=8.4. In patients with TgA‐IgA <100 U/ml, the decrease in HbA1c=7.75 suggests a possible awareness of the association of celiac disease in children with type 1 diabetes mellitus. In contrast, a parallel evolution of the HbA1c value=10.4 and TgA‐IgA values >100 U/ml is found, which shows an unfavorable evolution, i.e., an unsatisfactory diet control for both diseases. The analysis of positive TgA‐IgA values according to the presentation of type 1 diabetes mellitus reveals statistically and clinically significant differences. Patients without complications do not fully comply with the treatment of diabetes (HbA1c=8.11) and patients are not aware of the importance of the gluten‐free diet either (TgA‐IgA=111.8 U/ml). The same tendency is found in patients with type 1 diabetes mellitus with other manifestations, i.e., an unfavorable parallel evolution between HbA1c=11.6 and TgA‐IgA values=183.5 U/ml is found.
The evaluation of serological tests shows a high accuracy index of TgA‐IgA, which confirms the data regarding their role in the detection of untreated celiac disease 7 and implicitly in the screening of celiac disease in type 1 diabetes mellitus. The low DGP‐IgA+IgG values might be explained by the higher frequency of the distribution of positive values at the age of 8–10 years 8 and the absence of correlation of AGA and EmA values confirms the decreasing interest in them lately, owing to the fact that they also occur in healthy people 9.
Why were TgA‐IgA values lower and higher than 100 U/ml analyzed? The TgA‐IgA reactivity higher than 100 units has always been related to histological changes in intestinal biopsies, to the active phase of celiac disease 10, so that some recent studies recommend not to perform intestinal biopsy in patients with high TgA‐IgA levels 11. In this way, an invasive procedure would be avoided and a more rapid diagnosis and treatment of celiac disease would result 12. This aspect might be applied as a future solution in the management of type 1 diabetes mellitus associated with celiac disease, along with the development of health education programs regarding celiac disease in children with type 1 diabetes mellitus associated with this disease.
CONCLUSIONS
The study shows an increased prevalence, of 9.2%, of IgA antitissue transglutaminase antibodies in patients with type 1 diabetes mellitus, which evidences the need for the continuation and extension of the screening program for celiac disease in all children with type 1 diabetes mellitus. IgA antitissue transglutaminase antibodies are highly specific markers, Sp 82.6% (95% CI 69.9–91.7) and NPV 93.5% (95% CI 82.1–98.6) in the screening of celiac disease, but results must be confirmed by IgA antiendomysium antibodies, owing to the low positive predictive value, PPV 57.1% (95% CI 34–78.1).
The results obtained show that IgA antitissue transglutaminase antibodies are also markers of the unfavorable evolution of the celiac disease—type 1 diabetes mellitus association.
Acknowledgements
In Romania, this is the first study covering the population with diabetes mellitus in the north‐west and central part of our country.
REFERENCES
- 1. Zwolińska‐Wcisło M, Galicka‐Latała D, Rudnicka‐Sosin L, Rozpondek P. Coeliac disease and other autoimmunological disorders coexistence. Przegl Lek 2009;66:370–372. [PubMed] [Google Scholar]
- 2. Simell S, Hoppu S, Simell T, et al. Age at development of type 1 diabetes‐ and celiac disease‐associated antibodies and clinical disease in genetically susceptible children observed from birth. Diabetes Care 2010;33:774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ben Mami F, Ben Ammar I, El Felah B, Achour A. Association of type 1 diabetes and celiac disease: Features of this double pathology. Tunis Med 2010;88:18–22. [PubMed] [Google Scholar]
- 4. Narula P, Porter L, Langton J, et al. Gastrointestinal symptoms in children with type 1 diabetes screened for celiac disease. Pediatrics 2009;124:e489–e495. [DOI] [PubMed] [Google Scholar]
- 5. Dietert RR, Zelikoff JT. Identifying patterns of immune‐related disease: Use in disease prevention and management. World J Pediatr 2010;6:111–118. [DOI] [PubMed] [Google Scholar]
- 6. Vicuña Arregui M, Zozaya Urmeneta JM, Martínez de Esteban JP, et al. Study of celiac disease in adults with type 1 diabetes mellitus. Gastroenterol Hepatol 2010;33:6–11. [DOI] [PubMed] [Google Scholar]
- 7. Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme‐linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998;115:1322–1328. [DOI] [PubMed] [Google Scholar]
- 8. Samaşca G, Iancu M, Butnariu A, et al. New para‐clinical investigations in the celiac disease. Rev Rom Med Lab 2010;18:43–51. [Google Scholar]
- 9. Kaukinen K, Collin P, Laurila K, et al. Resurrection of gliadin antibodies in coeliac disease. Deamidated gliadin peptide antibody test provides additional diagnostic benefit. Scan J Gastroenterol 2007;42:1428–1433. [DOI] [PubMed] [Google Scholar]
- 10. Abrantes‐Lemos CP, Nakhle MC, Damiao AO, et al. Performance of two commercial ELISAs for detecting IgA anti‐human and anti‐guinea pig tissue transglutaminase antibodies. Clin Lab 2010;56:29–35. [PubMed] [Google Scholar]
- 11. Vivas S, Ruiz de Morales JG, Riestra S, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol 2009;15:4775–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill PG, Holmes GK. Coeliac disease: A biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther 2008;27:572–577. [DOI] [PubMed] [Google Scholar]


